Replication-Deficient Lymphocytic Choriomeningitis Virus-Vectored Vaccine Candidate for the Induction of T Cell Immunity against Mycobacterium tuberculosis
Abstract
:1. Introduction
2. Results
2.1. rLCMV Vector Delivering Ag85B-TB10.4 Elicits High Frequencies of Polyfunctional CD8 and CD4 T Cells
2.2. rLCMV-Induced CD8 T Cell Responses Are Augmented by Homologous Boosting
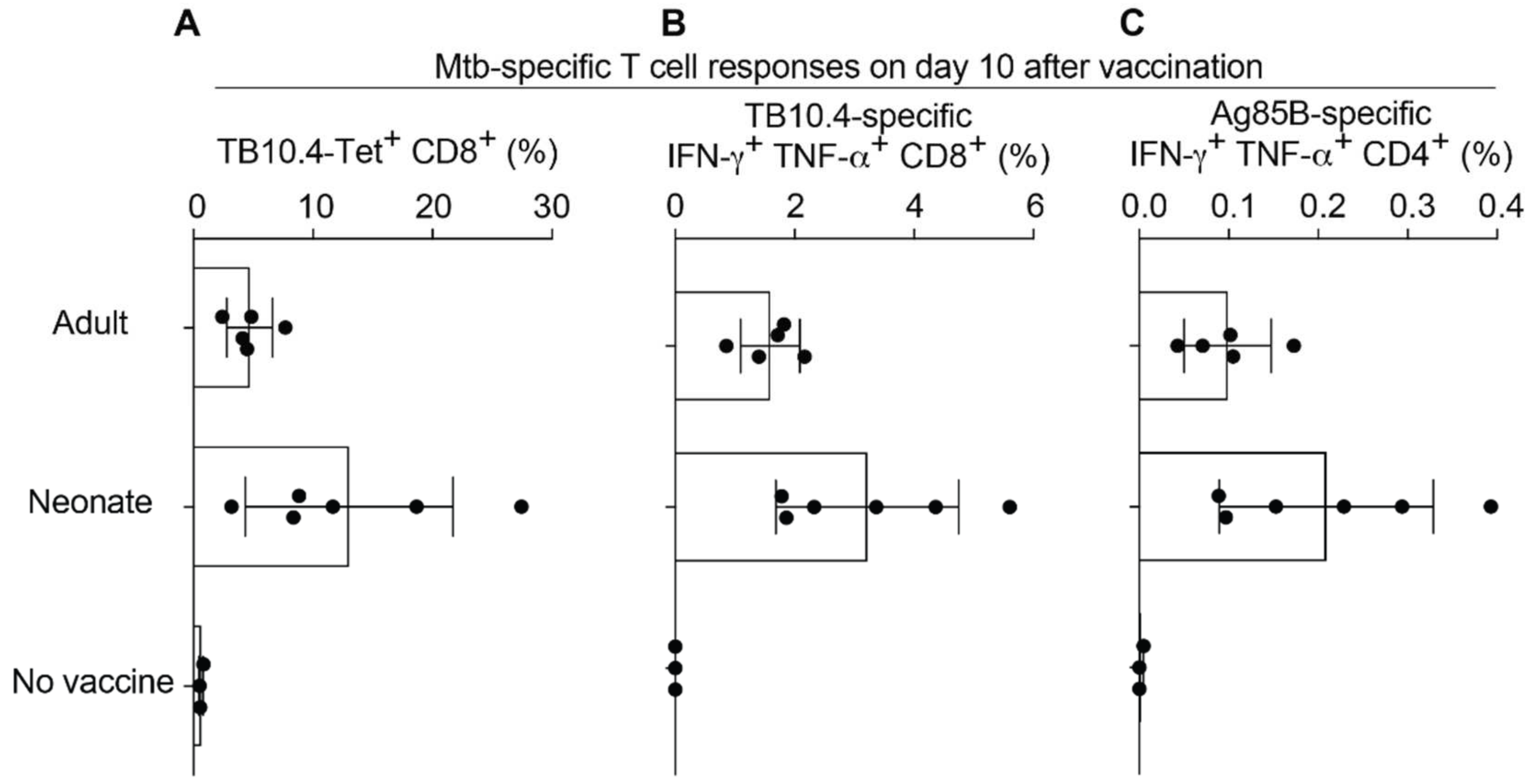
2.3. Neonatal Mice Mount Robust CD8 and CD4 T Cell Responses to rLCMV Vaccination
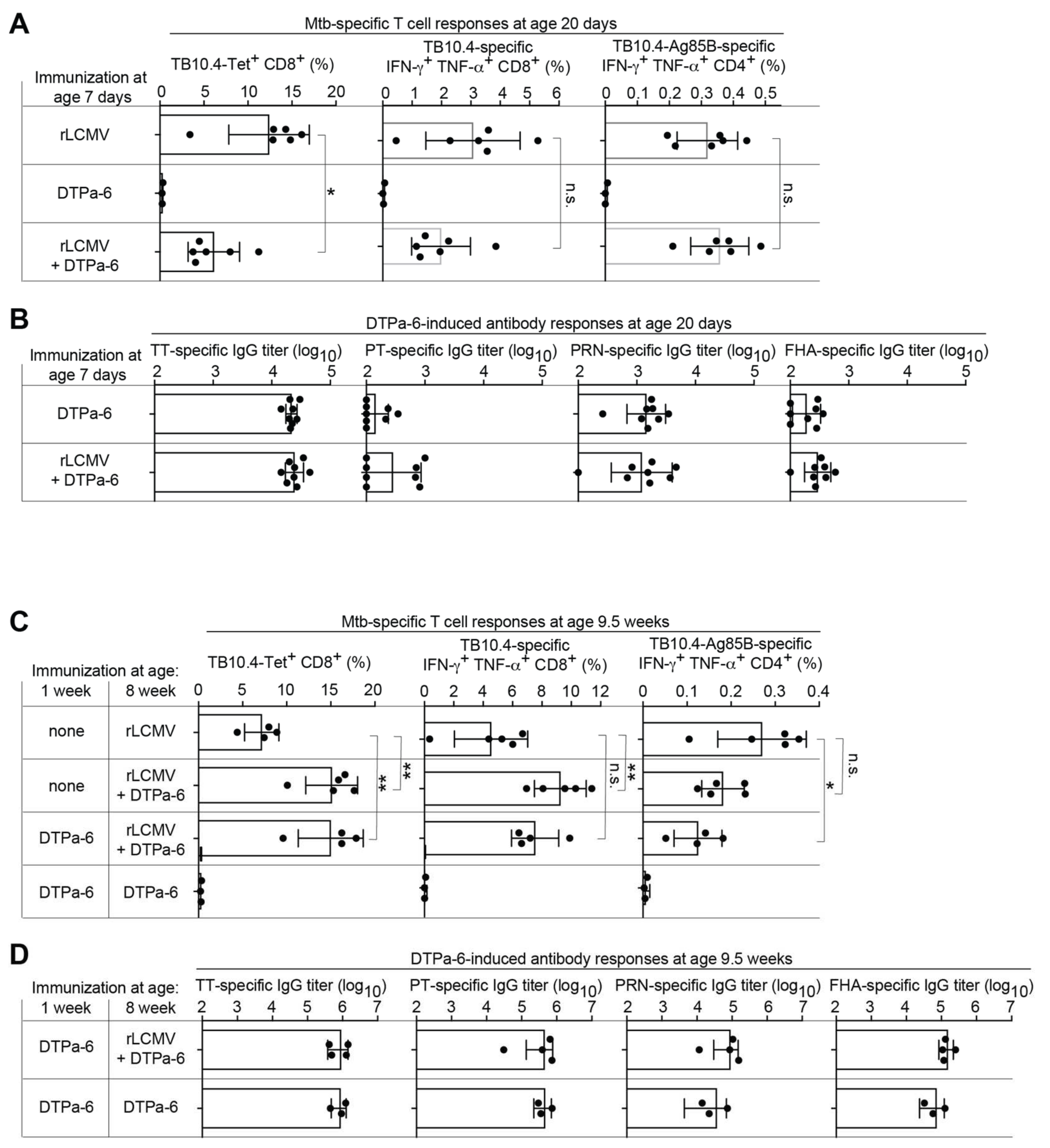
2.4. rLCMV Can Be Co-Administered with Human Infant Vaccines
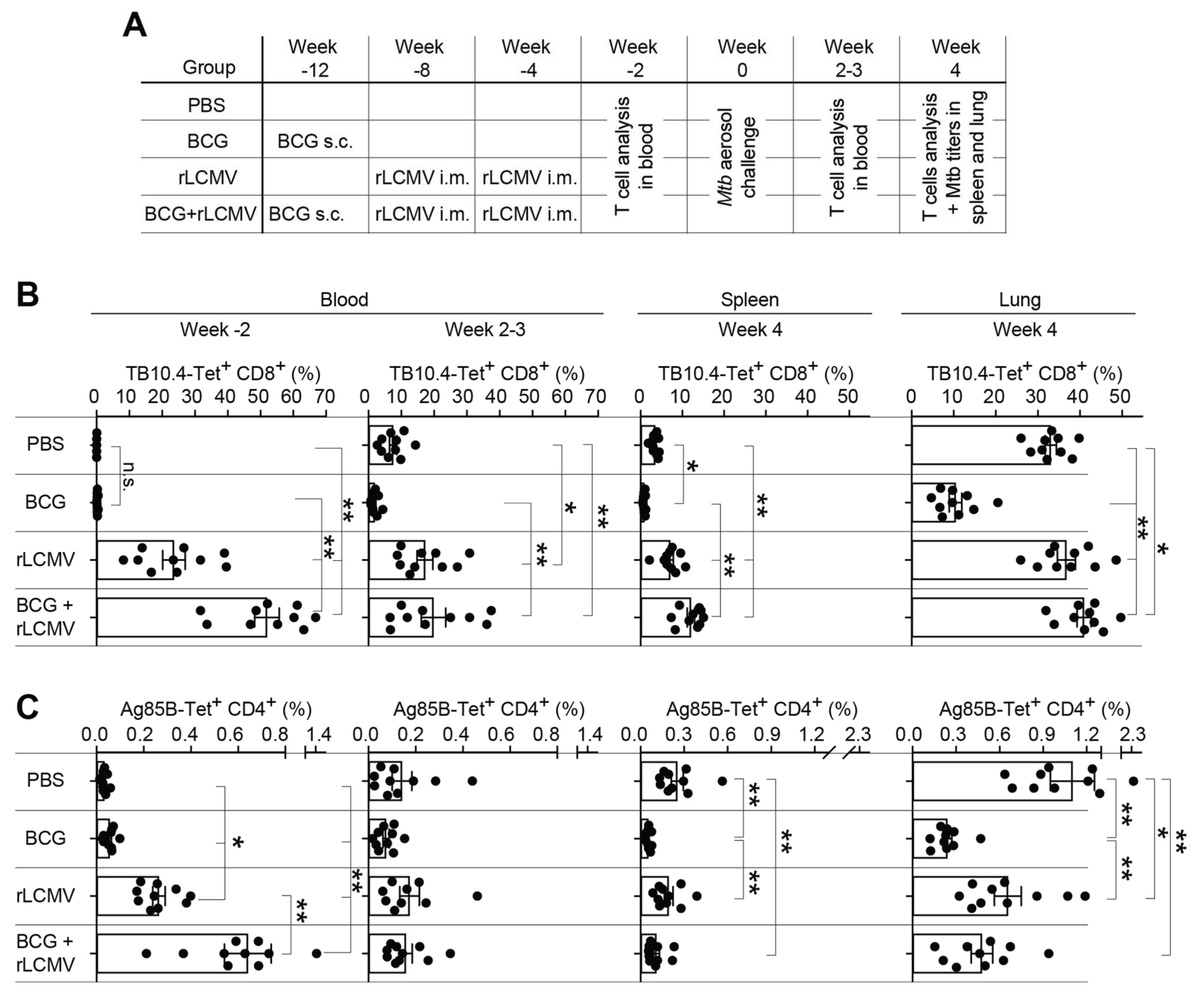
2.5. T Cell Responses upon rLCMV- and/or BCG Immunization and Subsequent Mtb Aerosol Challenge
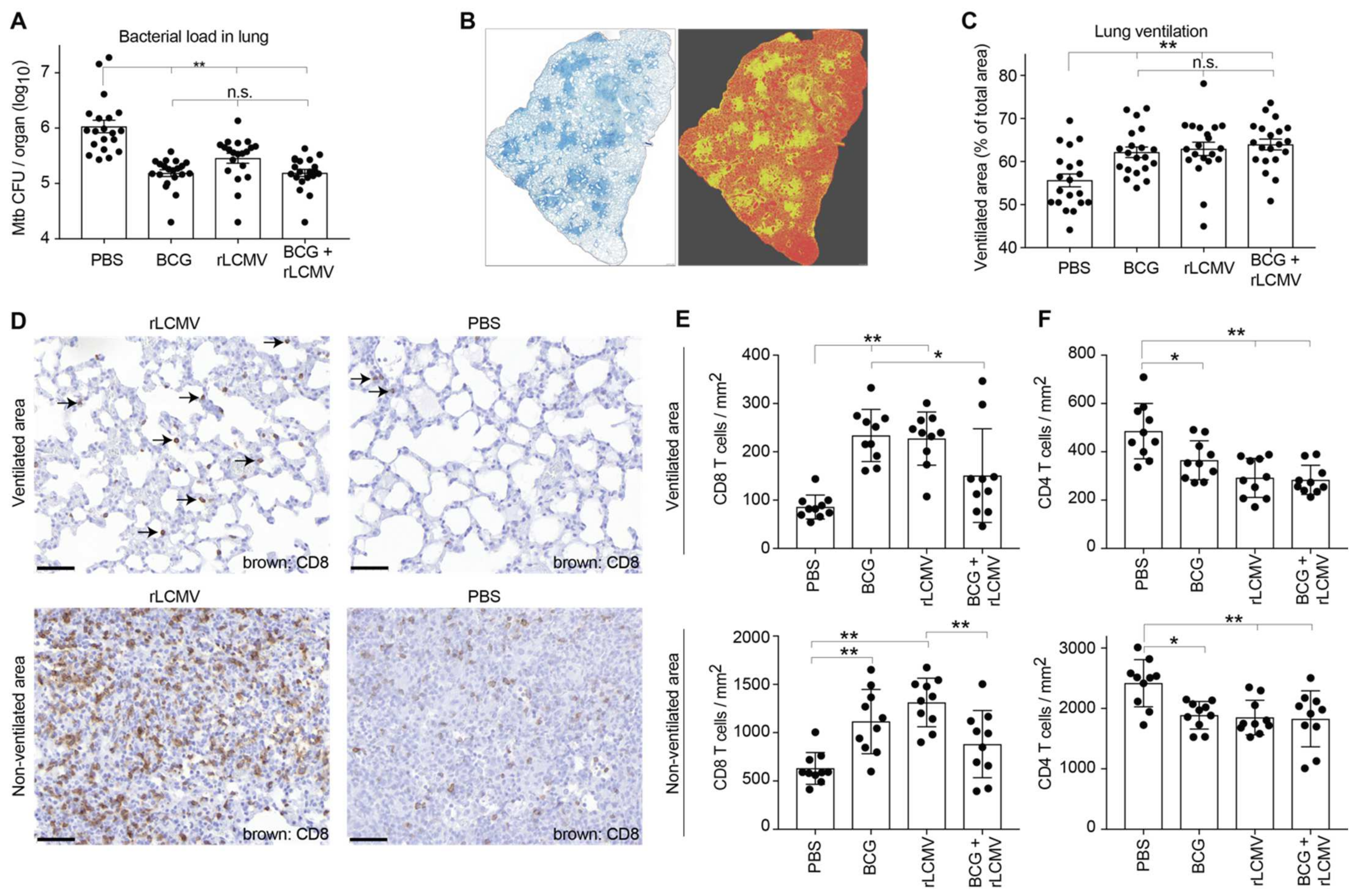
2.6. rLCMV Immunization Reduces Mtb Loads in Lung, Improves Lung Pathology, and Augments CD8 T Cell Recruitment to the Lung
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. BCG and Mtb Production, Mtb Challenge, and Bacterial Titer Determination
4.3. Viral Vectors and Vaccines
4.4. Determination of Antigen-Specific T Cell Responses
4.5. Histology, Immunohistochemistry, and Quantitative Assessment of T Cell Infiltration and Lung Ventilation
4.6. Determination of DTPa-6-Induced Antibody Responses
4.7. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- WHO. WHO Global Tuberculosis Report 2020. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 3 May 2021).
- Calmette, A.; Guérin, C.; Boquet, A.; Négre, L. La Vaccination Préventive Contre La Tuberculose Par Le “BCG”; Masson et Cie: Paris, France, 1927. [Google Scholar]
- Roy, A.; Eisenhut, M.; Harris, R.J.; Rodrigues, L.C.; Sridhar, S.; Habermann, S.; Snell, L.B.; Mangtani, P.; Adetifa, I.; Lalvani, A.; et al. Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: Systematic review and meta-analysis. BMJ 2014, 349, g4643. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, S.H.; Dockrell, H.M.; Drager, N.; Ho, M.M.; McShane, H.; Neyrolles, O.; Ottenhoff, T.H.; Patel, B.; Roordink, D.; Spertini, F.; et al. TBVAC2020: Advancing Tuberculosis Vaccines from Discovery to Clinical Development. Front. Immunol. 2017, 8, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tait, D.R.; Hatherill, M.; Van Der Meeren, O.; Ginsberg, A.M.; Van Brakel, E.; Salaun, B.; Scriba, T.J.; Akite, E.J.; Ayles, H.M.; Bollaerts, A.; et al. Final Analysis of a Trial of M72/AS01E Vaccine to Prevent Tuberculosis. N. Engl. J. Med. 2019, 381, 2429–2439. [Google Scholar] [CrossRef] [PubMed]
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [PubMed]
- Flynn, J.L.; Goldstein, M.M.; Triebold, K.J.; Koller, B.B.R.B.; Bloom, B.R. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA 1992, 89, 12013–12017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, A.O.; Mazzaccaro, R.J.; Russell, R.G.; Lee, F.K.; Turner, O.C.; Hong, S.; Van Kaer, L.; Bloom, B.R. Relative contributions of distinct MHC class I-dependent cell populations in protection to tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 4204–4208. [Google Scholar] [CrossRef] [Green Version]
- Müller, I.; Cobbold, S.P.; Waldmann, H.; Kaufmann, S.H. Impaired resistance to Mycobacterium tuberculosis infection after selective in vivo depletion of L3T4+ and Lyt-2+ T cells. Infect. Immun. 1987, 55, 2037–2041. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Y.; Huang, D.; Wang, R.C.; Shen, L.; Zeng, G.; Yao, S.; Shen, Y.; Halliday, L.; Fortman, J.; McAllister, M.; et al. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009, 5, e1000392. [Google Scholar] [CrossRef]
- Farinacci, M.; Weber, S.; Kaufmann, S.H. The recombinant tuberculosis vaccine rBCG DeltaureC::hly(+) induces apoptotic vesicles for improved priming of CD4(+) and CD8(+) T cells. Vaccine 2012, 30, 7608–7614. [Google Scholar] [CrossRef]
- Darrah, P.A.; Zeppa, J.J.; Maiello, P.; Hackney, J.A.; Ii, M.H.W.; Hughes, T.K.; Pokkali, S.; Ii, P.A.S.; Grant, N.L.; Rodgers, M.A.; et al. Prevention of tuberculosis in macaques after intravenous BCG immunization. Nature 2020, 577, 95–102. [Google Scholar] [CrossRef]
- Zinkernagel, R.M.; Doherty, P.C. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature 1974, 248, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Zinkernagel, R.M. Lymphocytic choriomeningitis virus and immunology. In Arenaviruses II. Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2002; Volume 263, pp. 1–5. [Google Scholar]
- Lau, L.L.; Jamieson, B.D.; Somasundaram, T.; Ahmed, R. Cytotoxic T-cell memory without antigen. Nature 1994, 369, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Homann, D.; Teyton, L.; Oldstone, M.B. Oldstone, Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001, 7, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Flatz, L.; Bergthaler, A.; de la Torre, J.C.; Pinschewer, D.D. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. USA 2006, 103, 4663–4668. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.B.; de la Torre, J.C. Rescue of the prototypic Arenavirus LCMV entirely from plasmid. Virology 2006, 350, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Pinschewer, D.D.; Perez, M.; Sanchez, A.B.; de la Torre, J.C. Recombinant lymphocytic choriomeningitis virus expressing vesicular stomatitis virus glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 7895–7900. [Google Scholar] [CrossRef] [Green Version]
- Flatz, L.; Hegazy, A.N.; Bergthaler, A.; Verschoor, A.; Claus, C.; Fernandez, M.; Gattinoni, L.; Johnson, S.; Kreppel, F.; Kochanek, S.; et al. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8(+) T cell immunity. Nat. Med. 2010, 16, 339–345. [Google Scholar] [CrossRef]
- Flatz, L.; Cheng, C.; Wang, L.; Foulds, K.E.; Ko, S.Y.; Kong, W.P.; Nabel, G.J.; Roychoudhuri, R.; Shi, W.; Bao, S.; et al. Gene-based vaccination with a mismatched envelope protects against simian immunodeficiency virus infection in nonhuman primates. J. Virol. 2012, 86, 7760–7770. [Google Scholar] [CrossRef] [Green Version]
- Schleiss, M.R.; Berka, U.; Watson, E.; Aistleithner, M.; Kiefmann, B.; Mangeat, B.; Swanson, E.C.; Gillis, P.A.; Hernandez-Alvarado, N.; Fernández-Alarcón, C.; et al. Additive Protection against Congenital Cytomegalovirus Conferred by Combined Glycoprotein B/pp65 Vaccination Using a Lymphocytic Choriomeningitis Virus Vector. Clin. Vaccine Immunol. 2017, 24, e00300-16. [Google Scholar] [CrossRef] [Green Version]
- Penaloza MacMaster, P.; Shields, J.L.; Alayo, Q.A.; Cabral, C.; Jimenez, J.; Mondesir, J.; Chandrashekar, A.; Cabral, J.M.; Lim, M.; Iampietro, M.J.; et al. Development of novel replication-defective lymphocytic choriomeningitis virus vectors expressing SIV antigens. Vaccine 2017, 35, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schwendinger, M.; Thiry, G.; De Vos, B.; Leroux-Roels, G.; Bruhwyler, J.; Huygens, A.; Ganeff, C.; Buchinger, H.; Orlinger, K.K.; Pinschewer, D.D.; et al. A Randomized Dose-Escalating Phase I Trial of a Replication-Deficient Lymphocytic Choriomeningitis Virus Vector-Based Vaccine Against Human Cytomegalovirus. J. Infect. Dis. 2020. [CrossRef] [PubMed] [Green Version]
- Sommerstein, R.; Flatz, L.; Remy, M.M.; Malinge, P.; Magistrelli, G.; Fischer, N.; Sahin, M.; Bergthaler, A.; Igonet, S.; Ter Meulen, J.; et al. Arenavirus Glycan Shield Promotes Neutralizing Antibody Evasion and Protracted Infection. PLoS Pathog. 2015, 11, e1005276. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.P.H.; Nørskov-Lauritsen, S.; Dolganov, G.M.; Schoolnik, G.K.; Lindenstrøm, T.; Andersen, P.; Agger, E.M.; Aagaard, C. Tuberculosis vaccine with high predicted population coverage and compatibility with modern diagnostics. Proc. Natl. Acad. Sci. USA 2014, 111, 1096–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babaki, M.K.Z.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef]
- Fletcher, H.A.; Schrager, L. TB vaccine development and the End TB Strategy: Importance and current status. Trans. R. Soc. Trop. Med. Hyg. 2016, 110, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Ernst, J.D.; Cornelius, A.; Bolz, M. Dynamics of Mycobacterium tuberculosis Ag85B Revealed by a Sensitive Enzyme-Linked Immunosorbent Assay. mBio 2019, 10, e00611-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belisle, J.T.; Vissa, V.D.; Sievert, T.; Takayama, K.; Brennan, P.J.; Besra, G.S. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science 1997, 276, 1420–1422. [Google Scholar] [CrossRef]
- Dietrich, J.; Aagaard, C.; Leah, R.; Olsen, A.W.; Stryhn, A.; Doherty, T.M.; Andersen, P. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: Efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J. Immunol. 2005, 174, 6332–6339. [Google Scholar]
- Siegrist, C.-A. Vaccination in the Neonatal Period and Early Infancy. Int. Rev. Immunol. 2000, 19, 195–219. [Google Scholar] [CrossRef]
- Ota, M.O.C.; Odutola, A.A.; Owiafe, P.K.; Donkor, S.; Owolabi, O.A.; Brittain, N.J.; Williams, N.; Rowland-Jones, S.; Hill, A.V.S.; Adegbola, R.A.; et al. Immunogenicity of the tuberculosis vaccine MVA85A is reduced by coadministration with EPI vaccines in a randomized controlled trial in Gambian infants. Sci. Transl. Med. 2011, 3, 88ra56. [Google Scholar] [CrossRef]
- Mittrücker, H.W.; Steinhoff, U.; Köhler, A.; Krause, M.; Lazar, D.; Mex, P.; Miekley, D.; Kaufmann, S.H. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. USA 2007, 104, 12434–12439. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.D.; Mott, D.; Sutiwisesak, R.; Lu, Y.-J.; Raso, F.; Stowell, B.; Babunovic, G.H.; Lee, J.; Carpenter, S.M.; Way, S.S.; et al. Mycobacterium tuberculosis-specific CD4+ and CD8+ T cells differ in their capacity to recognize infected macrophages. PLoS Pathog. 2018, 14, e1007060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grode, L.; Seiler, P.; Baumann, S.; Hess, J.; Brinkmann, V.; Eddine, A.N.; Mann, P.; Goosmann, C.; Bandermann, S.; Smith, D.; et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J. Clin. Investig. 2005, 115, 2472–2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denis, O.; Tanghe, A.; Palfliet, K.; Jurion, F.; Berg, T.P.V.D.; Vanonckelen, A.; Ooms, J.; Saman, E.; Ulmer, J.B.; Content, J.; et al. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 1998, 66, 1527–1533. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.; Bergthaler, A.; Graw, F.; Flatz, L.; Bonilla, W.V.; Siegrist, C.-A.; Lambert, P.-H.; Regoes, R.R.; Pinschewer, D.D. Protective efficacy of individual CD8+ T cell specificities in chronic viral infection. J. Immunol. 2015, 194, 1755–1762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, W.V.; Kirchhammer, N.; Marx, A.-F.; Kallert, S.M.; Krzyzaniak, M.A.; Lu, M.; Darbre, S.; Schmidt, S.; Raguz, J.; Berka, U.; et al. Heterologous arenavirus vector prime-boost overrules self-tolerance for efficient tumor-specific CD8 T cell attack. Cell Rep. Med. 2021, 2, 100209. [Google Scholar] [CrossRef]
- Auderset, F.; Ballester, M.; Mastelic-Gavillet, B.; Fontannaz, P.; Chabaud-Riou, M.; Reveneau, N.; Garinot, M.; Mistretta, N.; Liu, Y.; Lambert, P.-H.; et al. Reactivating Immunity Primed by Acellular Pertussis Vaccines in the Absence of Circulating Antibodies: Enhanced Bacterial Control by TLR9 Rather Than TLR4 Agonist-Including Formulation. Front. Immunol. 2019, 10, 1520. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belnoue, E.; Vogelzang, A.; Nieuwenhuizen, N.E.; Krzyzaniak, M.A.; Darbre, S.; Kreutzfeldt, M.; Wagner, I.; Merkler, D.; Lambert, P.-H.; Kaufmann, S.H.E.; et al. Replication-Deficient Lymphocytic Choriomeningitis Virus-Vectored Vaccine Candidate for the Induction of T Cell Immunity against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2022, 23, 2700. https://doi.org/10.3390/ijms23052700
Belnoue E, Vogelzang A, Nieuwenhuizen NE, Krzyzaniak MA, Darbre S, Kreutzfeldt M, Wagner I, Merkler D, Lambert P-H, Kaufmann SHE, et al. Replication-Deficient Lymphocytic Choriomeningitis Virus-Vectored Vaccine Candidate for the Induction of T Cell Immunity against Mycobacterium tuberculosis. International Journal of Molecular Sciences. 2022; 23(5):2700. https://doi.org/10.3390/ijms23052700
Chicago/Turabian StyleBelnoue, Elodie, Alexis Vogelzang, Natalie E. Nieuwenhuizen, Magdalena A. Krzyzaniak, Stephanie Darbre, Mario Kreutzfeldt, Ingrid Wagner, Doron Merkler, Paul-Henri Lambert, Stefan H. E. Kaufmann, and et al. 2022. "Replication-Deficient Lymphocytic Choriomeningitis Virus-Vectored Vaccine Candidate for the Induction of T Cell Immunity against Mycobacterium tuberculosis" International Journal of Molecular Sciences 23, no. 5: 2700. https://doi.org/10.3390/ijms23052700
APA StyleBelnoue, E., Vogelzang, A., Nieuwenhuizen, N. E., Krzyzaniak, M. A., Darbre, S., Kreutzfeldt, M., Wagner, I., Merkler, D., Lambert, P.-H., Kaufmann, S. H. E., Siegrist, C.-A., & Pinschewer, D. D. (2022). Replication-Deficient Lymphocytic Choriomeningitis Virus-Vectored Vaccine Candidate for the Induction of T Cell Immunity against Mycobacterium tuberculosis. International Journal of Molecular Sciences, 23(5), 2700. https://doi.org/10.3390/ijms23052700





