Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection
Abstract
1. Introduction
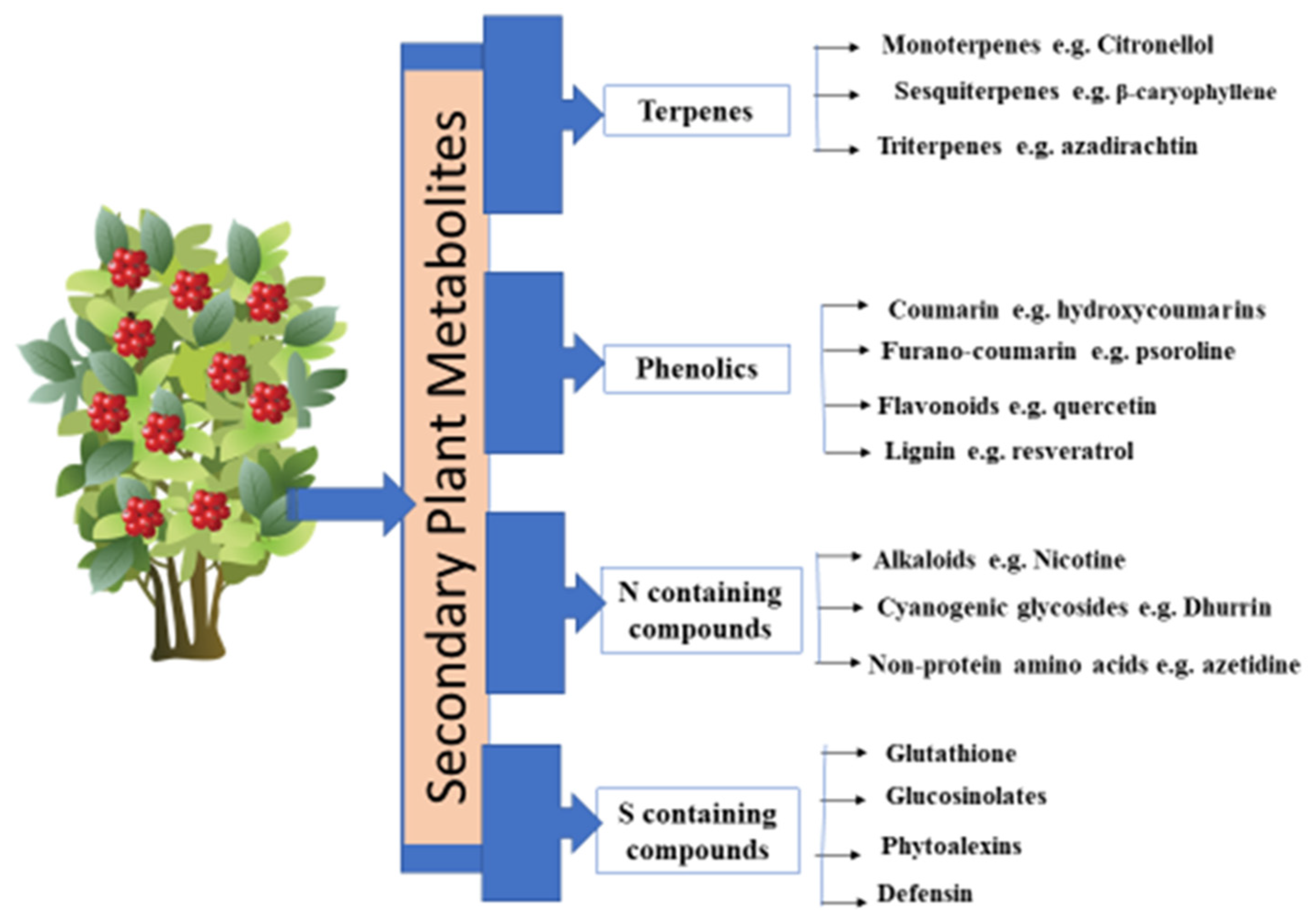
2. Types of Secondary Plant Metabolites
2.1. Terpenes
2.2. Phenolic Compounds
2.3. Sulfur-Containing Plant Secondary Metabolites
2.4. Nitrogen-Containing Compounds
- (a)
- true alkaloids (e.g., nicotine, morphine, quinine and atropine);
- (b)
- pseudo-alkaloids (e.g., capsaicin, solanidine and caffeine); and
- (c)
- proto-alkaloids (e.g., yohimbine, mescaline and hordenine).
3. Understanding the Biosynthesis of Secondary Plant Metabolites
- i.
- phenolic compounds (shikimate pathway),
- ii.
- terpenes (mevalonic or methylerythritol phosphate pathway), and
- iii.
- nitrogen and sulfur containing compounds (tricarboxylic acid cycle pathway) [9].
4. Secondary Metabolite Functional Role in the Regulation of Plant Defense and Early Detection of Herbivore Attack
5. Role of Phytohormones in Regulation of Induced Plant Defense through PSMs
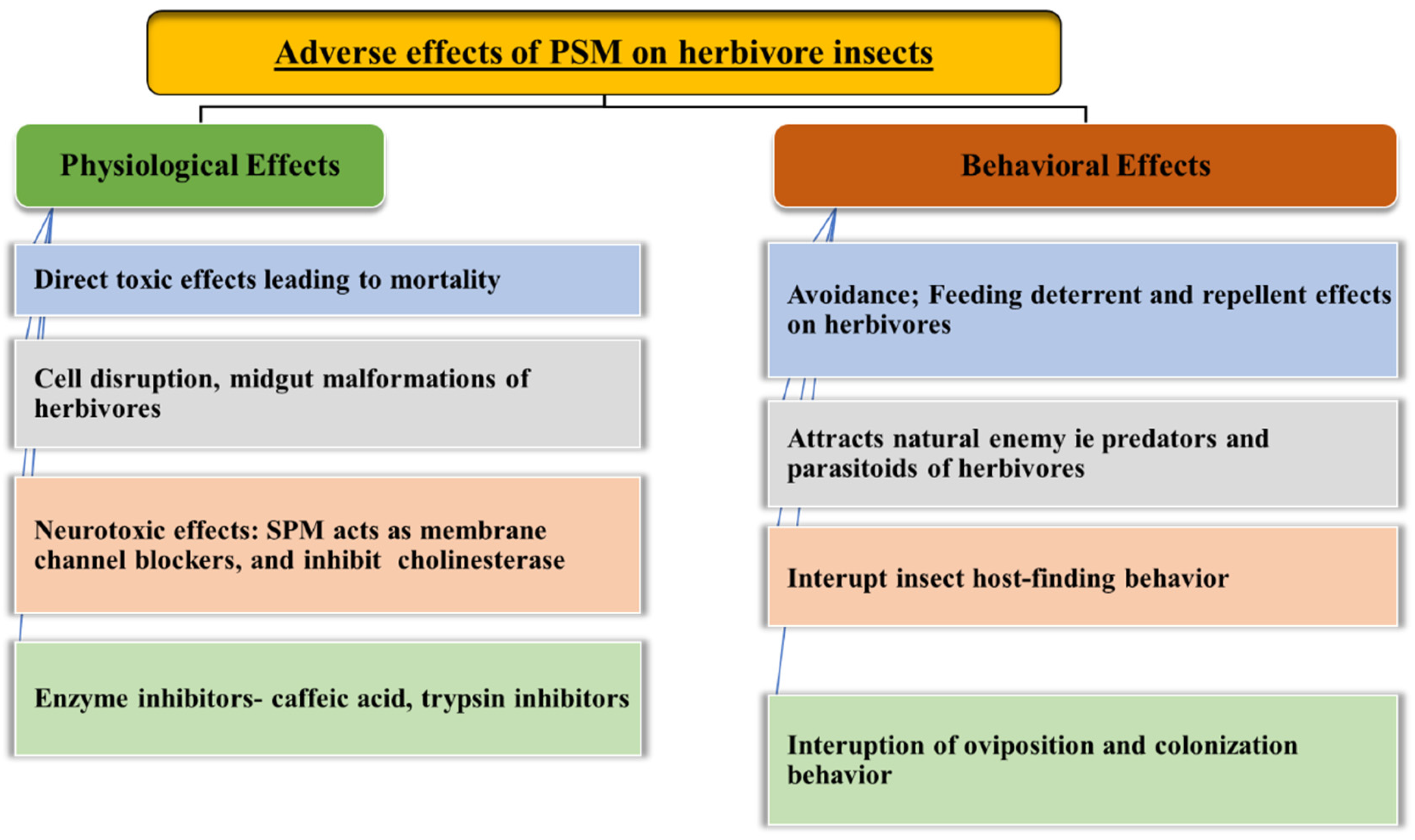
6. Effect of Plant Secondary Metabolites on the Physiology and Behavior of Herbivores
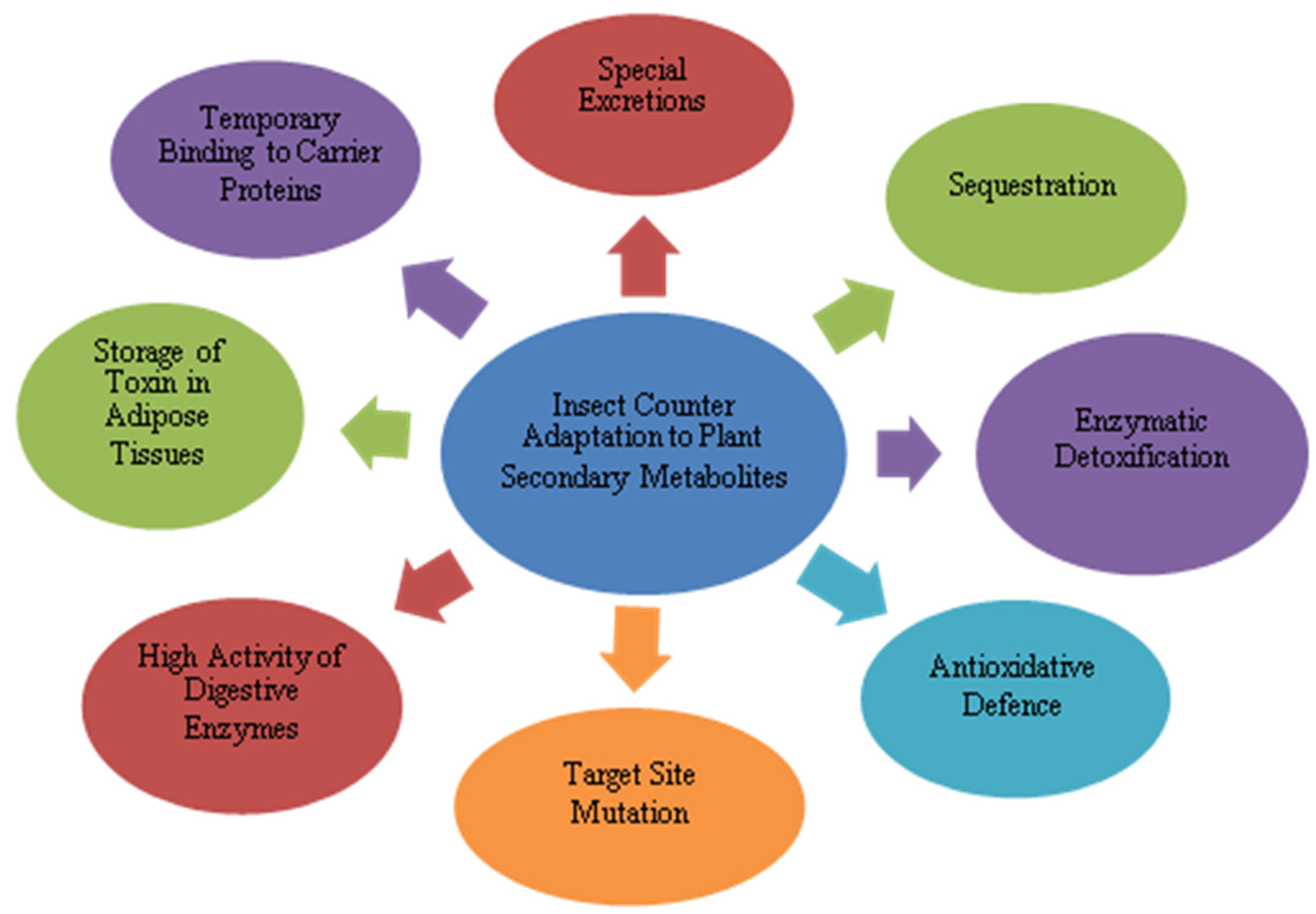
7. Adaptations of Herbivore Insects to Plant Secondary Metabolites
8. Functional Role of PSMs in Mediating the Multi-Trophic Interactions
9. Commercial Production of Secondary Metabolites: Barriers and Biotechnological Prospects
10. Role of Plant Secondary Metabolites in Sustainable Crop Protection
11. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Futuyma, D.J.; Moreno, G. The Evolution of Ecological Specialization. Annu. Rev. Ecol. Syst. 1988, 19, 207–233. [Google Scholar] [CrossRef]
- Weissing, F.J.; Edelaar, P.; van Doorn, G.S. Adaptive speciation theory: A conceptual review. Behav. Ecol. Sociobiol. 2011, 65, 461–480. [Google Scholar] [CrossRef]
- Freeman, B.; Beattie, G. Freeman an Overview of Plant Defenses against Pathogens and Herbivores. Plant Health Instr. 2008. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Kim, K.-M. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Integrated Pest Management; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; Volume 3, Available online: https://link.springer.com/book/10.1007/978-1-4615-7269-5 (accessed on 12 December 2021).
- Khare, S.; Singh, N.B.; Singh, A.; Hussain, I.; Niharika, K.; Yadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Willis, K.J. (Ed.) State of the World’s Plants; Report; Royal Botanic Gardens: London, UK, 2017; Available online: https://stateoftheworldsplants.org/ (accessed on 18 December 2021).
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- War, A.R.; Buhroo, A.A.; Hussain, B.; Ahmad, T.; Nair, R.M.; Sharma, H.C. Plant defense and insect adaptation with reference to secondary metabolites. In Co-Evolution of Secondary Metabolites, Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Hettenhausen, C.; Schuman, M.C.; Wu, J. MAPK signaling: A key element in plant defense response to insects. Insect Sci. 2014, 22, 157–164. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.-M.; Persicke, M.; Müller, C. Interactions between the jasmonic and salicylic acid pathway modulate the plant metabolome and affect herbivores of different feeding types. Plant, Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef]
- Katz, E.; Nisani, S.; Yadav, B.S.; Woldemariam, M.G.; Shai, B.; Obolski, U.; Ehrlich, M.; Shani, E.; Jander, G.; Chamovitz, D.A. The glucosinolate breakdown product indole-3-carbinol acts as an auxin antagonist in roots ofArabidopsis thaliana. Plant J. 2015, 82, 547–555. [Google Scholar] [CrossRef]
- Mason, P.A.; Singer, M.S. Defensive mixology: Combining acquired chemicals towards defence. Funct. Ecol. 2014, 29, 441–450. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Züst, T.; Robert, C.A.M. Using plant chemistry to improve interactions between plants, herbivores and their natural enemies: Challenges and opportunities. Curr. Opin. Biotechnol. 2021, 70, 262–265. [Google Scholar] [CrossRef]
- Stevenson, P.C.; Nicolson, S.; Wright, G.A. Plant secondary metabolites in nectar: Impacts on pollinators and ecological functions. Funct. Ecol. 2017, 31, 65–75. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Wink, M. Evolution of secondary metabolites from an ecological and molecular phylogenetic perspective. Phytochem. 2003, 64, 3–19. [Google Scholar] [CrossRef]
- Aharoni, A.; Jongsma, M.; Bouwmeester, H. Volatile science? Metabolic engineering of terpenoids in plants. Trends Plant Sci. 2005, 10, 594–602. [Google Scholar] [CrossRef]
- Mumm, R.; Posthumus, M.A.; Dicke, M. Significance of terpenoids in induced indirect plant defence against herbivorous arthropods. Plant Cell Environ. 2008, 31, 575–585. [Google Scholar] [CrossRef]
- Wouters, F.C.; Blanchette, B.; Gershenzon, J.; Vassão, D.G. Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef]
- Bhonwong, A.; Stout, M.J.; Attajarusit, J.; Tantasawat, P. Defensive Role of Tomato Polyphenol Oxidases against Cotton Bollworm (Helicoverpa armigera) and Beet Armyworm (Spodoptera exigua). J. Chem. Ecol. 2009, 35, 28–38. [Google Scholar] [CrossRef]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J.A. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Eigenbrode, S.; Stringam, G.R.; Thiagarajah, M.R. Feeding and Growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with Varying Glucosinolate Concentrations and Myrosinase Activities. J. Chem. Ecol. 2000, 26, 2401–2419. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B.; Paluch, G. Needles in the Haystack: Exploring Chemical Diversity of Botanical Insecticides. In Green Trends in Insect Control; López, O., Fernández- Bolaños, J.G., Eds.; RSC: Washington, DC, USA, 2011; pp. 248–265.28. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=d58bdf51886536483662e9bc4a1a89a2&site=xueshu_se (accessed on 12 December 2021).
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Aniszewski, T. Alkaloids—Secrets of Life: Alkaloid Chemistry, Biological Significance, Applications and Ecological Role; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Züst, T.; Agrawal, A.A. Mechanisms and evolution of plant resistance to aphids. Nat. Plants 2016, 2, 15206. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Schimmer, O. Molecular Modes of Action of Defensive Secondary Metabolites. Funct. Biotechnol. Plant Second. Metab. 2010, 39, 21–161. [Google Scholar] [CrossRef]
- Jørgensen, K.; Rasmussen, A.V.; Morant, M.; Nielsen, A.H.; Bjarnholt, N.; Zagrobelny, M.; Bak, S.; Møller, B.L. Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr. Opin. Plant Biol. 2005, 8, 280–291. [Google Scholar] [CrossRef]
- Kutchan, T.M. A role for intra- and intercellular translocation in natural product biosynthesis. Curr. Opin. Plant Biol. 2005, 8, 292–300. [Google Scholar] [CrossRef]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef]
- Ramírez-Gómez, X.S.; Jiménez-García, S.N.; Campos, V.B.; Campos, M.L.G. Plant Metabolites in Plant Defense against Pathogens; IntechOpen: London, UK, 2020. [Google Scholar]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Pichersky, E.; Lewinsohn, E. Convergent Evolution in Plant Specialized Metabolism. Annu. Rev. Plant Biol. 2011, 62, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Li, J.; Zhou, G.; Wang, Q.; Bian, W.; Erb, M.; Lou, Y. Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 2015, 4, e04805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Deng, L.; Shen, J.; Fang, M.; Chen, Q.; Lu, Y.; Wang, Q.; Li, C.; Zhai, Q. MYC2 Regulates the Termination of Jasmonate Signaling via an Autoregulatory Negative Feedback Loop. Plant Cell 2019, 31, 106–127. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Seidl-Adams, I.; Engelberth, J.; Hunter, C.T.; Alborn, H.; Tumlinson, J.H. Herbivorous Caterpillars Can Utilize Three Mechanisms to Alter Green Leaf Volatile Emission. Environ. Èntomol. 2019, 48, 419–425. [Google Scholar] [CrossRef]
- Quintana-Rodriguez, E.; Duran-Flores, D.; Heil, M.; Camacho, X. Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Schuurink, R.C.; Bleeker, P.M.; Schiestl, F. The role of volatiles in plant communication. Plant J. 2019, 100, 892–907. [Google Scholar] [CrossRef]
- Erb, M.; Veyrat, N.; Robert, C.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef]
- Ye, M.; Glauser, G.; Lou, Y.; Erb, M.; Hu, L. Molecular Dissection of Early Defense Signaling Underlying Volatile-Mediated Defense Regulation and Herbivore Resistance in Rice. Plant Cell 2019, 31, 687–698. [Google Scholar] [CrossRef]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef]
- Bonaventure, G. Perception of insect feeding by plants. Plant Biol. 2012, 14, 872–880. [Google Scholar] [CrossRef]
- Bonaventure, G. Plants Recognize Herbivorous Insects by Complex Signalling Networks. Annu. Plant Rev. Online 2018, 1–35. [Google Scholar] [CrossRef]
- Spiteller, D.; Oldham, N.J.; Boland, W. N-(17-Phosphonooxylinolenoyl)glutamine and N-(17-phosphonooxylinoleoyl)glutamine from Insect Gut: The First Backbone-Phosphorylated Fatty Acid Derivatives in Nature. J. Org. Chem. 2004, 69, 1104–1109. [Google Scholar] [CrossRef]
- Zunjarrao, S.S.; Tellis, M.B.; Joshi, S.N.; Joshi, R.S. Plant-Insect Interaction: The Saga of Molecular Coevolution. In Reference Series in Phytochemistry; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2020; pp. 19–45. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-319-96397-6_42?noAccess=true (accessed on 12 December 2021).
- Aljbory, Z.; Chen, M.-S. Indirect plant defense against insect herbivores: A review. Insect Sci. 2018, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Whitman, D.W.; Eller, F.J. Parasitic wasps orient to green leaf volatiles. Chemoecology 1990, 1, 69–76. [Google Scholar] [CrossRef]
- Mattiacci, L.; Dicke, M.; Posthumus, M.A. beta-Glucosidase: An elicitor of herbivore-induced plant odor that attracts host-searching parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 2036–2040. [Google Scholar] [CrossRef]
- Mithöfer, A.; Wanner, G.; Boland, W. Effects of Feeding Spodoptera littoralis on Lima Bean Leaves. II. Continuous Mechanical Wounding Resembling Insect Feeding is Sufficient to Elicit Herbivory-Related Volatile Emission. Plant Physiol. 2005, 137, 1160–1168. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, G.; Xiang, C.; Du, M.; Cheng, J.; Liu, S.; Lou, Y. β-Glucosidase treatment and infestation by the rice brown planthopper Nilaparvata lugens elicit similar signaling pathways in rice plants. Chin. Sci. Bull. 2008, 53, 53–57. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. BIOLOGY AND BIOCHEMISTRY OF GLUCOSINOLATES. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T. New Insights into Plant Responses to the Attack from Insect Herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef]
- Mithöfer, A.; Boland, W. Recognition of Herbivory-Associated Molecular Patterns. Plant Physiol. 2008, 146, 825–831. [Google Scholar] [CrossRef]
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.; Loughrin, J.H.; Tumlinson, J.H. An Elicitor of Plant Volatiles from Beet Armyworm Oral Secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Halitschke, R.; Schittko, U.; Pohnert, G.; Boland, W.; Baldwin, I.T. Molecular Interactions between the Specialist HerbivoreManduca sexta (Lepidoptera, Sphingidae) and Its Natural Host Nicotiana attenuata. III. Fatty Acid-Amino Acid Conjugates in Herbivore Oral Secretions Are Necessary and Sufficient for Herbivore-Specific Plant Responses. Plant Physiol. 2001, 125, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Von Dahl, C.C.; Winz, R.A.; Halitschke, R.; Kühnemann, F.; Gase, K.; Baldwin, I.T. Tuning the herbivore-induced ethylene burst: The role of transcript accumulation and ethylene perception inNicotiana attenuata. Plant J. 2007, 51, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Ekström, G.; Ekbom, B. Pest Control in Agro-ecosystems: An Ecological Approach. Crit. Rev. Plant Sci. 2011, 30, 74–94. [Google Scholar] [CrossRef]
- Checker, V.G.; Kushwaha, H.R.; Kumari, P.; Yadav, S. Role of Phytohormones in Plant Defense: Signaling and Cross Talk. In Molecular Aspects of Plant-Pathogen Interaction; Springer: Singapore, 2018. [Google Scholar]
- Rani, P.U.; Jyothsna, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–701. [Google Scholar] [CrossRef]
- Smith, J.L.; De Moraes, C.M.; Mescher, M.C. Jasmonate- and salicylate-mediated plant defense responses to insect herbivores, pathogens and parasitic plants. Pest Manag. Sci. 2009, 65, 497–503. [Google Scholar] [CrossRef]
- Balbi, V.; Devoto, A. Jasmonate signalling network in Arabidopsis thaliana: Crucial regulatory nodes and new physiological scenarios. New Phytol. 2008, 177, 301–318. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Chen, J.L.; Cheng, D.F.; Sun, J.R.; Liu, Y.; Tian, Z. Biochemical and molecular characterizations of Sitobion avenae-induced wheat defense responses. Crop. Prot. 2009, 28, 435–442. [Google Scholar] [CrossRef]
- Shivaji, R.; Camas, A.; Ankala, A.; Engelberth, J.; Tumlinson, J.H.; Williams, W.P.; Wilkinson, J.R.; Luthe, D.S. Plants on Constant Alert: Elevated Levels of Jasmonic Acid and Jasmonate-Induced Transcripts in Caterpillar-Resistant Maize. J. Chem. Ecol. 2010, 36, 179–191. [Google Scholar] [CrossRef]
- An, C.; Li, L.; Zhai, Q.; You, Y.; Deng, L.; Wu, F.; Chen, R.; Jiang, H.; Wang, H.; Chen, Q.; et al. Mediator subunit MED25 links the jasmonate receptor to transcriptionally active chromatin. Proc. Natl. Acad. Sci. USA 2017, 114, E8930–E8939. [Google Scholar] [CrossRef]
- Kumar, D.; Haq, I.; Chapagai, D.; Tripathi, D.; Donald, D.; Hossain, M.; Devaiah, S. Hormone Signaling: Current Perspectives on the Roles of Salicylic Acid and Its Derivatives in Plants. In The Formation, Structure and Activity of Phytochemicals; Springer: Berlin/Heidelberg, Germany, 2015; pp. 115–136. [Google Scholar]
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Signal. Behav. 2009, 4, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F. Salicylic Acid Biosynthesis and Metabolism. Arab. Book 2011, 9, e0156. [Google Scholar] [CrossRef] [PubMed]
- Walling, L.L. The Myriad Plant Responses to Herbivores. J. Plant Growth Regul. 2000, 19, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-N.; Wei, J.-N.; Kang, L. Feeding of pea leafminer larvae simultaneously activates jasmonic and salicylic acid pathways in plants to release a terpenoid for indirect defense. Insect Sci. 2021, 28, 811–824. [Google Scholar] [CrossRef]
- Argandoña, V.H.; Chaman, M.; Cardemil, L.; Muñoz, O.; Zúñiga, G.E.; Corcuera, L.J. Ethylene production and peroxidase activity in aphid-infested barley. J. Chem. Ecol. 2001, 27, 53–68. [Google Scholar] [CrossRef]
- Schmelz, E.A.; Alborn, H.T.; Banchio, E.; Tumlinson, J.H. Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 2003, 216, 665–673. [Google Scholar] [CrossRef]
- Stotz, H.U.; Pittendrigh, B.R.; Kroymann, J.; Weniger, K.; Fritsche, J.; Bauke, A.; Mitchell-Olds, T. Induced Plant Defense Responses against Chewing Insects. Ethylene Signaling Reduces Resistance of Arabidopsis against Egyptian Cotton Worm But Not Diamondback Moth. Plant Physiol. 2000, 124, 1007–1018. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Spivey, N.W.; Zeng, W.; Liu, P.-P.; Fu, Z.Q.; Klessig, D.F.; He, S.Y.; Dong, X. Coronatine Promotes Pseudomonas syringae Virulence in Plants by Activating a Signaling Cascade that Inhibits Salicylic Acid Accumulation. Cell Host Microbe 2012, 11, 587–596. [Google Scholar] [CrossRef]
- Liu, L.; Sonbol, F.-M.; Huot, B.; Gu, Y.; Withers, J.; Mwimba, M.; Yao, J.; He, S.Y.; Dong, X. Salicylic acid receptors activate jasmonic acid signalling through a non-canonical pathway to promote effector-triggered immunity. Nat. Commun. 2016, 7, 13099. [Google Scholar] [CrossRef]
- Erb, M.; Köllner, T.G.; Degenhardt, J.; Zwahlen, C.; Hibbard, B.E.; Turlings, T.C.J. The role of abscisic acid and water stress in root herbivore-induced leaf resistance. New Phytol. 2011, 189, 308–320. [Google Scholar] [CrossRef]
- Marrone, P.G. Market opportunities for biopesticides. In Proceedings of the American Chemical Society, 246th National Meeting and Exposition, Indianapolis, IN, USA, 8–12 September 2013; Volume 84, p. 104. [Google Scholar]
- Gilardoni, P.A.; Schuck, S.; Jüngling, R.; Rotter, B.; Baldwin, I.T.; Bonaventure, G. SuperSAGE analysis of the Nicotiana attenuate transcriptome after fatty acid-amino acid elicitation (FAC): Identification of early mediators of insect responses. BMC Plant Biol. 2010, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Dervinis, C.; Frost, C.J.; Lawrence, S.D.; Novak, N.G.; Davis, J.M. Cytokinin Primes Plant Responses to Wounding and Reduces Insect Performance. J. Plant Growth Regul. 2010, 29, 289–296. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Ferrieri, A.P.; Robert, C.A.; Glauser, G.; Kallenbach, M.; Baldwin, I.T.; Erb, M. Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol. 2013, 200, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Kitamura, H.; Kawarada, A.; Seta, Y.; Takai, M.; Tamura, S.; Sumiki, Y. Biochemical studies on “bakanae” fungus. Part XXXIV. Isolation of gibberellins and their properties. Bull. Agr. Chem. Soc. 1955, 19, 267–277. [Google Scholar]
- Buhl, C.; Strauss, S.H.; Lindroth, R.L. Down-regulation of gibberellic acid in poplar has negligible effects on host-plant suitability and insect pest response. Arthropod Plant Interact. 2015, 9, 85–95. [Google Scholar] [CrossRef]
- War, A.R.; Sharma, H.C. Effect of jasmonic acid and salicylic acid induced resistance in groundnut on Helicoverpa armigera. Physiol. Èntomol. 2014, 39, 136–142. [Google Scholar] [CrossRef]
- Bruinsma, M.; Posthumus, M.A.; Mumm, R.; Mueller, M.J.; van Loon, J.J.A.; Dicke, M. Jasmonic acid-induced volatiles of Brassica oleracea attract parasitoids: Effects of time and dose, and comparison with induction by herbivores. J. Exp. Bot. 2009, 60, 2575–2587. [Google Scholar] [CrossRef]
- Lou, Y.-G.; Du, M.-H.; Turlings, T.C.J.; Cheng, J.-A.; Shan, W.-F. Exogenous Application of Jasmonic Acid Induces Volatile Emissions in Rice and Enhances Parasitism of Nilaparvata lugens Eggs by theParasitoid Anagrus nilaparvatae. J. Chem. Ecol. 2005, 31, 1985–2002. [Google Scholar] [CrossRef]
- Sanches, P.; Santos, F.; Peñaflor, M.; Bento, J. Direct and indirect resistance of sugarcane to Diatraea saccharalis induced by jasmonic acid. Bull. Èntomol. Res. 2017, 107, 828–838. [Google Scholar] [CrossRef]
- Nouri-Ganbalani, G.; Borzoui, E.; Shahnavazi, M.; Nouri, A. Induction of Resistance Against Plutella xylostella (L.) (Lep.: Plutellidae) by Jasmonic Acid and Mealy Cabbage Aphid Feeding in Brassica napus L. Front. Physiol. 2018, 9, 859. [Google Scholar] [CrossRef]
- Menzel, T.R.; Weldegergis, B.T.; David, A.; Boland, W.; Gols, R.; Van Loon, J.J.A.; Dicke, M. Synergism in the effect of prior jasmonic acid application on herbivore-induced volatile emission by Lima bean plants: Transcription of a monoterpene synthase gene and volatile emission. J. Exp. Bot. 2014, 65, 4821–4831. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Vrieling, K.; Kim, H.K.; Mulder, P.P.; Klinkhamer, P.G. Application of methyl jasmonate and salicylic acid lead to contrasting effects on the plant’s metabolome and herbivory. Plant Sci. 2021, 303, 110784. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, T.F.S.; Stout, M.J.; Sant’Ana, J. Effects of exogenous methyl jasmonate and salicylic acid on rice resistance to Oebalus pugnax. Pest Manag. Sci. 2019, 75, 744–752. [Google Scholar] [CrossRef] [PubMed]
- James, D.G. Field Evaluation of Herbivore-Induced Plant Volatiles as Attractants for Beneficial Insects: Methyl Salicylate and the Green Lacewing, Chrysopa nigricornis. J. Chem. Ecol. 2003, 29, 1601–1609. [Google Scholar] [CrossRef]
- Damodaram, K.J.P.; Aurade, R.M.; Kempraj, V.; Roy, T.K.; Shivashankara, K.S.; Verghese, A. Salicylic Acid Induces Changes in Mango Fruit that Affect Oviposition Behavior and Development of the Oriental Fruit Fly, Bactrocera dorsalis. PLoS ONE 2015, 10, e0139124. [Google Scholar] [CrossRef]
- Haghighi, S.R.; Hosseininaveh, V.; Talebi, K.; Maali-Amiri, R.; Stelinski, L.L. Salicylic Acid Induced Resistance in Drought-Stressed Pistachio Seedlings Influences Physiological Performance of Agonoscena pistaciae (Hemiptera: Aphalaridae). J. Econ. Èntomol. 2021, 114, 2172–2188. [Google Scholar] [CrossRef]
- Shahabinejad, M.; Shojaaddini, M.; Maserti, B.; Arvin, S.M.J.; Seyedi, S.M. Exogenous application of methyl jasmonate and salicylic acid increases antioxidant activity in the leaves of pistachio (Pistacia vera L. cv. Fandoughi) trees and reduces the performance of the phloem-feeding psyllid Agonoscena pistaciae. Arthropod Plant Interact. 2014, 8, 525–530. [Google Scholar] [CrossRef]
- Alvarenga, R.; Moraes, J.C.; Auad, A.M.; Coelho, M.; Nascimento, A.M. Induction of resistance of corn plants to Spodoptera frugiperda (J. E. Smith, 1797) (Lepidoptera: Noctuidae) by application of silicon and gibberellic acid. Bull. Èntomol. Res. 2017, 107, 527–533. [Google Scholar] [CrossRef]
- Olson, D. The Role of Gibberellic Acid in Aphid-Plant-Arbuscular Mycorrhizal Fungus Interactions. Available online: https://digscholarship.unco.edu/theses/164 (accessed on 12 December 2021).
- Roberts, M.R.; Paul, N. Seduced by the dark side: Integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol. 2006, 170, 677–699. [Google Scholar] [CrossRef]
- Heil, M.; Baldwin, I.T. Fitness costs of induced resistance: Emerging experimental support for a slippery concept. Trends Plant Sci. 2002, 7, 61–67. [Google Scholar] [CrossRef]
- Sampedro, L.; Moreira, X.; Zas, R. Costs of constitutive and herbivore-induced chemical defences in pine trees emerge only under low nutrient availability. J. Ecol. 2011, 99, 818–827. [Google Scholar] [CrossRef]
- Santiago, R.; López-Malvar, A.; Souto, C.; Barros-Ríos, J. Methods for Determining Cell Wall-Bound Phenolics in Maize Stem Tissues. J. Agric. Food Chem. 2018, 66, 1279–1284. [Google Scholar] [CrossRef]
- Saunders, J.A.; O’Neill, N.R.; Romeo, J.T. Alkaloid Chemistry and Feeding Specificity of Insect Herbivores. In Alkaloids: Chemical and Biological Perspectives; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1992; pp. 151–196. Available online: https://link.springer.com/chapter/10.1007/978-1-4612-2908-7_2 (accessed on 12 December 2021).
- Wink, M. Medicinal Plants: A Source of Anti-Parasitic Secondary Metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, R.G.; Armstrong, J.W.; Campbell, E. Caffeine as a repellent for slugs and snails. Nature 2002, 417, 915–916. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Benelli, G.; Canale, A.; Toniolo, C.; Higuchi, A.; Murugan, K.; Pavela, R.; Nicoletti, M. Neem (Azadirachta indica): Towards the ideal insecticide? Nat. Prod. Res. 2016, 31, 369–386. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Divekar, P.; Kumar, P.; Suby, S.B. Screening of maize germplasm through antiobiosis mechanism of resistance against Chilo partellus (Swinhoe). J. Entomol. Zool. Stud. 2019, 7, 1111–1114. [Google Scholar]
- Divekar, P.; Kumar, P.; Suby, S.B. Antibiosis Effect of Phenolics (Ferulic and P-Coumaric Acid) on the Biology of Pink Stem Borer, Sesamia inferens. In Global Perspective in Agricultural and Applied Sciences for Food and Environmental Security. 2019. Available online: https://xueshu.baidu.com/usercenter/paper/show?paperid=196f04607f6x0cu0j61a08r05f051853&site=xueshu_se (accessed on 12 December 2021).
- Wink, M. Chapter 1 Molecular Modes of Action of Cytotoxic Alkaloids: From DNA Intercalation, Spindle Poisoning, Topoisomerase Inhibition to Apoptosis and Multiple Drug Resistance. Alkaloids Chem. Biol. 2007, 64, 1–47. [Google Scholar] [CrossRef]
- Schmeller, T.; Latz-Brüning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Jasmonic acid- mediated induced resistance in groundnut (Arachis hypogaea L.) against Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). J. Plant Growth Regul. 2011, 30, 512–523. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Erb, M. Tritrophic Interactions Mediated by Herbivore-Induced Plant Volatiles: Mechanisms, Ecological Relevance, and Application Potential. Annu. Rev. Èntomol. 2018, 63, 433–452. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.; Haring, M.A.; Schuurink, R.C. The Role of Specific Tomato Volatiles in Tomato-Whitefly Interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.; Kanakala, S.; Kliot, A.; Pakkianathan, B.C.; Abu Farich, B.; Santana-Magal, N.; Elimelech, M.; Kontsedalov, S.; Lebedev, G.; Cilia, M.; et al. Persistent, circulative transmission of begomoviruses by whitefly vectors. Curr. Opin. Virol. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Bleeker, P.M.; Mirabella, R.; Diergaarde, P.J.; VanDoorn, A.; Tissier, A.; Kant, M.; Prins, M.; de Vos, M.; Haring, M.A.; Schuurink, R.C. Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 2012, 109, 20124–20129. [Google Scholar] [CrossRef] [PubMed]
- Afroz, M.; Rahman, M.; Amin, R. Insect Plant Interaction with Reference to Secondary Metabolites: A Review. Agric. Rev. 2021, 42, 427–433. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Bos, J.I. Effector proteins that modulate plant–insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of Insect Olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant Reception in Insects: Roles of Receptors, Binding Proteins, and Degrading Enzymes. Annu. Rev. Èntomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Vieira, F.G.; Sánchez-Gracia, A.; Rozas, J. Comparative genomic analysis of the odorant-binding protein family in 12 Drosophila genomes: Purifying selection and birth-and-death evolution. Genome Biol. 2007, 8, R235. [Google Scholar] [CrossRef]
- Wanner, K.W.; Robertson, H.M. The gustatory receptor family in the silkworm moth Bombyx mori is characterized by a large expansion of a single lineage of putative bitter receptors. Insect Mol. Biol. 2008, 17, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Briscoe, A.D.; Macias-Muñoz, A.; Kozak, K.; Walters, J.R.; Yuan, F.; Jamie, G.A.; Martin, S.H.; Dasmahapatra, K.K.; Ferguson, L.C.; Mallet, J.; et al. Female Behaviour Drives Expression and Evolution of Gustatory Receptors in Butterflies. PLoS Genet. 2013, 9, e1003620. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Salzman, K.; Koiwa, H.; Salzman, R.A.; Shade, R.E.; Ahn, J.-E. Cowpea bruchid Callosobruchus maculatus uses a three-component strategy to overcome a plant defensive cysteine protease inhibitor. Insect Mol. Biol. 2003, 12, 135–145. [Google Scholar] [CrossRef]
- Pennisi, E. How do gut microbes help herbivores? Counting the ways. Science 2017, 355, 236. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Salzman, K.; Zeng, R. Insect Response to Plant Defensive Protease Inhibitors. Annu. Rev. Èntomol. 2015, 60, 233–252. [Google Scholar] [CrossRef]
- Eswaran, S.V.; Jindal, A. Grasshoppers—generalists to specialists? Resonance 2013, 18, 810–816. [Google Scholar] [CrossRef]
- Wright, G.A.; Baker, D.D.; Palmer, M.J.; Stabler, D.; Mustard, J.A.; Power, E.F.; Borland, A.M.; Stevenson, P.C. Caffeine in Floral Nectar Enhances a Pollinator’s Memory of Reward. Science 2013, 339, 1202–1204. [Google Scholar] [CrossRef]
- Koch, H.; Stevenson, P.C. Do linden trees kill bees? Reviewing the causes of bee deaths on silver linden (Tilia tomentosa). Biol. Lett. 2017, 13, 20170484. [Google Scholar] [CrossRef]
- Richardson, L.L.; Adler, L.S.; Leonard, A.S.; Andicoechea, J.; Regan, K.H.; Anthony, W.E.; Irwin, R.E. Secondary metabolites in floral nectar reduce parasite infections in bumblebees. Proc. R. Soc. B. 2015, 282, 20142471. [Google Scholar] [CrossRef]
- Cardel, Y.J.; Koptur, S. Effects of Florivory on the Pollination of Flowers: An Experimental Field Study with a Perennial Plant. Bot. Gaz. 2010, 171, 283–292. [Google Scholar] [CrossRef]
- Ck, P.A.H. Direct and indirect effects of herbivory: Feeding by spittlebugs affects pollinator visitation rates and seedset ofRudbeckia hirta. Écoscience 2001, 8, 45–50. [Google Scholar] [CrossRef]
- Lehtilä, K.; Strauss, S. Leaf damage by herbivores affects attractiveness to pollinators in wild radish, Raphanus raphanistrum. Oecologia 1997, 111, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, L.; Kesner, L.; Robert, C.A.M. Chemical cues shaping host-seeking by entomopathogenic nematodes. Curr. Opin. Insect Sci. 2021, 44, 72–81. [Google Scholar] [CrossRef]
- Opitz, S.E.W.; Müller, C. Plant chemistry and insect sequestration. Chemoecology 2009, 19, 117–154. [Google Scholar] [CrossRef]
- Rafter, J.L.; Gonda-King, L.; Niesen, D.; Seeram, N.P.; Rigsby, C.M.; Preisser, E.L. Impact of Consuming ‘Toxic’ Monarch Caterpillars on Adult Chinese Mantid Mass Gain and Fecundity. Insects 2017, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Jiang, X.; Reichelt, M.; Gershenzon, J.; Pandit, S.S.; Vassão, D.G. Tritrophic metabolism of plant chemical defenses and its effects on herbivore and predator performance. eLife 2019, 8, 51029. [Google Scholar] [CrossRef]
- Pappas, M.L.; Broekgaarden, C.; Broufas, G.D.; Kant, M.; Messelink, G.J.; Steppuhn, A.; Wäckers, F.; Van Dam, N.M. Induced plant defences in biological control of arthropod pests: A double-edged sword. Pest Manag. Sci. 2017, 73, 1780–1788. [Google Scholar] [CrossRef]
- Benrey, B.; Denno, R.F. The slow-growth–high-mortality hypothesis: A test using the cabbage butterfly. Ecology 1997, 78, 987–999. [Google Scholar]
- Cornelissen, T.; Stiling, P. Does low nutritional quality act as a plant defence? An experimental test of the slow-growth, high-mortality hypothesis. Ecol. Èntomol. 2006, 31, 32–40. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.J.; Ge, L.; Hu, L.; Li, J.; Han, X.; Zhang, T.; Lu, J.; et al. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef]
- Harvey, J.A.; van Nouhuys, S.; Biere, A. Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J. Chem. Ecol. 2005, 31, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Saraf, I.; Kumar, R.; Singh, I.P.; Kaur, S. Biological effects of secondary metabolites of Inula racemosa on the parasitoid Bracon hebetor. Èntomol. Exp. Appl. 2021, 169, 743–749. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef] [PubMed]
- Cuadrado, J.L.C.; Pinillos, E.O.; Tito, R.; Mirones, C.S.; Mendoza, N.N.G. Insecticidal Properties of Capsaicinoids and Glucosinolates Extracted from Capsicum chinense and Tropaeolum tuberosum. Insects 2019, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Ming, Q.-L.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L.-P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.E.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tiss. Organ Cult. 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A Tool for Enriching the Bioactive Composition of Foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Kang, S.-M.; Min, J.-Y.; Kim, Y.-D.; Kang, Y.-M.; Park, D.-J.; Jung, H.-N.; Kim, S.-W.; Choi, M.-S. Effects of methyl jasmonate and salicylic acid on the production of bilobalide and ginkgolides in cell cultures of Ginkgo biloba. In Vitro Cell. Dev. Biol. Plant. 2006, 42, 44–49. [Google Scholar] [CrossRef]
- DellaPenna, D. Plant Metabolic Engineering. Plant Physiol. 2001, 125, 160–163. [Google Scholar] [CrossRef][Green Version]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically Active Natural Product Synthesis and Supply via Plant Cell Culture Technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of Plant Secondary Metabolites: Examples, Tips and Suggestions for Biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [PubMed]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Nkechi, E.F.; Ejike, O.G.; Ihuoma, N.J.; Maria-goretti, O.C.; Francis, U.; Godwin, N.; Njokuocha, R. Effects of aqueous and oil leaf extracts of Pterocarpus santalinoides on the maize weevil, Sitophilus zeamais pest of stored maize grains. Afr. J. Agric. Res. 2018, 13, 617–626. [Google Scholar]
- Sande, D.; Mullen, J.; Wetzstein, M.; Houston, J. Environmental Impacts from Pesticide Use: A Case Study of Soil Fumigation in Florida Tomato Production. Int. J. Environ. Res. Public Health 2011, 8, 4649–4661. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, S.; Igaki, C. Characterization of acephate resistance in the diamondback moth Plutella xylostella. Pestic. Biochem. Physiol. 2010, 98, 121–127. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Defensive Function of Herbivore-Induced Plant Volatile Emissions in Nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef]
- Zhou, W.; Kügler, A.; McGale, E.; Haverkamp, A.; Knaden, M.; Guo, H.; Beran, F.; Yon, F.; Li, R.; Lackus, N.; et al. Tissue-Specific Emission of (E)-α-Bergamotene Helps Resolve the Dilemma When Pollinators Are Also Herbivores. Curr. Biol. 2017, 27, 1336–1341. [Google Scholar] [CrossRef]
- Steppuhn, A.; Gase, K.; Krock, B.; Halitschke, R.; Baldwin, I.T. Nicotine’s Defensive Function in Nature. PLoS Biol. 2004, 2, e217. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical insecticide research: Many publications, limited useful data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Alam, M.Z.; Haque, M.M.; Islam, M.S.; Hossain, E.; Hasan, S.B.; Hasan, S.B.; Hossain, M.S. Comparative study of integrated pest management and farmers practices on sustainable environment in the rice ecosystem. Int. J. Zool. 2016, 2016, 7286040. [Google Scholar] [CrossRef]
- Neeraj, G.S.; Kumar, A.; Ram, S.; Kumar, V. Evaluation of nematicidal activity of ethanolic extracts of medicinal plants to Meloidogyne incognita (kofoid and white) chitwood under lab conditions. Int. J. Pure Appl. Biosci. 2017, 5, 827–831. [Google Scholar] [CrossRef]
- Taylor, P. Plantwise Diagnostic Field Guide: A Tool to Diagnose Crop Problems and Make Recommendations for Their Management. Available online: https://www.gov.uk/research-for-development-outputs/plantwise-diagnostic-field-guide-a-tool-to-diagnose-crop-problems-and-make-recommendations-for-their-management (accessed on 12 December 2021).
- Agerbirk, N.; Olsen, C.E.; Bibby, B.M.; Frandsen, H.O.; Brown, L.D.; Nielsen, J.K.; Renwick, J.A.A. A saponin correlated with variable resistance of Barbarea vulgaris to the diamondback moth Plutella xylostella. J. Chem. Ecol. 2003, 29, 1417–1433. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kurashige, N.S. A Role for Isothiocyanates in Plant Resistance Against the Specialist Herbivore Pieris rapae. J. Chem. Ecol. 2003, 29, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Alquézar, B.; Volpe, H.; Magnani, R.F.; De Miranda, M.P.; Santos, M.A.; Wulff, N.A.; Bento, J.M.S.; Parra, J.R.P.; Bouwmeester, H.; Peña, L. β-caryophyllene emitted from a transgenic Arabidopsis or chemical dispenser repels Diaphorina citri, vector of Candidatus Liberibacters. Sci. Rep. 2017, 7, 5639. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Gershenzon, J.; Heckel, D.G. Insect Attraction versus Plant Defense: Young Leaves High in Glucosinolates Stimulate Oviposition by a Specialist Herbivore despite Poor Larval Survival due to High Saponin Content. PLoS ONE 2014, 9, e95766. [Google Scholar] [CrossRef] [PubMed]
- Baskar, K.; Ignacimuthu, S. Antifeedant, larvicidal and growth inhibitory effects of ononitol monohydrate isolated from Cassia tora L. against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Chemosphere 2012, 88, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Birkett, M.A.; Bruce, T.J.; Pickett, J.A. Repellent activity of Nepeta grandiflora and Nepeta clarkei (Lamiaceae) against the cereal aphid, Sitobion avenae (Homoptera: Aphididae). Phytochem. Lett. 2010, 3, 139–142. [Google Scholar] [CrossRef]
- Cespedes, C.; Torres, P.; Marín, J.C.; Arciniegas, A.; De Vivar, A.R.; Pérez-Castorena, A.L.; Aranda, E. Insect growth inhibition by tocotrienols and hydroquinones from Roldana barba-johannis. Phytochemistry 2004, 65, 1963–1975. [Google Scholar] [CrossRef]
- Colom, O.Á.; Neske, A.; Popich, S.; Bardón, A. Toxic effects of annonaceous acetogenins from Annona cherimolia (Magnoliales: Annonaceae) on Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Pest Sci. 2006, 80, 63–67. [Google Scholar] [CrossRef]
- Datta, R.; Kaur, A.; Saraf, I.; Singh, I.P.; Chadha, P.; Kaur, S. Assessment of genotoxic and biochemical effects of purified compounds of Alpinia galanga on a polyphagous lepidopteran pest Spodoptera litura (Fabricius). Phytoparasitica 2020, 48, 501–511. [Google Scholar] [CrossRef]
- Datta, R.; Kaur, A.; Saraf, I.; Singh, I.P.; Kaur, S. Effect of crude extracts and purified compounds of Alpinia galanga on nutritional physiology of a polyphagous lepidopteran pest, Spodoptera litura (Fabricius). Ecotoxicol. Environ. Saf. 2019, 168, 324–329. [Google Scholar] [CrossRef] [PubMed]
- De Souza, C.M.; Baldin, E.L.L.; do Prado Ribeiro, L.; dos Santos, T.L.B.; da Silva, I.F.; Morando, R.; Vendramim, J.D. Antifeedant and growth inhibitory effects of Annonaceae derivatives on Helicoverpa armigera (Hübner). Crop Prot. 2019, 121, 45–50. [Google Scholar] [CrossRef]
- El-Aswad, A.F.; Abdelgaleil, S.A.; Nakatani, M. Feeding deterrent and growth inhibitory properties of limonoids fromKhaya senegalensis against the cotton leafworm, Spodoptera littoralis. Pest Manag. Sci. 2004, 60, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-X.; Wang, Y.; You, C.-X.; Guo, S.-S.; Du, Y.-S.; Du, S.-S. Bioactivities of patchoulol and phloroacetophenone from Pogostemon cablin essential oil against three insects. Int. J. Food Prop. 2019, 22, 1365–1374. [Google Scholar] [CrossRef]
- Giongo, A.M.M.; Vendramim, J.D.; Freitas, S.D.L.; Silva, M.F.G.F. Toxicity of secondary metabolites from Meliaceae against Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2016, 45, 725–733. [Google Scholar] [CrossRef]
- Karlsson, M.F.; Birgersson, G.; Witzgall, P.; Lekfeldt, J.D.S.; Punyasiri, P.N.; Bengtsson, M. Guatemalan potato moth Tecia solanivora distinguish odour profiles from qualitatively different potatoes Solanum tuberosum L. Phytochemistry 2013, 85, 72–81. [Google Scholar] [CrossRef]
- Koul, O.; Singh, G.; Singh, R.; Multani, J. Bioefficacy and mode-of-action of aglaroxin A from Aglaia elaeagnoidea (syn. A. roxburghiana) against Helicoverpa armigera and Spodoptera litura. Èntomol. Exp. Appl. 2005, 114, 197–204. [Google Scholar] [CrossRef]
- Kundu, A.; Mishra, S.; Vadassery, J. Spodoptera litura-mediated chemical defense is differentially modulated in older and younger systemic leaves of Solanum lycopersicum. Planta 2018, 248, 981–997. [Google Scholar] [CrossRef]
- Lv, M.; Wu, W.; Liu, H. Effects of Fraxinellone on the Midgut Enzyme Activities of the 5th Instar Larvae of Oriental Armyworm, Mythimna separata Walker. Toxins 2014, 6, 2708–2718. [Google Scholar] [CrossRef]
- Movva, V.; Pathipati, U.R. Feeding-induced phenol production inCapsicum annuumL. influencesSpodoptera lituraF. larval growth and physiology. Arch. Insect Biochem. Physiol. 2017, 95, e21387. [Google Scholar] [CrossRef]
- Olivero-Verbel, J.; Tirado-Ballestas, I.; Caballero-Gallardo, K.; Stashenko, E.E. Essential oils applied to the food act as repellents toward Tribolium castaneum. J. Stored Prod. Res. 2013, 55, 145–147. [Google Scholar] [CrossRef]
- Pan, L.; Ren, L.; Chen, F.; Feng, Y.; Luo, Y. Antifeedant Activity of Ginkgo biloba Secondary Metabolites against Hyphantria cunea Larvae: Mechanisms and Applications. PLoS ONE 2016, 11, e0155682. [Google Scholar] [CrossRef] [PubMed]
- Selin-Rani, S.; Senthil-Nathan, S.; Thanigaivel, A.; Vasantha-Srinivasan, P.; Edwin, E.-S.; Ponsankar, A.; Lija-Escaline, J.; Kalaivani, K.; Abdel-Megeed, A.; Hunter, W.B.; et al. Toxicity and physiological effect of quercetin on generalist herbivore, Spodoptera litura Fab. and a non-target earthworm Eisenia fetida Savigny. Chemosphere 2016, 165, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Sohal, S.K. Bioefficacy of quercetin against melon fruit fly. Bull. Insectology 2013, 66, 79–83. [Google Scholar]
- Shinoda, T.; Nagao, T.; Nakayama, M.; Serizawa, H.; Koshioka, M.; Okabe, H.; Kawai, A. Identification of a triterpenoid saponin from a crucifer, Barbarea vulgaris, as a feeding deterrent to the diamondback moth, Plutella xylostella. J. Chem. Ecol. 2002, 28, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, P.; Simmonds, M.S.; Yule, M.A.; Veitch, N.C.; Kite, G.C.; Irwin, D.; Legg, M. Insect antifeedant furanocoumarins from Tetradium daniellii. Phytochemistry 2003, 63, 41–46. [Google Scholar] [CrossRef]
- Tattersall, D.B.; Bak, S.; Jones, P.R.; Olsen, C.E.; Nielsen, J.K.; Hansen, M.L.; Høj, P.B.; Møller, B.L. Resistance to an Herbivore Through Engineered Cyanogenic Glucoside Synthesis. Science 2001, 293, 1826–1828. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; Hao, N.; Su, X.; Du, J.; Hu, J.; Tian, X. The antifeedant, insecticidal and insect growth inhibitory activities of triterpenoid saponins from Clematis aethusifolia Turcz against Plutella xylostella (L.). Pest Manag. Sci. 2021, 77, 455–463. [Google Scholar] [CrossRef]
- Yi, X.; Liu, J.; Wang, P.; Hu, M.; Zhong, G. Contacting is essential for oviposition deterrence of rhodojaponin-iii in Spodoptera litura. Arch. Insect Biochem. Physiol. 2014, 86, 122–136. [Google Scholar] [CrossRef]

| Type of Insect | Plant Species | Induced Defense | Protective Function | Reference |
|---|---|---|---|---|
| Effect of JA and Its Derivatives | ||||
| Helicoverpa armigera | Arachis hypogaea | Increased activity of POD, PPO and total phenol, H2O2 and MDA | Host plant defense to herbivore | [87] |
| Pieris rapae and Plutella xylostella | Brassica oleracea | Emission of volatile compounds, such as β-ocimene, thuja 2,4(10)-diene, and terpinene | Attraction of parasitoids, such as Cotesia glomerata, C. rubecula, and Diadegma semiclausum | [88] |
| Nilaparvata lugens | Rice | Emission of plant volatiles, i.e., aliphatic aldehydes, alcohols, monoterpenes, sesquiterpenes, methyl salicylate, etc. | Attracts Anagrus nilaparvatae (egg parasitoid) | [89] |
| Diatraea saccharalis | Sugarcane | Emission of blend of sesquiterpenes | Attracts parasitoid Cotesia flavipes | [90] |
| Plutella xylostella | Brassica napus | Production of glucosinolates and trypsin inhibitor | Reduced survivorship of Plutella xylostella | [91] |
| Spider mites | Lima bean (Phaseolus lunatus) | Transcript levels of (E)-β-ocimene synthase (PlOS) increased and increased emission of (E)-β-ocimene | Enhanced biological control of spider mites due to increased volatiles emission | [92] |
| Effect of SA and Its Derivatives | ||||
| Frankliniella occidentalis | Jacobaea. aquatica | Increased levels of threonine, citric acid, and alanine | Reduced thrips population and inhibited feeding | [93] |
| Oebalus pugnax | Rice, Oryza sativa | Increased plant volatiles production | Reduced damage by stinkbug and prevented formation of spikelet sterility | [94] |
| Aphids/mites | Washington hop yard | Indirect defense | More attraction of Chrysopa nigricornis | [95] |
| Bactrocera dorsalis | Mango | Increased levels of anti-oxidative enzymes, such as catalase, peroxidase, poly phenoloxidase, along with phenol and flavonoid | Reduced oviposition, larval and adult emergence of Bactrocera dorsalis | [96] |
| Helicoverpa armigera | Ground nut | Increased glutathione s-transferase activity | Reduction in larval weight and survival | [87] |
| Psyllid, Agonoscena pistaciae | Pistachio | High phenol and H2O2production | Reduced survival of A. pistaciae | [97] |
| Psyllid, Agonoscena pistaciae | Pistacia vera | Increased production of anti-oxidative enzymes, such as polypheloxidase, and peroxidase | Reduction in the number of eggs and in nymphal density | [98] |
| Effect of Gibberellic Acid(GA) | ||||
| Spodoptera frugiperda | Maize | Increased silicon uptake in plants | Decreased fecundity and reduced feeding on corn in S. frugiperda | [99] |
| Pea aphid, Acyrthosiphon pisum | Medicago truncatula | Increased levels of JA and SA and JA-related plant gene expression | Decreased fitness of A. pisum | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K.; et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. Int. J. Mol. Sci. 2022, 23, 2690. https://doi.org/10.3390/ijms23052690
Divekar PA, Narayana S, Divekar BA, Kumar R, Gadratagi BG, Ray A, Singh AK, Rani V, Singh V, Singh AK, et al. Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. International Journal of Molecular Sciences. 2022; 23(5):2690. https://doi.org/10.3390/ijms23052690
Chicago/Turabian StyleDivekar, Pratap Adinath, Srinivasa Narayana, Bhupendra Adinath Divekar, Rajeev Kumar, Basana Gowda Gadratagi, Aishwarya Ray, Achuit Kumar Singh, Vijaya Rani, Vikas Singh, Akhilesh Kumar Singh, and et al. 2022. "Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection" International Journal of Molecular Sciences 23, no. 5: 2690. https://doi.org/10.3390/ijms23052690
APA StyleDivekar, P. A., Narayana, S., Divekar, B. A., Kumar, R., Gadratagi, B. G., Ray, A., Singh, A. K., Rani, V., Singh, V., Singh, A. K., Kumar, A., Singh, R. P., Meena, R. S., & Behera, T. K. (2022). Plant Secondary Metabolites as Defense Tools against Herbivores for Sustainable Crop Protection. International Journal of Molecular Sciences, 23(5), 2690. https://doi.org/10.3390/ijms23052690







