Comparison of L- and D-Amino Acids for Bacterial Imaging in Lung Infection Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Conditions
2.2. Human Cell Line and Culture Conditions
2.3. Accumulation of 3H-L- and D-Met and Ala in E. coli EC-14
2.4. Accumulation of 3H-L- and D-Met and Ala in HaCaT Cells
2.5. E. coli EC-14 Lung-Infection-Model Mice
2.6. Biological Distribution of E. coli EC-14 Lung-Infection-Model Mice Using 3H-L-Met, 3H-D-Met, and 18F-FDG
3. Results
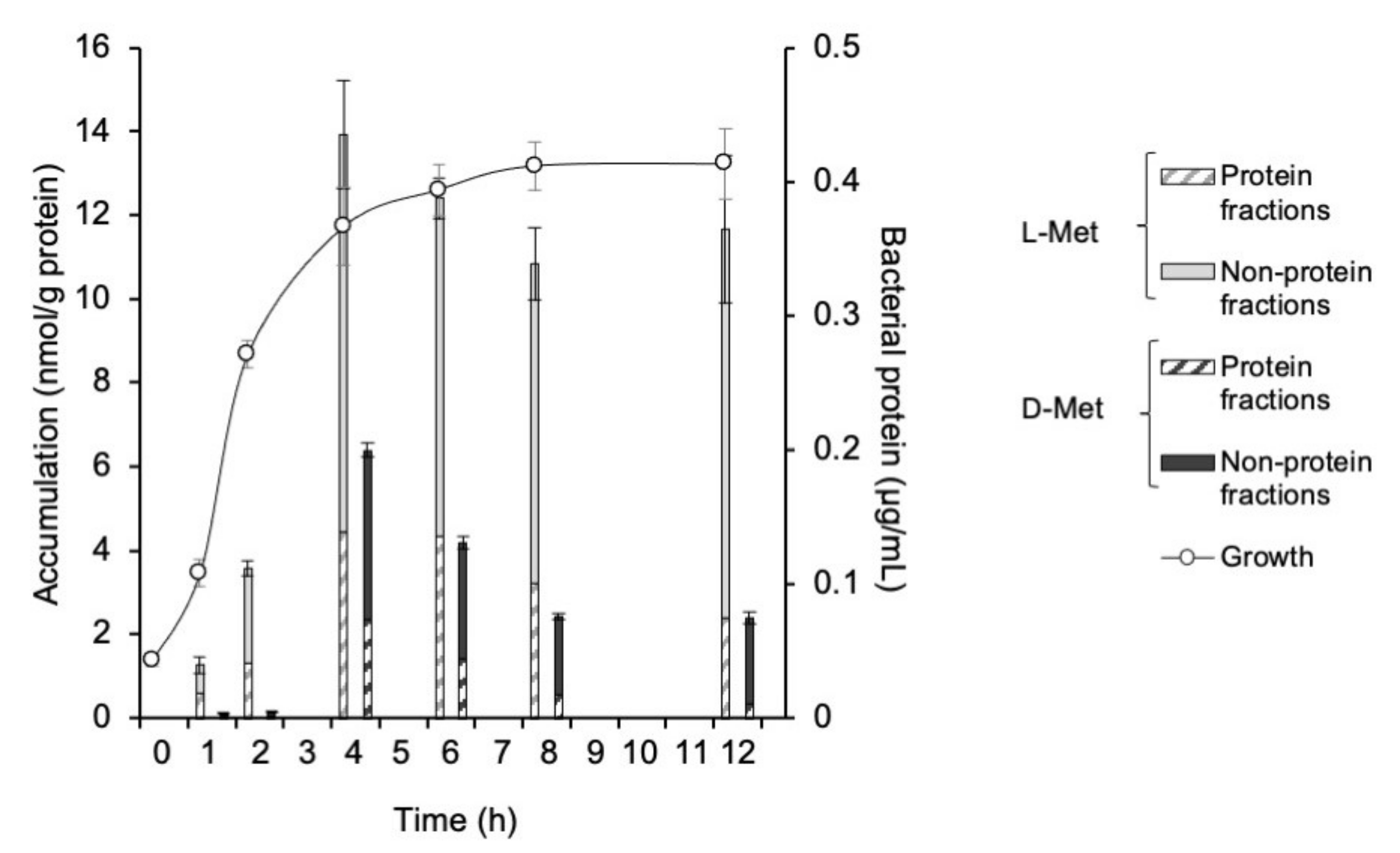
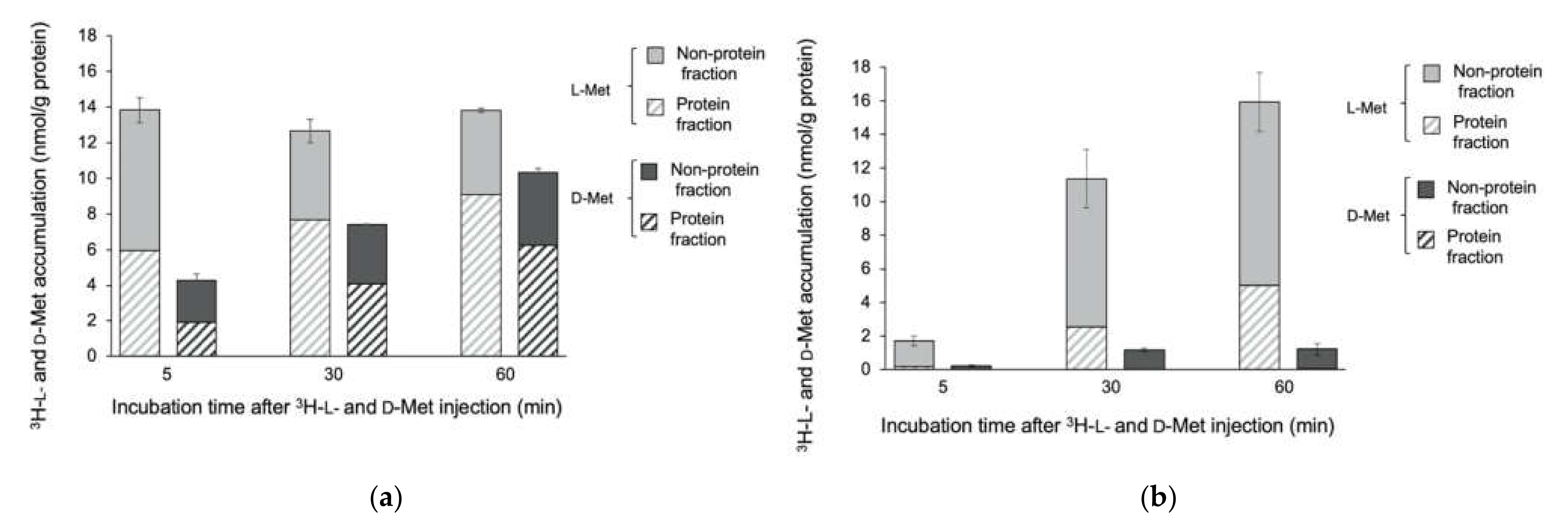
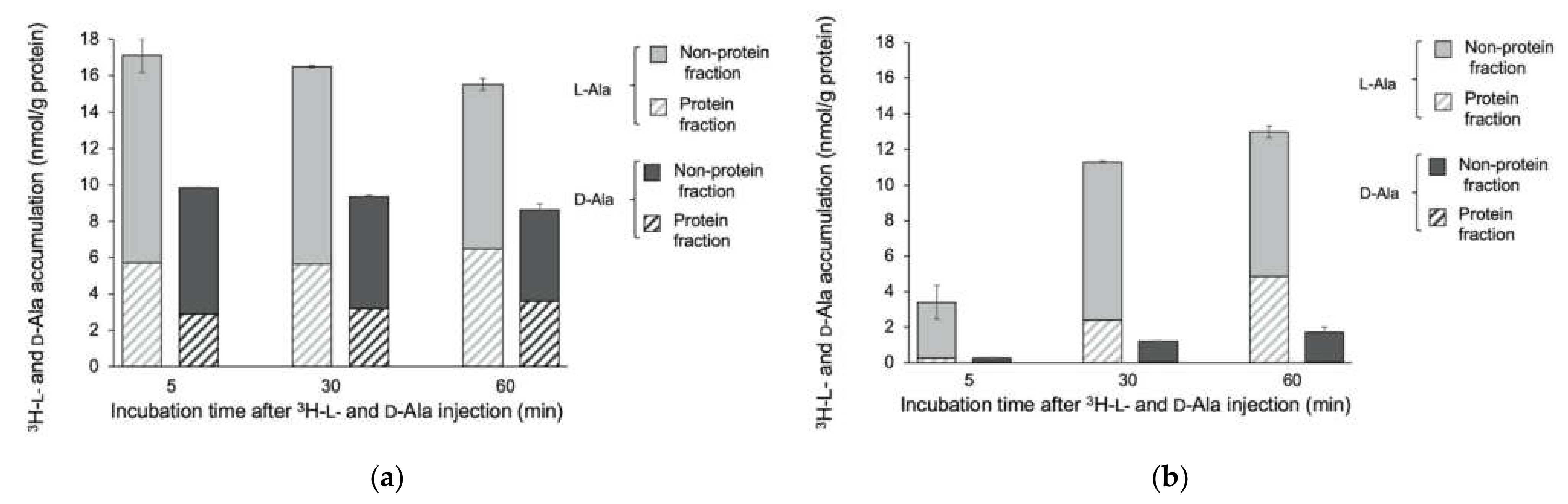
3.1. Accumulation of 3H-L- and D-Met and -Ala in E. coli EC-14
3.2. Accumulation of 3H-L- and D-Met and -Ala in E. coli EC-14 and HaCaT Cells
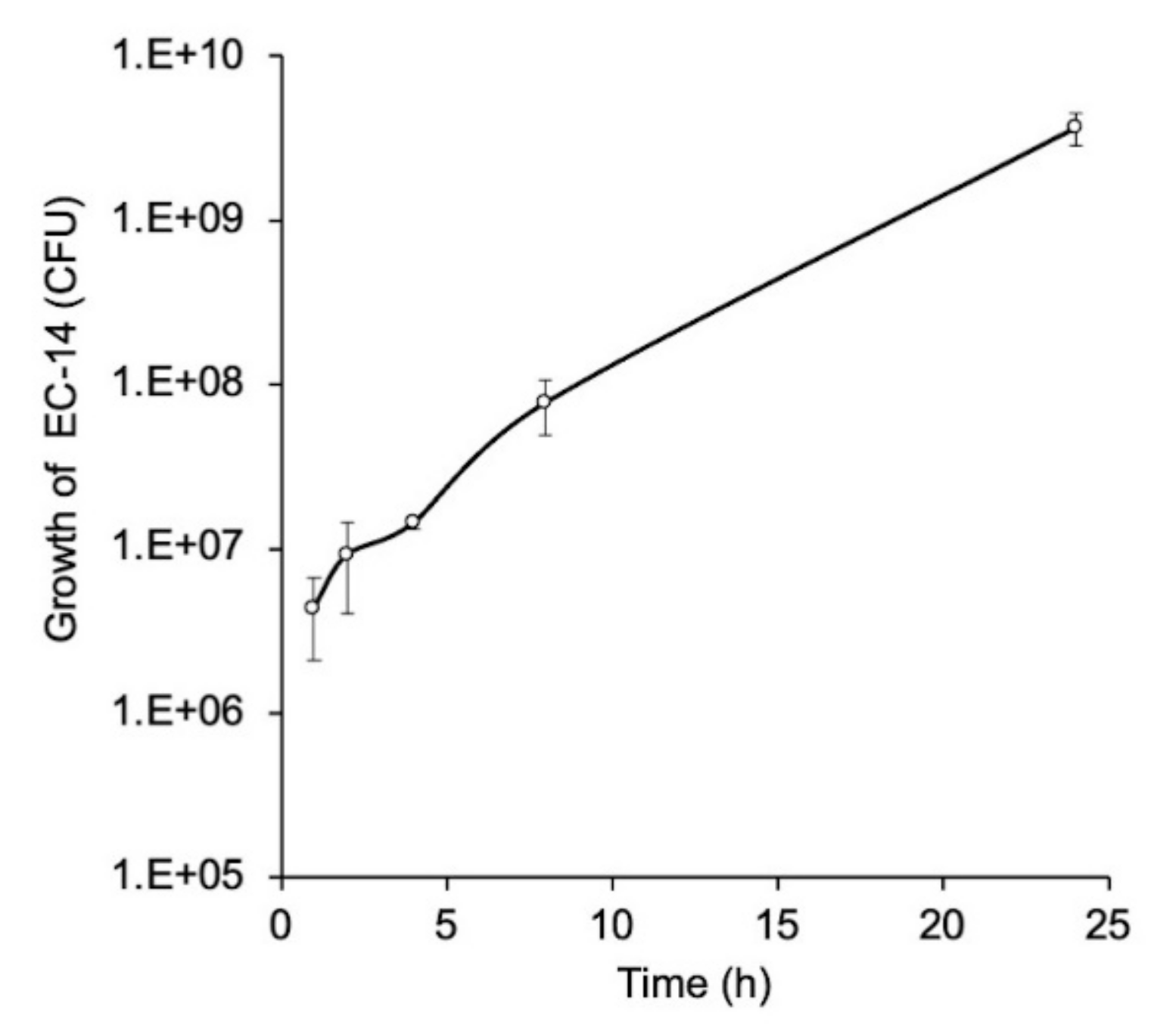
3.3. Growth Curve of E. coli EC-14 in Lung of Lung-Infection-Model Mice
3.4. Biological Distribution of 3H-L-Met, 3H-D-Met, and 18F-FDG in EC-14 Lung-Infection-Model Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tsuzuki, S.; Matsunaga, N.; Yahara, K.; Gu, Y.; Hayakawa, K.; Hirabayashi, A.; Kajihara, T.; Sugai, M.; Shibayama, K.; Ohmagari, N. National trend of blood-stream infection attributable deaths caused by Staphylococcus aureus and Escherichia coli in Japan. J. Infect. Chemother. 2020, 26, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Van Oosten, M.; Hahn, M.; Crane, L.M.; Pleijhuis, R.G.; Francis, K.P.; van Diji, J.M.; van Dam, G.M. Targeted imaging of bacterial infections: Advances, hurdles and hopes. FEMS Microbiol. Rev. 2015, 39, 892–916. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, S.; Barnett, T.C.; Rivera-Hernandez, T.; Rohde, M.; Walker, M.J. Streptococcus pyogenes adhesion and colonization. FEBS Lett. 2016, 590, 3739–3757. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Lagier, J.C.; Edouard, S.; Pagnier, I.; Mediannikov, O.; Drancourt, M.; Raoult, D. Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 2015, 28, 208–236. [Google Scholar] [CrossRef]
- Paolucci, M.; Landimi, M.P.; Sambri, V. Conventional and molecular techniques for the early diagnosis of bacteraemia. Int. J. Antimicrob. Agents. 2010, 36, S6–S16. [Google Scholar] [CrossRef]
- Signore, A.; Artiko, V.; Conserva, M.; Ferro-Flores, G.; Welling, M.; Jain, S.; Hess, S.; Sathekge, M. Imaging Bacteria with Radiolabelled Probes: Is It Feasible? J. Clin. Med. 2020, 9, 2372. [Google Scholar] [CrossRef]
- Mota, F.; Ordonez, A.A.; Firth, G.; Ruiz-Bedoya, C.A.; Ma, M.T.; Jain, S.K. Radiotracer Development for Bacterial Imaging. J. Med. Chem. 2020, 63, 1964–1977. [Google Scholar] [CrossRef]
- Kato, T.; Shinoda, J.; Nakayama, N.; Miwa, K.; Okumura, A.; Yano, H.; Yoshimura, S.; Maruyama, T.; Muragaki, Y.; Iwama, T. Metabolic assessment of gliomas using 11C-methionine, [18F] fluorodeoxyglucose, and 11C-choline positron-emission tomography AJNR Am. J. Neuroradiol. 2008, 29, 1176–1182. [Google Scholar] [CrossRef]
- Love, C.; Tomas, M.B.; Tronco, G.G.; Palestro, C.J. FDG PET of infection and inflammation. Radiographics 2005, 25, 1357–1368. [Google Scholar] [CrossRef]
- Neumann, K.D.; Villanueva-Meyer, J.E.; Mutch, C.A.; Flavell, R.R.; Blecha, J.E.; Kwak, T.; Sriram, R.; VanBrocklin, H.F.; Rosenberg, O.S.; Ohliger, M.A.; et al. Imaging active infection in vivo using D-amino acid derived PET radiotracers. Sci. Rep. 2017, 7, 7903. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Lagier, J.C.; Raoult, D.; Khelaifia, S. Bacterial culture through selective and non-selective conditions: The evolution of culture media in clinical microbiology. New Microbes New Infect. 2020, 30, 100622. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Blanot, D.; de Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mizutani, A.; Nishii, K.; Nakajima, S.; Shikano, N.; Nishii, R.; Fukuchi, K.; Kawai, K. Differences in accumulation and the transport mechanism of L- and D-methionine in high- and low-grade human glioma cells. Nucl. Med. Biol. 2017, 44, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hashimoto, F.; Ohe, K.; Nadamura, T.; Nishi, K.; Shikano, N.; Nishii, R.; Higashi, T.; Okazawa, H.; Kawai, K. Transport mechanism of 11C-labeled L-and D-methionine in human-derived tumor cells. Nucl. Med. Biol. 2012, 39, 1213–1218. [Google Scholar] [CrossRef]
- Kagawa, S.; Nishii, R.; Higashi, T.; Yamauchi, H.; Ogawa, E.; Okudaira, H.; Kobayashi, M.; Yoshimoto, M.; Shikano, N.; Kawai, K. Relationship between [14C]MeAIB uptake and amino acid transporter family gene expression levels or proliferative activity in a pilot study in human carcinoma cells: Comparison with [3H]methionine uptake. Nucl. Med. Biol. 2017, 49, 8–15. [Google Scholar] [CrossRef][Green Version]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Aliashkevich, A.; Alvarez, L.; Cava, F. New insights into the mechanisms and biological roles of D-amino acids in complex eco-systems. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Lam, H.; Oh, D.C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science 2009, 18, 1552–1555. [Google Scholar] [CrossRef]
- Cava, F.; Lam, H.; de Pedro, M.A.; Waldor, M.K. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol. Life Sci. 2011, 68, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Radkov, A.D.; Moe, L.A. Bacterial synthesis of D-amino acids. applied microbiology and biotechnology. Appl. Microbiol. Biotechnol. 2014, 98, 5363–5374. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Santoni-Rugiu, E.; Ralfkiaer, E.; Porse, B.T.; Moser, C.; Høiby, N.; Borregaard, N.; Cowland, J.B. Lipocalin 2 is protective against E. coli pneumonia. Respir. Res. 2010, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Dietert, K.; Gutbier, B.; Wienhold, S.M.; Reppe, K.; Jiang, X.; Yao, L.; Chaput, C.; Naujoks, J.; Brack, M.; Kupke, A.; et al. Spectrum of pathogen-and model-specific histopathologies in mouse models of acute pneumonia. PLoS ONE 2017, 12, e0188251. [Google Scholar] [CrossRef]
- Casali, M.; Lauri, C.; Altini, C.; Bertagna, F.; Cassarino, G.; Cistaro, A.; Erba, A.P.; Ferrari, C.; Mainolfi, C.G.; Palucci, A.; et al. State of the art of 18F-FDG PET/CT application in inflammation and infection: A guide for image acquisition and interpretation. Clin. Transl. Imaging 2021, 9, 299–339. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tsujikawa, T.; Kondo, C.; Maki, M.; Momose, M.; Nagai, M.; Ohnuki, T.; Nishikawa, T.; Kusakabe, K. Accuracy of PET for Diagnosis of Solid Pulmonary Lesions with 18F-FDG Uptake Below the Standardized Uptake Value of 2.5. J. Nucl. Med. 2006, 47, 426–431. [Google Scholar]

| Time after 3H-L-Met Injection | 15 min | 60 min | 15 min | 60 min | 15 min | 60 min |
|---|---|---|---|---|---|---|
| Organ (%ID/g) | Control | 4 h after Infection | 24 h after Infection | |||
| Blood | 0.50 ± 0.18 | 0.50 ± 0.43 | 1.61 ± 0.82 * | 1.51±0.07 † | 2.74 ± 0.86 † | 1.24 ± 0.86 * |
| Heart | 1.65 ± 0.74 | 1.89 ± 0.31 | 2.96 ± 0.96 | 3.74± 0.10 † | 1.35 ± 0.19 | 1.40 ± 0.27 |
| Lung | 1.49 ± 0.19 | 1.57 ± 0.26 | 4.16 ± 0.60 † | 4.06 ± 0.12 † | 3.13 ± 1.09 * | 2.47 ± 0.10 † |
| Liver | 1.66 ± 0.53 | 2.09 ± 0.84 | 3.16 ± 0.20 † | 4.74 ± 0.14 † | 6.19 ± 0.93 † | 9.43 ± 0.82 † |
| Spleen | 1.39 ± 0.57 | 2.47 ± 0.63 | 7.06 ± 1.81 † | 6.09 ± 2.79 * | 3.01 ± 0.55 † | 3.23 ± 1.81 |
| Pancreas | 4.29 ± 4.68 | 12.47 ± 4.68 | 22.01 ± 6.38 † | 21.69 ± 3.40 * | 14.39 ± 2.53 † | 11.94 ± 0.94 |
| Kidney | 1.67 ± 0.62 | 3.17 ± 0.49 | 5.13 ± 2.83 * | 5.92± 0.73 † | 5.15 ± 0.40 † | 4.43 ± 0.72 * |
| Brain | 4.10 ± 4.61 | 0.51 ± 0.10 | 2.80 ± 3.39 | 1.11 ± 0.48 | 1.41 ± 0.17 | 1.36 ± 0.18 † |
| Bladder | 0.20 ± 0.17 | 0.35 ± 0.27 | 0.55 ± 0.36 | 1.15± 0.42 * | 0.46 ± 0.41 | 0.46 ± 0.41 * |
| Lung/heart | 1.03 ± 0.39 | 0.83 ± 0.03 | 1.51 ± 0.51 | 1.09 ± 0.02 † | 2.27 ± 0.47 † | 1.82 ± 0.41 † |
| Lung/liver | 0.94 ± 0.20 | 0.85 ± 0.38 | 1.32 ± 0.20 * | 0.86 ± 0.03 | 0.50 ± 0.13 * | 0.26 ± 0.04 * |
| Infected lung /control lung | – | – | 2.80 | 2.58 | 2.11 | 1.57 |
| Time after 3H-D-Met Injection | 15 min | 60 min | 15 min | 60 min | 15 min | 60 min |
|---|---|---|---|---|---|---|
| Organ (%ID/g) | Control | 4 h after Infection | 24 h after Infection | |||
| Blood | 0.34 ± 0.14 | 0.27 ± 0.07 | 1.71 ± 0.17 † | 1.09 ± 0.08 † | 2.54 ± 0.83 † | 2.27 ± 0.66 † |
| Heart | 1.01 ± 0.25 | 0.92 ± 0.31 | 2.32 ± 0.07 † | 2.18 ± 0.38 † | 1.25 ± 0.09 | 1.28 ± 0.30 |
| Lung | 1.01 ± 0.10 | 1.02 ± 0.33 | 2.87 ± 0.28 † | 2.52 ± 0.55 † | 2.34 ± 0.09 † | 2.34 ± 0.62 † |
| Liver | 1.14 ± 0.13 | 1.64 ± 0.80 | 3.01 ± 0.26 † | 3.80 ± 0.26 † | 4.32 ± 0.24 † | 7.12 ± 0.75 † |
| Spleen | 1.29 ± 0.25 | 2.01 ± 0.30 | 4.70 ± 0.37 † | 6.78 ± 2.16 † | 2.06 ± 0.42 * | 4.05 ± 1.12 * |
| Pancreas | 7.70 ± 1.25 | 8.77 ± 3.45 | 19.14 ± 2.18 † | 16.56 ± 4.03 * | 12.07 ± 1.57 † | 15.68 ± 3.51 * |
| Kidney | 3.36 ± 0.89 | 3.10 ± 0.42 | 8.79 ± 0.67 † | 8.15 ± 1.26 † | 9.11 ± 1.55 † | 10.25 ± 1.43 † |
| Brain | 0.44 ± 0.06 | 0.59 ± 0.13 | 1.21 ± 0.17 † | 1.28 ± 0.36 * | 0.92 ± 0.15 † | 1.60 ± 0.15 † |
| Bladder | 0.62 ± 0.36 | 0.70 ± 0.49 | 1.94 ± 0.41 † | 0.69 ± 0.13 | 2.01 ± 0.85 * | – |
| Lung/heart | 1.06 ± 0.33 | 1.20 ± 0.50 | 1.24 ± 0.14 | 1.17 ± 0.23 | 1.88 ± 0.18 † | 1.88 ± 0.52 |
| Lung/liver | 0.91 ± 0.20 | 0.68 ± 0.24 | 0.96 ± 0.10 | 0.67 ± 0.18 | 0.54 ± 0.03 * | 0.33 ± 0.10 * |
| Infected lung /control lung | – | – | 2.84 | 2.48 | 2.31 | 2.30 |
| Time after 18F-FDG Injection | 15 min | 60 min | 15 min | 60 min | 15 min | 60 min |
|---|---|---|---|---|---|---|
| Organ (%ID/g) | Control | 4 h after Infection | 24 h after Infection | |||
| Blood | 1.98 ± 0.58 | 0.45 ± 0.09 | 1.84 ± 0.85 | 0.41 ± 0.06 | 2.59 ± 0.72 | 0.49 ± 0.15 |
| Heart | 13.14 ± 7.34 | 11.23 ± 6.51 | 18.28 ± 8.61 | 17.07 ± 7.99 | 5.72 ± 3.23 * | 9.85 ± 5.13 |
| Lung | 3.41 ± 0.34 | 3.57 ± 0.80 | 4.58 ± 0.91 † | 3.99 ± 0.71 | 7.82 ± 1.21 † | 9.31 ± 1.79 † |
| Liver | 2.85 ± 1.39 | 0.99 ± 0.21 | 2.47 ± 0.54 | 0.89 ± 0.13 | 4.65 ± 0.94 † | 2.34 ± 1.04 † |
| Spleen | 2.68 ± 0.68 | 3.42 ± 0.47 | 3.44 ± 0.63 * | 3.53 ± 1.24 | 5.92 ± 2.90 † | 9.86 ± 6.01 † |
| Pancreas | 2.42 ± 1.57 | 1.69 ± 0.31 | 1.64 ± 0.30 | 1.61 ± 0.11 | 2.66 ± 1.79 | 2.16 ± 0.35 * |
| Kidney | 5.97 ± 3.73 | 1.90 ± 0.51 | 5.40 ± 2.77 | 3.30 ± 1.82 | 6.69 ± 0.74 | 3.14 ± 0.54 † |
| Brain | 5.12 ± 2.35 | 6.39 ± 1.03 | 6.97 ± 1.46 | 5.11 ± 1.49 | 12.46 ± 1.53 † | 11.51 ± 2.78 † |
| Bladder | 1.50 ± 0.84 | 2.04 ± 1.19 | 1.86 ± 0.87 | 1.76 ± 1.13 | 1.67 ± 1.13 | - |
| Lung/heart | 0.36 ± 0.22 | 0.43 ± 0.33 | 0.36 ± 0.34 | 0.30 ± 0.18 | 1.72 ± 0.76 † | 1.15 ± 0.52 † |
| Lung/liver | 1.34 ± 0.37 | 3.86 ± 1.60 | 1.88 ± 0.31 † | 4.52 ± 0.84 | 1.71 ± 0.23 * | 4.49 ± 1.49 |
| Infected lung /control lung | - | - | 1.34 | 1.12 | 2.29 | 2.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muranaka, Y.; Mizutani, A.; Kobayashi, M.; Nakamoto, K.; Matsue, M.; Nishi, K.; Yamazaki, K.; Nishii, R.; Shikano, N.; Okamoto, S.; et al. Comparison of L- and D-Amino Acids for Bacterial Imaging in Lung Infection Mouse Model. Int. J. Mol. Sci. 2022, 23, 2467. https://doi.org/10.3390/ijms23052467
Muranaka Y, Mizutani A, Kobayashi M, Nakamoto K, Matsue M, Nishi K, Yamazaki K, Nishii R, Shikano N, Okamoto S, et al. Comparison of L- and D-Amino Acids for Bacterial Imaging in Lung Infection Mouse Model. International Journal of Molecular Sciences. 2022; 23(5):2467. https://doi.org/10.3390/ijms23052467
Chicago/Turabian StyleMuranaka, Yuka, Asuka Mizutani, Masato Kobayashi, Koya Nakamoto, Miki Matsue, Kodai Nishi, Kana Yamazaki, Ryuichi Nishii, Naoto Shikano, Shigefumi Okamoto, and et al. 2022. "Comparison of L- and D-Amino Acids for Bacterial Imaging in Lung Infection Mouse Model" International Journal of Molecular Sciences 23, no. 5: 2467. https://doi.org/10.3390/ijms23052467
APA StyleMuranaka, Y., Mizutani, A., Kobayashi, M., Nakamoto, K., Matsue, M., Nishi, K., Yamazaki, K., Nishii, R., Shikano, N., Okamoto, S., & Kawai, K. (2022). Comparison of L- and D-Amino Acids for Bacterial Imaging in Lung Infection Mouse Model. International Journal of Molecular Sciences, 23(5), 2467. https://doi.org/10.3390/ijms23052467








