Recent Advances of Microneedles and Their Application in Disease Treatment
Abstract
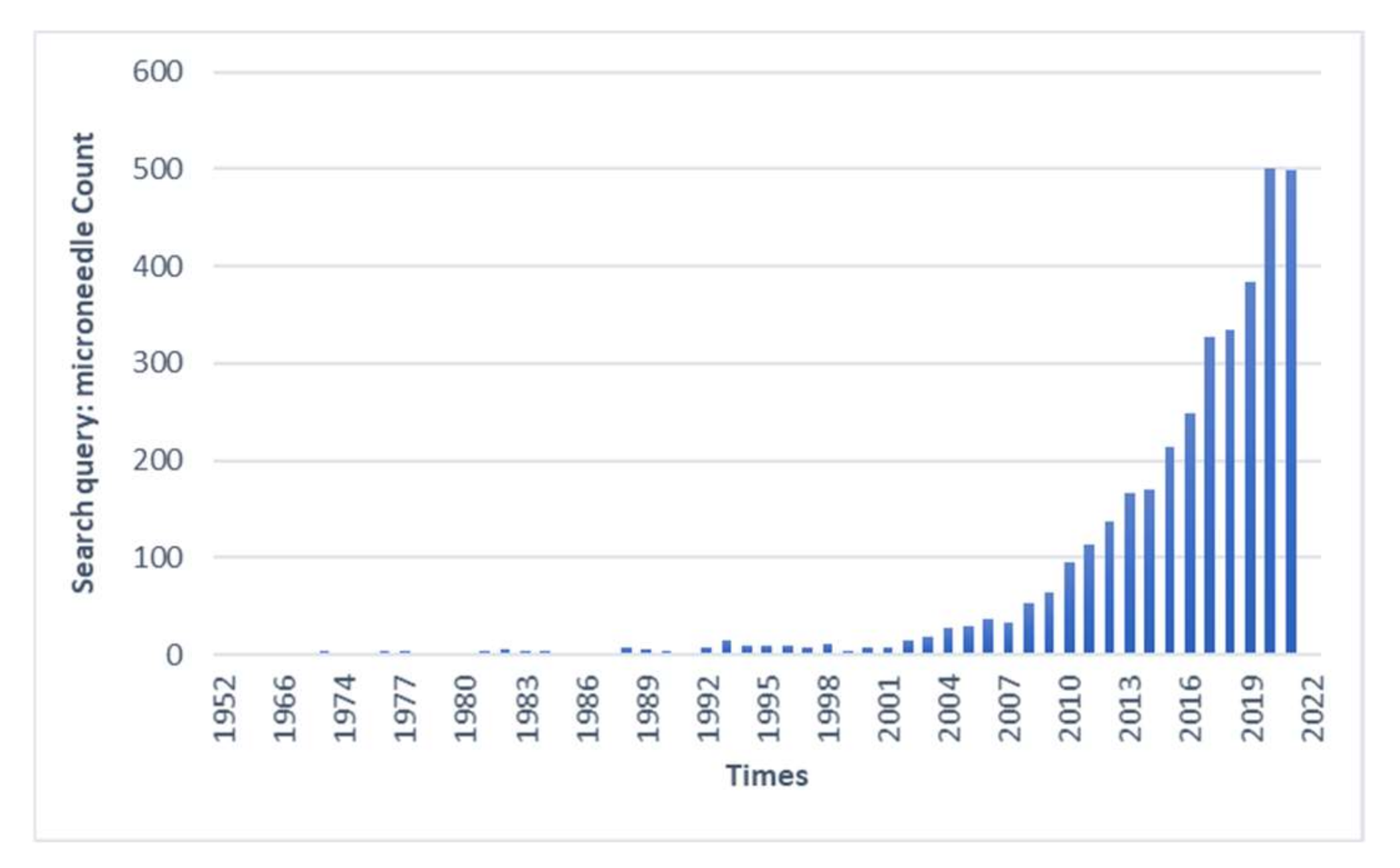
1. Introduction
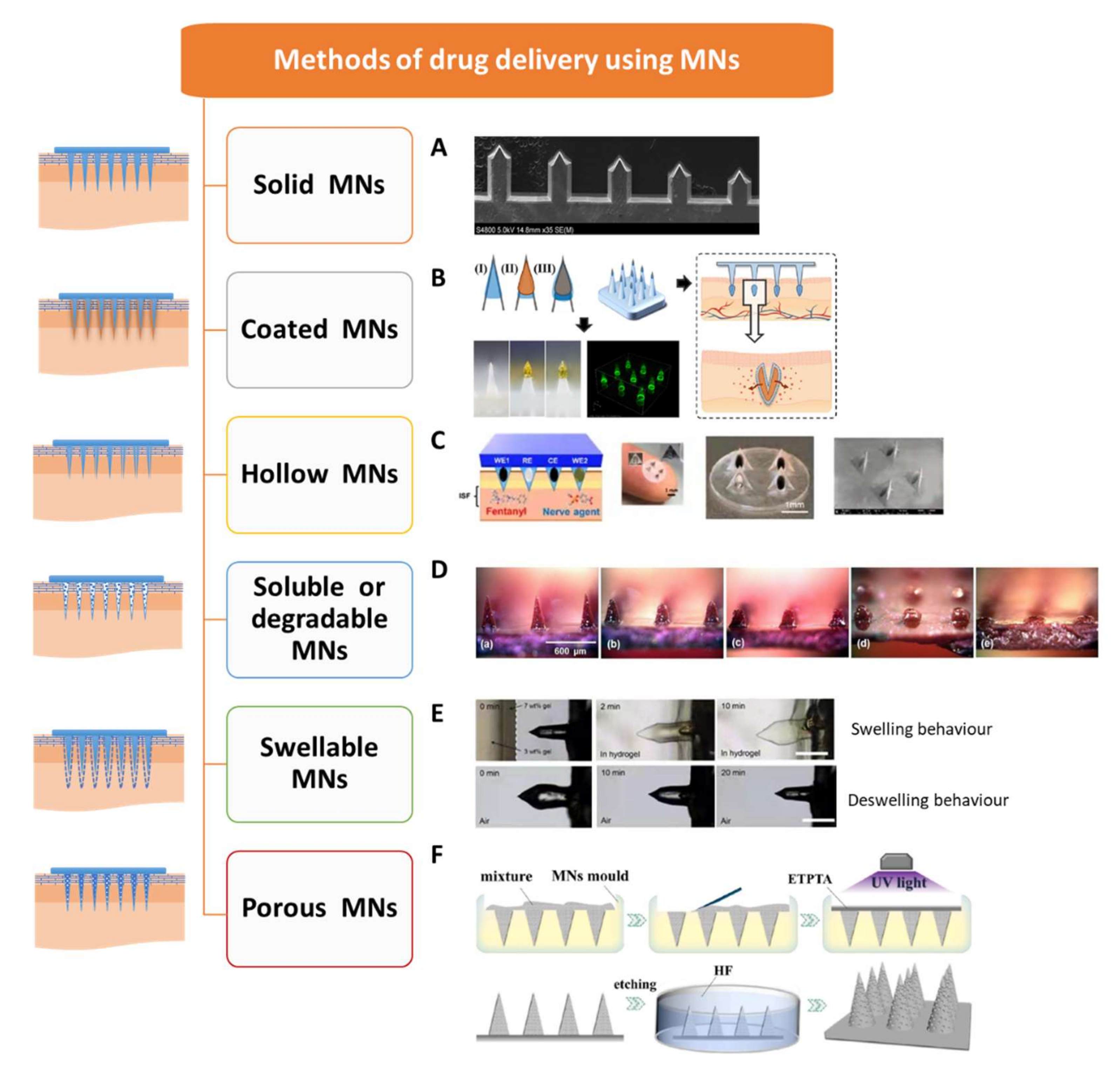
2. Types of MNs
2.1. Solid MNs
2.2. Coated MNs
2.3. Hollow MNs
2.4. Soluble or Degradable MNs
2.5. Swellable MNs
2.6. Porous MNs
3. Fabrication of MNs
3.1. Materials and Properties of MNs
3.2. Design of MNs
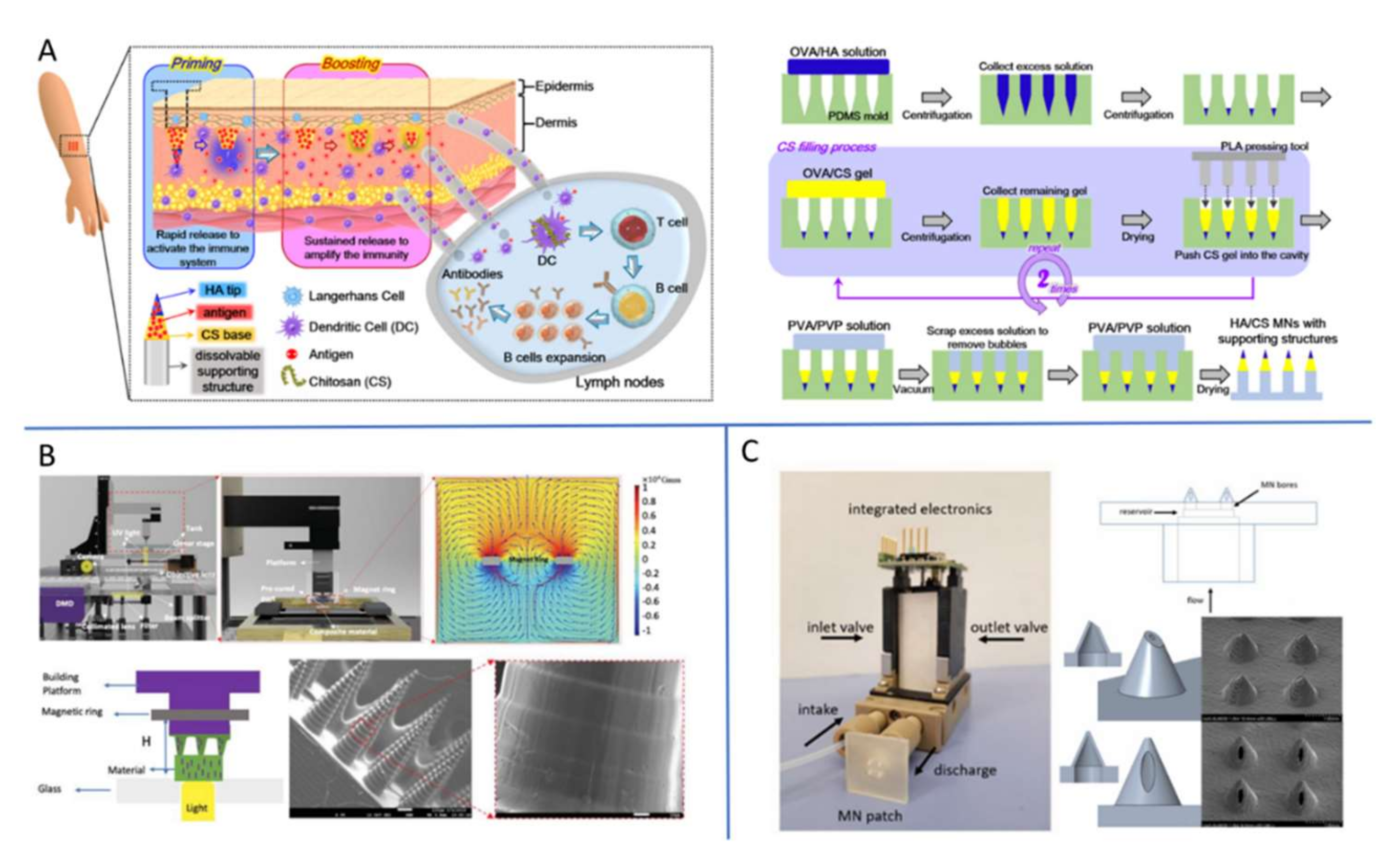
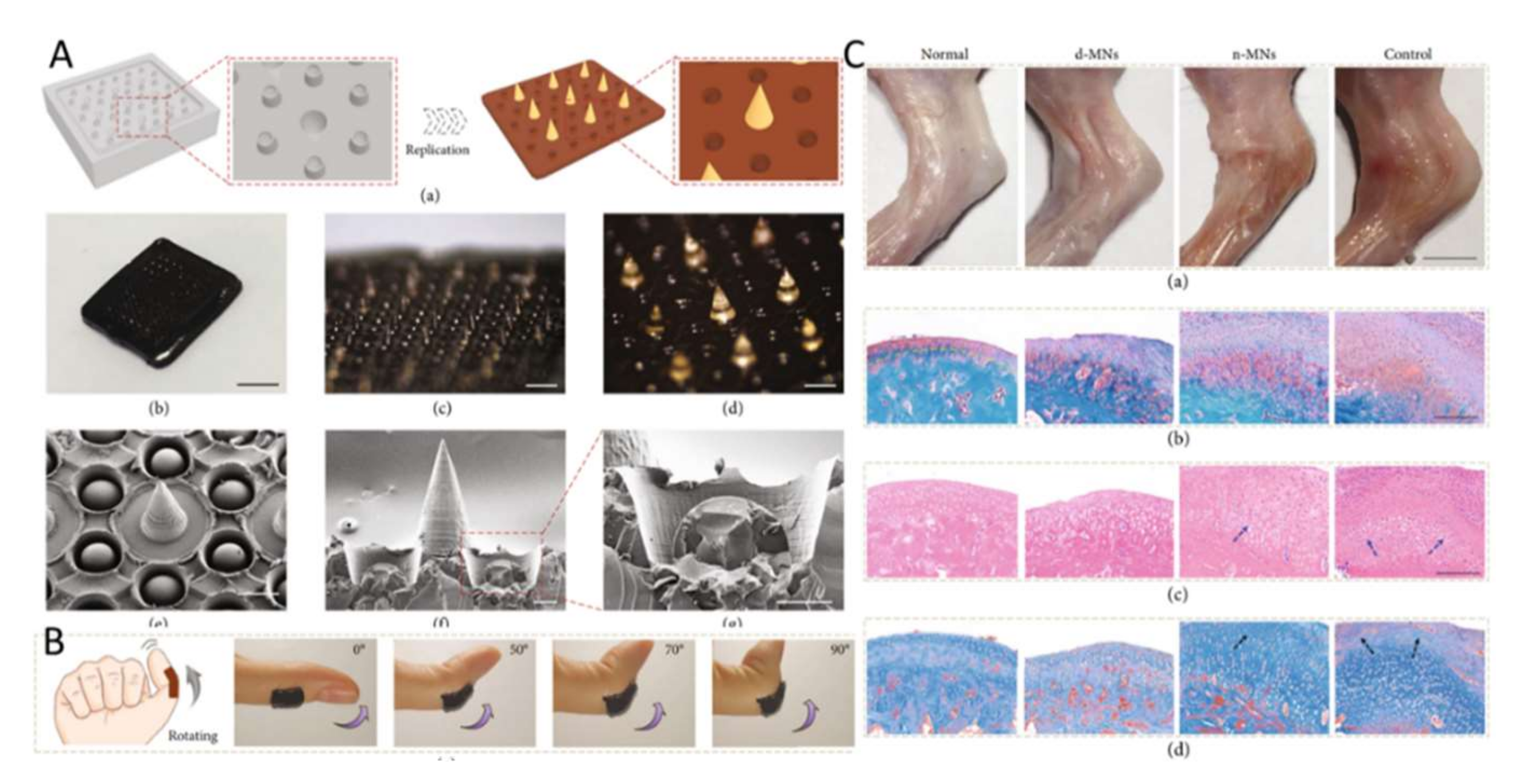
3.3. Fabrication Technology of MNs
4. Biomedical Applications and Latest Research Progress of MNs
4.1. The Treatment of Osteoarthritis
4.2. The Treatment of Rheumatoid Arthritis
4.3. The Treatment of Dermatology Dermatosis
4.4. The Delivery of Vaccine
4.5. The Treatment of Cancer
4.6. Usage in the Contraception Field
4.7. The Treatment of Diabetes
4.8. Other Applications
5. Outlook: Nanotechnology Potentiates MNs in Biomedicine
Author Contributions
Funding
Conflicts of Interest
References
- McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated Microneedles for Gene and Drug Delivery. Annu. Rev. Biomed. Eng. 2000, 2, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, J.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliver. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.J.; Bonilla-Martinez, D.; Villegas-Gonzalez, M.A.; Molina-Trinidad, E.; Casas-Alancaster, N.; Revilla-Vazquez, A.L. Microneedles: A valuable physical enhancer to increase transdermal drug delivery. J. Clin. Pharmacol. 2011, 51, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.; Banga, A.K. Microneedles and transdermal drug delivery. J. Drug Deliv. Sci. Tec. 2009, 19, 303–310. [Google Scholar] [CrossRef]
- Yan, L.; Alba, M.; Tabassum, N.; Voelcker, N.H. Micro-and Nanosystems for Advanced Transdermal Delivery. Adv. Therap. 2019, 2, 1900141. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.O.; Fathe, K.R.; Brunaugh, A.; Ferrati, S.; Li, S.; Montenegro-Nicolini, M.; Mousavikhamene, Z.; McConville, J.T.; Prausnitz, M.R.; Smyth, H.D.C. Challenges and Future Prospects for the Delivery of Biologics: Oral Mucosal, Pulmonary, and Transdermal Routes. AAPS J. 2017, 19, 652–668. [Google Scholar] [CrossRef]
- Hegde, N.R.; Kaveri, S.V.; Bayry, J. Recent advances in the administration of vaccines for infectious diseases: Microneedles as painless delivery devices for mass vaccination. Drug Discov. Today 2011, 16, 1061–1068. [Google Scholar] [CrossRef]
- Teo, A.L.; Shearwood, C.; Ng, K.C.; Lu, J.; Moochhala, S. Transdermal microneedles for drug delivery applications. Mater. Sci. Eng. B 2006, 132, 151–154. [Google Scholar] [CrossRef]
- Rzhevskiy, A.S.; Singh, T.R.R.; Donnelly, R.F.; Anissimov, Y.G. Microneedles as the technique of drug delivery enhancement in diverse organs and tissues. J. Control. Release 2018, 270, 184–202. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.H.; Laurent, P.E. Intradermal vaccine delivery: Will new delivery systems transform vaccine administration? Vaccine 2008, 26, 3197–3208. [Google Scholar] [CrossRef] [PubMed]
- Gniadecka, M.; Jemec, G.B. Quantitative evaluation of chronological ageing and photoageing in vivo: Studies on skin echogenicity and thickness. Br. J. Dermatol. 1998, 139, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, W.; Huang, Z.; Liu, S.; Ye, Y.; Li, Q.; Huang, M. Fabrication of Tip-Dissolving Microneedles for Transdermal Drug Delivery of Meloxicam. AAPS Pharmscitech. 2017, 19, 1141–1151. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Garland, M.J.; Morrow, D.I.; Migalska, K.; Singh, T.R.; Majithiya, R.; Woolfson, A.D. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J. Control. Release 2010, 147, 333–941. [Google Scholar] [CrossRef]
- Li, W.; Tang, J.; Terry, R.N.; Li, S.; Brunie, A.; Callahan, R.L.; Noel, R.K.; Rodríguez, C.A.; Schwendeman, S.P.; Prausnitz, M.R. Long-acting reversible contraception by effervescent microneedle patch. Sci. Adv. 2019, 5, eaaw8145. [Google Scholar] [CrossRef]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef]
- Amodwala, S.; Kumar, P.; Thakkar, H.P. Statistically optimized fast dissolving microneedle transdermal patch of meloxicam: A patient friendly approach to manage arthritis. Eur. J. Pharm. Sci. 2017, 104, 114–123. [Google Scholar] [CrossRef]
- Yang, G.; Chen, Q.; Wen, D.; Chen, Z.; Wang, J.; Chen, G.; Wang, Z.; Zhang, X.; Zhang, Y.; Hu, Q.; et al. A Therapeutic Microneedle Patch Made from Hair-Derived Keratin for Promoting Hair Regrowth. ACS Nano 2019, 13, 4354–4360. [Google Scholar] [CrossRef]
- Yang, J.; Liu, X.; Fu, Y.; Song, Y. Recent advances of microneedles for biomedical applications: Drug delivery and beyond. Acta Pharm. Sin. B 2019, 9, 469–483. [Google Scholar] [CrossRef]
- Bao, L.; Park, J.; Bonfante, G.; Kim, B. Recent advances in porous microneedles: Materials, fabrication, and transdermal applications. Drug Deliv. Transl. Res. 2022, 12, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Howells, O.; Blayney, G.J.; Gualeni, B.; Birchall, J.C.; Eng, P.F.; Ashraf, H.; Sharma, S.; Guy, O.J. Design, fabrication, and characterisation of a silicon microneedle array for transdermal therapeutic delivery using a single step wet etch process. Eur. J. Pharm. Biopharm. 2021, 171, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, S.; Yang, G.; Gao, Y. Enhanced delivery efficiency and sustained release of biopharmaceuticals by complexation-based gel encapsulated coated microneedles: rhIFNα-1β example. Asian J. Pharm. Sci. 2021, 16, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Goud, K.Y.; Li, Z.; Moonla, C.; Mohamed, M.A.; Tehrani, F.; Teymourian, H.; Wang, J. Continuous Opioid Monitoring along with Nerve Agents on a Wearable Microneedle Sensor Array. J. Am. Chem. Soc. 2020, 142, 5991–5995. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef]
- Seong, K.; Seo, M.; Hwang, D.Y.; O’Cearbhaill, E.D.; Sreenan, S.; Karp, J.M.; Yang, S.Y. A self-adherent, bullet-shaped microneedle patch for controlled transdermal delivery of insulin. J. Control. Release 2017, 265, 48–56. [Google Scholar] [CrossRef]
- Yi, K.; Wang, Y.; Shi, K.; Chi, J.; Lyu, J.; Zhao, Y. Aptamer-decorated porous microneedles arrays for extraction and detection of skin interstitial fluid biomarkers. Biosens. Bioelectron. 2021, 190, 113404. [Google Scholar] [CrossRef]
- Lee, K.; Goudie, M.J.; Tebon, P.; Sun, W.; Luo, Z.; Lee, J.; Zhang, S.; Fetah, K.; Kim, H.; Xue, Y.; et al. Non-transdermal microneedles for advanced drug delivery. Adv. Drug Deliver. Rev. 2019, 165–166, 41–59. [Google Scholar] [CrossRef]
- Jamaledin, R.; Di Natale, C.; Onesto, V.; Taraghdari, Z.B.; Zare, E.N.; Makvandi, P.; Vecchione, R.; Netti, P.A. Progress in Microneedle-Mediated Protein Delivery. J. Clin. Med. 2020, 9, 542. [Google Scholar] [CrossRef]
- Zeng, Q.; Gammon, J.M.; Tostanoski, L.H.; Chiu, Y.; Jewell, C.M. In Vivo Expansion of Melanoma-Specific T Cells Using Microneedle Arrays Coated with Immune-Polyelectrolyte Multilayers. ACS Biomater. Sci. Eng. 2016, 3, 195–205. [Google Scholar] [CrossRef]
- Garland, M.J.; Migalska, K.; Mahmood, T.M.; Singh, T.R.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as medical devices for enhanced transdermal drug delivery. Expert Rev. Med. Devices 2011, 8, 459–482. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Kole, P.L.; Mahmood, T.M.; McCarthy, H.O.; Woolfson, A.D. Hydrogel-Forming Microneedle Arrays for Enhanced Transdermal Drug Delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ling, M.; Lai, K.; Pramudityo, E. Chitosan Microneedle Patches for Sustained Transdermal Delivery of Macromolecules. Biomacromolecules 2012, 13, 4022–4031. [Google Scholar] [CrossRef] [PubMed]
- DeMuth, P.C.; Garcia-Beltran, W.F.; Ai-Ling, M.L.; Hammond, P.T.; Irvine, D.J. Composite Dissolving Microneedles for Coordinated Control of Antigen and Adjuvant Delivery Kinetics in Transcutaneous Vaccination. Adv. Funct. Mater. 2013, 23, 161–172. [Google Scholar] [CrossRef]
- Chang, H.; Zheng, M.; Yu, X.; Than, A.; Seeni, R.Z.; Kang, R.; Tian, J.; Khanh, D.P.; Liu, L.; Chen, P.; et al. A Swellable Microneedle Patch to Rapidly Extract Skin Interstitial Fluid for Timely Metabolic Analysis. Adv. Mater. 2017, 29, 1702243. [Google Scholar] [CrossRef]
- Takeuchi, K.; Takama, N.; Sharma, K.; Paul, O.; Ruther, P.; Suga, T.; Kim, B. Microfluidic chip connected to porous microneedle array for continuous ISF sampling. Drug Deliv. Transl. Res. 2022, 12, 435–443. [Google Scholar] [CrossRef]
- Liu, L.; Kai, H.; Nagamine, K.; Ogawa, Y.; Nishizawa, M. Porous polymer microneedles with interconnecting microchannels for rapid fluid transport. RSC Adv. 2016, 6, 48630–48635. [Google Scholar] [CrossRef]
- Li, J.; Liu, B.; Zhou, Y.; Chen, Z.; Jiang, L.; Yuan, W.; Liang, L. Fabrication of a Ti porous microneedle array by metal injection molding for transdermal drug delivery. PLoS ONE 2017, 12, e0172043. [Google Scholar] [CrossRef]
- Kusama, S.; Sato, K.; Matsui, Y.; Kimura, N.; Abe, H.; Yoshida, S.; Nishizawa, M. Transdermal electroosmotic flow generated by a porous microneedle array patch. Nat. Commun. 2021, 12, 658. [Google Scholar] [CrossRef]
- Yang, H.; Lin, W.; Zheng, Y. Advances and perspective on the translational medicine of biodegradable metals. Biomater. Transl. 2021, 2, 177–187. [Google Scholar]
- Long, J.; Teng, B.; Zhang, W.; Li, L.; Zhang, M.; Chen, Y.; Yao, Z.; Meng, X.; Wang, X.; Qin, L.; et al. Preclinical evaluation of acute systemic toxicity of magnesium incorporated poly(lactic-co-glycolic acid) porous scaffolds by three-dimensional printing. Biomater. Transl. 2021, 2, 272–284. [Google Scholar]
- Larraneta, E.; Moore, J.; Vicente-Perez, E.M.; Gonzalez-Vazquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Mir, M.; Utomo, E.; Donnelly, R.F. Bacterially sensitive nanoparticle-based dissolving microneedles of doxycycline for enhanced treatment of bacterial biofilm skin infection: A proof of concept study. Int. J. Pharm. X 2020, 2, 100047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.; Xian, Y.; Singh, P.; Feng, J.; Cui, S.; Carrier, A.; Oakes, K.; Luan, T.; Zhang, X. Multifunctional Graphene-Oxide-Reinforced Dissolvable Polymeric Microneedles for Transdermal Drug Delivery. ACS Appl. Mater Interfaces 2020, 12, 352–360. [Google Scholar] [CrossRef]
- Li, Z.; He, Y.; Deng, L.; Zhang, Z.; Lin, Y. A fast-dissolving microneedle array loaded with chitosan nanoparticles to evoke systemic immune responses in mice. J. Mater. Chem. B. 2020, 8, 216–225. [Google Scholar] [CrossRef]
- Mao, J.; Wang, H.; Xie, Y.; Fu, Y.; Li, Y.; Liu, P.; Du, H.; Zhu, J.; Dong, L.; Hussain, M.; et al. Transdermal delivery of rapamycin with poor water-solubility by dissolving polymeric microneedles for anti-angiogenesis. J. Mater. Chem. B 2020, 8, 928–934. [Google Scholar] [CrossRef]
- Derakhshandeh, H.; Aghabaglou, F.; McCarthy, A.; Mostafavi, A.; Wiseman, C.; Bonick, Z.; Ghanavati, I.; Harris, S.; Kreikemeier Bower, C.; Moosavi Basri, S.M.; et al. A Wirelessly Controlled Smart Bandage with 3D-Printed Miniaturized Needle Arrays. Adv. Funct. Mater. 2020, 30, 1905544. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Yang, X.; Feng, P.; Cao, J.; Liu, W.; Tang, Y. Composition-Engineered Metal–Organic Framework-Based Microneedles for Glucose-Mediated Transdermal Insulin Delivery. ACS Appl. Mater. Inter. 2020, 12, 13613–13621. [Google Scholar] [CrossRef]
- Yu, J.; Wang, J.; Zhang, Y.; Chen, G.; Mao, W.; Ye, Y.; Kahkoska, A.R.; Buse, J.B.; Langer, R.; Gu, Z. Glucose-responsive insulin patch for the regulation of blood glucose in mice and minipigs. Nat. Biomed. Eng. 2020, 4, 499–506. [Google Scholar] [CrossRef]
- Moreira, A.F.; Rodrigues, C.F.; Jacinto, T.A.; Miguel, S.P.; Costa, E.C.; Correia, I.J. Poly (vinyl alcohol)/chitosan layer-by-layer microneedles for cancer chemo-photothermal therapy. Int. J. Pharm. 2020, 576, 118907. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Tiew, W.J.; Zhang, J.; Ho, P.C.; Kachouie, N.N.; Kang, L. Geometrical optimisation of a personalised microneedle eye patch for transdermal delivery of anti-wrinkle small peptide. Biofabrication 2020, 12, 035003. [Google Scholar] [CrossRef] [PubMed]
- Vora, L.K.; Courtenay, A.J.; Tekko, I.A.; Larraneta, E.; Donnelly, R.F. Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules. Int. J. Biol. Macromol. 2019, 146, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, G.; Yu, Y.; Sun, L.; Zhao, Y. Bioinspired Adhesive and Antibacterial Microneedles for Versatile Transdermal Drug Delivery. Research 2020, 2020, 3672120. [Google Scholar] [CrossRef]
- Vecchione, R.; Coppola, S.; Esposito, E.; Casale, C.; Vespini, V.; Grilli, S.; Ferraro, P.; Netti, P.A. Electro-Drawn Drug-Loaded Biodegradable Polymer Microneedles as a Viable Route to Hypodermic Injection. Adv. Funct. Mater. 2014, 24, 3515–3523. [Google Scholar] [CrossRef]
- Chiu, Y.; Chen, M.; Wan, S. Sodium Hyaluronate/Chitosan Composite Microneedles as a Single-Dose Intradermal Immunization System. Biomacromolecules 2018, 19, 2278–2285. [Google Scholar] [CrossRef]
- Zheng, Z.; Eglin, D.; Alini, M.; Richards, G.R.; Qin, L.; Lai, Y. Visible Light-Induced 3D Bioprinting Technologies and Corresponding Bioink Materials for Tissue Engineering: A Review. Engineering 2021, 7, 966–978. [Google Scholar] [CrossRef]
- Qiu, G.; Ding, W.; Tian, W.; Qin, L.; Zhao, Y.; Zhang, L.; Lu, J.; Chen, D.; Yuan, G.; Wu, C.; et al. Medical Additive Manufacturing: From a Frontier Technology to the Research and Development of Products. Engineering 2020, 6, 1217–1221. [Google Scholar] [CrossRef]
- Di Prima, M.; Coburn, J.; Hwang, D.; Kelly, J.; Khairuzzaman, A.; Ricles, L. Additively manufactured medical products–the FDA perspective. 3D Print. Med. 2016, 2, 1. [Google Scholar] [CrossRef]
- Economidou, S.N.; Uddin, M.J.; Marques, M.J.; Douroumis, D.; Sow, W.T.; Li, H.; Reid, A.; Windmill, J.F.C.; Podoleanu, A. A novel 3D printed hollow microneedle microelectromechanical system for controlled, personalized transdermal drug delivery. Addit. Manuf. 2021, 38, 101815. [Google Scholar] [CrossRef]
- Li, X.; Shan, W.; Yang, Y.; Joralmon, D.; Zhu, Y.; Chen, Y.; Yuan, Y.; Xu, H.; Rong, J.; Dai, R.; et al. Limpet Tooth-Inspired Painless Microneedles Fabricated by Magnetic Field-Assisted 3D Printing. Adv. Funct. Mater. 2021, 31, 2003725. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, Y.; Hu, K.; Li, F. Enhancement of skin permeation of docetaxel: A novel approach combining microneedle and elastic liposomes. J. Control. Release 2008, 129, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.P.; Murthy, N.; Prausnitz, M.R. Minimally Invasive Protein Delivery with Rapidly Dissolving Polymer Microneedles. Adv. Mater. 2008, 20, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharmaceut. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Z.; Huo, M.R.; Zhou, J.P.; Zhou, Y.Q.; Hao, B.H.; Liu, T.; Zhang, Y. Super-short solid silicon microneedles for transdermal drug delivery applications. Int. J. Pharm. 2010, 389, 122–129. [Google Scholar]
- Gill, H.S.; Prausnitz, M.R. Coated microneedles for transdermal delivery. J. Control. Release 2007, 117, 227–237. [Google Scholar] [CrossRef]
- Fomani, A.A.; Mansour, R.R. Fabrication and characterization of the flexible neural microprobes with improved structural design. Sens. Actuators A Phys. 2011, 168, 233–241. [Google Scholar] [CrossRef]
- Kim, M.J.; Park, S.C.; Rizal, B.; Guanes, G.; Baek, S.K.; Park, J.H.; Betz, A.R.; Choi, S.O. Fabrication of Circular Obelisk-Type Multilayer Microneedles Using Micro-Milling and Spray Deposition. Front. Bioeng. Biotechnol. 2018, 6, 54. [Google Scholar] [CrossRef]
- Demir, Y.K.; Akan, Z.; Kerimoglu, O. Characterization of polymeric microneedle arrays for transdermal drug delivery. PLoS ONE 2013, 8, e77289. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.C.; Lee, D.S.; Jung, H. Drawing lithography: Three-dimensional fabrication of an ultrahigh-aspect-ratio microneedle. Adv. Mater. 2010, 22, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.K.; Lee, K.J.; Youn, Y.N.; Jang, E.H.; Kim, W.; Min, B.K.; Ryu, W. Spatially discrete thermal drawing of biodegradable microneedles for vascular drug delivery. Eur. J. Pharm. Biopharm. 2013, 83, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, F.; Vecchione, R.; Bhowmick, S.; Coppola, G.; Coppola, S.; Esposito, E.; Lettera, V.; Ferraro, P.; Netti, P.A. Electro-drawn polymer microneedle arrays with controlled shape and dimension. Sens. Actuators B Chem. 2018, 255, 1553–1560. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, L.; Li, J.; Yao, L.; Chen, Y.; Liu, B.; Jiang, L. Rapid fabrication of microneedles using magnetorheological drawing lithography. Acta Biomater. 2018, 65, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Kim, M.; Yang, H.; Lee, K.; Jung, H. Droplet-born air blowing: Novel dissolving microneedle fabrication. J. Control. Release 2013, 170, 430–436. [Google Scholar] [CrossRef]
- Han, M.; Hyun, D.; Park, H.; Lee, S.S.; Kim, C.; Kim, C. A novel fabrication process for out-of-plane microneedle sheets of biocompatible polymer. J. Micromech. Microeng. 2007, 17, 1184–1191. [Google Scholar] [CrossRef]
- Moon, S.-J.; Lee, S.S. Fabrication of microneedle array using inclined LIGA Process. In TRANSDUCERS ’03. 12th International Conference on Solid-State Sensors, Actuators and Microsystems; Digest of Technical Papers (Cat. No.03TH8664); IEEE: Boston, MA, USA, 2003; Volume 2, pp. 1546–1549. [Google Scholar]
- Pere, C.; Economidou, S.N.; Lall, G.; Ziraud, C.; Boateng, J.S.; Alexander, B.D.; Lamprou, D.A.; Douroumis, D. 3D printed microneedles for insulin skin delivery. Int. J. Pharm. 2018, 544, 425–432. [Google Scholar] [CrossRef]
- Luzuriaga, M.A.; Berry, D.R.; Reagan, J.C.; Smaldone, R.A.; Gassensmith, J.J. Biodegradable 3D printed polymer microneedles for transdermal drug delivery. Front. Bioeng. Biotechnol. 2018, 18, 1223–1230. [Google Scholar] [CrossRef]
- Lim, S.H.; Ng, J.Y.; Kang, L. Three-dimensional printing of a microneedle array on personalized curved surfaces for dual-pronged treatment of trigger finger. Biofabrication 2017, 9, 015010. [Google Scholar] [CrossRef]
- Moussi, K.; Bukhamsin, A.; Hidalgo, T.; Kosel, J. Biocompatible 3D Printed Microneedles for Transdermal, Intradermal, and Percutaneous Applications. Adv. Eng. Mater. 2019, 22, 1901358. [Google Scholar] [CrossRef]
- Harvey, E.C.; Rumsby, P.T. Fabrication techniques and their application to produce novel micromachined structures and devices using excimer laser projection. In Proceedings of the SPIE1997, Austin, TX, USA, 5 September 1997; Volume 3223, pp. 26–33. [Google Scholar]
- Li, J.; Zhou, Y.; Yang, J.; Ye, R.; Gao, J.; Ren, L.; Liu, B.; Liang, L.; Jiang, L. Fabrication of gradient porous microneedle array by modified hot embossing for transdermal drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Janphuang, P.; Laebua, M.; Sriphung, C.; Taweewat, P.; Sirichalarmkul, A.; Sukjantha, K.; Promsawat, N.; Leuasoongnoen, P.; Suphachiaraphan, S.; Phimol, K.; et al. Polymer based microneedle patch fabricated using microinjection moulding. In Proceedings of the 4th International Conference on Engineering, Applied Sciences and Technology (ICEAST 2018), Phuket, Thailand, 4–7 July 2018; Volume 192, p. 01039. [Google Scholar]
- Yung, K.L.; Xu, Y.; Kang, C.; Liu, H.; Tam, K.F.; Ko, S.M.; Kwan, F.Y.; Lee, T.M.H. Sharp tipped plastic hollow microneedle array by microinjection moulding. J. Micromech. Microeng. 2012, 22, 015016. [Google Scholar] [CrossRef]
- Duong, H.; Yin, Y.; Thambi, T.; Nguyen, T.L.; Giang, P.V.; Lee, M.S.; Lee, J.E.; Kim, J.; Jeong, J.H.; Lee, D.S. Smart vaccine delivery based on microneedle arrays decorated with ultra-pH-responsive copolymers for cancer immunotherapy. Biomaterials 2018, 185, 13–24. [Google Scholar] [CrossRef] [PubMed]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef]
- Gill, H.S.; Prausnitz, M.R. Coating formulations for microneedles. Pharm. Res. 2007, 24, 1369–1380. [Google Scholar] [CrossRef]
- REAUME, S.E. The use of hydrofluoric acid in making glass microneedles. Science 1952, 116, 641. [Google Scholar] [CrossRef]
- Robinson, N. Enzyme response of traumatized tissue after intracortical injection into 5 day old rat brain. J. Neurol. Neurosurg. Psychiatry 1972, 35, 865–872. [Google Scholar] [CrossRef][Green Version]
- Szubinska, B. “New membrane” formation in Amoeba proteus upon injury of individual cells. Electron microscope observations. J. Cell Biol. 1971, 49, 747–772. [Google Scholar] [CrossRef]
- Joel, D.D.; Hess, M.W.; Cottier, H. Magnitude and pattern of thymic lymphocyte migration in neonatal mice. J. Exp. Med. 1972, 135, 907–923. [Google Scholar] [CrossRef]
- DOSSEL, W.E. Preparation of tungsten micro-needles for use in embryologic research. Lab. Invest. 1958, 7, 171–173. [Google Scholar]
- De Jonge, A.J.; Vermeulen, W.; Keijzer, W.; Hoeijmakers, J.H.; Bootsma, D. Microinjection of Micrococcus luteus UV-endonuclease restores UV-induced unscheduled DNA synthesis in cells of 9 xeroderma pigmentosum complementation groups. Mutat. Res. 1985, 150, 99–105. [Google Scholar] [CrossRef][Green Version]
- Edds, K.T. Motility in Echinosphaerium nucleofilum. I. An analysis of particle motions in the axopodia and a direct test of the involvement of the axoneme. J. Cell Biol. 1975, 66, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. Characterization of genes and proteins involved in excision repair of human cells. J. Cell Sci. Suppl. 1987, 6, 111–125. [Google Scholar] [CrossRef]
- Riedel, H.; Kondor-Koch, C.; Garoff, H. Cell surface expression of fusogenic vesicular stomatitis virus G protein from cloned cDNA. EMBO J. 1984, 3, 1477–1483. [Google Scholar] [CrossRef]
- Poirier, D.; Renaud, F.; Dewar, V.; Strodiot, L.; Wauters, F.; Janimak, J.; Shimada, T.; Nomura, T.; Kabata, K.; Kuruma, K.; et al. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials 2017, 145, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Zhao, J.; Li, B.; Cai, P.; Loh, X.J.; Xu, C.; Chen, P.; Kai, D.; Zheng, L. Implantable and degradable antioxidant poly(epsilon-caprolactone)-lignin nanofiber membrane for effective osteoarthritis treatment. Biomaterials 2020, 230, 119601. [Google Scholar] [CrossRef] [PubMed]
- Gerwin, N.; Hops, C.; Lucke, A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliver. Rev. 2006, 58, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Koul, V. Transdermal delivery of methotrexate: Past, present and future prospects. Ther. Deliv. 2012, 3, 315–325. [Google Scholar] [CrossRef]
- Abla, M.J.; Chaturvedula, A.; O Mahony, C.; Banga, A.K. Transdermal delivery of methotrexate for pediatrics using silicon microneedles. Ther. Deliv. 2013, 4, 543–551. [Google Scholar] [CrossRef]
- Shende, P.; Salunke, M. Transepidermal microneedles for co-administration of folic acid with methotrexate in the treatment of rheumatoid arthritis. Biomed. Phys. Eng. Express 2019, 5, 025023. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, C.; Zhang, S.; Yang, G.; He, M.; Gao, Y. Systemic delivery of artemether by dissolving microneedles. Int. J. Pharm. 2016, 508, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H.; Jing, Q.; Wang, Z.; He, Z.; Wu, T.; Feng, N.P. Improved Biosafety and Transdermal Delivery of Aconitine via Diethylene Glycol Monoethyl Ether-Mediated Microemulsion Assisted with Microneedles. Pharmaceutics 2020, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, Y.; Li, Z.; Zhao, J.; Feng, N. Microneedle-mediated transdermal delivery of nanostructured lipid carriers for alkaloids from Aconitum sinomontanum. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1541–1551. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Cheng, N.; Zhao, J.; Hou, X.; Zhang, Y.; Feng, N. Novel nanostructured lipid carriers-loaded dissolving microneedles for controlled local administration of aconitine. Int. J. Pharm. 2019, 572, 118741. [Google Scholar] [CrossRef] [PubMed]
- Dangol, M.; Yang, H.; Li, C.G.; Lahiji, S.F.; Kim, S.; Ma, Y.; Jung, H. Innovative polymeric system (IPS) for solvent-free lipophilic drug transdermal delivery via dissolving microneedles. J. Control. Release 2016, 223, 118–125. [Google Scholar] [CrossRef]
- Yao, W.; Tao, C.; Zou, J.; Zheng, H.; Zhu, J.; Zhu, Z.; Zhu, J.; Liu, L.; Li, F.; Song, X. Flexible two-layer dissolving and safing microneedle transdermal of neurotoxin: A biocomfortable attempt to treat Rheumatoid Arthritis. Int. J. Pharm. 2019, 563, 91–100. [Google Scholar] [CrossRef]
- Chen, G.; Hao, B.; Ju, D.; Liu, M.; Zhao, H.; Du, Z.; Xia, J. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm. Sin. B 2015, 5, 569–576. [Google Scholar] [CrossRef]
- Cui, Y.; Mo, Y.; Zhang, Q.; Tian, W.; Xue, Y.; Bai, J.; Du, S. Microneedle-Assisted Percutaneous Delivery of Paeoniflorin-Loaded Ethosomes. Molecules 2018, 23, 3371. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Gui, S.; Wu, X.; Chen, L.; Cao, Y.; Yin, D.; Ma, P. Sinomenine hydrochloride-loaded dissolving microneedles enhanced its absorption in rabbits. Pharm. Dev. Technol. 2016, 21, 787–793. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, N.; Wang, Z.; Su, J.; Yang, J.; Han, J.; Zhao, Y. Microneedle-Assisted Transdermal Delivery of Etanercept for Rheumatoid Arthritis Treatment. Pharmaceutics 2019, 11, 235. [Google Scholar] [CrossRef]
- Ali, A.A.; McCrudden, C.M.; McCaffrey, J.; McBride, J.W.; Cole, G.; Dunne, N.J.; Robson, T.; Kissenpfennig, A.; Donnelly, R.F.; McCarthy, H.O. DNA vaccination for cervical cancer; a novel technology platform of RALA mediated gene delivery via polymeric microneedles. Nanomed. UK 2017, 13, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zeng, M.; Shan, H.; Tong, C. Microneedle Patches as Drug and Vaccine Delivery Platform. Curr. Med. Chem. 2017, 24, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Erdos, G.; Huang, S.; Kenniston, T.W.; Balmert, S.C.; Carey, C.D.; Raj, V.S.; Epperly, M.W.; Klimstra, W.B.; Haagmans, B.L.; et al. Microneedle array delivered recombinant coronavirus vaccines: Immunogenicity and rapid translational development. EBioMedicine 2020, 55, 102743. [Google Scholar] [CrossRef] [PubMed]
- Arya, J.; Prausnitz, M.R. Microneedle patches for vaccination in developing countries. J. Control. Release 2016, 240, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Chew, S.W.T.; Zheng, M.; Lio, D.C.S.; Wiraja, C.; Mei, Y.; Ning, X.; Cui, M.; Than, A.; Shi, P.; et al. Cryomicroneedles for transdermal cell delivery. Nat. Biomed. Eng. 2021, 5, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Duong, H.; Yin, Y.; Thambi, T.; Kim, B.S.; Jeong, J.H.; Lee, D.S. Highly potent intradermal vaccination by an array of dissolving microneedle polypeptide cocktails for cancer immunotherapy. J. Mater. Chem. B 2020, 8, 1171–1181. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Li, W.; Tang, J.; Schwendeman, S.P.; Prausnitz, M.R. Immediate detachment of microneedles by interfacial fracture for sustained delivery of a contraceptive hormone in the skin. J. Control. Release 2021, 337, 676–685. [Google Scholar] [CrossRef]
- Chen, W.; Tian, R.; Xu, C.; Yung, B.C.; Wang, G.; Liu, Y.; Ni, Q.; Zhang, F.; Zhou, Z.; Wang, J.; et al. Microneedle-array patches loaded with dual mineralized protein/peptide particles for type 2 diabetes therapy. Nat. Commun. 2017, 8, 1777. [Google Scholar] [CrossRef]
- Wang, J.; Yu, J.; Zhang, Y.; Kahkoska, A.R.; Wang, Z.; Fang, J.; Whitelegge, J.P.; Li, S.; Buse, J.B.; Gu, Z. Glucose transporter inhibitor-conjugated insulin mitigates hypoglycemia. Proc. Natl. Acad. Sci. USA 2019, 116, 10744–10748. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Y.; Ye, Y.; DiSanto, R.; Sun, W.; Ranson, D.; Ligler, F.S.; Buse, J.B.; Gu, Z. Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery. Proc. Natl. Acad. Sci. USA 2015, 112, 8260–8265. [Google Scholar] [CrossRef]
- Ye, Y.; Yu, J.; Wang, C.; Nguyen, N.Y.; Walker, G.M.; Buse, J.B.; Gu, Z. Microneedles Integrated with Pancreatic Cells and Synthetic Glucose-Signal Amplifiers for Smart Insulin Delivery. Adv. Mater. 2016, 28, 3115–3121. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jiang, G.; Song, G.; Zhu, J.; Yang, Y. Fabrication of separable microneedles with phase change coating for NIR-triggered transdermal delivery of metformin on diabetic rats. Biomed. Microdevices 2020, 22, 12. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wainer, J.; Ryoo, S.W.; Qi, X.; Chang, R.; Li, J.; Lee, S.H.; Min, S.; Wentworth, A.; Collins, J.E.; et al. Dynamic omnidirectional adhesive microneedle system for oral macromolecular drug delivery. Sci. Adv. 2022, 8, eabk1792. [Google Scholar] [CrossRef]
- Chen, H.; Qin, Z.; Zhao, J.; He, Y.; Ren, E.; Zhu, Y.; Liu, G.; Mao, C.; Zheng, L. Cartilage-targeting and dual MMP-13/pH responsive theranostic nanoprobes for osteoarthritis imaging and precision therapy. Biomaterials 2019, 225, 119520. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, T.E.; Werfel, T.A.; Cho, H.; Hasty, K.A.; Duvall, C.L. Particle-based technologies for osteoarthritis detection and therapy. Drug. Deliv. Transl. Res. 2016, 6, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Rothenfluh, D.A.; Bermudez, H.; O’Neil, C.P.; Hubbell, J.A. Biofunctional polymer nanoparticles for intra-articular targeting and retention in cartilage. Nat. Mater. 2008, 7, 248–254. [Google Scholar] [CrossRef] [PubMed]

| Materials | Application | Morphology | Mechanical Strength | Insertion Capability | Reference |
|---|---|---|---|---|---|
| PVP, PVA | Bacterial biofilm skin infection | Needle density of 16 × 16, pyramidal needles; 850 μm height [600 μm pyramidal tip, 250 μm base column] and 300 μm width at the base and 300 μm interspacing | After the application of 32 N/MN array, the reductions in MN height were found to be 12.36 ± 3.12%, 13.03 ± 2.71%, 12.65 ± 3.22%, 12.98 ± 2.09%, 13.16 ± 3.10%, 12.91 ± 2.98% and 13.21 ± 2.11%, respectively. The percentage of height reduction of MNs after the application of 32 N/MN arrays, equivalent to human manual compression pressure [42]. | In the full-thickness porcine skin, the penetration depth of 503.65 ± 12.43 μm, 500.43 ± 10.12 μm, 506.43 ± 21.11 μm, 498.43 ± 10.41 μm, 502.11 ± 10.03 μm and 499.87 ± 10.03 μm. | 2020 [43] |
| PVP (30,000 Da), CMC (250,000 Da), HA (200,000–300,000 Da) | Melanoma | 10 × 10 square pyramidal MNs, and geometry, that is, 350 μm base width, 700 μm height, about 15 μm tip width, and 500 μm needle center-to-center spacing | Transdermal applications (threshold, 0.15 N). Go (500 μg/mL) increasing CMC failure force the up to about 0.2 N | Complete insertion (98–100%) of PVP MNs by incorporating 500 μg/mL GO | 2020 [44] |
| PVP(K29/32) | Immune | An 8 mm × 8 mm array, and uniformly distributed with 225 (15 × 15) square pyramid needles having a bottom edge of 200 μm and a height of 600 μm | The depths of the holes created by MN needles were about 150 μm. | 2020 [45] | |
| PVP(10 kDa) | Anti-angiogenesis, skin tumors and vascular anomalies (vas) | Had a uniform structure of pyramidal shape and contained 100 needles (i.e., a 10 × 10 array) with a length of 800 ± 15 μm. | The compression force increased to 0.6 N/needle when the displacement increased to 0.6 mm. | Deliver RAPA to a depth of 200 μm. | 2020 [46] |
| resin | chronic wounds | With different needle spacings (1.5–3 mm), needle lengths (0.8–3 mm), base sizes (0.5–1.5 mm), and opening diameters (0.2–0.5 mm). a length of about 2 mm was selected | Mechanical tester: the MNAs did not break and were only bent under a compressive force of about 78 N. Pigskin: no deformation or breakage was observed upon penetration and removal from the pigskin. | The majority of the MNAs penetrated fresh pigskin with less than 2N of force and full penetration was achieved with about 7N. | 2020 [47] |
| Chitosan (180 kDa) | Wound healing | A 20 × 20 mn array and each MN possessed a conical shape with a tip diameter of 5 μm, a height of 600 μm, and a base diameter of 300 μm | 2020 [48] | ||
| PVA (Mw: 31,000–50,000, 87–89% hydrolyzed) | Diabetes | 15 × 15 array, 650 μm needle length, the total area of 11 × 11 mm2. A base of 315 μm and a height of 650 μm | 0.71 N/needle, which is efficient for skin penetration | 2020 [49] | |
| N-vinylpyrrolidone (NVP) | Insulin | a 20 × 20 array; each needle had a pyramidal shape, with a width of 400 µm at the base and a height of 900 µm | The fracture force of the MNs to be 0.90 ± 0.35 N/per needle using a tensile compression machine | The MN patch could effectively penetrate the skin of the minipig. | 2020 [50] |
| PVA (Mw: 31,000 g/mol), PVP (Mw: 360,000 g/mol), chitosan (Mw: 50,000–190,000 Da) | Cancer | A 16 (4 by 4) MN array, a bevel-like structure with 425 µm, 1420 µm, and 1740 µm of width, height, and tip-to-tip distance, respectively. | 2020 [51] | ||
| resin | Anti-wrinkle | The MN illustrated here are that of MN height 800 μm; MN tip diameter 100 μm; MN base diameter of 400 μm and MN interspacing of 800 μm. | able to withstand breakage from a typical thumb force of about 20N | The approximate depth of penetration for the intact skin is about 220 μm; FMNP about 480 μm and PMNP about 750 μm. | 2020 [52] |
| pullulan (viscosity: 133 mm2/s, 10% w/w, Ubbelohde type viscometer, average Mw 200 kDa) | Small molecule drugs and biomolecules | Displayed well-formed DMNs with sharp tips, a complete array of needles. | An insertion force of 0.089 N per needle for 30 s may be suitable for penetrating the skin | 403 ± 35.8 μm inserted out of the total height of 504 ± 6.4 μm which is 80 ± 7.2% insertion. | 2019 [53] |
| MeHA | Timely metabolic analysis | The obtained MN patch displayed a height of about 800 µm with a base width of about 250 µm and inter needle spacing of about 450 µm. | A thumb press (about 1.5 N) could easily penetrate the MNs into porcine skin. | The efficient penetration depth of MN was about 300 µm. | 2017 [35] |
| Fabrication Methods | Reference | |
|---|---|---|
| Mold-free methods/master structures | Photolithography | 2008 [63] |
| Dry etch | 1998 [64], 2010 [65] | |
| Wet etch | 2010 [66] | |
| Laser cutting | 2007 [67] | |
| Electroplating or electroless-plating | 2011 [68] | |
| Micro milling and micro grinding | 2018 [69], 2013 [70] | |
| Drawing lithography | 2010 [71], 2013 [72] | |
| Electro-drawing | 2018 [73], 2014 [55] | |
| Magnetorheological drawing lithography | 2018 [74] | |
| Droplet-born air blowing (DAB) method | 2013 [75] | |
| Lithography, electroplating, and molding (LIGA) technique | 2007 [76], 2003 [77] | |
| Stereolithography (STL) | 2018 [78] | |
| Fused deposition modeling (FDM) | 2018 [79] | |
| Micro-stereolithographic 3D printing | 2017 [80],2021 [60] | |
| A two-photon polymerization (TPP) 3D printing methodology | 2019 [81] | |
| magnetic field-assisted 3D printing (MF-3DP) | 2021 [61] | |
| Laser ablation and cutting | 1997 [82] | |
| Mold-based methods | Hot embossing | 2018 [83] |
| Injection molding | 2018 [84], 2012 [85] | |
| Solvent casting | 2012 [33], 2018 [56,86] | |
| Coated method | Spraying | 2014 [87] |
| Dipping | 2007 [88] | |
| Method for forming porous structure | Electrochemical anodization | 2022 [21] |
| Wet etching | ||
| Mild micro-molding | ||
| Sintering process | ||
| Porogen leaching | ||
| Hot embossing | ||
| Phase separation | ||
| Emulsion and bonding | ||
| Biomedical Application | Latest Research |
|---|---|
| Osteoarthritis | Glucocorticoid [54], Meloxicam [14,18] |
| Rheumatoid arthritis | Methotrexate [101,102,103] Artemether [104,105] Alkaloids [106,107] Capsaicin [108] Neurotoxin [109] Triptolide [110] Paeoniflorin [111] Sinomenine [112] Etanercept (EN) [113] |
| Dermatosis | Exosomes and small molecule drug UK5099 and keratin [19] |
| Delivery of vaccine | MERS-CoV vaccine and SARS-CoV-2 vaccine [117] |
| Cancer | Delivery of ovalbumin-pulsed dendritic cells for subcutaneous melanoma tumors [119] Bioresorbable polypeptide matrix with a nanopolyplex, loaded ovalbumin-expressing plasmid OVA (pOVA) and immunostimulant-polyinosinic:polycytidylic acid (poly(I:C)), for B16/OVA melanoma tumors [120] Mediating the delivery of doxorubicin and aumss nanorods (Dox@micron) to cancer cells [51] Loaded rapamycin for skin tumors and vascular anomalies [51] |
| Contraception | Levonorgestrel [16] |
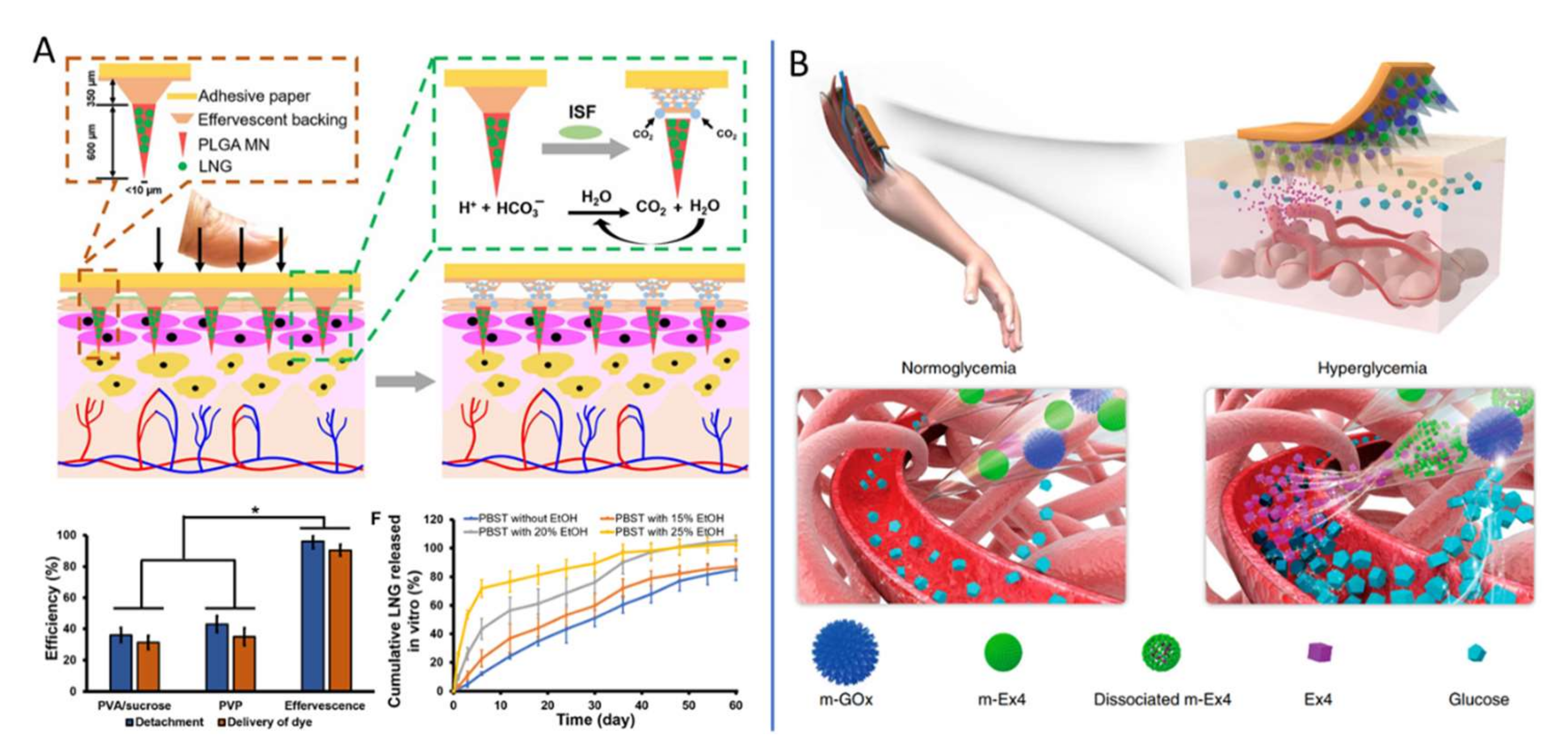
| Diabetics | Insulin delivery [50,122,123,124] Exendin-4 (Ex4) and glucose oxidase (GOx) delivery [125] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zhang, W.; Li, C.; Zhang, J.; Qin, L.; Lai, Y. Recent Advances of Microneedles and Their Application in Disease Treatment. Int. J. Mol. Sci. 2022, 23, 2401. https://doi.org/10.3390/ijms23052401
Zhang W, Zhang W, Li C, Zhang J, Qin L, Lai Y. Recent Advances of Microneedles and Their Application in Disease Treatment. International Journal of Molecular Sciences. 2022; 23(5):2401. https://doi.org/10.3390/ijms23052401
Chicago/Turabian StyleZhang, Wenjing, Wei Zhang, Cairong Li, Jianhua Zhang, Ling Qin, and Yuxiao Lai. 2022. "Recent Advances of Microneedles and Their Application in Disease Treatment" International Journal of Molecular Sciences 23, no. 5: 2401. https://doi.org/10.3390/ijms23052401
APA StyleZhang, W., Zhang, W., Li, C., Zhang, J., Qin, L., & Lai, Y. (2022). Recent Advances of Microneedles and Their Application in Disease Treatment. International Journal of Molecular Sciences, 23(5), 2401. https://doi.org/10.3390/ijms23052401







