Angiotensin Type-2 Receptors: Transducers of Natriuresis in the Renal Proximal Tubule
Abstract
1. Introduction
2. Renal AT1R and AT2R Expression
3. Evidence for Opposing Actions of AT2Rs on AT1R-Induced Renal Na+ Reabsorption
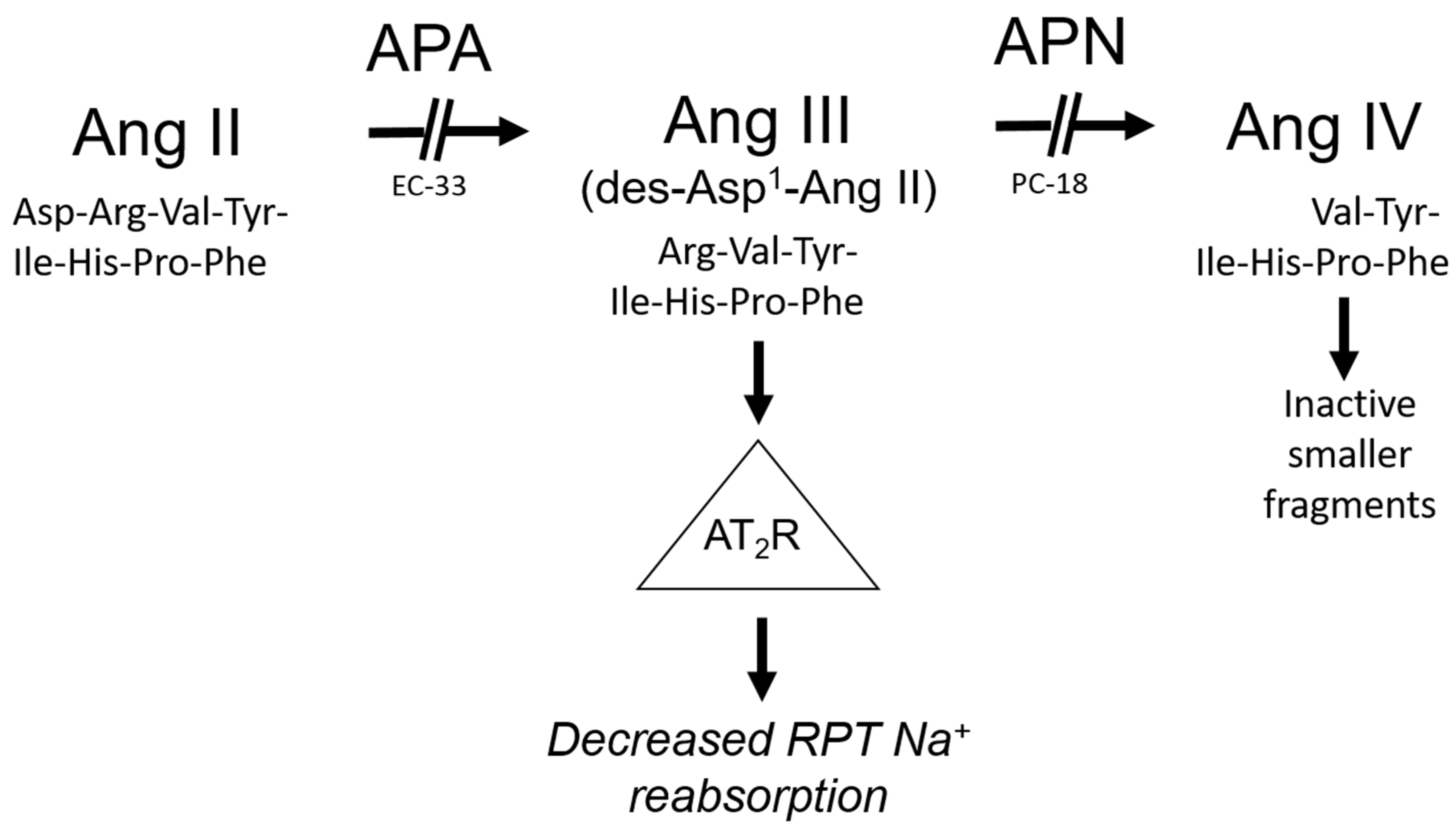
4. Ang III Is the Major Endogenous AT2R Agonist for the Natriuretic Response
5. Nephron Site of AT2R Inhibition of Na+ Reabsorption
6. Renal Mechanism of AT2R-Induced Natriuresis
7. Primary Cell Signaling Pathways Mediating the AT2R Natriuretic Response
8. Dopamine-1 Receptor (D1R)-AT2R Interactions in Natriuresis
9. Sex Determinants of AT2R-Induced Natriuresis
10. AT2R-Induced BP Reduction in Angiotensin-Dependent Hypertension
11. Defective AT2R-Induced Natriuresis in Primary Hypertension
12. Enhanced AT2R-Induced Natriuresis in Obese Zucker Rats
13. Potential for AT2R Agonists as Natriuretic/Diuretic Agents Acting at the Renal Proximal Tubule
14. Summary
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muntner, P.; Carey, R.M.; Gidding, S.S.; Jones, D.W.; Taler, S.J.; Wright, J.T.; Whelton, P.K. Potential impact of the 2017 American College of Cardiology (ACC)/American Heart Association (AHA) Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults in the US population. J. Am. Coll. Cardiol. 2018, 71, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and control of hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison-Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. A guideline for the prevention, detection, evaluation and management of high blood pressure. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef] [PubMed]
- Karnik, S.S.; Unal, H.; Kemp, J.R.; Tirupula, K.C.; Eguchi, S.; Vanderheyden, P.M.L.; Thomas, W.G. International Union of basic and clinical pharmacology. XCIX. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli. Pharmacol. Rev. 2015, 67, 754–819. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M. The intrarenal renin-angiotensin and dopaminergic systems: Control of renal sodium excretion and blood pressure. Hypertension 2013, 61, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Ozono, R.; Wang, Z.Q.; Moore, A.F.; Inagami, T.; Siragy, H.M.; Carey, R.M. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension 1997, 30, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Miyata, N.; Park, F.; Li, X.F.; Cowley , A.W., Jr. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am. J. Physiol. 1999, 277, F437–F446. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Padia, S.H.; Carey, R.M. AT₂ receptor activation induces natriuresis and lowers blood pressure. Circ. Res. 2014, 115, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Abadir, P.M.; Foster, D.B.; Crow, M.; Cooke, C.A.; Rucker, J.J.; Jain, A.; Smith, B.J.; Furks, T.N.; Cohn, R.D.; Fedarko, N.S.; et al. Identification and characterization of a functional mitochondrial angiotensin system. Proc. Natl. Acad. Sci. USA 2011, 108, 14849–14854. [Google Scholar] [CrossRef]
- Friederich-Persson, M.; Persson, P. Mitochondrial angiotensin II receptors regulate oxygen consumption in kidney mitochondria from healthy and type 1 diabetic rats. Am. J. Physiol. Renal Physiol. 2020, 318, F683–F688. [Google Scholar] [CrossRef]
- Siragy, H.M.; Inagami, T.; Ichiki, T.; Carey, R.M. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc. Natl. Acad. Sci. USA 1999, 96, 6506–6510. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Coleman, T.G.; Young, D.B.; Lohmeier, T.E.; DeClue, J.W. Salt balance and longterm blood pressure control. Annu. Rev. Med. 1980, 31, 15–27. [Google Scholar] [CrossRef]
- Guyton, A.C.; Coleman, T.G.; Cowley, A.V., Jr.; Scheel, K.W.; Manning, R.D., Jr.; Norman, R.A., Jr. Arterial pressure regulation: Overriding dominance of the kidneys in long- term regulation and in hypertension. Am. J. Med. 1972, 52, 584–594. [Google Scholar] [CrossRef]
- Gross, V.; Schunck, W.H.; Honeck, H.; Mills, A.F.; Karger, E.; Walther, T.; Bader, M.; Inagami, T.; Schneider, W.; Luft, F.C. Inhibition of pressure natriuresis in mice lacking the AT2 receptor. Kidney Int. 2000, 57, 191–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Obst, M.; Gross, V.; Janke, J.; Wellner, M.; Schneider, W.; Luft, F.C. Pressure natriuresis in AT(2) receptor-deficient mice with L-NAME hypertension. J. Am. Soc. Nephrol. 2003, 14, 303–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Padia, S.H.; Howell, N.L.; Siragy, H.M.; Carey, R.M. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension 2006, 47, 537–544. [Google Scholar] [CrossRef]
- Padia, S.H.; Kemp, B.A.; Howell, N.L.; Siragy, H.M.; Fournie-Zaluski, M.C.; Roques, B.C.; Carey, R.M. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type-1 receptor-blocked rats. Hypertension 2007, 43, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Padia, S.H.; Kemp, B.A.; Howell, N.L.; Fournie-Zaluski, M.C.; Roques, B.P.; Carey, R.M. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension 2008, 51, 460–465. [Google Scholar] [CrossRef]
- Kemp, B.A.; Bell, J.F.; Rottkamp, D.M.; Howell, N.L.; Shao, W.; Navar, L.G.; Padia, S.H.; Carey, R.M. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type-2 receptors. Hypertension 2012, 60, 387–395. [Google Scholar] [CrossRef]
- Bosnyak, S.; Jones, E.S.; Christopolous, A.; Aguilar, M.I.; Thomas, W.G.; Widdop, R.E. Relative affinity of angiotensin peptides and novel ligands. Clin. Sci. 2011, 121, 297–303. [Google Scholar] [CrossRef]
- Hilliard-Krause, L.M.; Kemp, B.A.; Tan, A.S.J.; Jones, E.S.; Del Borgo, M.P.; Aguilar, M.-I.; Denton, K.M.; Carey, R.M.; Widdop, R.E. Renal functional effects of the highly selective AT2R agonist, ß-Pro7Ang III, in normotensive rats. Clin. Sci. 2020, 134, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.I.; Purcell, A.W.; Devi, R.; Lew, R.; Rossjohn, J.; Smith, A.I.; Perlmutter, P. Beta-amino acid-containing hybrid peptides–new opportunities in peptidomimetics. Org. Biomol. Chem 2007, 5, 2884–2890. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Garvin, J.L. Angiotensin II stimulates thick ascending limb NO production via AT(2) receptors and Akt1-dependent nitric-oxide synthase (NOS3) activation. J. Biol. Chem. 2010, 285, 14932–14940. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C.; Ren, H.; Han, Y.; He, D.; Zhou, L.; Hopfer, U.; Jose, P.A.; Zeng, C. Angiotensin II AT(2) receptor decreases AT(1) receptor expression and function via nitric oxdide/cGMP/Sp1 in renal proximal tubule cells from Wistar-Kyoto rats. J. Hypertens. 2012, 30, 1176–1184. [Google Scholar] [CrossRef]
- Gildea, J.J.; Peng, X.; Kemp, B.A.; Carey, R.M.; Jose, P.A.; Felder, R.A. The dopamine D1 receptor and angiotensin type-2 receptor are both required for inhibition of sodium transport through a protein phosphatase 2A pathway. Hypertension 2019, 72, 1258–1265. [Google Scholar] [CrossRef]
- Kemp, B.A.; Gildea, J.J.; Howell, N.L.; Keller, S.R.; Brautigan, D.G.; Carey, R.M. Renal AT2 receptor-PP2A signaling mediates natriuresis. Circ. Res. 2022, 130, 96–111. [Google Scholar] [CrossRef]
- Gildea, J.J.; Shah, N.; Tran, H.; Spinosa, M.; Van Sciver, R.; Sasaki, M.; Yatabe, J.; Carey, R.M.; Jose, P.A.; Felder, R.A. Dopamine and angiotensin receptors cooperatively inhibit sodium transport in human renal proximal tubule cells. Hypertension 2012, 60, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Salomone, L.J.; Howell, N.L.; McGrath, H.E.; Kemp, B.A.; Keller, S.R.; Gildea, J.J.; Felder, R.A.; Carey, R.M. Intrarenal dopamine D1-like receptor stimulation induces natriuresis via an angiotensin type-2 receptor mechanism. Hypertension 2007, 49, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Padia, S.H.; Kemp, B.A.; Howell, N.L.; Keller, S.R.; Gildea, J.J.; Carey, R.M. Mechanisms of dopamine D1 and angiotensin AT2 receptor interaction in natriuresis. Hypertension 2012, 59, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, Y.; Zheng, S.; Kuo, X.; Ascico, L.D.; Huang, H.; Gao, Z.; Jose, P.A.; Zeng, C. Enhanced natriuresis and diuresis in Wistar rats caused by costimulation of renal dopamine D3 and angiotensin type 2 receptors. Am. J. Hypertens. 2015, 28, 1267–1276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhargava, A.; Arnold, A.P.; Bangasser, D.A.; Denton, K.M.; Gupta, A.; Hilliard Krause, L.M.; Mayer, E.A.; McCarthy, M.; Miller, W.L.; Raznahan, A.; et al. Considering sex as a biological variable in basic and clinical studies: An Endocrine Society Scientific Statement. Endocr. Rev. 2021, 42, 219–258. [Google Scholar] [CrossRef] [PubMed]
- Colafella, K.M.M.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Hilliard, L.M.; Mirabito, K.M.; Denton, K.M. Unmasking the potential of the angiotensin AT2 receptor as a therapeutic target in hypertension in men and women: What we know and what we need to find out. Clin. Exp. Pharm. Phys. 2013, 40, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Sampson, A.K.; Moritz, K.M.; Jones, E.S.; Flower, R.L.; Widdop, R.E.; Denton, K.M. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008, 52, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, L.M.; Nematbakhsh, M.; Kett, M.M.; Teichman, E.; Sampson, A.K.; Widdop, R.E.; Evans, G.; Denton, K.M. Gender differences in pressure-natriuresis and renal autoregulation: Role of the angiotensin type 2 receptor. Hypertension 2011, 57, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D.; Hilliard, L.M.; Head, G.A.; Jones, E.S.; Widdop, R.E.; Denton, K.M. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 2012, 59, 129–135. [Google Scholar] [CrossRef]
- Wang, H.; Garvin, J.L.; Carretero, O.A. Angiotensin II enhances tubuloglomerular feedback via luminal AT(1) receptors on the macula densa. Kidney Int. 2001, 60, 1851–1857. [Google Scholar] [CrossRef]
- Dilley, J.R.; Arendshorst, W.J. Enhanced tubuloglomerular feedback activity in rats developing spontaneous hypertension. Am. J. Physiol. 1984, 247, F672–F679. [Google Scholar] [CrossRef]
- Hilliard, L.M.; Jones, E.S.; Steckelings, M.; Unger, T.; Widdop, R.E.; Denton, K.M. Sex-specific influence of angiotensin type 2 receptor stimulation on renal function: A novel therapeutic target for hypertension. Hypertension 2012, 59 Pt 2, 409–414. [Google Scholar] [CrossRef]
- Kemp, B.A.; Howell, N.L.; Keller, S.R.; Gildea, J.J.; Padia, S.H.; Carey, R.M. AT2 receptor activation prevents sodium retention and reduces blood pressure in angiotensin II-dependent hypertension. Circ. Res. 2016, 119, 532–543. [Google Scholar] [CrossRef]
- Trippodo, N.C.; Frohlich, E.D. Similarities of genetic (spontaneous) hypertension: Man and rat. Circ. Res. 1981, 48, 309–319. [Google Scholar] [CrossRef]
- Nagaoka, A.; Kakihana, M.; Shibota, M.; Fujiwara, K.; Shimakawa, K. Reduced sodium excretory ability in young spontaneously hypertensive rats. Jpn. J. Pharmacol. 1982, 32, 839–844. [Google Scholar]
- Mozaffari, M.S.; Jirakulsomchok, S.; Shao, Z.H.; Wyss, J.M. High-NaCl diets increase natriuretic and diuretic responses in salt-resistant but not salt-sensitive, S.H.R. Am. J. Physiol. 1991, 260, F890–F897. [Google Scholar]
- Heckmann, U.; Zidek, W.; Schurek, H.J. Sodium reabsorption in the isolated perfused kidney of normotensive and spontaneously hypertensive rats. J. Hypertens. Suppl. 1989, 7, S172–S173. [Google Scholar] [CrossRef]
- Firth, J.D.; Raine, A.E.; Ledingham, J.G. Sodium and lithium handling in the isolated hypertensive rat kidney. Clin. Sci. 1989, 76, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, R.E.; Roald, A.B.; Tenstad, O.; Iversen, B.M. Renal hemodynamics during development of hypertension in young spontaneously hypertensive rats. Kidney Blood Press Res. 2002, 25, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Biollaz, J.; Waeber, B.; Diezi, J.; Burnier, M.; Brunner, H.R. Lithium infusion to study sodium handling in unanesthetized hypertensive rats. Hypertension 1986, 8, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Beierwaltes, W.H.; Arendshorst, W.J.; Klemmer, P.J. Electrolyte and water balance in young spontaneously hypertensive rats. Hypertension 1982, 4, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Renal dysfunction, rather than nonrenal vascular dysfunction, mediates salt-induced hypertension. Circulation 2016, 133, 894–906. [Google Scholar] [CrossRef]
- Kawabe, K.; Watanabe, T.X.; Shiono, K.; Sokabe, H. Influence on blood pressure of renal isografts between spontaneously hypertensive and normotensive rats, utilizing the F1 hybrids. Jpn. Heart J. 1978, 19, 886–894. [Google Scholar] [CrossRef]
- Omvik, P.; Tarazi, R.C.; Bravo, E.L. Regulation of sodium balance in hypertension. Hypertension 1980, 2, 515–523. [Google Scholar] [CrossRef][Green Version]
- Nagaoka, A.; Kakihana, M.; Suno, M.; Hamajo, K. Renal hemodynamics and sodium excretion in stroke-prone spontaneously hypertensive rats. Am. J. Physiol. Renal Physiol. 1981, 241, F244–F249. [Google Scholar] [CrossRef]
- Arendshorst, W.J.; Beierwaltes, W.H. Renal tubular reabsorption in spontaneously hypertensive rats. Am. J. Physiol. 1979, 237, F38–F47. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.A., Jr.; Enobakhare, J.A.; DeClue, J.W.; Douglas, B.H.; Guyton, A.C. Arterial pressure-urinary output relationship in hypertensive rats. Am. J. Physiol. Reg. Integr. Comp. Physiol. 1978, 234, R98–R103. [Google Scholar] [CrossRef]
- Padia, S.H.; Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Carey, R.M. Intrarenal angiotensin III infusion induces natriuresis and angiotensin type-2 receptor translocation in Wistar-Kyoto but not in spontaneously hypertensive rats. Hypertension 2009, 53, 338–343. [Google Scholar] [CrossRef][Green Version]
- Kemp, B.A.; Howell, N.L.; Keller, S.R.; Gildea, J.J.; Padia, S.H.; Shao, W.; Navar, L.G.; Carey, R.M. Defective renal angiotensin III and AT2 receptor signaling in pre-hypertensive spontaneously hypertensive rats. JAHA 2019, 8, e012016. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.A.; Howell, N.L.; Gildea, J.J.; Keller, S.R.; Carey, R.M. Identification of a primary renal AT2 receptor defect in spontaneously hypertensive rats. Circ. Res. 2020, 126, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, L.M.; Chow, C.L.E.; Mirabito, K.M.; Steckelings, U.M.; Unger, T.; Widdop, R.E.; Denton, K.M. Angiotensin Type 2 Receptor Stimulation Increases Renal Function in Female, but Not Male, Spontaneously Hypertensive Rats. Hypertension 2014, 64, 378–383. [Google Scholar] [CrossRef]
- Hakam, A.C.; Hussain, T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension 2005, 45, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Hakam, A.C.; Hussain, T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension 2006, 47, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.H.; Ali, Q.; Hussain, T. Protective role of angiotensin II type 2 receptor in blood pressure increase in obese Zucker rats. Hypertension 2009, 53, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Hussain, T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens. Res. 2012, 35, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Patel, S.; Hussain, T. Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese Zucker rats. Am. J. Physiol. Renal Physiol. 2015, 308, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Siragy, H.M.; Inagami, T.; Carey, R.M. NO and cGMP mediate angiotensin AT2 receptor-induced renin inhibition in young rats. Am. J. Physiol. Renal Physiol. 2007, 293, R1461–R1467. [Google Scholar]
- Kassamali, R.; Sicca, D.A. Acetazolamide: A forgotten diuretic agent. Cardiol. Rev. 2011, 19, 276–278. [Google Scholar] [CrossRef]

| Experimental Rodent Model | Natriuretic Response to AT2R Agonist | Natriuretic Response Abolished by AT2R Antagonist | Natriuretic Response Abolished by Inhibition of NO/cGMP | Natriuretic Response Abolished by Calyculin A (PP2A Inhibitor) |
|---|---|---|---|---|
| Sprague-Dawley rat | ||||
| Normal baseline | Yes | Yes | Yes | - |
| AT1R blockade (acute) | - | Yes | - | - |
| Ang II infusion (chronic) | Yes | Yes | - | - |
| Wistar-Kyoto rat | Yes | Yes | Yes | Yes |
| Spontaneously hypertensive rat | ||||
| Prehypertensive | No | NA | NA | - |
| Hypertensive | No | NA | NA | - |
| Zucker rat | ||||
| Obese | Yes | Yes | Yes | - |
| Lean | No | NA | NA | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carey, R.M.; Siragy, H.M.; Gildea, J.J.; Keller, S.R. Angiotensin Type-2 Receptors: Transducers of Natriuresis in the Renal Proximal Tubule. Int. J. Mol. Sci. 2022, 23, 2317. https://doi.org/10.3390/ijms23042317
Carey RM, Siragy HM, Gildea JJ, Keller SR. Angiotensin Type-2 Receptors: Transducers of Natriuresis in the Renal Proximal Tubule. International Journal of Molecular Sciences. 2022; 23(4):2317. https://doi.org/10.3390/ijms23042317
Chicago/Turabian StyleCarey, Robert M., Helmy M. Siragy, John J. Gildea, and Susanna R. Keller. 2022. "Angiotensin Type-2 Receptors: Transducers of Natriuresis in the Renal Proximal Tubule" International Journal of Molecular Sciences 23, no. 4: 2317. https://doi.org/10.3390/ijms23042317
APA StyleCarey, R. M., Siragy, H. M., Gildea, J. J., & Keller, S. R. (2022). Angiotensin Type-2 Receptors: Transducers of Natriuresis in the Renal Proximal Tubule. International Journal of Molecular Sciences, 23(4), 2317. https://doi.org/10.3390/ijms23042317






