Circumventing Doxorubicin Resistance Using Elastin-like Polypeptide Biopolymer-Mediated Drug Delivery
Abstract
:1. Introduction
2. Results
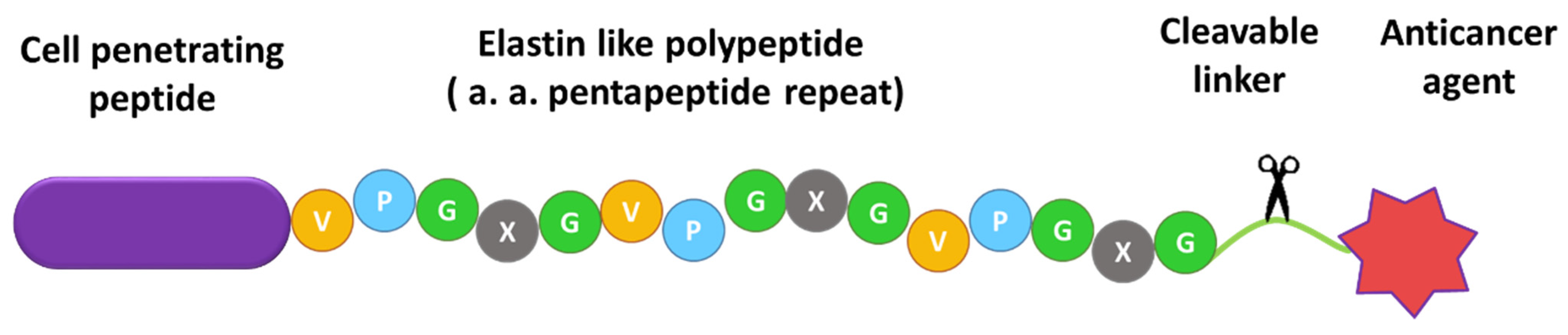
2.1. Design of ELP-Based Drug Delivery Macromolecule
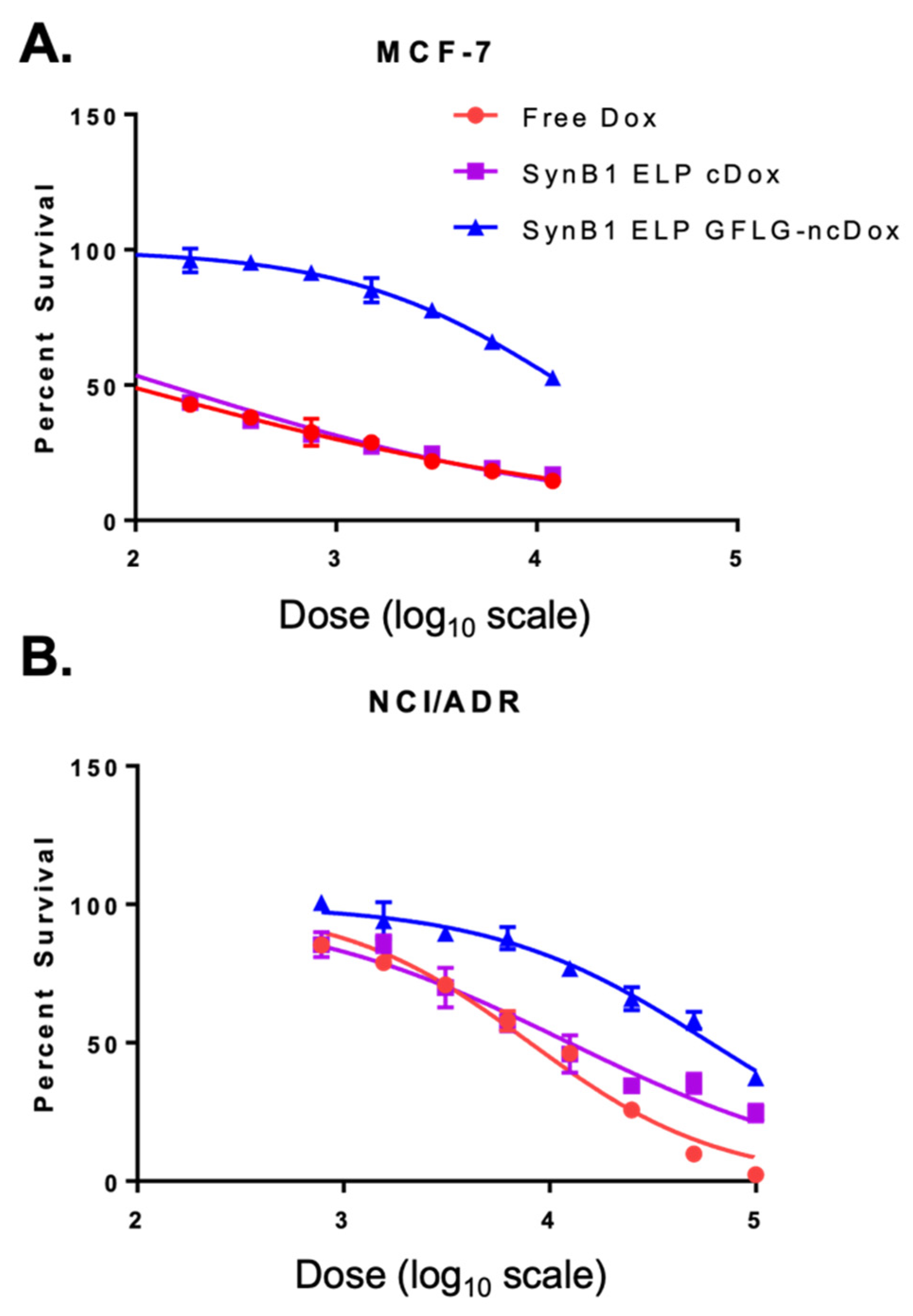
2.2. Comparison of Cytotoxic Effects of Several Drug Delivery Systems vs. Free Dox
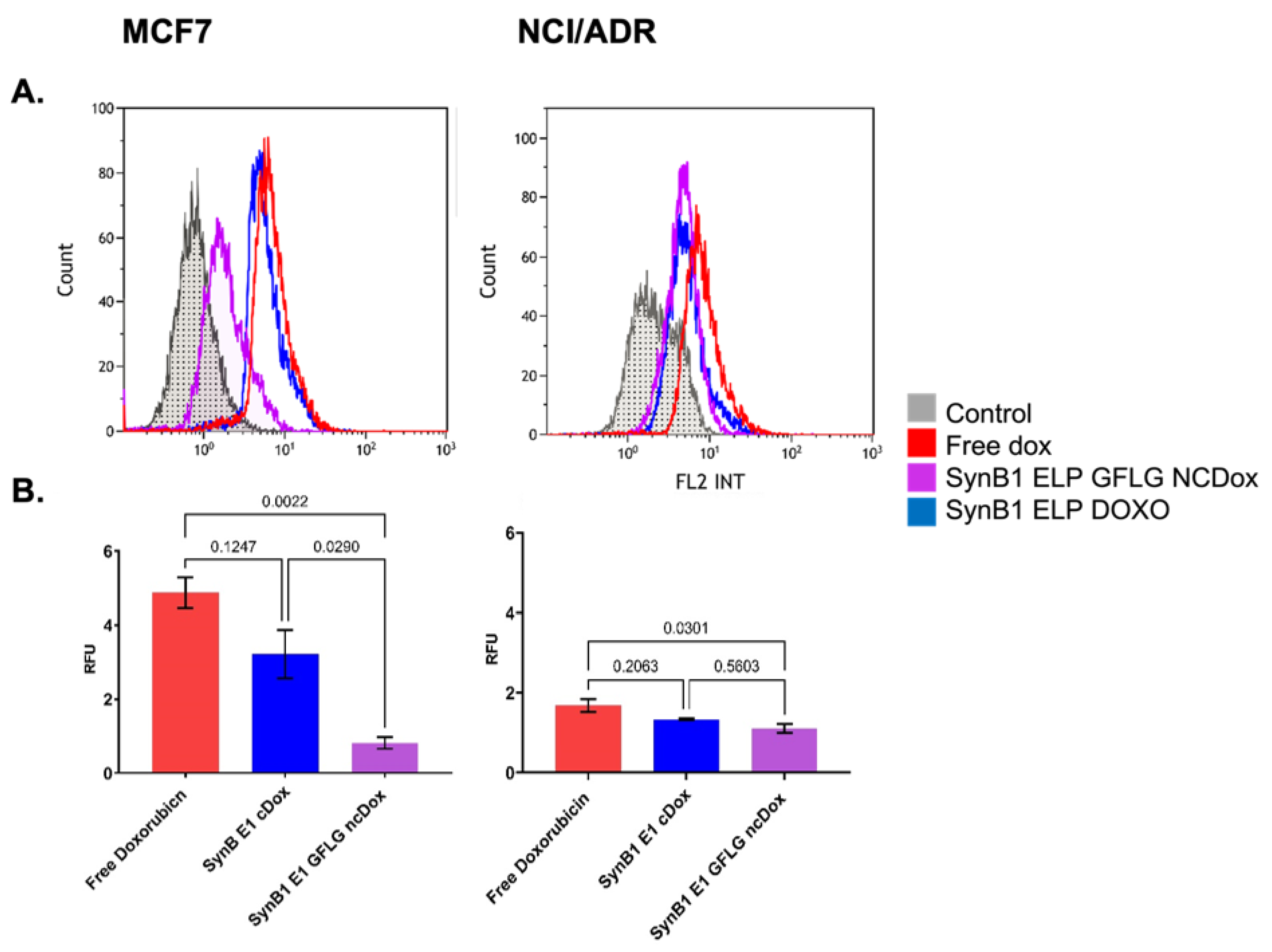
2.3. Cellular Uptake of ELP-Delivered Doxorubicin
2.4. Cell Cycle Distribution
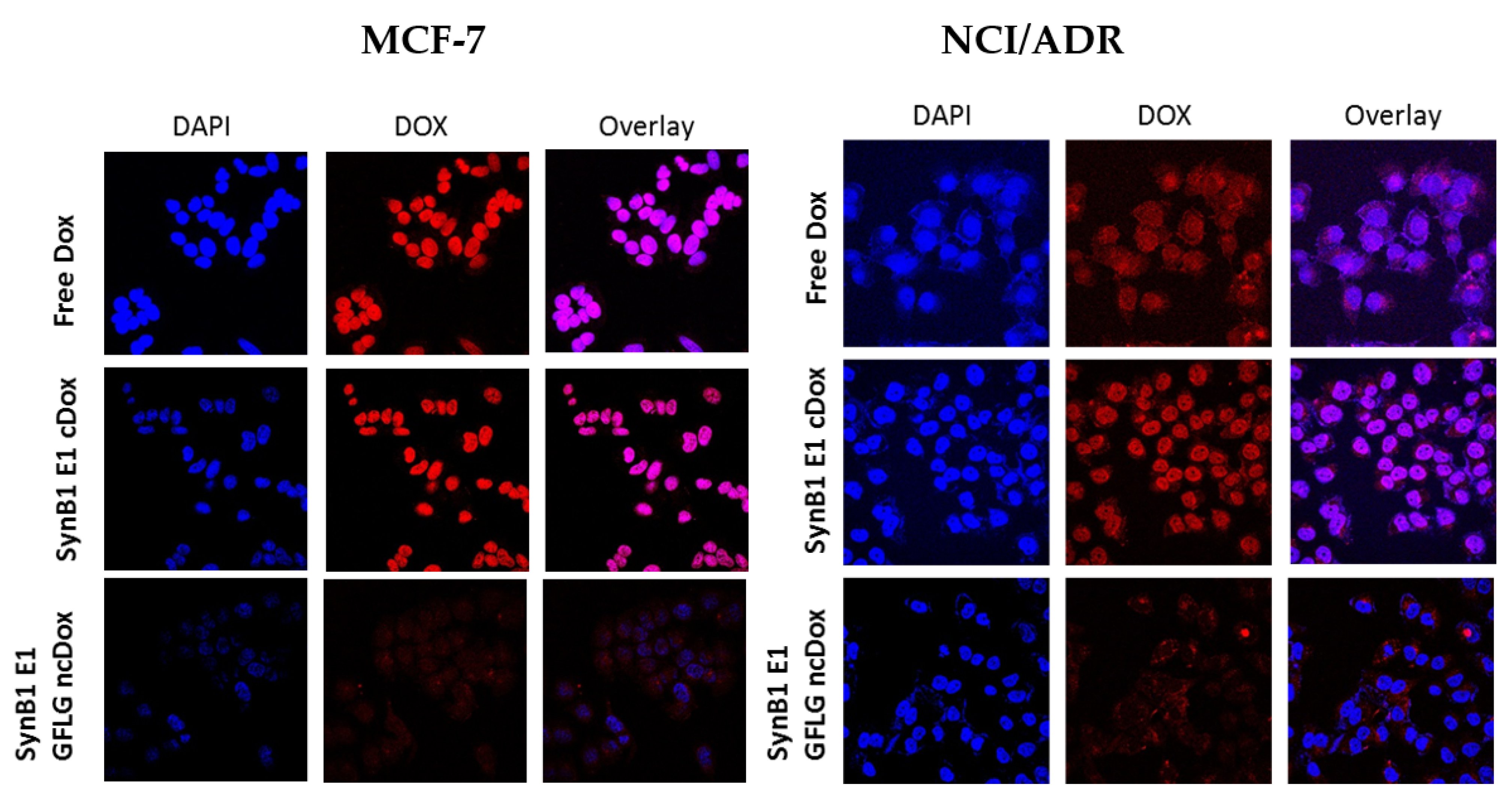
2.5. Intracellular Localization
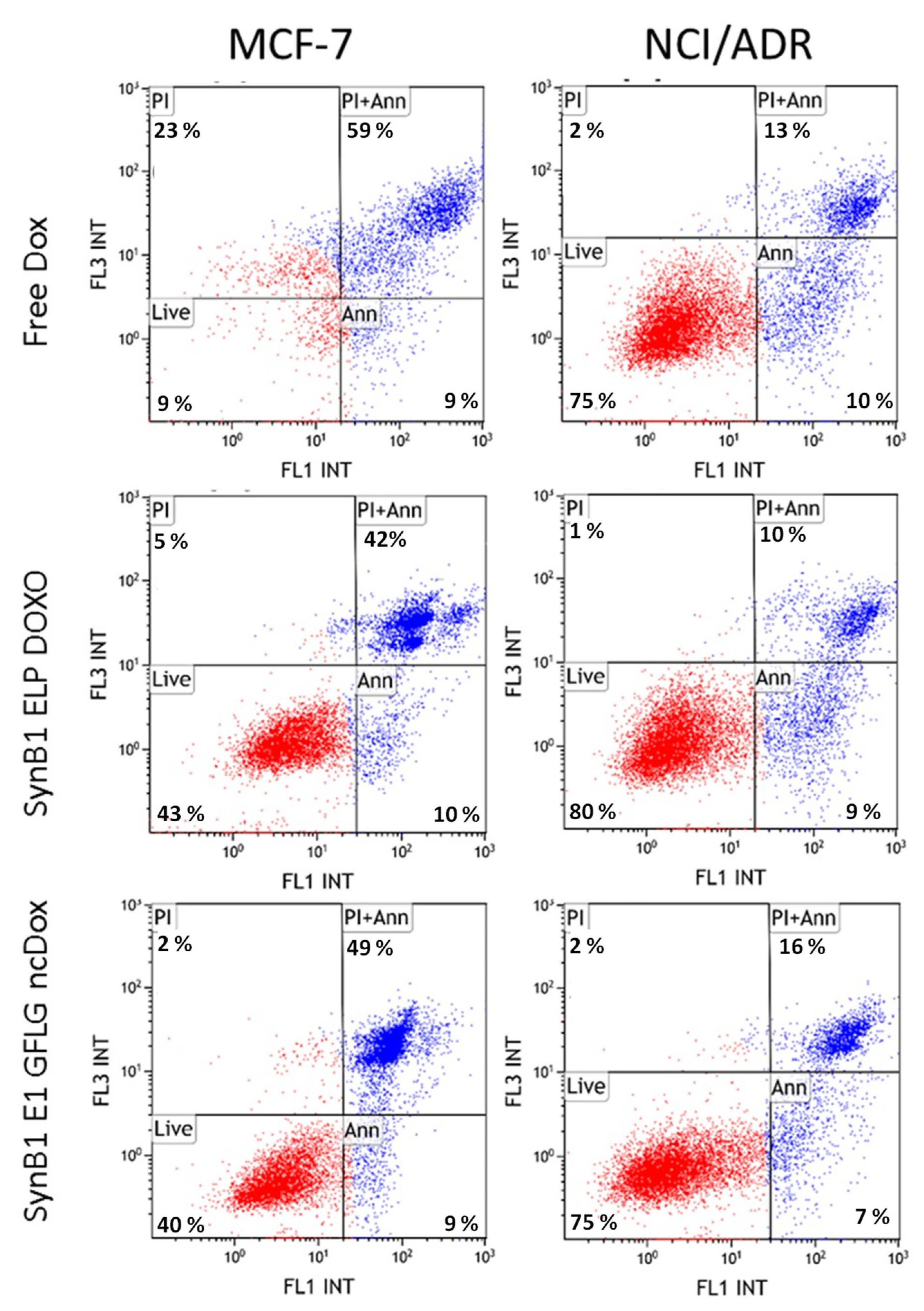
2.6. Apoptosis
3. Discussion
4. Materials and Methods
4.1. Polypeptide Expression and Purification
4.2. Conjugation of Doxorubicin Derivatives to ELP
4.3. Cell Lines
4.4. Cytotoxicity Assay
4.5. Apoptosis Assay
4.6. Cell Cycle Analysis
4.7. Cellular Localization
4.8. Cellular Uptake Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zahreddine, H.; Borden, K.L. Mechanisms and insights into drug resistance in cancer. Front. Pharmacol. 2013, 4, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Hamon, Y.; Chimini, G. The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 2001, 42, 1007–1017. [Google Scholar] [CrossRef]
- Zhou, X.W.; Xia, Y.-Z.; Zhang, Y.-L.; Luo, J.-G.; Han, C.; Zhang, C.; Yang, L.; Kong, L.-Y. Tomentodione M sensitizes multidrug resistant cancer cells by decreasing P-glycoprotein via inhibition of p38 MAPK signaling. Oncotarget 2017, 8, 101965–101983. [Google Scholar] [CrossRef] [Green Version]
- Doyle, L.A.; Yang, W.; Abruzzo, L.V.; Krogmann, T.; Gao, Y.; Rishi, A.K.; Ross, D.D. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc. Natl. Acad. Sci. USA 1998, 95, 15665–15670. [Google Scholar] [CrossRef] [Green Version]
- Palmeira, A.; Sousa, M.E.; Vasconcelos, M.H.; Pinto, M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef] [PubMed]
- Baekelandt, M.; Lehne, G.; Tropé, C.G.; Szántó, I.; Pfeiffer, P.; Gustavssson, B.; Kristensen, G.B. Phase I/II trial of the multidrug-resistance modulator valspodar combined with cisplatin and doxorubicin in refractory ovarian cancer. J. Clin. Oncol. 2001, 19, 2983–2993. [Google Scholar] [CrossRef]
- Fracasso, P.M.; Brady, M.F.; Moore, D.H.; Walker, J.L.; Rose, P.G.; Letvak, L.; Grogan, T.M.; McGuire, W.P. Phase II study of paclitaxel and valspodar (PSC 833) in refractory ovarian carcinoma: A gynecologic oncology group study. J. Clin. Oncol. 2001, 19, 2975–2982. [Google Scholar] [CrossRef]
- Seiden, M.V.; Swenerton, K.D.; Matulonis, U.; Campos, S.; Rose, P.; Batist, G.; Ette, E.; Garg, V.; Fuller, A.; Harding, M.W.; et al. A phase II study of the MDR inhibitor biricodar (INCEL, VX-710) and paclitaxel in women with advanced ovarian cancer refractory to paclitaxel therapy. Gynecol. Oncol. 2002, 86, 302–310. [Google Scholar] [CrossRef]
- Lhomme, C.; Joly, F.; Walker, J.L.; Lissoni, A.A.; Nicoletto, M.O.; Manikhas, G.M.; Baekelandt, M.M.; Gordon, A.N.; Fracasso, P.M.; Mietlowski, W.L.; et al. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J. Clin. Oncol. 2008, 26, 2674–2682. [Google Scholar] [CrossRef]
- Kaye, S.B. Reversal of drug resistance in ovarian cancer: Where do we go from here? J. Clin. Oncol. 2008, 26, 2616–2618. [Google Scholar] [CrossRef] [PubMed]
- Nyrop, K.A.; Ms, A.M.D.; Reeder-Hayes, K.E.; Shachar, S.S.; Reeve, B.B.; Basch, E.; Choi, S.K.; Lee, J.T.; Wood, W.A.; Anders, C.K.; et al. Patient-reported and clinician-reported chemotherapy-induced peripheral neuropathy in patients with early breast cancer: Current clinical practice. Cancer 2019, 125, 2945–2954. [Google Scholar] [CrossRef] [PubMed]
- Tempfer, C.B.; Kern, P.; Dogan, A.; Hilal, Z.; Rezniczek, G.A. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for endometrial cancer-derived peritoneal metastases: A systematic review. Clin. Exp. Metastasis 2019, 36, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Javia, A.; Shetty, S.; Bardoliwala, D.; Maiti, K.; Banerjee, S.; Khopade, A.; Misra, A.; Sawant, K.; Bhowmick, S. Triple negative breast cancer and non-small cell lung cancer: Clinical challenges and nano-formulation approaches. J. Control Release 2021, 337, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, G.L., 3rd; Davis, A.N.; Fokt, I.; Priebe, W.; Raucher, D. A thermally targeted elastin-like polypeptide-doxorubicin conjugate overcomes drug resistance. Investig. New Drugs 2007, 25, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Garraway, L.A.; Widlund, H.; Rubin, M.; Getz, G.; Berger, A.J.; Ramaswamy, S.; Beroukhim, R.; Milner, J.D.A.; Granter, S.R.; Du, J.; et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 2005, 436, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Roschke, A.V.; Tonon, G.; Gehlhaus, K.S.; Mctyre, N.; Bussey, K.J.; Lababidi, S.; Scudiero, D.A.; Weinstein, J.N.; Kirsch, I.R. Karyotypic Complexity of the NCI-60 Drug-Screening Panel. Cancer Res. 2003, 63, 8634–8647. [Google Scholar]
- Moktan, S.; Ryppa, C.; Kratz, F.; Raucher, A. A thermally responsive biopolymer conjugated to an acid-sensitive derivative of paclitaxel stabilizes microtubules, arrests cell cycle, and induces apoptosis. Investig New Drugs 2012, 30, 236–248. [Google Scholar] [CrossRef]
- Erasimus, H.; Gobin, M.; Niclou, S.; Van Dyck, E. DNA repair mechanisms and their clinical impact in glioblastoma. Mutat. Res. Rev. Mutat. Res. 2016, 769, 19–35. [Google Scholar] [CrossRef]
- Dragojevic, S.; Ryu, J.S.; Raucher, D. Polymer-Based Prodrugs: Improving Tumor Targeting and the Solubility of Small Molecule Drugs in Cancer Therapy. Molecules 2015, 20, 21750–21769. [Google Scholar] [CrossRef] [PubMed]
- Bidwell, G.L., 3rd; Fokt, I.; Priebe, W.; Raucher, D. Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem. Pharmacol. 2007, 73, 620–631. [Google Scholar]
- Raucher, D.; Massodi, I.; Bidwell, G.L. Thermally targeted delivery of chemotherapeutics and anti-cancer peptides by elastin-like polypeptide. Expert Opin. Drug Deliv. 2008, 5, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef] [PubMed]
- Kasaian, J.; Mosaffa, F.; Behravan, J.; Masullo, M.; Piacente, S.; Ghandadi, M.; Iranshahi, M. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpene coumarins. Fitoterapia 2015, 103, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.; Perkins, E.; Kratz, F.; Raucher, D. Cell penetrating peptides fused to a thermally targeted biopolymer drug carrier improve the delivery and antitumor efficacy of an acid-sensitive doxorubicin derivative. Int. J. Pharm. 2012, 436, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Kratz, F.; Ehling, G.; Kauffmann, H.-M.; Unger, C. Acute and repeat-dose toxicity studies of the (6-maleimidocaproyl)hydrazone derivative of doxorubicin (DOXO-EMCH), an albumin-binding prodrug of the anticancer agent doxorubicin. Hum. Exp. Toxicol. 2007, 26, 19–35. [Google Scholar] [CrossRef]
- Dreher, M.R.; Raucher, D.; Balu, N.; Colvin, O.M.; Ludeman, S.M.; Chilkoti, A. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J. Control Release 2003, 91, 31–43. [Google Scholar] [CrossRef]
- Moktan, S.; Perkins, E.; Kratz, F.; Raucher, D. Thermal targeting of an acid-sensitive doxorubicin conjugate of elastin-like polypeptide enhances the therapeutic efficacy compared with the parent compound in vivo. Mol. Cancer Ther. 2012, 11, 1547–1556. [Google Scholar] [CrossRef] [Green Version]
- Daniell, H.; Guda, C.; McPherson, D.T.; Zhang, X.; Xu, J.; Urry, D.W.; Tuan, R.S. Hyperexpression of a Synthetic Protein-Based Polymer Gene, in Recombinant Protein Protocols: Detection and Isolation; Tuan, R.S., Ed.; Humana Press: Totowa, NJ, USA, 1997; pp. 359–371. [Google Scholar]
- Bidwell, G.L., 3rd; Raucher, D. Application of thermally responsive polypeptides directed against c-Myc transcriptional function for cancer therapy. Mol. Cancer Ther. 2005, 4, 1076–1085. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Cheng, A.-C.; Wang, M.-S.; Peng, X. Detection of apoptosis induced by new type gosling viral enteritis virus in vitro through fluorescein annexin V-FITC/PI double labeling. World J. Gastroenterol. 2008, 14, 2174–2178. [Google Scholar] [CrossRef] [PubMed]


| MCF-7 | NCI/ADR | |
|---|---|---|
| Free Doxorubicin | 0.089 ± 0.0182 | 8.029 ± 1.046 |
| SynB1 ELP DOXO | 0.143 ± 0.0387 | 12.414 ± 2.761 |
| SynB1 ELP GFLG-NCDox | 13.82 ± 1.638 | 60.169 ± 9.934 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragojevic, S.; Turner, L.; Raucher, D. Circumventing Doxorubicin Resistance Using Elastin-like Polypeptide Biopolymer-Mediated Drug Delivery. Int. J. Mol. Sci. 2022, 23, 2301. https://doi.org/10.3390/ijms23042301
Dragojevic S, Turner L, Raucher D. Circumventing Doxorubicin Resistance Using Elastin-like Polypeptide Biopolymer-Mediated Drug Delivery. International Journal of Molecular Sciences. 2022; 23(4):2301. https://doi.org/10.3390/ijms23042301
Chicago/Turabian StyleDragojevic, Sonja, Lindsay Turner, and Drazen Raucher. 2022. "Circumventing Doxorubicin Resistance Using Elastin-like Polypeptide Biopolymer-Mediated Drug Delivery" International Journal of Molecular Sciences 23, no. 4: 2301. https://doi.org/10.3390/ijms23042301
APA StyleDragojevic, S., Turner, L., & Raucher, D. (2022). Circumventing Doxorubicin Resistance Using Elastin-like Polypeptide Biopolymer-Mediated Drug Delivery. International Journal of Molecular Sciences, 23(4), 2301. https://doi.org/10.3390/ijms23042301






