Capsaicin and Zinc Promote Glucose Uptake in C2C12 Skeletal Muscle Cells through a Common Calcium Signalling Pathway
Abstract
1. Introduction
2. Results
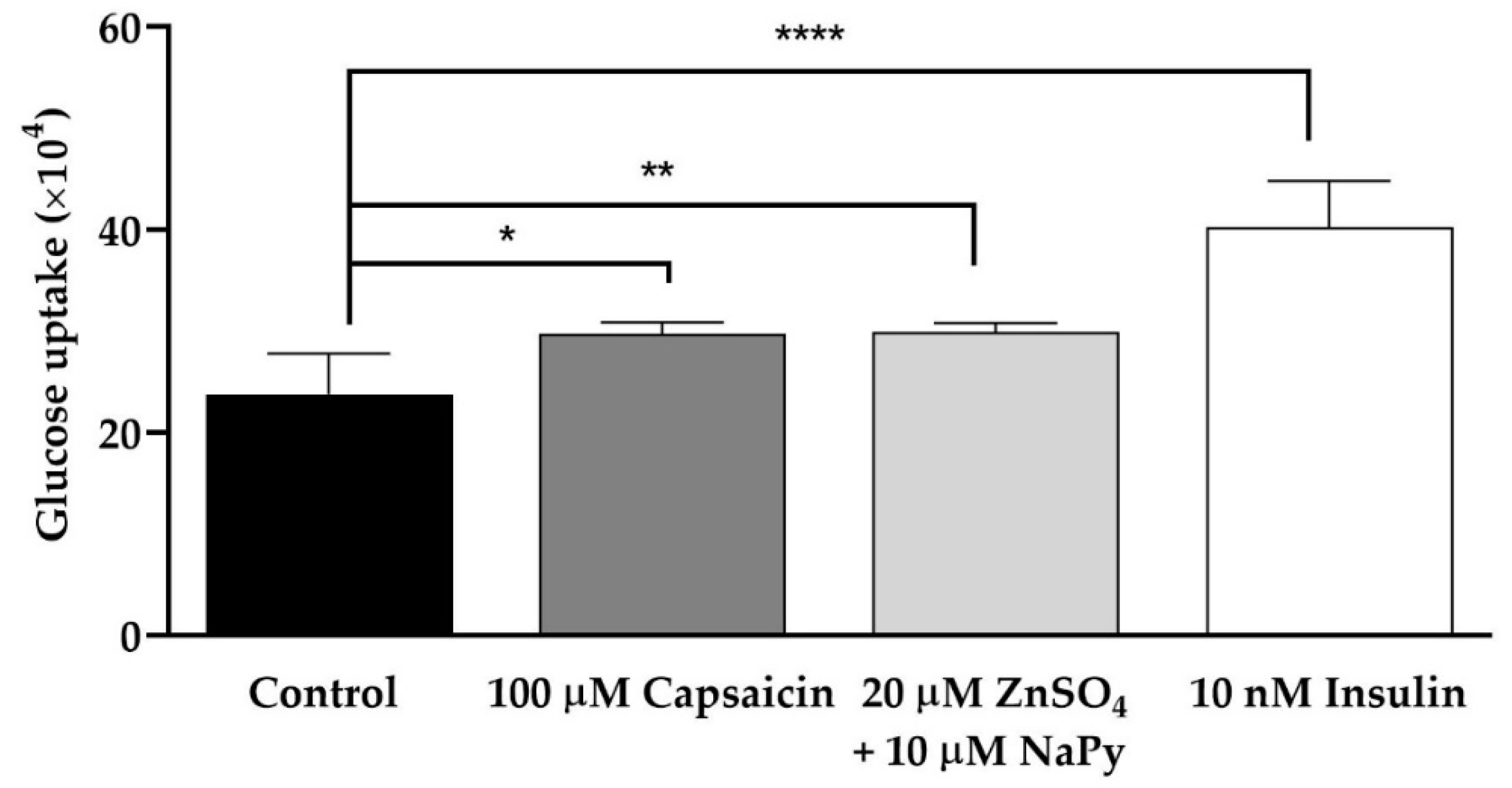
2.1. Capsaicin and Zinc Elevate Glucose Uptake in C2C12 Skeletal Muscle Cells
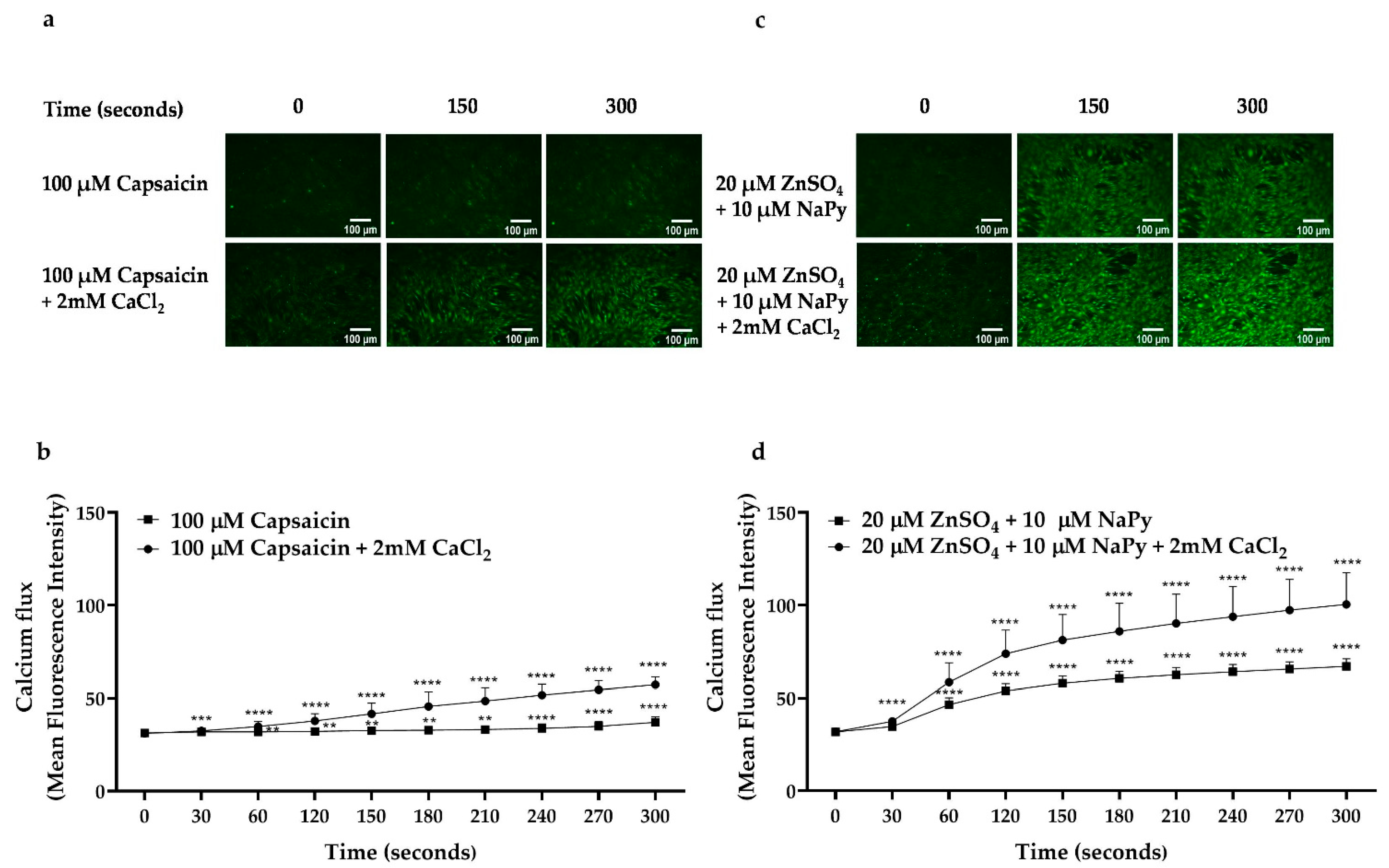
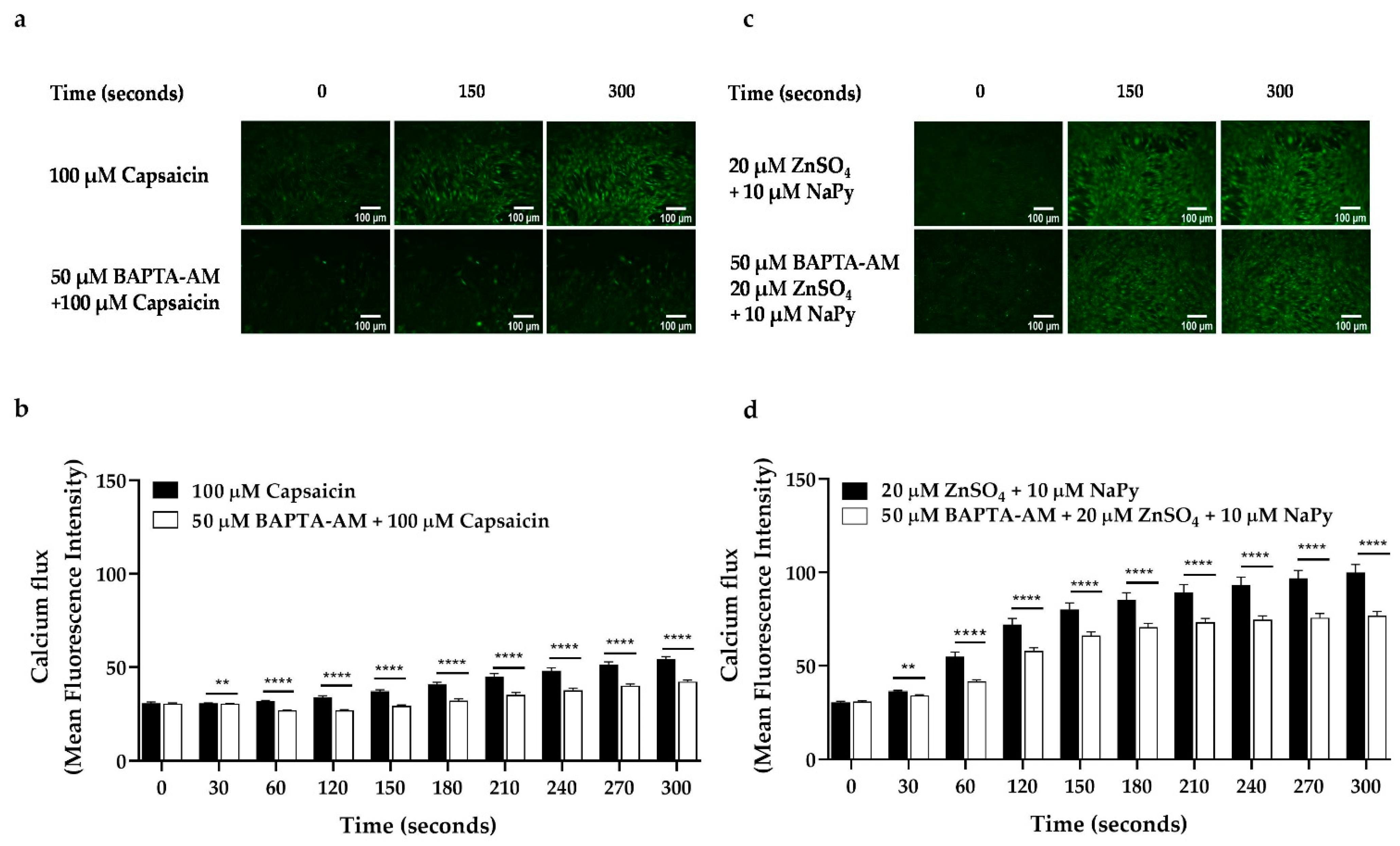
2.2. Capsaicin and Zinc Stimulate Calcium Flux in C2C12 Skeletal Muscle Cells
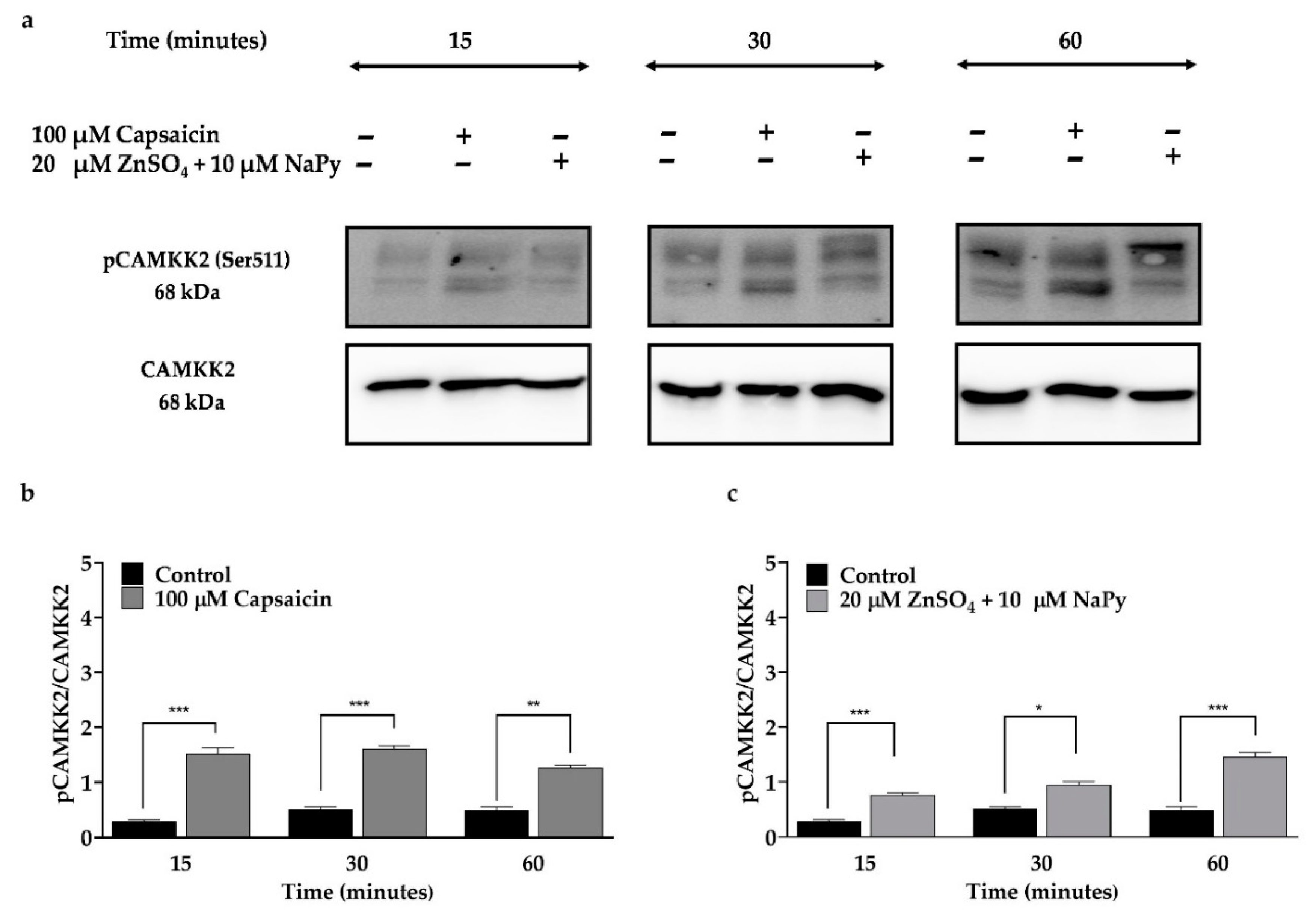
2.3. Capsaicin and Zinc Phosphorylate CAMKK2 in C2C12 Skeletal Muscle Cells
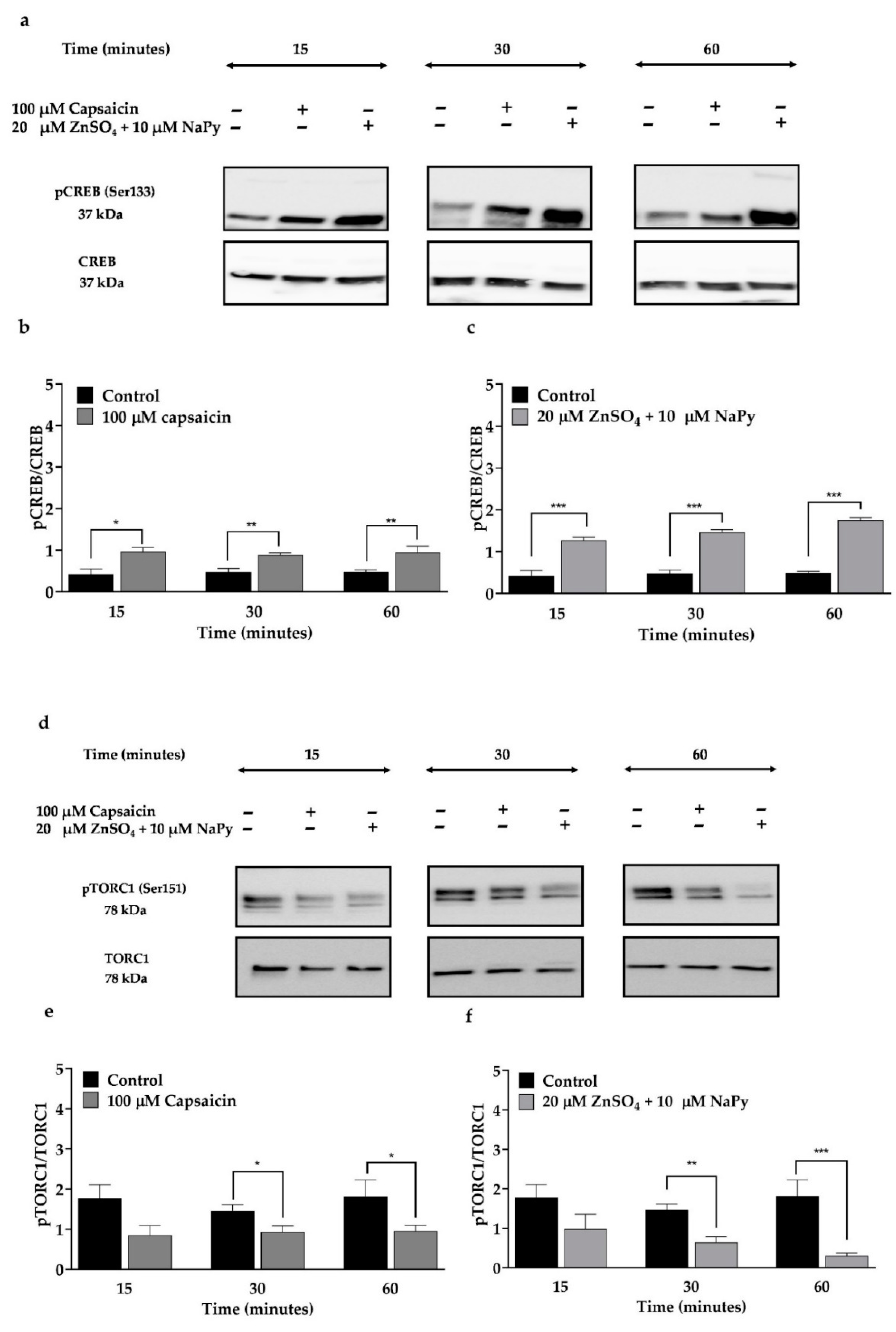
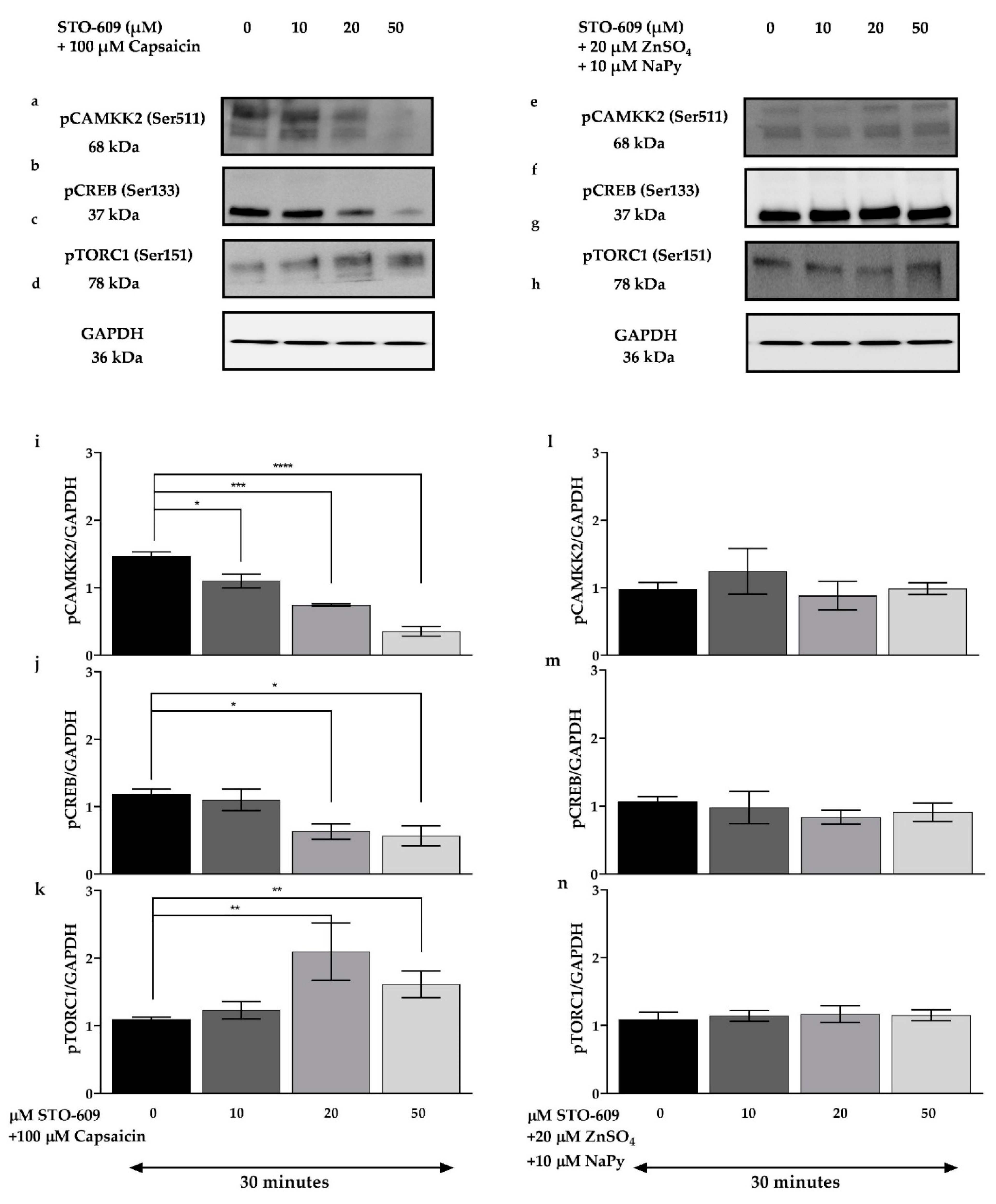
2.4. Capsaicin and Zinc Phosphorylate CREB and TORC1 in C2C12 Skeletal Muscle Cells
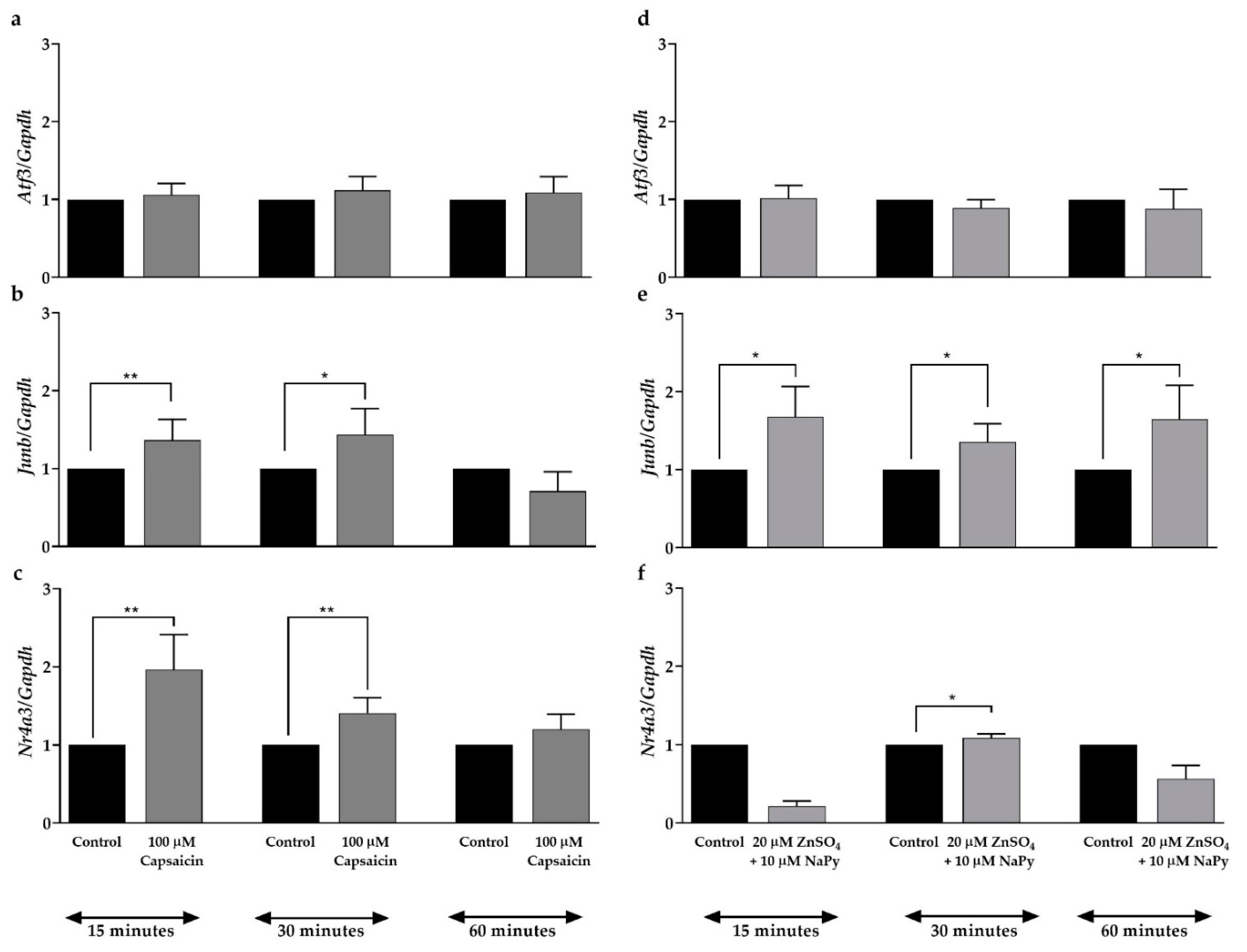
2.5. Capsaicin and Zinc Stimulate Junb and Nr4a3 Expression in C2C12 Skeletal Muscle Cells
2.6. Calcium Activates Signalling Molecules Involved in Glucose Metabolism
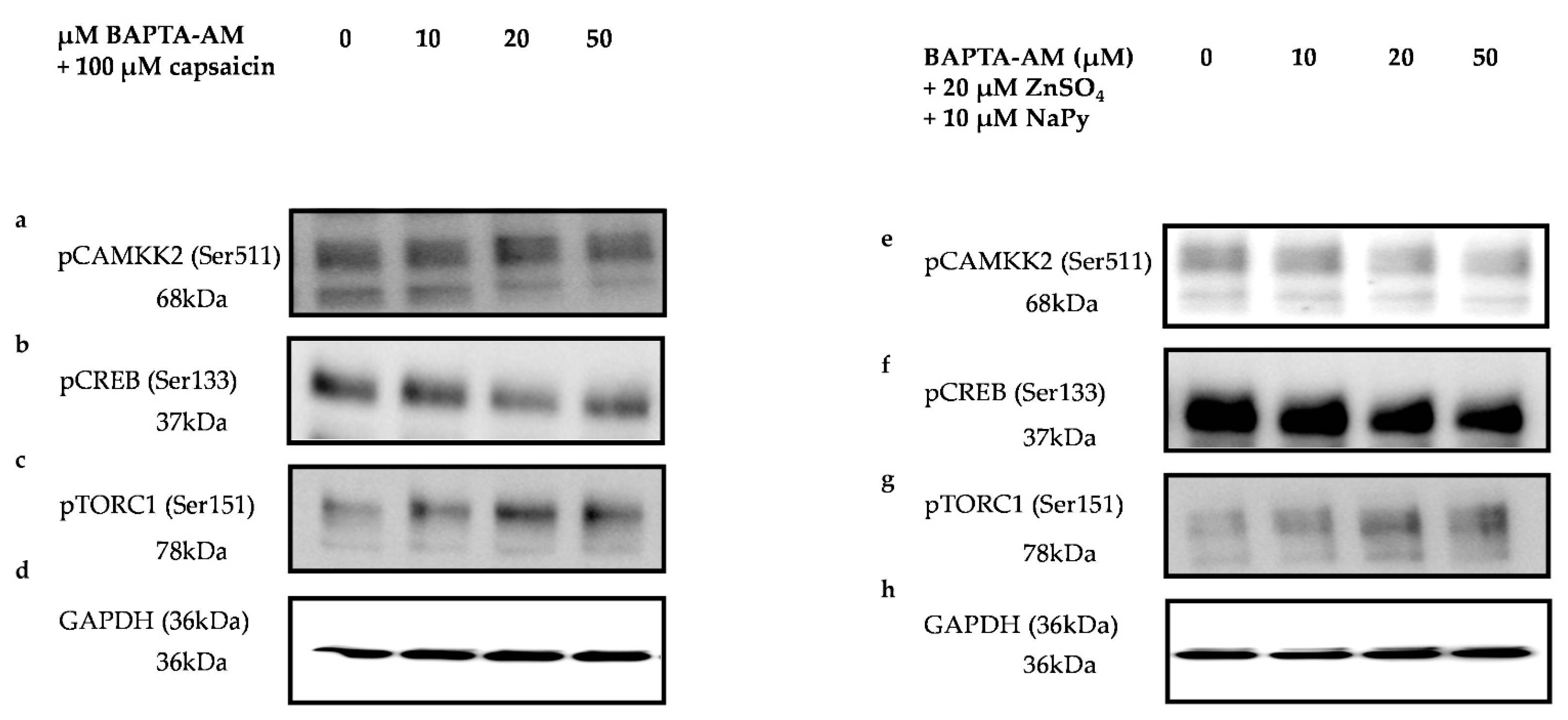
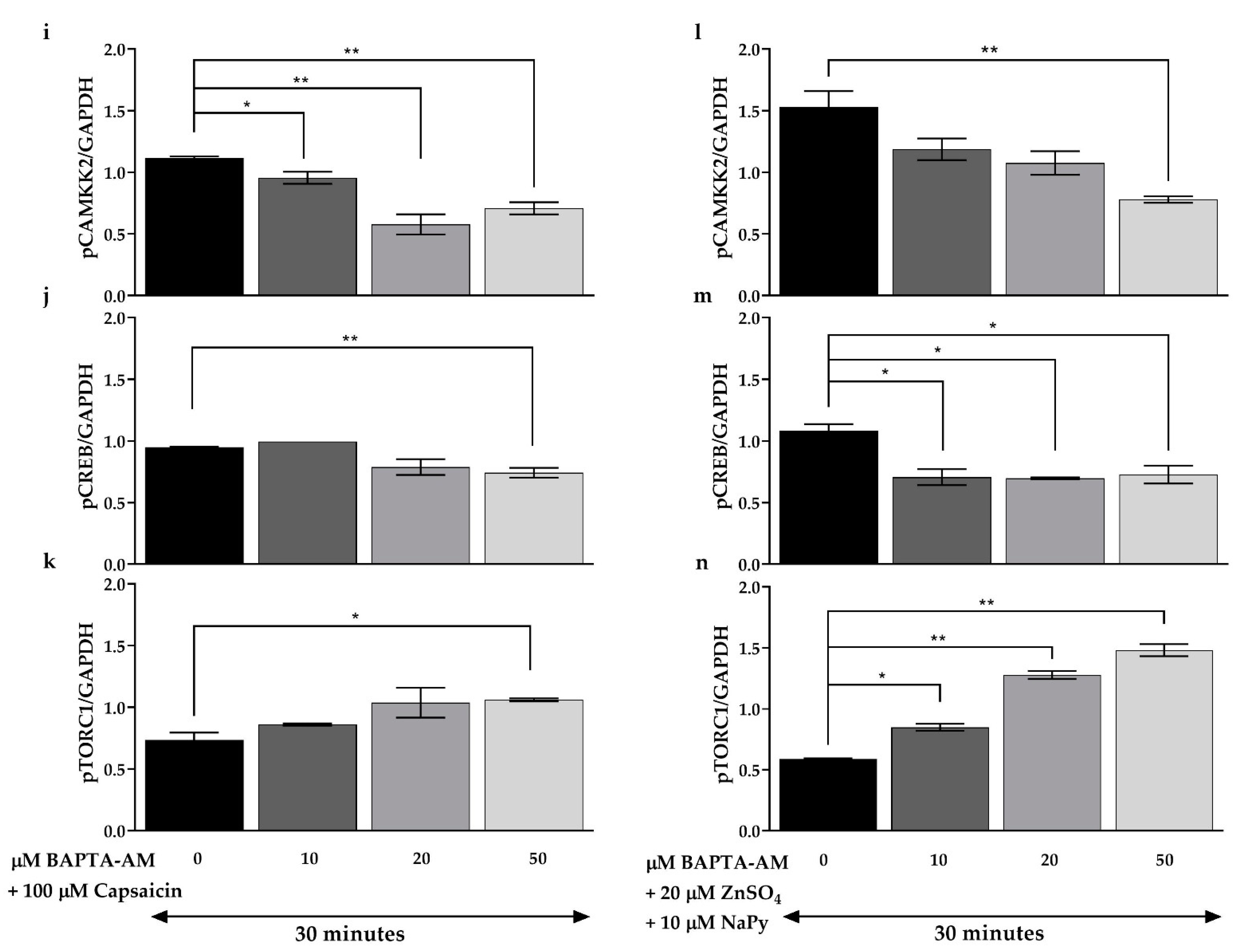
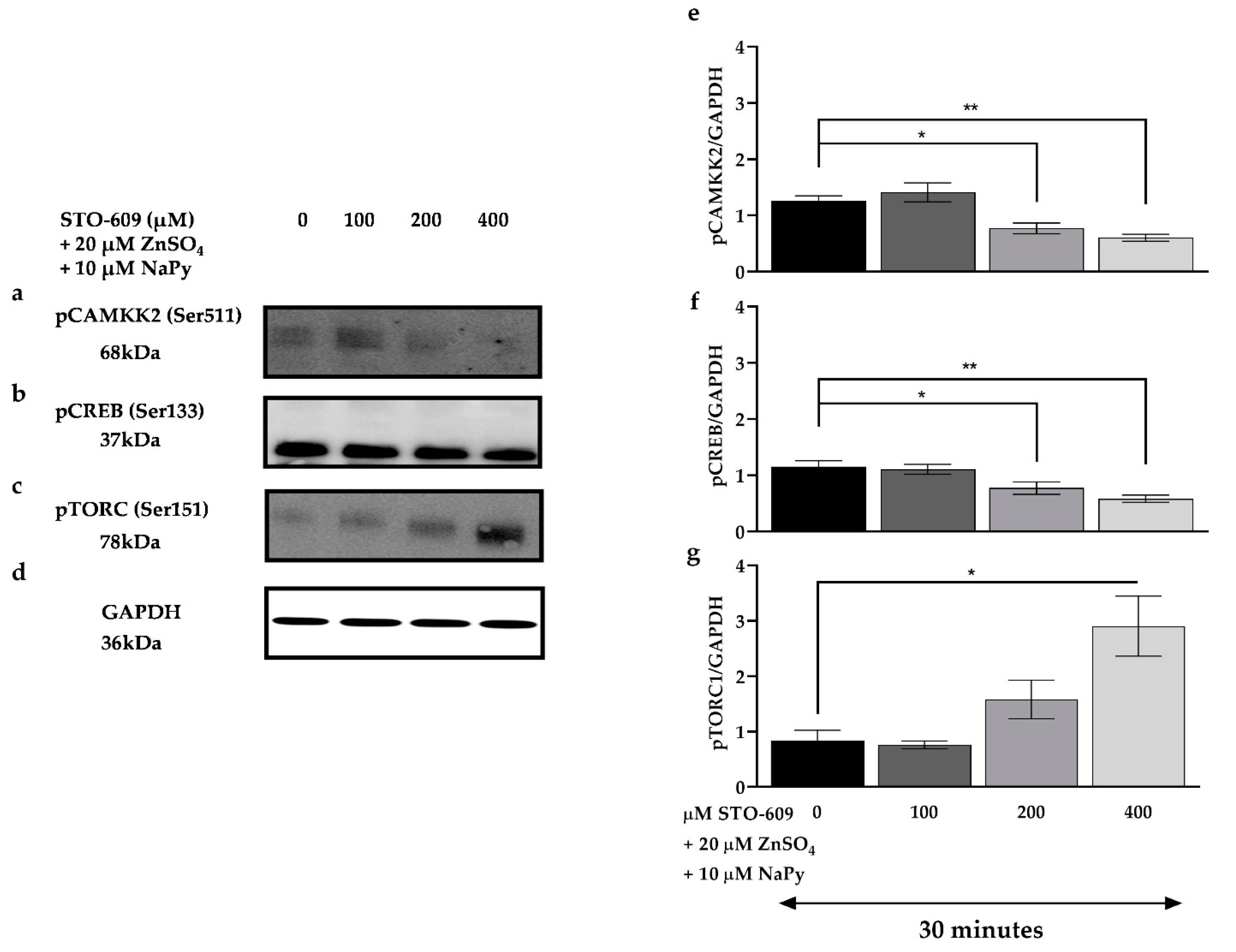
2.7. CAMKK2 Phosphorylation Is Involved in Calcium-Induced Activation of Signalling Molecules by Capsaicin and Zinc
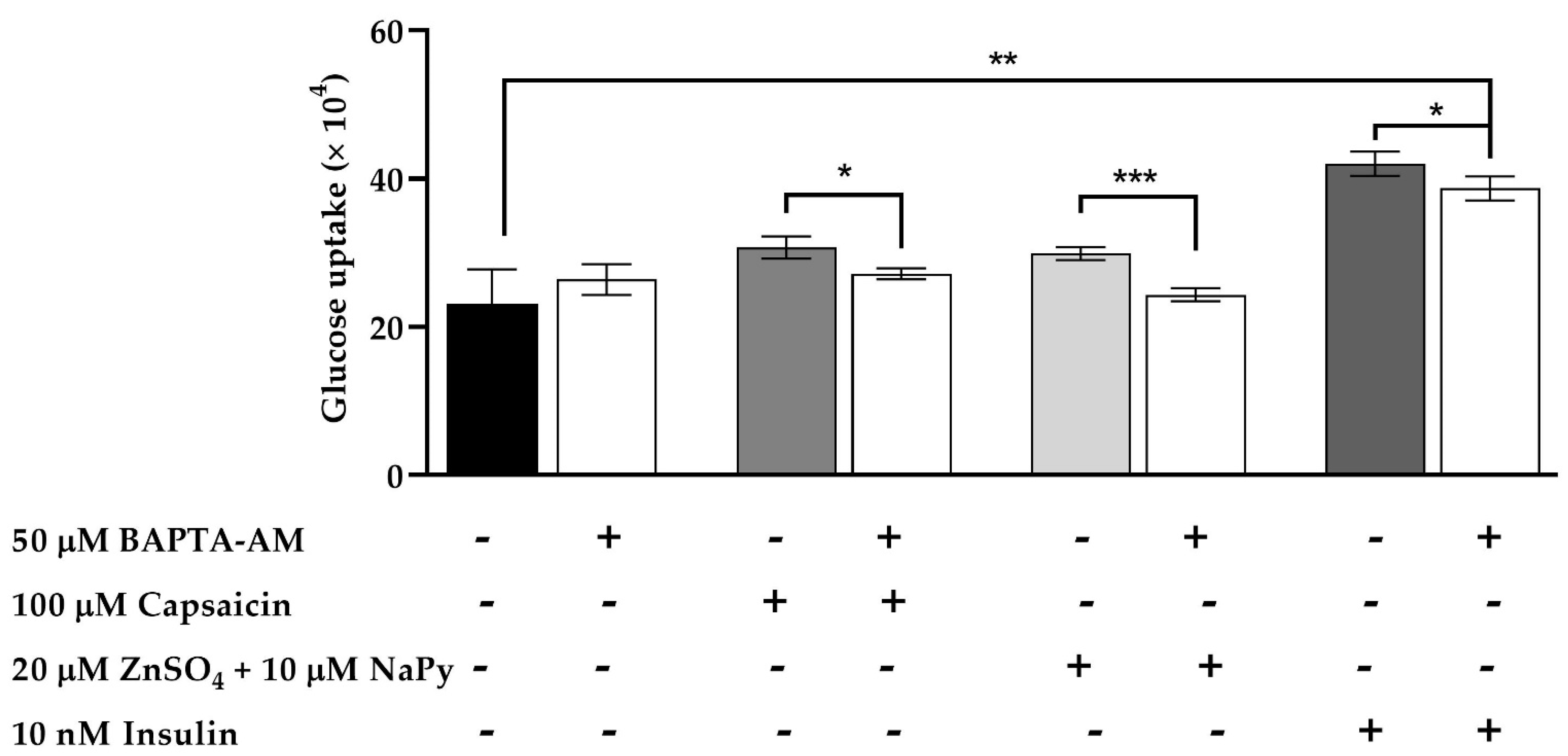
2.8. Effect of Cytosolic Calcium Level in Glucose Uptake by Capsaicin and Zinc in Skeletal Muscle Cells
2.9. Capsaicin and Zinc Elevate Intracellular cAMP Levels in C2C12 Skeletal Muscle Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Immunoblot Analysis of Protein Expression
4.4. Calcium Imaging
4.5. cAMP Measurement
4.6. CAMKK2 Inhibition Study
4.7. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qPCR)
4.8. Glucose Uptake Assay
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAMKK2 | Calcium/calmodulin-dependent protein kinase 2 |
| CREB | cAMP-response element-binding protein |
| TORC1 | Target of rapamycin kinase complex 1 |
| TRPV1 | Transient receptor potential cation channel subfamily V member |
| IR | Insulin resistance |
| T2DM | Type 2 diabetes mellitus |
| Atf1 | Activating transcription factor 1 |
| Jun | V-jun avian sarcoma virus 17 oncogene homolog |
| Nr4a3 | Nuclear receptor 4 A3 |
| SR | Sarcoplasmic reticulum |
| NaPy | Pyrithione sodium |
| HBSS | Hanks’ balanced salt solution |
| GLUT4 | Glucose transporter type 4 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| AKT | Protein kinase B |
| SHP | Small heterodimer partner |
| ERK1/2 | Extracellular signal-regulated protein kinases 1 and 2 |
| AMPK | 5’ AMP-activated protein kinase |
| PLC | G proteins activate phospholipase-C |
| PGC-1 alpha | Pparg coactivator 1 alpha |
| IL-6 | Interleukin 6 |
| SIK-1 | Salt Inducible Kinase 1 |
References
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in metabolic syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Vahidi Ferdowsi, P.; Ng, R.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc Modulates Several Transcription-Factor Regulated Pathways in Mouse Skeletal Muscle Cells. Molecules 2020, 25, 5098. [Google Scholar] [CrossRef] [PubMed]
- Vahidi Ferdowsi, P.; Ahuja, K.D.K.; Beckett, J.M.; Myers, S. TRPV1 Activation by Capsaicin Mediates Glucose Oxidation and ATP Production Independent of Insulin Signalling in Mouse Skeletal Muscle Cells. Cells 2021, 10, 1560. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S. Effect of zinc supplementation on insulin resistance and metabolic risk factors in obese Korean women. Nutr. Res. Pract. 2012, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, X.-F.; Zhang, B.; Wang, Z.-H.; Zhang, J.-G.; Huang, F.-F.; Su, C.; Ouyang, Y.-F.; Zhao, J.; Du, W.-W. Dietary zinc intake and its association with metabolic syndrome indicators among Chinese adults: An analysis of the China Nutritional Transition Cohort Survey 2015. Nutrients 2018, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.-X. Glucose Homeostatis and the Pathogenesis of Diabetes Mellitus; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am. J. Clin. Nutr. 2006, 84, 63–69. [Google Scholar] [CrossRef]
- Hashemipour, M.; Kelishadi, R.; Shapouri, J.; Sarrafzadegan, N.; Amini, M.; Tavakoli, N.; Movahedian-Attar, A.; Mirmoghtadaee, P.; Poursafa, P. Effect of zinc supplementation on insulin resistance and components of the metabolic syndrome in prepubertal obese children. Hormones 2009, 8, 279–285. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Tinkov, A.; Skalny, A.; Suliburska, J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J. Physiol. Sci. 2018, 68, 19–31. [Google Scholar] [CrossRef]
- Kang, J.H.; Tsuyoshi, G.; Han, I.S.; Kawada, T.; Kim, Y.M.; Yu, R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity 2010, 18, 780–787. [Google Scholar] [CrossRef]
- Wang, P.; Yan, Z.; Zhong, J.; Chen, J.; Ni, Y.; Li, L.; Ma, L.; Zhao, Z.; Liu, D.; Zhu, Z. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptide-1 secretion and improves glucose homeostasis. Diabetes 2012, 61, 2155–2165. [Google Scholar] [CrossRef]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zeng, Y.; Chang, H.; Wang, J.; Wang, B.; Wan, J.; Chen, S.H.; Zhang, Q.Y.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Weerapan Khovidhunkit, M. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai 2009, 92, 108–113. [Google Scholar]
- Ranasinghe, P.; Wathurapatha, W.; Ishara, M.; Jayawardana, R.; Galappatthy, P.; Katulanda, P.; Constantine, G. Effects of zinc supplementation on serum lipids: A systematic review and meta-analysis. Nutr. Metab. 2015, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Zhang, Z.; Liu, S.; Aluo, Z.; Zhang, L.; Yu, L.; Li, Y.; Song, Z.; Zhou, L. Zinc supplementation alleviates lipid and glucose metabolic disorders induced by a high-fat diet. J. Agric. Food Chem. 2020, 68, 5189–5200. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Sen, S. Status of zinc and magnesium levels in type 2 diabetes mellitus and its relationship with glycemic status. Int. J. Diabetes Dev. Ctries. 2014, 34, 220–223. [Google Scholar] [CrossRef]
- Cruz, K.J.C.; Morais, J.B.S.; de Oliveira, A.R.S.; Severo, J.S.; do Nascimento Marreiro, D. The effect of zinc supplementation on insulin resistance in obese subjects: A systematic review. Biol. Trace Elem. Res. 2017, 176, 239–243. [Google Scholar] [CrossRef]
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.-R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, J.T.; Park, H.S.; Kwon, D.Y.; Kim, M.S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013, 439, 66–70. [Google Scholar] [CrossRef]
- Jayawardena, R.; Ranasinghe, P.; Galappatthy, P.; Malkanthi, R.; Constantine, G.; Katulanda, P. Effects of zinc supplementation on diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2012, 4, 1–12. [Google Scholar] [CrossRef]
- Cooper-Capetini, V.; De Vasconcelos, D.A.A.; Martins, A.R.; Hirabara, S.M.; Donato, J., Jr.; Carpinelli, A.R.; Abdulkader, F. Zinc supplementation improves glucose homeostasis in high fat-fed mice by enhancing pancreatic β-cell function. Nutrients 2017, 9, 1150. [Google Scholar] [CrossRef]
- Norouzi, S.; Adulcikas, J.; Sohal, S.S.; Myers, S. Zinc stimulates glucose oxidation and glycemic control by modulating the insulin signaling pathway in human and mouse skeletal muscle cell lines. PLoS ONE 2018, 13, e0191727. [Google Scholar] [CrossRef] [PubMed]
- Singla, N.; Dhawan, D. Influence of zinc on calcium-dependent signal transduction pathways during aluminium-induced neurodegeneration. Mol. Neurobiol. 2014, 50, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Czeh, G.; Varga, A.; Sandor, Z.; Szolcsanyi, J. Capsaicin-induced changes in the cytosolic calcium level and mitochondrial membrane potential. Neurophysiology 2005, 37, 76–87. [Google Scholar] [CrossRef]
- Guerrero-Hernandez, A.; Verkhratsky, A. Calcium signalling in diabetes. Cell Calcium 2014, 56, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Lanner, J.T.; Katz, A.; Tavi, P.; Sandström, M.E.; Zhang, S.-J.; Wretman, C.; James, S.; Fauconnier, J.; Lännergren, J.; Bruton, J.D. The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes 2006, 55, 2077–2083. [Google Scholar] [CrossRef]
- Ito, N.; Ruegg, U.T.; Kudo, A.; Miyagoe-Suzuki, Y.; Takeda, S.I. Capsaicin mimics mechanical load-induced intracellular signaling events: Involvement of TRPV1-mediated calcium signaling in induction of skeletal muscle hypertrophy. Channels 2013, 7, 221–224. [Google Scholar] [CrossRef]
- Yang, H.-W.; Jiang, Y.-F.; Lee, H.-G.; Jeon, Y.-J.; Ryu, B. Ca2+-Dependent Glucose Transport in Skeletal Muscle by Diphlorethohydroxycarmalol, an Alga Phlorotannin: In Vitro and In Vivo Study. Oxid. Med. Cell. Longev. 2021, 2021, 8893679. [Google Scholar] [CrossRef]
- Bruno, N.E.; Kelly, K.A.; Hawkins, R.; Bramah-Lawani, M.; Amelio, A.L.; Nwachukwu, J.C.; Nettles, K.W.; Conkright, M.D. Creb coactivators direct anabolic responses and enhance performance of skeletal muscle. EMBO J. 2014, 33, 1027–1043. [Google Scholar] [CrossRef]
- Spencer, R.L.; Weiser, M.J. TORC: A new twist on corticotropin-releasing hormone gene expression. Endocrinology 2010, 151, 855–858. [Google Scholar] [CrossRef][Green Version]
- Junho, C.V.C.; Caio-Silva, W.; Trentin-Sonoda, M.; Carneiro-Ramos, M.S. An overview of the role of calcium/calmodulin-dependent protein kinase in cardiorenal syndrome. Front. Physiol. 2020, 11, 735. [Google Scholar] [CrossRef]
- Kovács, K.A.; Steullet, P.; Steinmann, M.; Do, K.Q.; Magistretti, P.J.; Halfon, O.; Cardinaux, J.-R. TORC1 is a calcium-and cAMP-sensitive coincidence detector involved in hippocampal long-term synaptic plasticity. Proc. Natl. Acad. Sci. USA 2007, 104, 4700–4705. [Google Scholar] [CrossRef] [PubMed]
- Berdeaux, R.; Hutchins, C. Anabolic and Pro-metabolic Functions of CREB-CRTC in Skeletal Muscle: Advantages and Obstacles for Type 2 Diabetes and Cancer Cachexia. Front. Endocrinol. 2019, 10, 535. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Zhang, Y. Activating transcription factor 3 in immune response and metabolic regulation. Liver Res. 2017, 1, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, L. Targeting cAMP/PKA pathway for glycemic control and type 2 diabetes therapy. J. Mol. Endocrinol. 2016, 57, R93–R108. [Google Scholar] [CrossRef] [PubMed]
- Park, D.-R.; Park, K.-H.; Kim, B.-J.; Yoon, C.-S.; Kim, U.-H. Exercise ameliorates insulin resistance via Ca2+ signals distinct from those of insulin for GLUT4 translocation in skeletal muscles. Diabetes 2015, 64, 1224–1234. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP response element-binding protein (CREB): A possible signaling molecule link in the pathophysiology of schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef]
- Wright, D.C.; Hucker, K.A.; Holloszy, J.O.; Han, D.H. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes 2004, 53, 330–335. [Google Scholar] [CrossRef]
- Najar, M.A.; Rex, D.; Modi, P.K.; Agarwal, N.; Dagamajalu, S.; Karthikkeyan, G.; Vijayakumar, M.; Chatterjee, A.; Sankar, U.; Prasad, T.K. A complete map of the Calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) signaling pathway. J. Cell Commun. Signal. 2021, 15, 283–290. [Google Scholar] [CrossRef]
- Russell, W.R.; Baka, A.; Björck, I.; Delzenne, N.; Gao, D.; Griffiths, H.R.; Hadjilucas, E.; Juvonen, K.; Lahtinen, S.; Lansink, M. Impact of diet composition on blood glucose regulation. Crit. Rev. Food Sci. Nutr. 2016, 56, 541–590. [Google Scholar] [CrossRef]
- Cardenas, D. What is clinical nutrition? Understanding the epistemological foundations of a new discipline. Clin. Nutr. ESPEN 2016, 11, e63–e66. [Google Scholar] [CrossRef]
- Olatunji, T.L.; Afolayan, A.J. The suitability of chili pepper (Capsicum annuum L.) for alleviating human micronutrient dietary deficiencies: A review. Food Sci. Nutr. 2018, 6, 2239–2251. [Google Scholar] [CrossRef] [PubMed]
- Zsombok, A. Vanilloid receptors—Do they have a role in whole body metabolism? Evidence from TRPV1. J. Diabetes Complicat. 2013, 27, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Zaldivia, M.T.; Wallert, M.; Loseff-Silver, J.; Al-Aryahi, S.; Zamani, J.; Thurgood, P.; Salim, A.; Htun, N.M.; Stub, D. Transcatheter aortic valve implantation represents an anti-inflammatory therapy via reduction of shear stress–induced, piezo-1–mediated monocyte activation. Circulation 2020, 142, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Baratchi, S.; Keov, P.; Darby, W.G.; Lai, A.; Khoshmanesh, K.; Thurgood, P.; Vahidi, P.; Ejendal, K.; McIntyre, P. The TRPV4 Agonist GSK1016790A Regulates the Membrane Expression of TRPV4 Channels. Front. Pharmacol. 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Tolan, I.; Ragoobirsingh, D.; Morrison, E.Y.S.A. The effect of capsaicin on blood glucose, plasma insulin levels and insulin binding in dog models. Phytother. Res. 2001, 15, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Schultze, S.M.; Hemmings, B.A.; Niessen, M.; Tschopp, O. PI3K/AKT, MAPK and AMPK signalling: Protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012, 14, E1. [Google Scholar] [CrossRef]
- Hershfinkel, M.; Moran, A.; Grossman, N.; Sekler, I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA 2001, 98, 11749–11754. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Legrand, C.; Giacomello, E.; Berthier, C.; Allard, B.; Sorrentino, V.; Jacquemond, V. Spontaneous and voltage-activated Ca2+ release in adult mouse skeletal muscle fibres expressing the type 3 ryanodine receptor. J. Physiol. 2008, 586, 441–457. [Google Scholar] [CrossRef]
- Jessen, N.; Goodyear, L.J. Contraction signaling to glucose transport in skeletal muscle. J. Appl. Physiol. 2005, 99, 330–337. [Google Scholar] [CrossRef]
- Woodier, J.; Rainbow, R.D.; Stewart, A.J.; Pitt, S.J. Intracellular zinc modulates cardiac ryanodine receptor-mediated calcium release. J. Biol. Chem. 2015, 290, 17599–17610. [Google Scholar] [CrossRef] [PubMed]
- Worrall, D.S.; Olefsky, J.M. The effects of intracellular calcium depletion on insulin signaling in 3T3-L1 adipocytes. Mol. Endocrinol. 2002, 16, 378–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, P.; Tian, D.; Song, G.; Ming, Q.; Liu, J.; Shen, J.; Liu, Q.-H.; Yang, X. Neferine promotes GLUT4 expression and fusion with the plasma membrane to induce glucose uptake in L6 cells. Front. Pharmacol. 2019, 10, 999. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.N.; Sankar, U. CaMKK2 Signaling in Metabolism and Skeletal Disease: A New Axis with Therapeutic Potential. Curr. Osteoporos. Rep. 2019, 17, 169–177. [Google Scholar] [CrossRef]
- Altarejos, J.Y.; Goebel, N.; Conkright, M.D.; Inoue, H.; Xie, J.; Arias, C.M.; Sawchenko, P.E.; Montminy, M. The Creb1 coactivator Crtc1 is required for energy balance and fertility. Nat. Med. 2008, 14, 1112–1117. [Google Scholar] [CrossRef]
- Dalle, S.; Quoyer, J.; Varin, E.; Costes, S. Roles and regulation of the transcription factor CREB in pancreatic β-cells. Curr. Mol. Pharmacol. 2011, 4, 187–195. [Google Scholar] [CrossRef]
- Bittinger, M.A.; McWhinnie, E.; Meltzer, J.; Iourgenko, V.; Latario, B.; Liu, X.; Chen, C.H.; Song, C.; Garza, D.; Labow, M. Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr. Biol. 2004, 14, 2156–2161. [Google Scholar] [CrossRef]
- Kim, G.H.; Szabo, A.; King, E.M.; Ayala, J.; Ayala, J.E.; Altarejos, J.Y. Leptin recruits Creb-regulated transcriptional coactivator 1 to improve hyperglycemia in insulin-deficient diabetes. Mol. Metab. 2015, 4, 227–236. [Google Scholar] [CrossRef]
- Watson, P.A.; Nesterova, A.; Burant, C.F.; Klemm, D.J.; Reusch, J.E.-B. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J. Biol. Chem. 2001, 276, 46142–46150. [Google Scholar] [CrossRef]
- Altarejos, J.Y.; Montminy, M. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 2011, 12, 141–151. [Google Scholar] [CrossRef]
- Ku, H.-C.; Cheng, C.-F. Master regulator activating transcription factor 3 (ATF3) in metabolic homeostasis and cancer. Front. Endocrinol. 2020, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Ding, D.; Li, Y. Regulation of Hepatic Metabolism and Cell Growth by the ATF/CREB Family of Transcription Factors. Diabetes 2021, 70, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, L.; Liu, Z.-P.; Chen, L. Identifying module biomarker in type 2 diabetes mellitus by discriminative area of functional activity. BMC Bioinform. 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, B.; Zhang, X.; Sun, G.; Sun, X. Targeting Orphan Nuclear Receptors NR4As for Energy Homeostasis and Diabetes. Front. Pharmacol. 2020, 11, 1867. [Google Scholar] [CrossRef]
- Kida, R.; Noguchi, T.; Murakami, M.; Hashimoto, O.; Kawada, T.; Matsui, T.; Funaba, M. Supra-pharmacological concentration of capsaicin stimulates brown adipogenesis through induction of endoplasmic reticulum stress. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Zhang, J.-W.; Li, L.; Ye, Z.-Y.; Zhang, Y.; Gao, X.; Li, F.; Yan, X.-S.; Liu, Z.-G.; Liu, L.-J. Complex regulation of capsaicin on intracellular second messengers by calcium dependent and independent mechanisms in primary sensory neurons. Neurosci. Lett. 2012, 517, 30–35. [Google Scholar] [CrossRef]
- von Bülow, V.; Rink, L.; Haase, H. Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-α and IL-1β production in monocytes by elevation of guanosine 3′, 5′-cyclic monophosphate. J. Immunol. 2005, 175, 4697–4705. [Google Scholar] [CrossRef]
- Maywald, M.; Wessels, I.; Rink, L. Zinc signals and immunity. Int. J. Mol. Sci. 2017, 18, 2222. [Google Scholar] [CrossRef]
- Myers, S.A.; Nield, A.; Myers, M. Zinc transporters, mechanisms of action and therapeutic utility: Implications for type 2 diabetes mellitus. J. Nutr. Metab. 2012, 2012, 173712. [Google Scholar] [CrossRef]
- Hershfinkel, M.; Silverman, W.F.; Sekler, I. The zinc sensing receptor, a link between zinc and cell signaling. Mol. Med. 2007, 13, 331–336. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, H.; Yang, H.; Li, C.; Sang, Q.; Liu, X.; Liu, Y.; Wang, Y.; Sun, Z. Zinc stimulates glucose consumption by modulating the insulin signaling pathway in L6 myotubes: Essential roles of Akt–GLUT4, GSK3β and mTOR–S6K1. J. Nutr. Biochem. 2016, 34, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdowsi, P.V.; Ahuja, K.D.K.; Beckett, J.M.; Myers, S. Capsaicin and Zinc Promote Glucose Uptake in C2C12 Skeletal Muscle Cells through a Common Calcium Signalling Pathway. Int. J. Mol. Sci. 2022, 23, 2207. https://doi.org/10.3390/ijms23042207
Ferdowsi PV, Ahuja KDK, Beckett JM, Myers S. Capsaicin and Zinc Promote Glucose Uptake in C2C12 Skeletal Muscle Cells through a Common Calcium Signalling Pathway. International Journal of Molecular Sciences. 2022; 23(4):2207. https://doi.org/10.3390/ijms23042207
Chicago/Turabian StyleFerdowsi, Parisa Vahidi, Kiran D. K. Ahuja, Jeffrey M. Beckett, and Stephen Myers. 2022. "Capsaicin and Zinc Promote Glucose Uptake in C2C12 Skeletal Muscle Cells through a Common Calcium Signalling Pathway" International Journal of Molecular Sciences 23, no. 4: 2207. https://doi.org/10.3390/ijms23042207
APA StyleFerdowsi, P. V., Ahuja, K. D. K., Beckett, J. M., & Myers, S. (2022). Capsaicin and Zinc Promote Glucose Uptake in C2C12 Skeletal Muscle Cells through a Common Calcium Signalling Pathway. International Journal of Molecular Sciences, 23(4), 2207. https://doi.org/10.3390/ijms23042207





