Review of the Mechanisms of Snake Venom Induced Pain: It’s All about Location, Location, Location
Abstract
:1. Introduction
2. Location: Molecular Mechanisms of Venom Mediated Pain
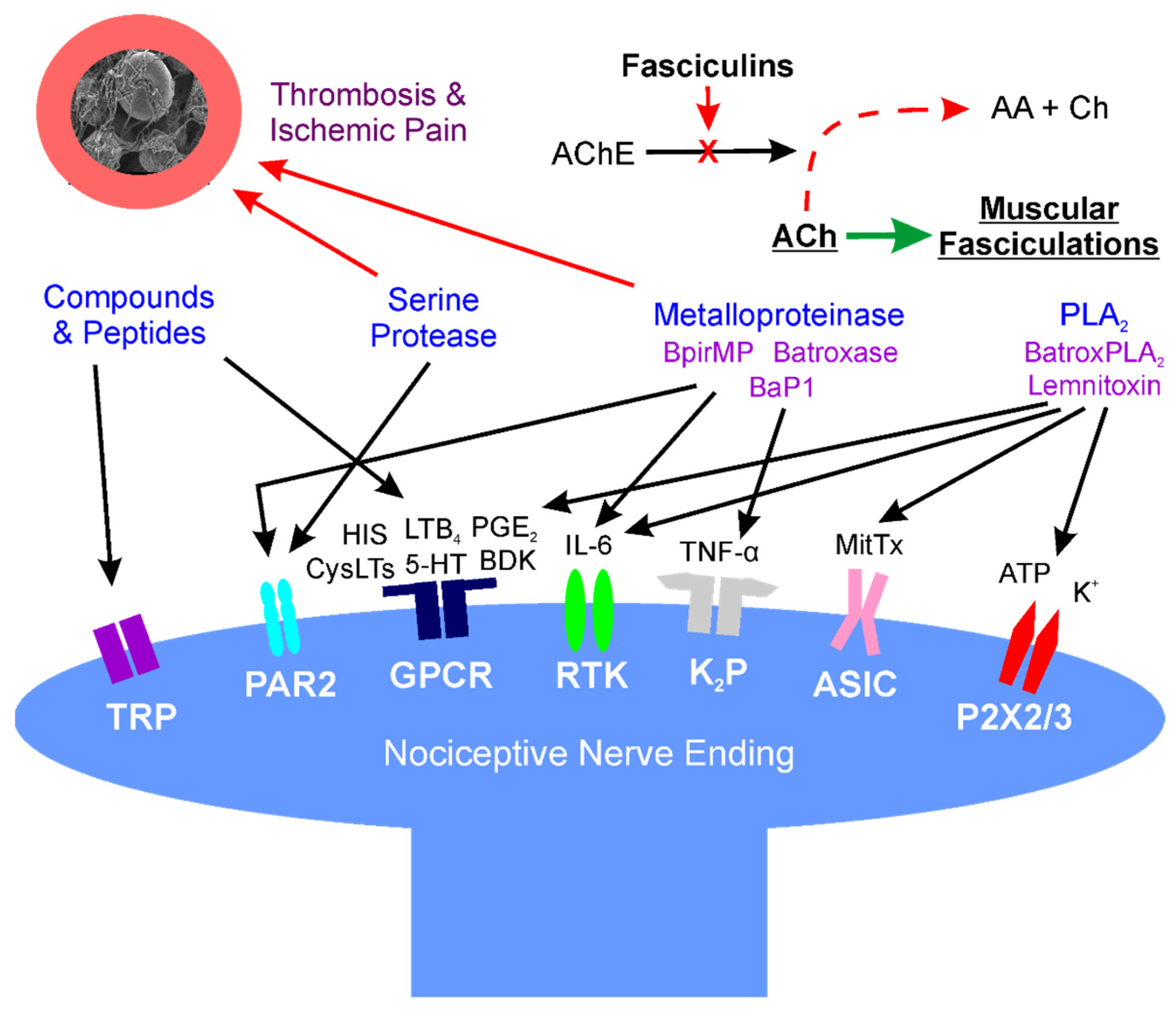
2.1. Overview
2.2. Small Molecular Weight, Non-Enzymatic Compounds (Direct Effects)
2.3. Phospholipase A2 (PLA2) (Direct Effects)
2.4. Serine Proteases (Direct and Indirect Effects)
2.5. Metalloproteinases (Direct and Indirect Effects)
2.6. Fasciculins (Indirect Effects)
3. Location: Sites of Pain after Envenomation
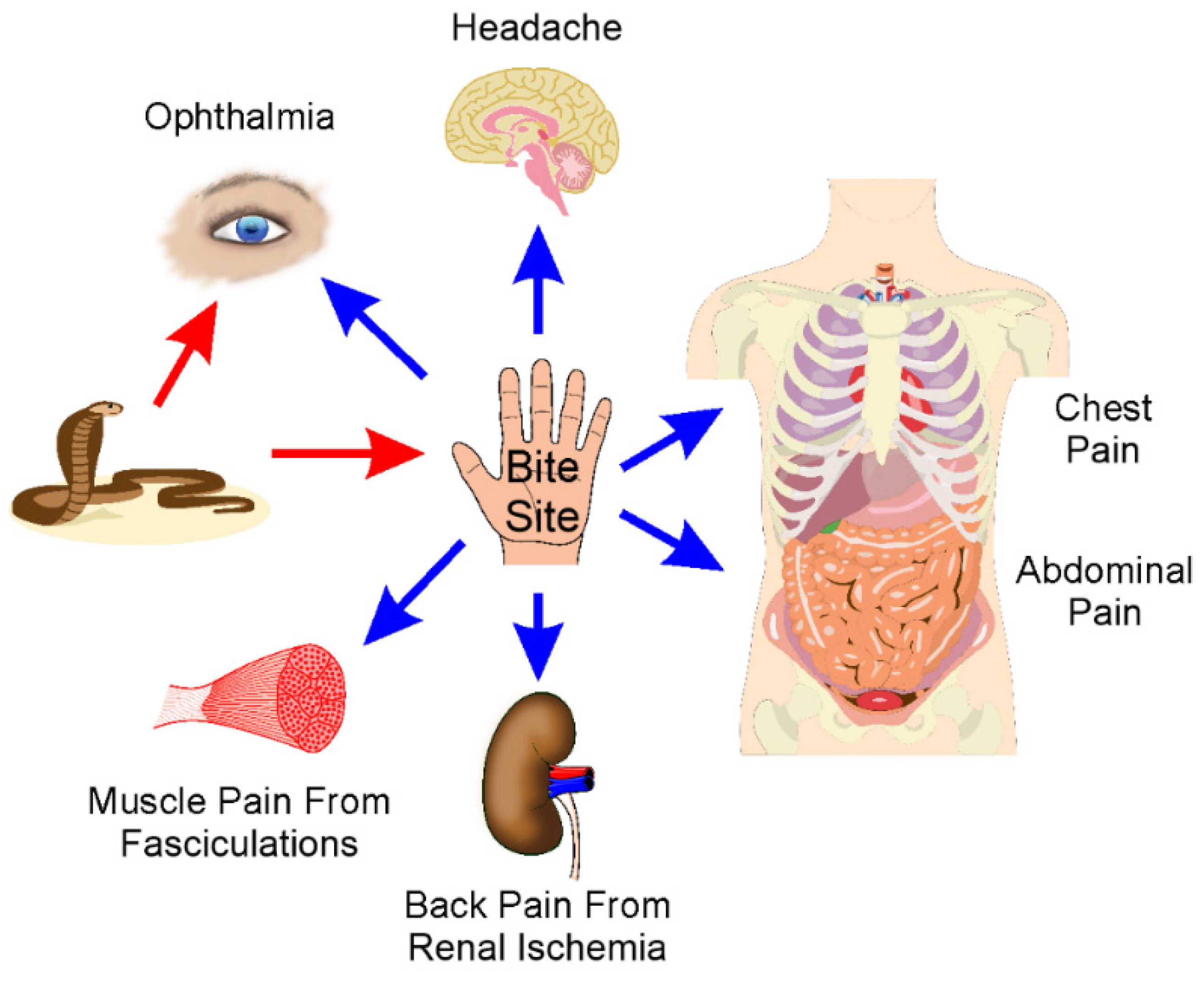
3.1. Overview
3.2. Bite Site and Surrounding Tissue Pain
3.3. Eye Pain
3.4. Headache
3.5. Chest Pain
3.6. Abdominal Pain and General Systemic Pain
3.7. Back Pain
3.8. Complex and Chronic Pain Syndromes
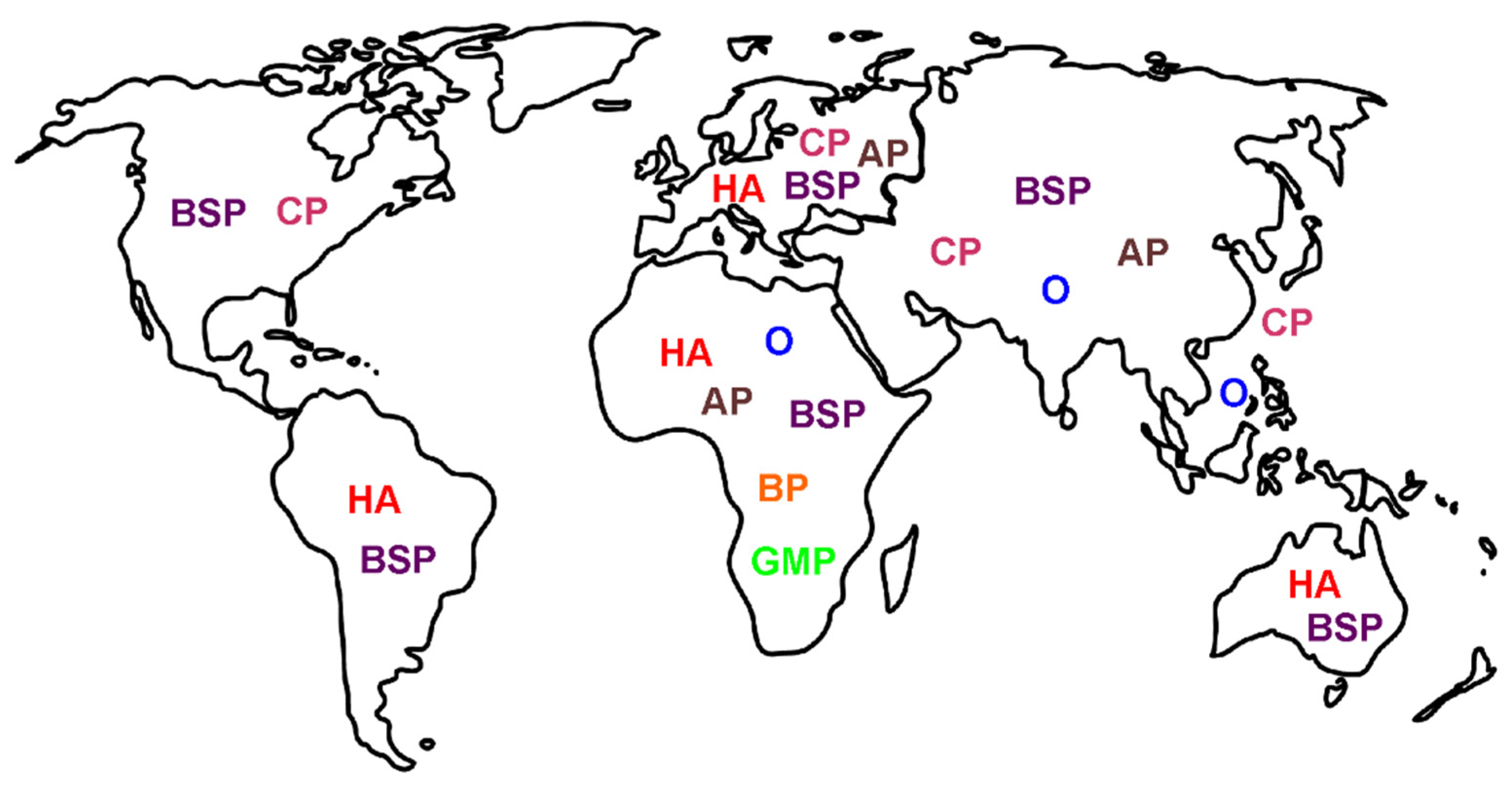
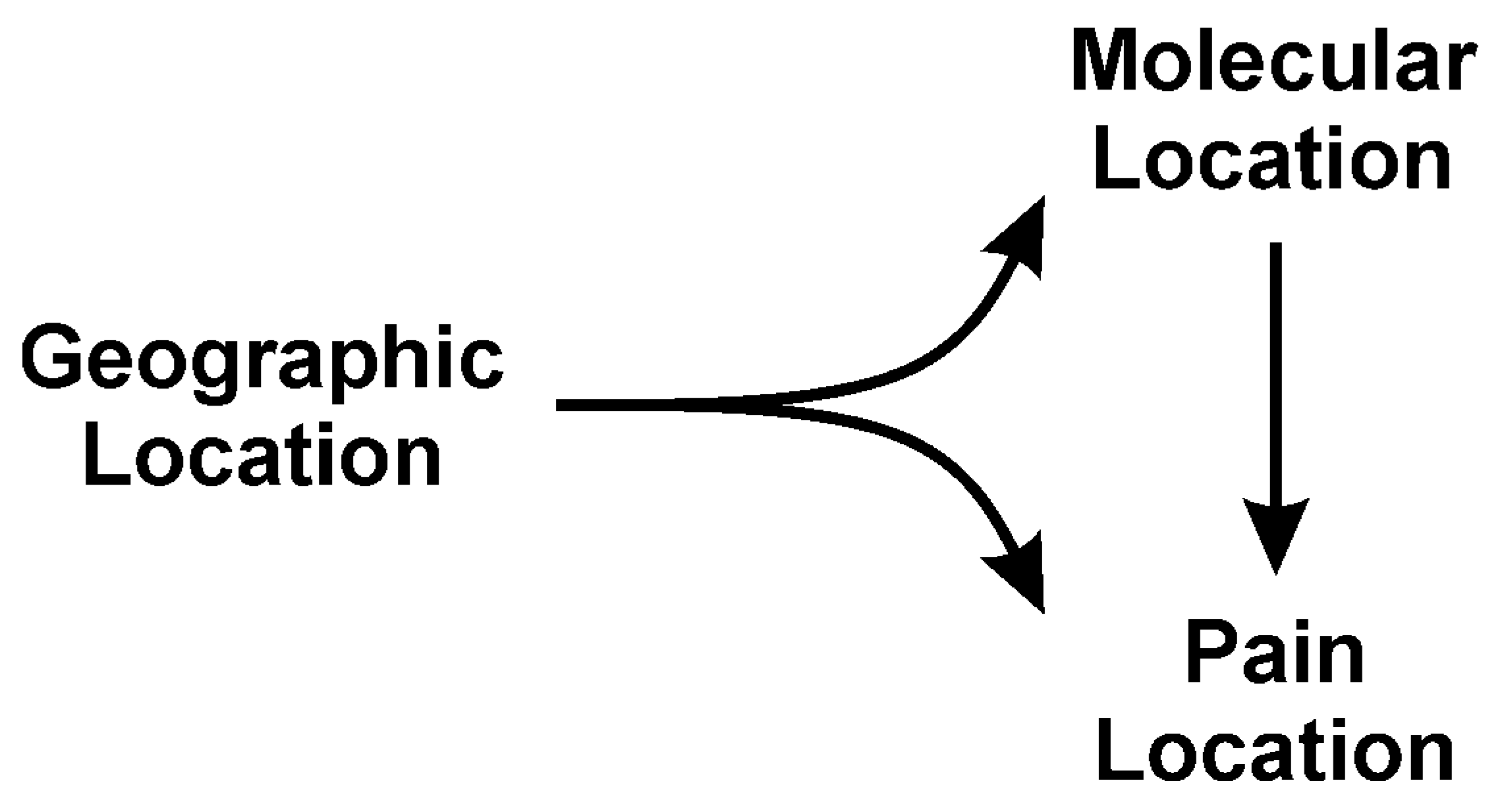
4. Location: Nature of Venom Mediated Pain as a Function of Geographic Origin of the Snake—“It’s Not Where the Snake Bites You, but Where You Are Bitten, That Matters”
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kumar, S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venom: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V.I. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Villar-Briones, A.; Aird, S.D. Organic and Peptidyl Constituents of Snake Venoms: The Picture Is Vastly More Complex Than We Imagined. Toxins 2018, 10, 392. [Google Scholar] [CrossRef] [Green Version]
- Ward-Smith, H.; Arbuckle, K.; Naude, A.; Wüster, W. Fangs for the Memories? A Survey of Pain in Snakebite Patients Does Not Support a Strong Role for Defense in the Evolution of Snake Venom Composition. Toxins 2020, 12, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chippaux, J.P.; Amri, K. Severe Heloderma spp. envenomation: A review of the literature. Clin. Toxicol. 2021, 59, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Tibballs, J. Diagnosis and treatment of confirmed and suspected snake bite. Implications from an analysis of 46 paediatric cases. Med. J. Aust. 1992, 156, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, S.; Arambepola, C.; Chang, T.; Gnanathasan, A. Long-term health complications following snake envenoming. J. Multidiscip. Healthc. 2018, 11, 279–285. [Google Scholar] [CrossRef] [Green Version]
- Alves, E.C.; Sachett, J.A.G.; Sampaio, V.S.; Sousa, J.D.B.; Oliveira, S.S.; Nascimento, E.F.D.; Santos, A.D.S.; da Silva, I.M.; da Silva, A.M.M.; Wen, F.H.; et al. Predicting acute renal failure in Bothrops snakebite patients in a tertiary reference center, Western Brazilian Amazon. PLoS ONE 2018, 13, e0202361. [Google Scholar] [CrossRef]
- Razavi, S.; Weinstein, S.A.; Bates, D.J.; Alfred, S.; White, J. The Australian mulga snake (Pseudechis australis: Elapidae): Report of a large case series of bites and review of current knowledge. Toxicon 2014, 85, 17–26. [Google Scholar] [CrossRef]
- Tilbury, C.R.; Branch, W.R. Observations on the bite of the southern burrowing asp (Atractaspis bibronii) in Natal. S. Afr. Med. J. 1989, 75, 327–331. [Google Scholar]
- Jansen, M.; McLeod, M.; White, J.; Isbister, G.K. Spotted black snake (Pseudechis guttatus) envenoming. Med. J. Aust. 2007, 186, 41–42. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, C.R.; Souza, S.N.; Silva, M.C.D.; Ventura, J.S.; Piorelli, R.O.; Puorto, G. Bites by Tomodon dorsatus (serpentes, dipsadidae): Clinical and epidemiological study of 86 cases. Toxicon 2019, 162, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Gan, M.; O’Leary, M.A.; Brown, S.G.; Jacoby, T.; Spain, D.; Tankel, A.; Gavaghan, C.; Garrett, P.; Isbister, G.K. Envenoming by the rough-scaled snake (Tropidechis carinatus): A series of confirmed cases. Med. J. Aust. 2009, 191, 183–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlo, R.; Dželalija, B.; Zupančić, B.; Bačić, I.; Dunatov, T.; Kanjer, A.; Skarica, R.; Sabalić, S.; Bukvic, N.; Nikolić, H.; et al. Venomous snakebites in the Croatian North Dalmatia region. Wien. Klin. Wochenschr. 2011, 123, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Nascimento da Costa, T.; Mota-da-Silva, A.; Colombini, M.; Moura-da-Silva, A.M.; Medeiros de Souza, R.; Monteiro, W.M.; Bernarde, P.S. Relationship between snake size and clinical, epidemiological and laboratory aspects of Bothrops atrox snakebites in the Western Brazilian Amazon. Toxicon 2020, 186, 160–167. [Google Scholar] [CrossRef]
- Hansen, E.A.; Stein, E.A.; Mader, T.H.; Mazzoli, R.A. Spitting cobra ophthalmia in United Nations Forces in Somalia. Am. J. Ophthalmol. 1994, 117, 671. [Google Scholar] [CrossRef]
- Chu, E.R.; Weinstein, S.A.; White, J.; Warrell, D.A. Venom ophthalmia caused by venoms of spitting elapid and other snakes: Report of ten cases with review of epidemiology, clinical features, pathophysiology and management. Toxicon 2010, 56, 259–272. [Google Scholar] [CrossRef]
- Ang, L.J.; Sanjay, S.; Sangtam, T. Ophthalmia due to spitting cobra venom in an urban setting--a report of three cases. Middle East Afr. J. Ophthalmol. 2014, 21, 259–261. [Google Scholar] [CrossRef]
- Lanzetta, M.A.; Cirtita, M.; Aziebu, E.; Cham, M.; Lanzetta, P. Ophthalmia Secondary to Cobra Venom Spitting in the Volta Region, Ghana: A Case Report. Case Rep. Ophthalmol. 2017, 8, 99–103. [Google Scholar] [CrossRef]
- Tsai, T.H.; Lin, C.C.; Mao, Y.C.; Hung, C.L.; Yang, Y.C.; Yang, C.C.; Jeng, M.J. Naja atra venom-spit ophthalmia in Taiwan: An epidemiological survey from 1990 to 2016. J. Chin. Med. Assoc. 2020, 83, 77–83. [Google Scholar] [CrossRef]
- Sai-Sein-Lin-Oo; Myat-Thet-New; Khin-Maung-Gyi; Than-Aye; Mi-Mi-Khine; Myat-Myat-Thein; Myo-Thant; Pyae-Phyo-Aung; Oakkar-Kyaw-Khant; Aye-Zarchi-San; et al. Clinical importance of the Mandalay spitting cobra (Naja mandalayensis) in Upper Myanmar-Bites, envenoming and ophthalmia. Toxicon 2020, 184, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Huang, Y.K.; Chen, Y.W.; Chen, M.H.; Tu, A.T.; Chen, Y.C. Venom Ophthalmia and Ocular Complications Caused by Snake Venom. Toxins 2020, 12, 576. [Google Scholar] [CrossRef] [PubMed]
- Handford, C. Case of venom ophthalmia following contact with Naja pallida: The red spitting cobra. J. R. Army Med. Corps. 2018, 164, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Ophthalmic Exposure to Crotalid Venom. J. Emerg. Med. 2009, 36, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yen, D.H.; Chen, Y.W.; Huang, M.S.; Huang, C.I.; Chen, M.H. Toxin ophthalmia caused by nuchal gland secretion of the Taiwan tiger keelback (Rhabdophis tigrinus formosanus). J. Formos. Med. Assoc. 2014, 113, 750–753. [Google Scholar] [CrossRef] [Green Version]
- Chu, E.R.; White, J.; Weinstein, S. Is there any role for intravenous antivenom for snake venom ophthalmia? J. Emerg. Med. 2010, 39, 659–660. [Google Scholar] [CrossRef]
- Dissanayake, P.; Sellahewa, K.H. Acute myocardial infarction in a patient with Russell’s viper bite. Ceylon Med. J. 1996, 41, 67–68. [Google Scholar]
- Kazandjian, T.D.; Petras, D.; Robinson, S.D.; van Thiel, J.; Greene, H.W.; Arbuckle, K.; Barlow, A.; Carter, D.A.; Wouters, R.M.; Whiteley, G.; et al. Convergent evolution of pain-inducing defensive venom components in spitting cobras. Science 2021, 371, 386–390. [Google Scholar] [CrossRef]
- Simpson, C.H.; Richardson, W.H.; Swartzentruber, G.S.; Lloyd, V.J. ST Segment Elevation Myocardial Infarction Following a Crotalus horridus Envenomation. Wilderness Environ. Med. 2018, 29, 383–387. [Google Scholar] [CrossRef] [Green Version]
- Frangides, C.; Kouni, S.; Niarchos, C.; Koutsojannis, C. Hypersersensitivity and Kounis syndrome due to a viper bite. Eur. J. Intern. Med. 2006, 17, 215–216. [Google Scholar] [CrossRef]
- Saadeh, A.M. Case report: Acute myocardial infarction complicating a viper bite. Am. J. Trop. Med. Hyg. 2001, 64, 280–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satish, R.; Kanchan, R.; Yashawant, R.; Ashish, D.; Kedar, R. Acute MI in a stented patient following snake bite-possibility of stent thrombosis-A case report. Indian Heart J. 2013, 65, 327–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bawaskar, H.S.; Bawaskar, P.H.; Bawaskar, P.H. Premonitory signs and symptoms of envenoming by common krait (Bungarus caeruleus). Trop. Doct. 2014, 44, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Valenta, J.; Stach, Z.; Fricova, D.; Zak, J.; Balik, M. Envenoming by the viperid snake Proatheris superciliaris: A case report. Toxicon 2008, 52, 392–394. [Google Scholar] [CrossRef]
- Pourreau, F.; Pinsard, M.; Goyffon, M.; Plasse, F.; Desport, E.; Thierry, A.; Touchard, G.; Bridoux, F. Bilateral renal cortical necrosis with end-stage renal failure following envenoming by Proatheris superciliaris: A case report. Toxicon 2014, 84, 36–40. [Google Scholar] [CrossRef]
- Sarkhel, S.; Ghosh, R.; Mana, K.; Gantait, K. A hospital based epidemiological study of snakebite in Paschim Medinipur district, West Bengal, India. Toxicol. Rep. 2017, 4, 415–419. [Google Scholar] [CrossRef]
- Atkinson, P.M.; Bradlow, B.A.; White, J.A.; Greig, H.B.; Gaillard, M.C. Clinical features of twig snake (Thelotornis capensis) envenomation. S. Afr. Med. J. 1980, 58, 1007–1011. [Google Scholar]
- Kularatne, S.A. Common krait (Bungarus caeruleus) bite in Anuradhapura, Sri Lanka: A prospective clinical study, 1996–1998. Postgrad. Med. J. 2002, 78, 276–280. [Google Scholar] [CrossRef]
- Mao, Y.C.; Liu, P.Y.; Chiang, L.C.; Liao, S.C.; Su, H.Y.; Hsieh, S.Y.; Yang, C.C. Bungarus multicinctus multicinctus Snakebite in Taiwan. Am. J. Trop. Med. Hyg. 2017, 96, 1497–1504. [Google Scholar] [CrossRef] [Green Version]
- Bucaretchi, F.; Herrera, S.R.; Hyslop, S.; Baracat, E.C.; Vieira, R.J. Snakebites by Bothrops spp. in children in Campinas, São Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2001, 43, 329–333. [Google Scholar] [CrossRef]
- Singh, J.; Bhoi, S.; Gupta, V.; Goel, A. Clinical profile of venomous snake bites in north Indian Military Hospital. J. Emerg. Trauma Shock 2008, 1, 78–80. [Google Scholar] [PubMed]
- Pivko-Levy, D.; Munchnak, I.; Rimon, A.; Balla, U.; Scolnik, D.; Hoyte, C.; Voliovitch, Y.; Glatstein, M. Evaluation of antivenom therapy for Vipera palaestinae bites in children: Experience of two large, tertiary care pediatric hospitals. Clin. Toxicol. 2017, 55, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Kularatne, S.A.; Silva, A.; Weerakoon, K.; Maduwage, K.; Walathara, C.; Paranagama, R.; Mendis, S. Revisiting Russell’s viper (Daboia russelii) bite in Sri Lanka: Is abdominal pain an early feature of systemic envenoming? PLoS ONE 2014, 9, e90198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermansen, M.N.; Krug, A.H.; Tjønnfjord, E.; Brabrand, M. Envenomation by the common European adder (Vipera berus): A case series of 219 patients. Eur. J. Emerg. Med. 2019, 26, 362–365. [Google Scholar] [CrossRef]
- Paolino, G.; Di Nicola, M.R.; Pontara, A.; Didona, D.; Moliterni, E.; Mercuri, S.R.; Grano, M.; Borgianni, N.; Kumar, R.; Pampena, R. Vipera snakebite in Europe: A systematic review of a neglected disease. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2247–2260. [Google Scholar] [CrossRef]
- Reid, H.A. Adder bites in Britain. Br. Med. J. 1976, 2, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, B.; Shrestha, B.P.; Rahman, T.R.; Sharma, S.K.; Tripathi, M. Complex regional pain syndrome (CRPS) type-1 following snake bite: A case report. Nepal Med. Coll. J. 2008, 10, 278–280. [Google Scholar]
- Seo, Y.H.; Park, M.R.; Yoo, S.H. Development of complex regional pain syndrome after a snake bite: A case report. Korean J. Pain. 2014, 27, 68–71. [Google Scholar] [CrossRef]
- Pachowicz, M.; Nocuń, A.; Postępski, J.; Olesińska, E.; Emeryk, A.; Chrapko, B. Complex Regional Pain Syndrome type I with atypical scintigraphic pattern–diagnosis and evaluation of the entity with three phase bone scintigraphy. A case report. Nucl. Med. Rev. Cent. East. Eur. 2014, 17, 115–119. [Google Scholar] [CrossRef]
- Kleggetveit, I.P.; Skulberg, P.K.; Jørum, E. Complex regional pain syndrome following viper-bite. Scand. J. Pain. 2016, 10, 15–18. [Google Scholar] [CrossRef]
- Cruz Salcedo, E.M.; Blanco, A.; Reed, J. Complex Regional Pain Syndrome Developing After a Coral Snake Bite: A Case Report. Cureus 2020, 12, e9787. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, R.P. Complex Regional Pain Syndrome Following Snakebite: A Putatively Rare Complication of Envenomation and Review of the Literature. Int. Med. Case Rep. J. 2020, 13, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.H. Serotonin and related tryptamine derivatives in snake venoms. Mem. Inst. Butantan. 1966, 33, 509–518. [Google Scholar] [PubMed]
- Lopes, P.H.; Rocha, M.M.T.; Kuniyoshi, A.K.; Portaro, F.C.V.; Gonçalves, L.R.C. Edema and Nociception Induced by Philodryas patagoniensis Venom in Mice: A Pharmacological Evaluation with Implications for the Accident Treatment. J. Pharm. Exp. Ther. 2017, 361, 349–354. [Google Scholar] [CrossRef] [Green Version]
- Maia-Marques, R.; Nascimento, I.M.R.; Lauria, P.S.S.; Silva, E.C.P.D.; Silva, D.F.; Casais-E-Silva, L.L. Inflammatory mediators in the pronociceptive effects induced by Bothrops leucurus snake venom: The role of biogenic amines, nitric oxide, and eicosanoids. Toxicology 2021, 448, 152649. [Google Scholar] [CrossRef]
- Freedman, J.E.; Snyder, S.H. Vipoxin. A protein from Russell’s viper venom with high affinity for biogenic amine receptors. J. Biol. Chem. 1981, 256, 13172–13179. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional Toxins in Snake Venoms and Therapeutic Implications: From Pain to Hemorrhage and Necrosis. Front. Ecol. Evol. 2019, 7, 218. [Google Scholar] [CrossRef] [Green Version]
- Jami, S.; Erickson, A.; Brierley, S.M.; Vetter, I. Pain-Causing Venom Peptides: Insights into Sensory Neuron Pharmacology. Toxins 2017, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers. 2017, 3, 17079. [Google Scholar] [CrossRef]
- Geron, M.; Kumar, R.; Matzner, H.; Lahiani, A.; Gincberg, G.; Cohen, G.; Lazarovici, P.; Priel, A. Protein toxins of the Echis coloratus viper venom directly activate TRPV1. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 615–623. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Chesler, A.T.; Sharif-Naeini, R.; Medzihradszky, K.F.; Zhou, S.; King, D.; Sánchez, E.E.; Burlingame, A.L.; Basbaum, A.I.; Julius, D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 2011, 479, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.K.P.; Camargo, E.A.; Antunes, E. Inflammatory Action of Secretory Phospholipases A2 from Snake Venoms. In Toxins and Drug Discovery; Gopalakrishnakone, P., Cruz, L., Luo, S., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 35–52. [Google Scholar]
- Câmara, P.R.; Esquisatto, L.C.; Camargo, E.A.; Ribela, M.T.; Toyama, M.H.; Marangoni, S.; De Nucci, G.; Antunes, E. Inflammatory oedema induced by phospholipases A2 isolated from Crotalus durissus sp. in the rat dorsal skin: A role for mast cells and sensory C-fibers. Toxicon 2003, 41, 823–829. [Google Scholar] [CrossRef]
- Camargo, E.A.; Ferreira, T.; Ribela, M.T.; de Nucci, G.; Landucci, E.C.; Antunes, E. Role of substance P and bradykinin in acute pancreatitis induced by secretory phospholipase A2. Pancreas 2008, 37, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Casais-E-Silva, L.L.; Teixeira, C.F.; Lebrun, I.; Lomonte, B.; Alape-Girón, A.; Gutiérrez, J.M. Lemnitoxin, the major component of Micrurus lemniscatus coral snake venom, is a myotoxic and pro-inflammatory phospholipase A2. Toxicol. Lett. 2016, 257, 60–71. [Google Scholar] [CrossRef]
- Menaldo, D.L.; Bernardes, C.P.; Zoccal, K.F.; Jacob-Ferreira, A.L.; Costa, T.R.; Del Lama, M.P.; Naal, R.M.; Frantz, F.G.; Faccioli, L.H.; Sampaio, S.V. Immune cells and mediators involved in the inflammatory responses induced by a P-I metalloprotease and a phospholipase A2 from Bothrops atrox venom. Mol. Immunol. 2017, 85, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Menaldo, D.L.; Bernardes, C.P.; Pereira, J.C.; Silveira, D.S.; Mamede, C.C.; Stanziola, L.; de Oliveira, F.; Pereira-Crott, L.S.; Faccioli, L.H.; Sampaio, S.V. Effects of two serine proteases from Bothrops pirajai snake venom on the complement system and the inflammatory response. Int. Immunopharmacol. 2013, 15, 764–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zychar, B.C.; Dale, C.S.; Demarchi, D.S.; Gonçalves, L.R. Contribution of metalloproteases, serine proteases and phospholipases A2 to the inflammatory reaction induced by Bothrops jararaca crude venom in mice. Toxicon 2010, 55, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.M.; Teixeira, P.C.F.; Leite, A.C.; Gutiérrez, J.M.; Rocha, F.A. The snake venom metalloproteinase BaP1 induces joint hypernociception through TNF-α and PGE2-dependent mechanisms. Br. J. Pharmacol. 2007, 151, 1254–1261. [Google Scholar] [CrossRef] [Green Version]
- Bernardes, C.P.; Menaldo, D.L.; Mamede, C.C.; Zoccal, K.F.; Cintra, A.C.; Faccioli, L.H.; Stanziola, L.; de Oliveira, F.; Sampaio, S.V. Evaluation of the local inflammatory events induced by BpirMP, a metalloproteinase from Bothrops pirajai venom. Mol. Immunol. 2015, 68, 456–464. [Google Scholar] [CrossRef]
- De Toni, L.G.; Menaldo, D.L.; Cintra, A.C.; Figueiredo, M.J.; de Souza, A.R.; Maximiano, W.M.; Jamur, M.C.; Souza, G.E.; Sampaio, S.V. Inflammatory mediators involved in the paw edema and hyperalgesia induced by Batroxase, a metalloproteinase isolated from Bothrops atrox snake venom. Int. Immunopharmacol. 2015, 28, 199–207. [Google Scholar] [CrossRef]
- Moreira, V.; Leiguez, E.; Janovits, P.M.; Maia-Marques, R.; Fernandes, C.M.; Teixeira, C. Inflammatory Effects of Bothrops Phospholipases A2: Mechanisms Involved in Biosynthesis of Lipid Mediators and Lipid Accumulation. Toxins 2021, 13, 868. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, V.O.; Picolo, G.; Fernandes, C.A.H.; Fontes, M.R.M.; Cury, Y. Secreted Phospholipases A2 from Animal Venoms in Pain and Analgesia. Toxins 2017, 9, 406. [Google Scholar] [CrossRef] [Green Version]
- Mamede, C.C.; de Sousa, B.B.; Pereira, D.F.; Matias, M.S.; de Queiroz, M.R.; de Morais, N.C.; Vieira, S.A.; Stanziola, L.; de Oliveira, F. Comparative analysis of local effects caused by Bothrops alternatus and Bothrops moojeni snake venoms: Enzymatic contributions and inflammatory modulations. Toxicon 2016, 117, 37–45. [Google Scholar] [CrossRef]
- Zhang, C.; Medzihradszky, K.F.; Sánchez, E.E.; Basbaum, A.I.; Julius, D. Lys49 myotoxin from the Brazilian lancehead pit viper elicits pain through regulated ATP release. Proc. Natl. Acad. Sci. USA 2017, 114, E2524–E2532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cintra-Francischinelli, M.; Caccin, P.; Chiavegato, A.; Pizzo, P.; Carmignoto, G.; Angulo, Y.; Lomonte, B.; Gutiérrez, J.M.; Montecucco, C. Bothrops snake myotoxins induce a large efflux of ATP and potassium with spreading of cell damage and pain. Proc. Natl. Acad. Sci. USA 2010, 107, 14140–14145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quindlen-Hotek, J.C.; Kartha, S.; Winkelstein, B.A. Immediate inhibition of spinal secretory phospholipase A2 prevents the pain and elevated spinal neuronal hyperexcitability and neuroimmune regulatory genes that develop with nerve root compression. Neuroreport 2020, 31, 1084–1089. [Google Scholar] [CrossRef]
- Kokotos, G.; Six, D.A.; Loukas, V.; Smith, T.; Constantinou-Kokotou, V.; Hadjipavlou-Litina, D.; Kotsovolou, S.; Chiou, A.; Beltzner, C.C.; Dennis, E.A. Inhibition of group IVA cytosolic phospholipase A2 by novel 2-oxoamides in vitro, in cells, and in vivo. J. Med. Chem. 2004, 47, 3615–36128. [Google Scholar] [CrossRef]
- Lam, D.K.; Schmidt, B.L. Serine proteases and protease-activated receptor 2-dependent allodynia: A novel cancer pain pathway. Pain 2010, 149, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Cattaruzza, F.; Amadesi, S.; Carlsson, J.F.; Murphy, J.E.; Lyo, V.; Kirkwood, K.; Cottrell, G.S.; Bogyo, M.; Knecht, W.; Bunnett, N.W. Serine proteases and protease-activated receptor 2 mediate the proinflammatory and algesic actions of diverse stimulants. Br. J. Pharm. 2014, 171, 3814–3826. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef]
- Li, X.; Tai, H.H. Thromboxane A2 receptor-mediated release of matrix metalloproteinase-1 (MMP-1) induces expression of monocyte chemoattractant protein-1 (MCP-1) by activation of protease-activated receptor 2 (PAR2) in A549 human lung adenocarcinoma cells. Mol. Carcinog. 2014, 53, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Quarch, V.; Brander, L.; Cioccari, L. An Unexpected Case of Black Mamba (Dendroaspis polylepis) Bite in Switzerland. Case Rep. Crit. Care. 2017, 2017, 5021924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, E.; Mbugua, P.M.; Rodriguez-Ithurralde, D. Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angusticeps. J. Physiol. 1984, 79, 232–240. [Google Scholar]
- Wong, S.F.; Chung, F. Succinylcholine-associated postoperative myalgia. Anaesthesia 2000, 55, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Paniagua, D.; Vergara, I.; Román, R.; Romero, C.; Benard-Valle, M.; Calderón, A.; Jiménez, L.; Bernas, M.J.; Witte, M.H.; Boyer, L.V.; et al. Antivenom effect on lymphatic absorption and pharmacokinetics of coral snake venom using a large animal model. Clin. Toxicol. 2019, 57, 727–734. [Google Scholar] [CrossRef]
- Curry, S.C.; Kunkel, D.B. Toxicology rounds. Death from a rattlesnake bite. Am. J. Emerg. Med. 1985, 3, 227–235. [Google Scholar] [CrossRef]
- Warrell, D.A. Proatheris superciliaris: The deadly venom of a rare and elusive snake revealed. Toxicon 2008, 52, 833–835. [Google Scholar] [CrossRef]
- Kessler, A.; Yoo, M.; Calisoff, R. Complex regional pain syndrome: An updated comprehensive review. NeuroRehabilitation 2020, 47, 253–264. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, V.G.; Wagner, M.T. Review of the Mechanisms of Snake Venom Induced Pain: It’s All about Location, Location, Location. Int. J. Mol. Sci. 2022, 23, 2128. https://doi.org/10.3390/ijms23042128
Nielsen VG, Wagner MT. Review of the Mechanisms of Snake Venom Induced Pain: It’s All about Location, Location, Location. International Journal of Molecular Sciences. 2022; 23(4):2128. https://doi.org/10.3390/ijms23042128
Chicago/Turabian StyleNielsen, Vance G., and Michael T. Wagner. 2022. "Review of the Mechanisms of Snake Venom Induced Pain: It’s All about Location, Location, Location" International Journal of Molecular Sciences 23, no. 4: 2128. https://doi.org/10.3390/ijms23042128
APA StyleNielsen, V. G., & Wagner, M. T. (2022). Review of the Mechanisms of Snake Venom Induced Pain: It’s All about Location, Location, Location. International Journal of Molecular Sciences, 23(4), 2128. https://doi.org/10.3390/ijms23042128





