Neurosecretory Protein GL Accelerates Liver Steatosis in Mice Fed Medium-Fat/Medium-Fructose Diet
Abstract
:1. Introduction
2. Results
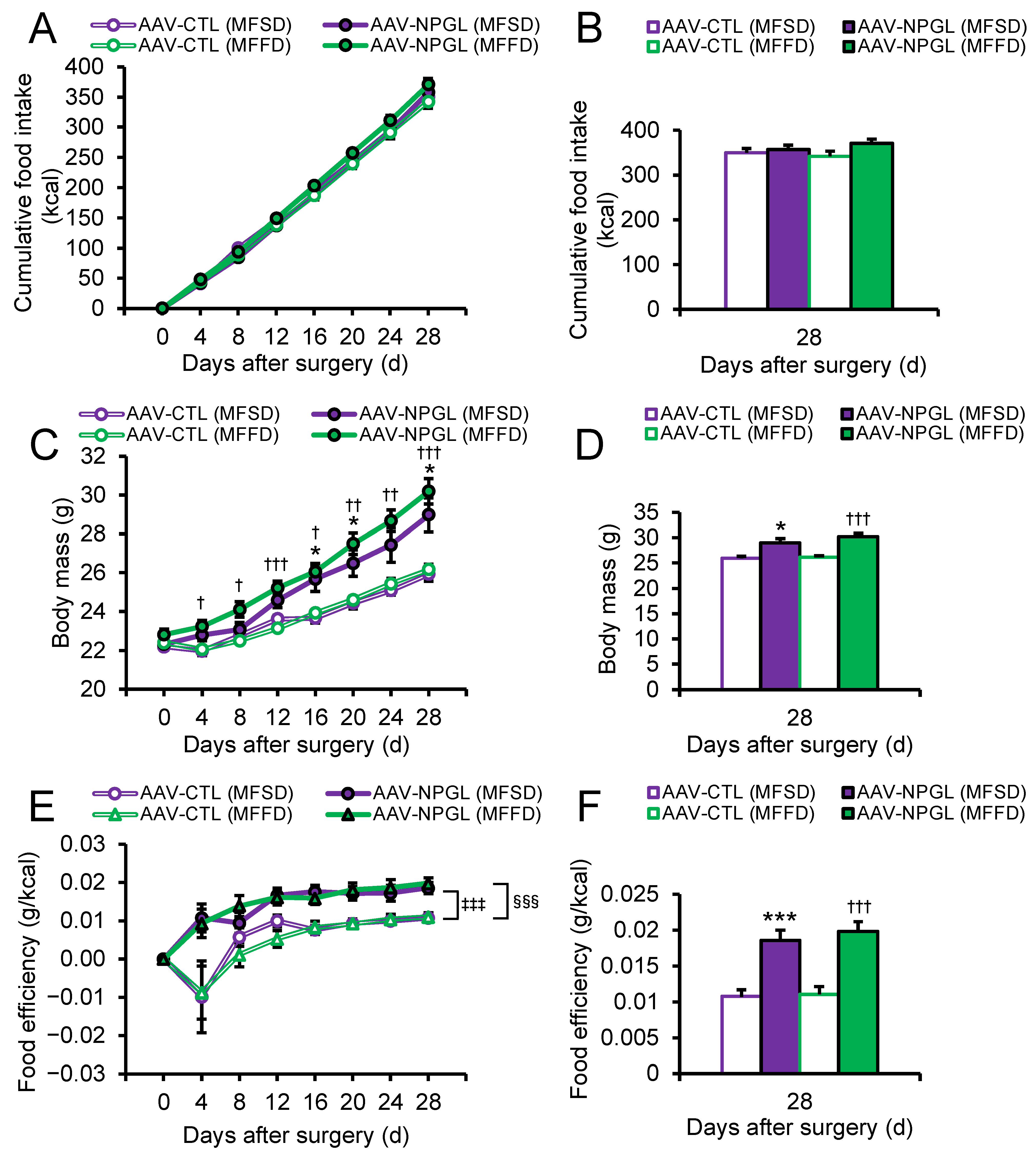
2.1. Effects of NPGL-Precursor Gene Overexpression on Food Intake, Body Mass, and Food Efficiency
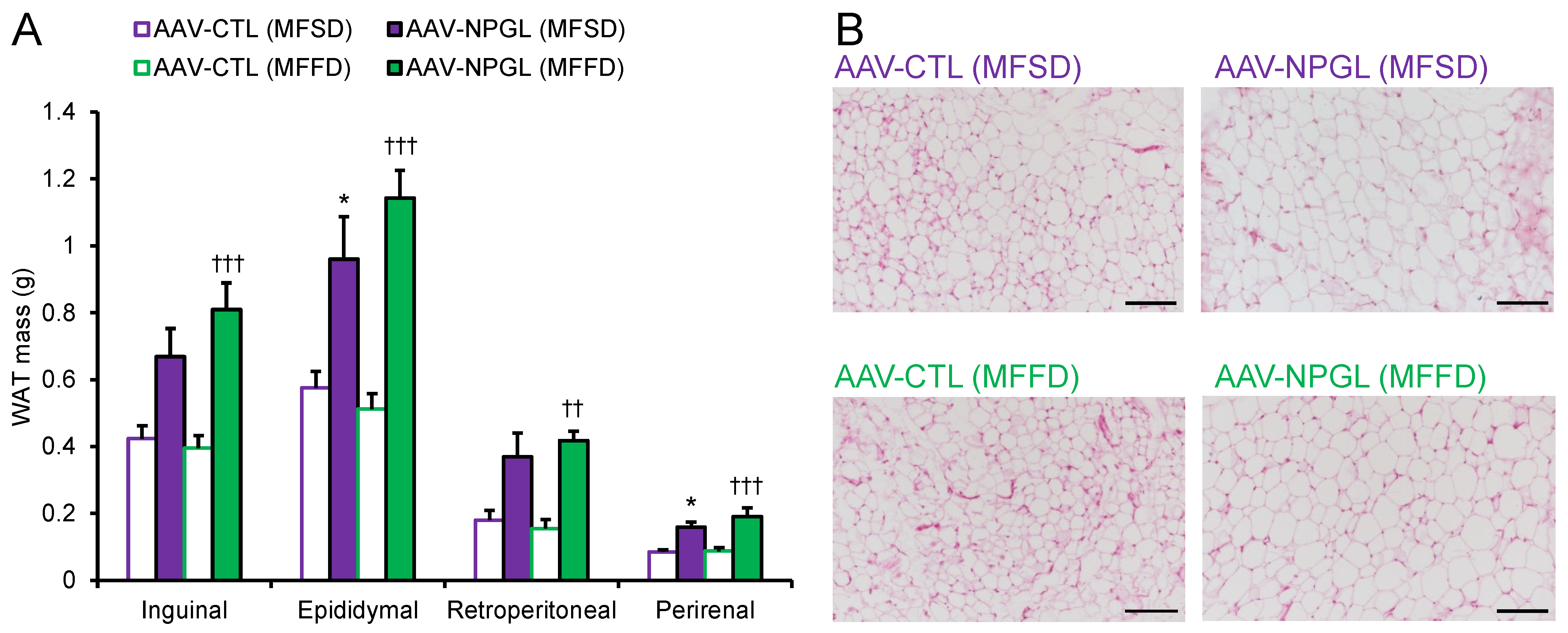
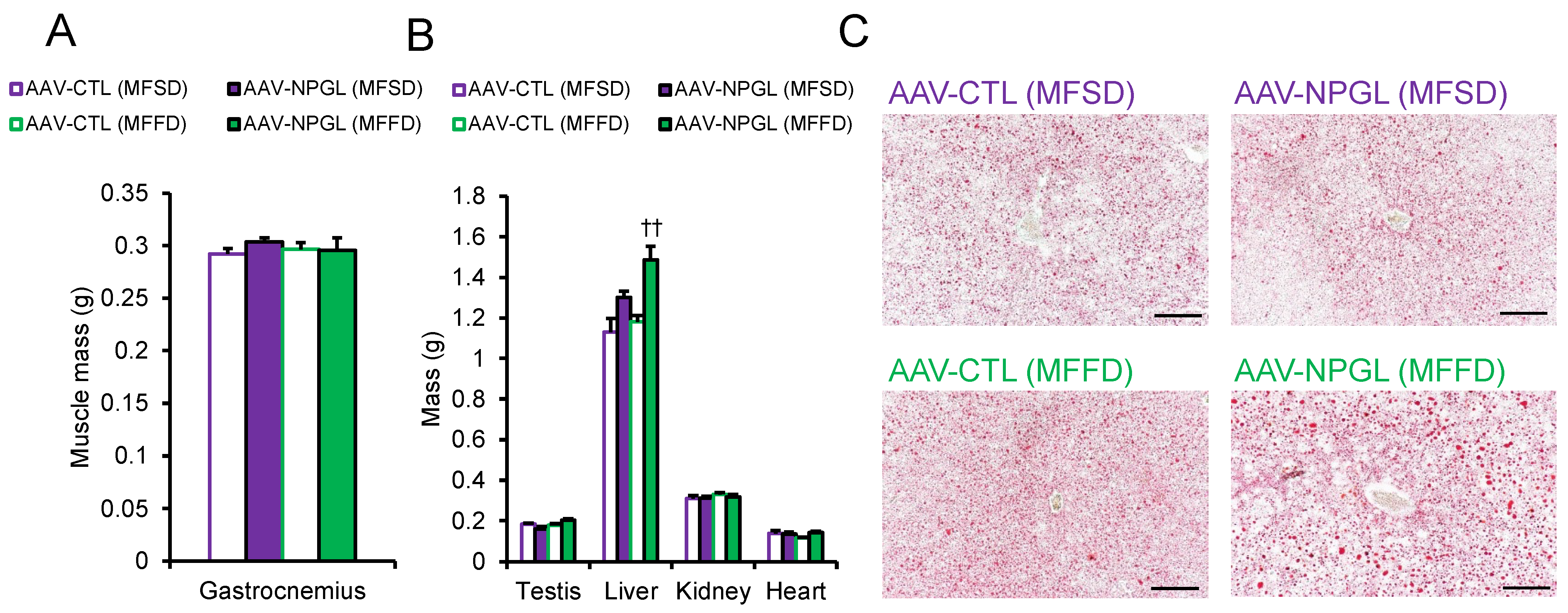
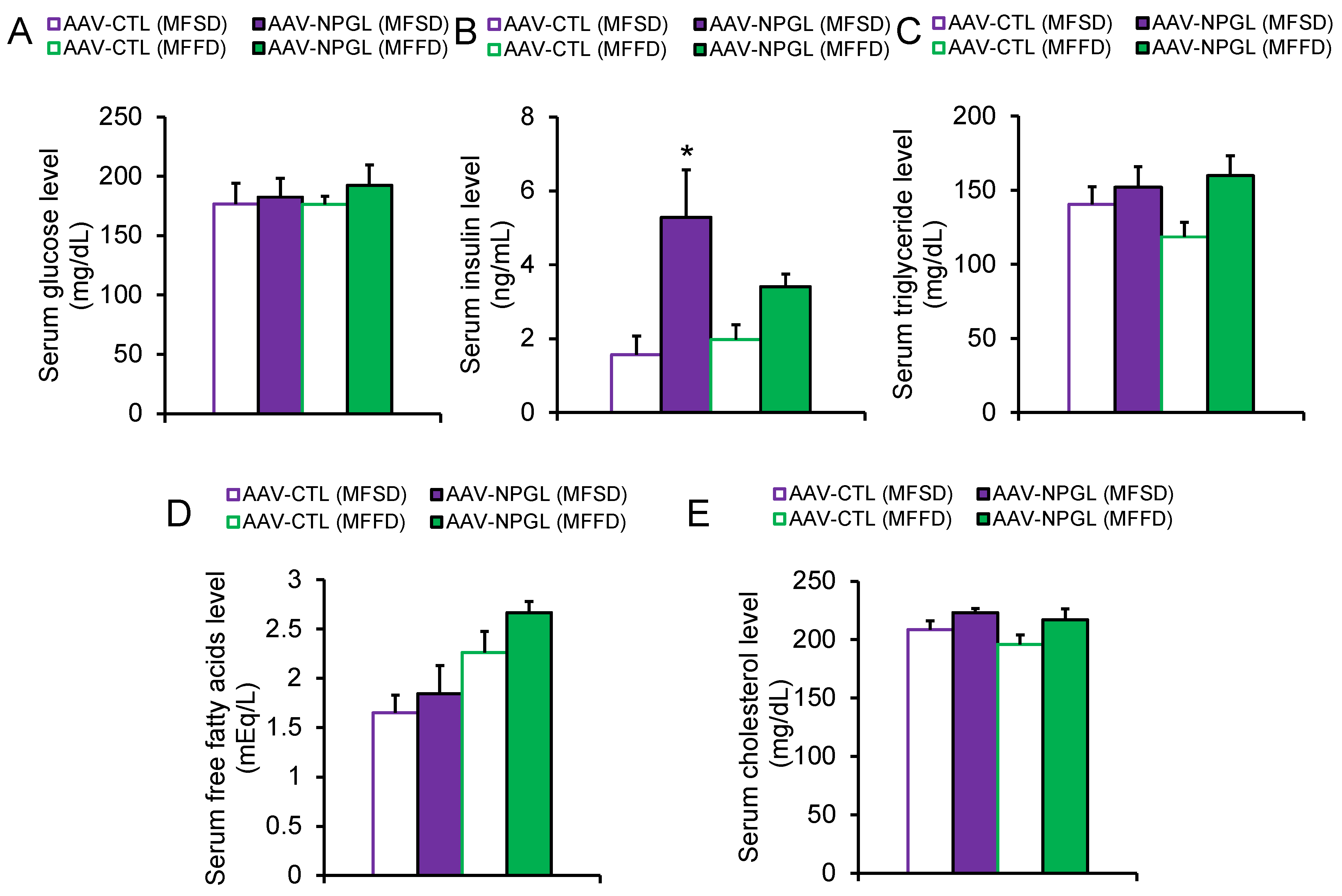
2.2. Effects of NPGL-Precursor Gene Overexpression on Body Composition and Serum Parameters
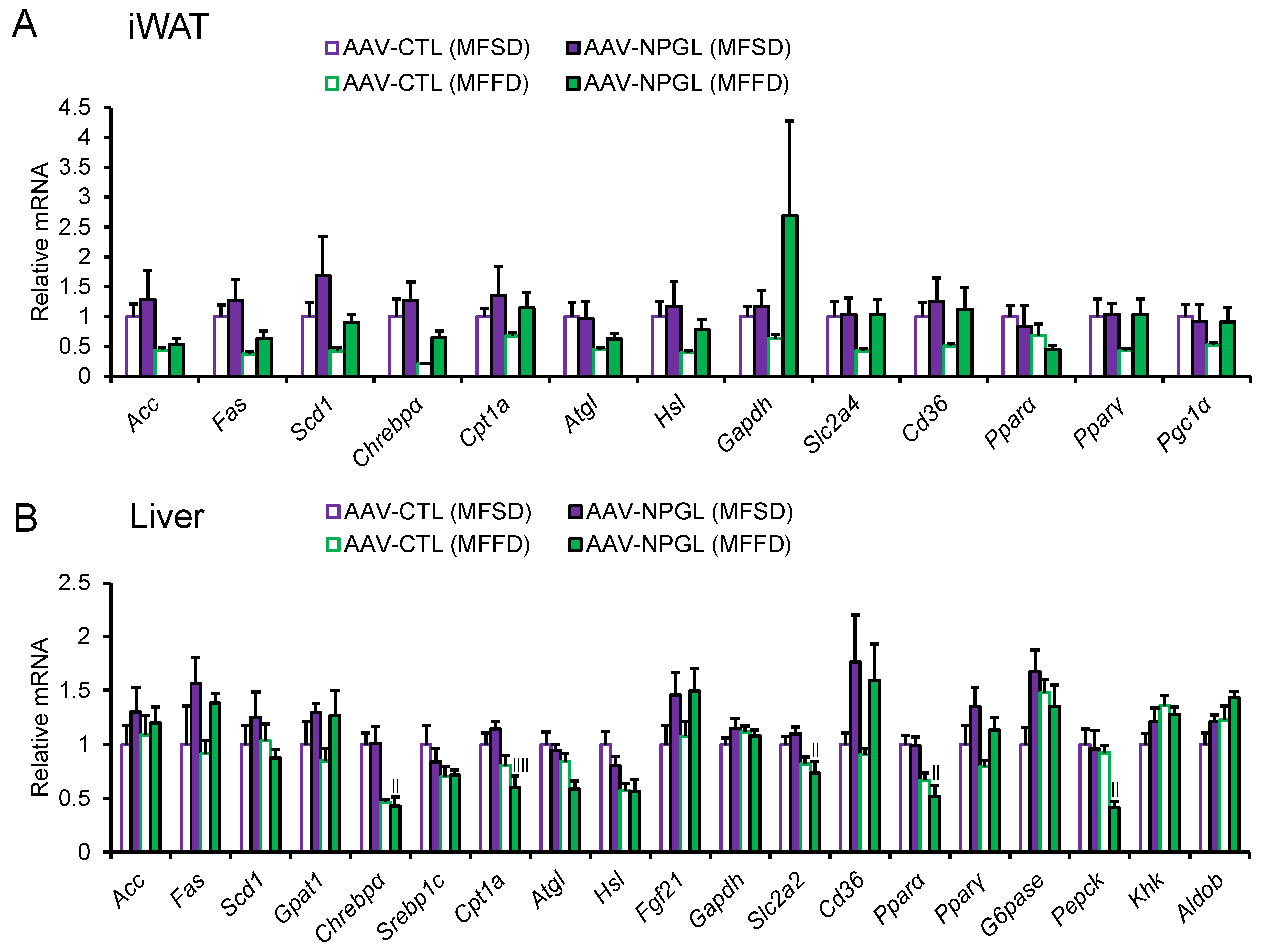
2.3. Effects of NPGL-Precursor Gene Overexpression on the mRNA Expression of Lipid Metabolism-Related Genes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Production of AAV-Based Vectors
4.3. Stereotaxic Surgery
4.4. Measurement of Body Mass, Food Intake, and Body Composition
4.5. qRT-PCR
4.6. Hematoxylin and Eosin Staining
4.7. Oil Red O Staining
4.8. Serum Biochemical Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T.; et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippe, J.M.; Angelopoulos, T.J. Sugars, obesity, and cardiovascular disease: Results from recent randomized control trials. Eur. J. Nutr. 2016, 55, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, T.; Abdelmalek, M.F.; Sullivan, S.; Nadeau, K.J.; Green, M.; Roncal, C.; Nakagawa, T.; Kuwabara, M.; Sato, Y.; Kang, D.H.; et al. Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 2018, 68, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Tappy, L.; Le, K.A. Metabolic effects of fructose and the worldwide increase in obesity. Physiol. Rev. 2010, 90, 23–46. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.W.; Woods, S.C.; Porte, D.; Seeley, R.J.; Baskin, D.G. Central nervous system control of food intake. Nature 2000, 404, 661–671. [Google Scholar] [CrossRef]
- Valassi, E.; Scacchi, M.; Cavagnini, F. Neuroendocrine control of food intake. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 158–168. [Google Scholar] [CrossRef]
- Sohn, J.W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015, 48, 229–233. [Google Scholar] [CrossRef]
- Baldini, G.; Phelan, K.D. The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 2019, 241, R1–R33. [Google Scholar] [CrossRef]
- Wilson, B.D.; Bagnol, D.; Kaelin, C.B.; Ollmann, M.M.; Gantz, I.; Watson, S.J.; Barsh, G.S. Physiological and anatomical circuitry between agouti-related protein and leptin signaling. Endocrinology 1999, 140, 2387–2397. [Google Scholar] [CrossRef]
- Koch, L.; Wunderlich, F.T.; Seibler, J.; Könner, A.C.; Hampel, B.; Irlenbusch, S.; Brabant, G.; Kahn, C.R.; Schwenk, F.; Brüning, J.C. Central insulin action regulates peripheral glucose and fat metabolism in mice. J. Clin. Investig. 2008, 118, 2132–2147. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Sato, T.; Tateyama, M.; Kageyama, H.; Maejima, Y.; Nakata, M.; Hirako, S.; Matsuo, T.; Kyaw, S.; Shiuchi, T.; et al. Activation of AMPK-regulated CRH neurons in the PVH is sufficient and necessary to induce dietary preference for carbohydrate over fat. Cell Rep. 2018, 22, 706–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Holstein-Rathlou, S.; Bondurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukena, K.; Iwakoshi-Ukena, E.; Taniuchi, S.; Bessho, Y.; Maejima, S.; Masuda, K.; Shikano, K.; Kondo, K.; Furumitsu, M.; Tachibana, T. Identification of a cDNA encoding a novel small secretory protein, neurosecretory protein GL, in the chicken hypothalamic infundibulum. Biochem. Biophys. Res. Commun. 2014, 446, 298–303. [Google Scholar] [CrossRef]
- Iwakoshi-Ukena, E.; Shikano, K.; Kondo, K.; Taniuchi, S.; Furumitsu, M.; Ochi, Y.; Sasaki, T.; Okamoto, S.; Bentley, G.E.; Kriegsfeld, L.J.; et al. Neurosecretory protein GL stimulates food intake, de novo lipogenesis, and onset of obesity. ELife 2017, 6, e28527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuura, D.; Shikano, K.; Saito, T.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ochi, Y.; Sato, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Neurosecretory protein GL, a hypothalamic small secretory protein, participates in energy homeostasis in male mice. Endocrinology 2017, 158, 1120–1129. [Google Scholar] [CrossRef]
- Ukena, K. Avian and murine neurosecretory protein GL participates in the regulation of feeding and energy metabolism. Gen. Comp. Endocrinol. 2018, 260, 164–170. [Google Scholar] [CrossRef]
- Shikano, K.; Iwakoshi-Ukena, E.; Kato, M.; Furumitsu, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Neurosecretory protein GL induces fat accumulation in chicks. Front. Endocrinol. 2019, 10, 392. [Google Scholar] [CrossRef]
- Fukumura, K.; Shikano, K.; Narimatsu, Y.; Iwakoshi-Ukena, E.; Furumitsu, M.; Naito, M.; Ukena, K. Effects of neurosecretory protein GL on food intake and fat accumulation under different dietary nutrient compositions in rats. Biosci. Biotechnol. Biochem. 2021, 85, 1514–1520. [Google Scholar] [CrossRef]
- Narimatsu, Y.; Iwakoshi-Ukena, E.; Fukumura, K.; Shikano, K.; Furumitsu, M.; Morishita, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Hypothalamic overexpression of neurosecretory protein GL leads to obesity in male C57BL/6J mice. Neuroendocrinology 2021. [Google Scholar] [CrossRef]
- Ter Horst, K.W.; Serlie, M.J. Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients 2017, 9, 981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vrána, A.; Fábry, P.; Slabochová, Z.; Kazdová, L. Effect of dietary fructose on free fatty acid release from adipose tissue and serum free fatty acid concentration in the rat. Ann. Nutr. Metab. 1974, 17, 74–83. [Google Scholar] [CrossRef]
- Prager, G.N.; Ontko, J.A. Direct effects of fructose metabolism on fatty acid oxidation in a recombined rat liver mitochondria-high speed supernatant system. Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1976, 424, 386–395. [Google Scholar] [CrossRef]
- Topping, D.L.; Mayes, P.A. The immediate effects of insulin and fructose on the metabolism of the perfused liver. Changes in lipoprotein secretion, fatty acid oxidation and esterification, lipogenesis and carbohydrate metabolism. Biochem. J. 1972, 126, 295–311. [Google Scholar] [CrossRef] [Green Version]
- Shikano, K.; Kato, M.; Iwakoshi-Ukena, E.; Furumitsu, M.; Matsuura, D.; Masuda, K.; Tachibana, T.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Effects of chronic intracerebroventricular infusion of neurosecretory protein GL on body mass and food and water intake in chicks. Gen. Comp. Endocrinol. 2018, 256, 37–42. [Google Scholar] [CrossRef]
- Fortino, M.A.; Lombardo, Y.B.; Chicco, A. The reduction of dietary sucrose improves dyslipidemia, adiposity, and insulin secretion in an insulin-resistant rat model. Nutrition 2007, 23, 489–497. [Google Scholar] [CrossRef]
- Hattori, H.; Hanai, Y.; Oshima, Y.; Kataoka, H.; Eto, N. Excessive intake of high-fructose corn syrup drinks induces impaired glucose tolerance. Biomedicines 2021, 9, 541. [Google Scholar] [CrossRef]
- Shikano, K.; Iwakoshi-Ukena, E.; Saito, T.; Narimatsu, Y.; Kadota, A.; Furumitsu, M.; Bentley, G.E.; Kriegsfeld, L.J.; Ukena, K. Neurosecretory protein GL induces fat accumulation in mice. J. Endocrinol. 2020, 244, 1–12. [Google Scholar] [CrossRef]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef] [Green Version]
- Iizuka, K. The role of carbohydrate response element binding protein in intestinal and hepatic fructose metabolism. Nutrients 2017, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Uyeda, K.; Yamashita, H.; Kawaguchi, T. Carbohydrate responsive element-binding protein (ChREBP): A key regulator of glucose metabolism and fat storage. Biochem. Pharmacol. 2002, 63, 2075–2080. [Google Scholar] [CrossRef]
- Fukumura, K.; Narimatsu, Y.; Moriwaki, S.; Iwakoshi-Ukena, E.; Furumitsu, M.; Ukena, K. Effects of overexpression of neurosecretory protein GL-precursor gene on glucose homeostasis and insulin sensitivity in mice. Int. J. Mol. Sci. 2021, 22, 4681. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Suen, P.K.; Zhang, C.Q.; Mak, W.S.; Gu, M.H.; Liu, Q.Q.; Sun, S.S.M. Improved growth performance, food efficiency, and lysine availability in growing rats fed with lysine-biofortified rice. Sci. Rep. 2017, 7, 1389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Gene | Treatment | Diet | Interaction | |||
|---|---|---|---|---|---|---|
| Acc | F(1, 19) = 0.39 | 0.538 | F(1, 19) = 4.55 | <0.05 | F(1, 19) = 0.10 | 0.755 |
| Fas | F(1, 19) = 1.20 | 0.287 | F(1, 19) = 6.96 | <0.05 | F(1, 19) = 0.001 | 0.979 |
| Scd1 | F(1, 19) = 2.02 | 0.172 | F(1, 19) = 2.78 | 0.112 | F(1, 19) = 0.07 | 0.795 |
| Chrebpα | F(1, 19) = 2.16 | 0.158 | F(1, 19) = 8.31 | <0.01 | F(1, 19) = 0.12 | 0.737 |
| Cpt1a | F(1, 19) = 1.70 | 0.208 | F(1, 19) = 0.70 | 0.415 | F(1, 19) = 0.03 | 0.853 |
| Atgl | F(1, 19) = 0.11 | 0.740 | F(1, 19) = 4.35 | 0.051 | F(1, 19) = 0.23 | 0.634 |
| Hsl | F(1, 19) = 1.01 | 0.327 | F(1, 19) = 3.03 | 0.098 | F(1, 19) = 0.14 | 0.710 |
| Gapdh | F(1, 19) = 2.05 | 0.168 | F(1, 19) = 0.55 | 0.486 | F(1, 19) = 1.45 | 0.243 |
| Slc2a4 | F(1, 19) = 1.85 | 0.189 | F(1, 19) = 1.45 | 0.244 | F(1, 19) = 1.40 | 0.251 |
| Cd36 | F(1, 19) = 1.88 | 0.186 | F(1, 19) = 0.95 | 0.341 | F(1, 19) = 0.32 | 0.576 |
| Pparα | F(1, 19) = 0.58 | 0.455 | F(1, 19) = 1.87 | 0.187 | F(1, 19) = 0.15 | 0.906 |
| Pparγ | F(1, 19) = 1.94 | 0.180 | F(1, 19) = 1.47 | 0.240 | F(1, 19) = 1.49 | 0.237 |
| Pgc1α | F(1, 19) = 0.41 | 0.531 | F(1, 19) = 1.04 | 0.321 | F(1, 19) = 1.00 | 0.329 |
| Gene | Treatment | Diet | Interaction | |||
|---|---|---|---|---|---|---|
| Acc | F(1, 19) = 0.96 | 0.339 | F(1, 19) = 0.01 | 0.972 | F(1, 19) = 0.22 | 0.644 |
| Fas | F(1, 19) = 3.94 | 0.062 | F(1, 19) = 0.27 | 0.606 | F(1, 19) = 0.04 | 0.848 |
| Scd1 | F(1, 19) = 0.05 | 0.818 | F(1, 19) = 0.78 | 0.389 | F(1, 19) = 1.15 | 0.297 |
| Gpat1 | F(1, 19) = 3.74 | 0.068 | F(1, 19) = 0.23 | 0.638 | F(1, 19) = 0.11 | 0.739 |
| Chrebpα | F(1, 19) = 0.01 | 0.913 | F(1, 19) = 23.54 | <0.005 | F(1, 19) = 0.04 | 0.841 |
| Srebp1c | F(1, 19) = 0.26 | 0.613 | F(1, 19) = 2.29 | 0.147 | F(1, 19) = 0.41 | 0.532 |
| Cpt1a | F(1, 19) = 0.10 | 0.758 | F(1, 19) = 12.49 | <0.005 | F(1, 19) = 2.76 | 0.113 |
| Atgl | F(1, 19) = 2.76 | 0.113 | F(1, 19) = 7.72 | <0.05 | F(1, 19) = 1.20 | 0.286 |
| Hsl | F(1, 19) = 0.88 | 0.361 | F(1, 19) = 9.68 | <0.01 | F(1, 19) = 0.78 | 0.389 |
| Fgf21 | F(1, 19) = 4.62 | <0.05 | F(1, 19) = 0.08 | 0.785 | F(1, 19) = 0.01 | 0.922 |
| Gapdh | F(1, 19) = 0.58 | 0.454 | F(1, 19) = 0.09 | 0.764 | F(1, 19) = 1.40 | 0.252 |
| Slc2a2 | F(1, 19) = 0.01 | 0.935 | F(1, 19) = 9.61 | <0.01 | F(1, 19) = 1.05 | 0.319 |
| Cd36 | F(1, 19) = 5.64 | <0.05 | F(1, 19) = 0.19 | 0.671 | F(1, 19) = 0.02 | 0.902 |
| Pparα | F(1, 19) = 0.78 | 0.389 | F(1, 19) = 19.13 | <0.005 | F(1, 19) = 0.59 | 0.451 |
| Pparγ | F(1, 19) = 4.87 | <0.05 | F(1, 19) = 1.85 | 0.190 | F(1, 19) = 0.002 | 0.961 |
| G6pase | F(1, 19) = 2.09 | 0.164 | F(1, 19) = 0.15 | 0.704 | F(1, 19) = 4.48 | <0.05 |
| Pepck | F(1, 19) = 4.22 | 0.054 | F(1, 19) = 5.42 | <0.05 | F(1, 19) = 3.07 | 0.096 |
| Khk | F(1, 19) = 0.34 | 0.565 | F(1, 19) = 3.60 | 0.073 | F(1, 19) = 1.78 | 0.199 |
| Aldob | F(1, 19) = 4.01 | 0.060 | F(1, 19) = 4.44 | <0.05 | F(1, 19) = 0.001 | 0.977 |
| Diet | Protein (kcal%) | Carbohydrate (kcal%) | Fat (kcal%) | kcal/g |
|---|---|---|---|---|
| Sucrose/Fructose (kcal%) | ||||
| Medium-fat/medium-sucrose diet (MFSD) | 20 | 48 | 32 | 4.4 |
| 19/0 | ||||
| Medium-fat/medium-fructose diet (MFFD) | 20 | 48 | 32 | 4.4 |
| 0/19 |
| Gene | Sense Primer (5′ to 3′) | Antisense Primer (5′ to 3′) |
|---|---|---|
| Acc | TCCGCACTGACTGTAACCACAT | TGCTCCGCACAGATTCTTCA |
| Fas | AGGGGTCGACCTGGTCCTCA | GCCATGCCCAGAGGGTGGTT |
| Scd1 | CTGTACGGGATCATACTGGTTC | GCCGTGCCTTGTAAGTTCTG |
| Gpat1 | TCATCCAGTATGGCATTCTCACA | GCAAGGCCAGGACTGACATC |
| Chrebpα | CGACACTCACCCACCTCTTC | TTGTTCAGCCGGATCTTGTC |
| Srebp1c | GGAGCCATGGATTGCACATT | GGCCCGGGAAGTCACTGT |
| Cpt1a | CCTGGGCATGATTGCAAAG | GGACGCCACTCACGATGTT |
| Atgl | AACACCAGCATCCAGTTCAA | GGTTCAGTAGGCCATTCCTC |
| Hsl | GCTGGGCTGTCAAGCACTGT | GTAACTGGGTAGGCTGCCAT |
| Fgf21 | CCTCTAGGTTTCTTTGCCAACAG | AAGCTGCAGGCCTCAGGAT |
| Gapdh | AAGGTCATCCCAGAGCTGAA | CTGCTTCACCACCTTCTTGA |
| Slc2a2 | GGCTAATTTCAGGACTGGTT | TTTCTTTGCCCTGACTTCCT |
| Slc2a4 | GTAACTTCATTGTCGGCATGG | AGCTGAGATCTGGTCAAACG |
| Cd36 | TCCTCTGACATTTGCAGGTCTATC | AAAGGCATTGGCTGGAAGAA |
| Pparα | TCGAATATGTGGGGACAAGG | GACAGGCACTTGTGAAAACG |
| Pparγ | GCCCTTTGGTGACTTTATGGA | GCAGCAGGTTGTCTTGGATG |
| Pgc1α | GCAACATGCTCAAGCCAAAC | TGCAGTTCCAGAGAGTTCCA |
| G6pase | ACTGTGGGCATCAATCTCCTC | CGGGACAGACAGACGTTCAGC |
| Pepck | GTGCTGGAGTGGATGTTCGG | CTGGCTGATTCTCTGTTTCAGG |
| Khk | CCTGCCAGATGTGTCTGCTA | TGCAGCATCTTCACCTGTTC |
| Aldob | AGAGGATGGAGAAGGGCATT | ATGCAGGATCCCTCAACAAG |
| Npgl | TATGTAGACTGTGTCCTCTC | TCTAAGGAGCTGAGAATATGCA |
| Rps18 | CCTGAGAAGTTCCAGCACAT | TTCTCCAGCCCTCTTGGTG |
| Actb | GGCACCACACCTTCTACAAT | AGGTCTCAAACATGATCTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narimatsu, Y.; Iwakoshi-Ukena, E.; Naito, M.; Moriwaki, S.; Furumitsu, M.; Ukena, K. Neurosecretory Protein GL Accelerates Liver Steatosis in Mice Fed Medium-Fat/Medium-Fructose Diet. Int. J. Mol. Sci. 2022, 23, 2071. https://doi.org/10.3390/ijms23042071
Narimatsu Y, Iwakoshi-Ukena E, Naito M, Moriwaki S, Furumitsu M, Ukena K. Neurosecretory Protein GL Accelerates Liver Steatosis in Mice Fed Medium-Fat/Medium-Fructose Diet. International Journal of Molecular Sciences. 2022; 23(4):2071. https://doi.org/10.3390/ijms23042071
Chicago/Turabian StyleNarimatsu, Yuki, Eiko Iwakoshi-Ukena, Mana Naito, Shogo Moriwaki, Megumi Furumitsu, and Kazuyoshi Ukena. 2022. "Neurosecretory Protein GL Accelerates Liver Steatosis in Mice Fed Medium-Fat/Medium-Fructose Diet" International Journal of Molecular Sciences 23, no. 4: 2071. https://doi.org/10.3390/ijms23042071
APA StyleNarimatsu, Y., Iwakoshi-Ukena, E., Naito, M., Moriwaki, S., Furumitsu, M., & Ukena, K. (2022). Neurosecretory Protein GL Accelerates Liver Steatosis in Mice Fed Medium-Fat/Medium-Fructose Diet. International Journal of Molecular Sciences, 23(4), 2071. https://doi.org/10.3390/ijms23042071






