Immature Testicular Tissue Engineered from Weaned Mice to Adults for Prepubertal Fertility Preservation—An In Vivo Translational Study
Abstract
1. Introduction
2. Results
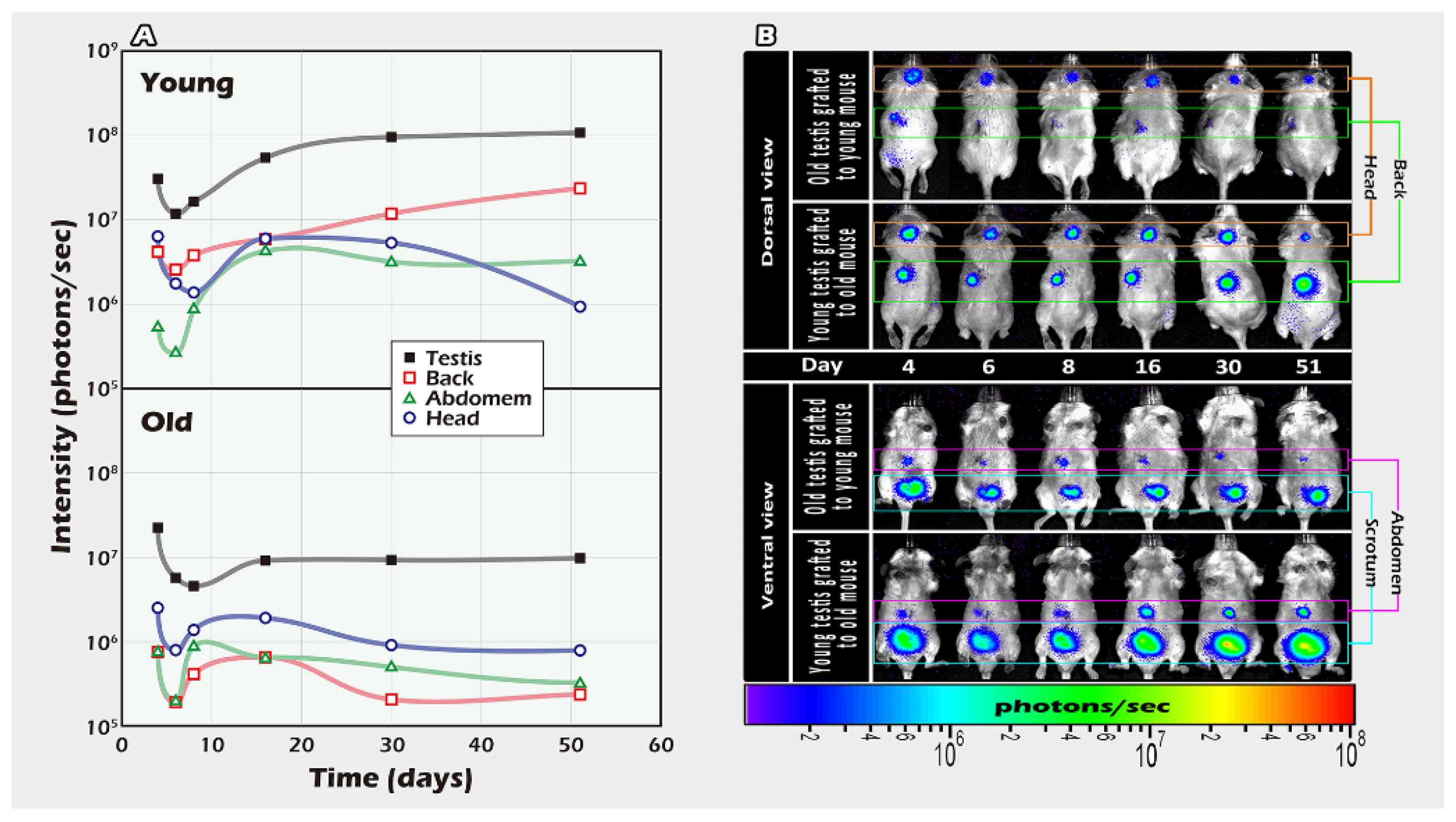
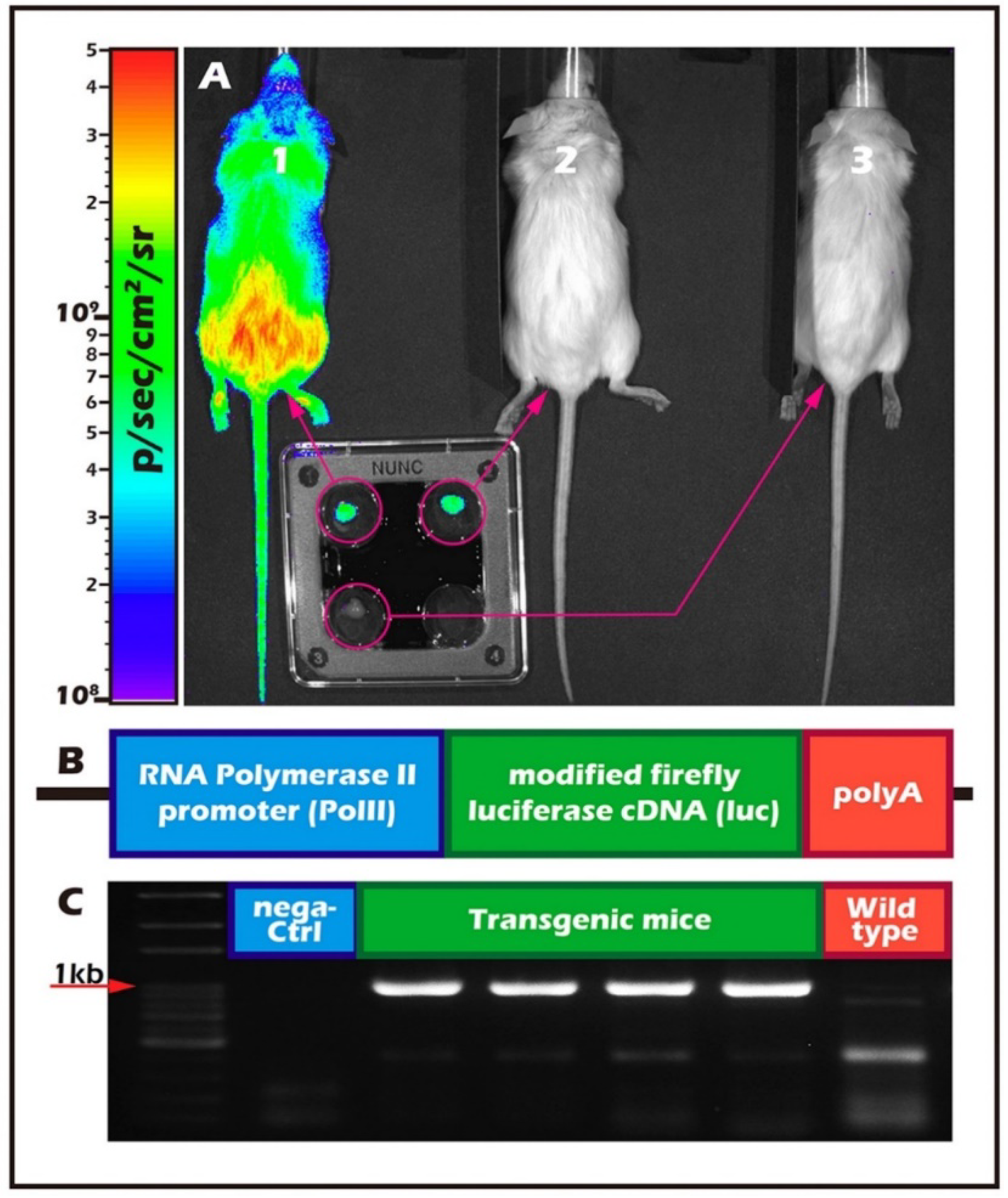
2.1. Different Ages of Donor Testicular Tissues Transplanted into Various Recipient Sites
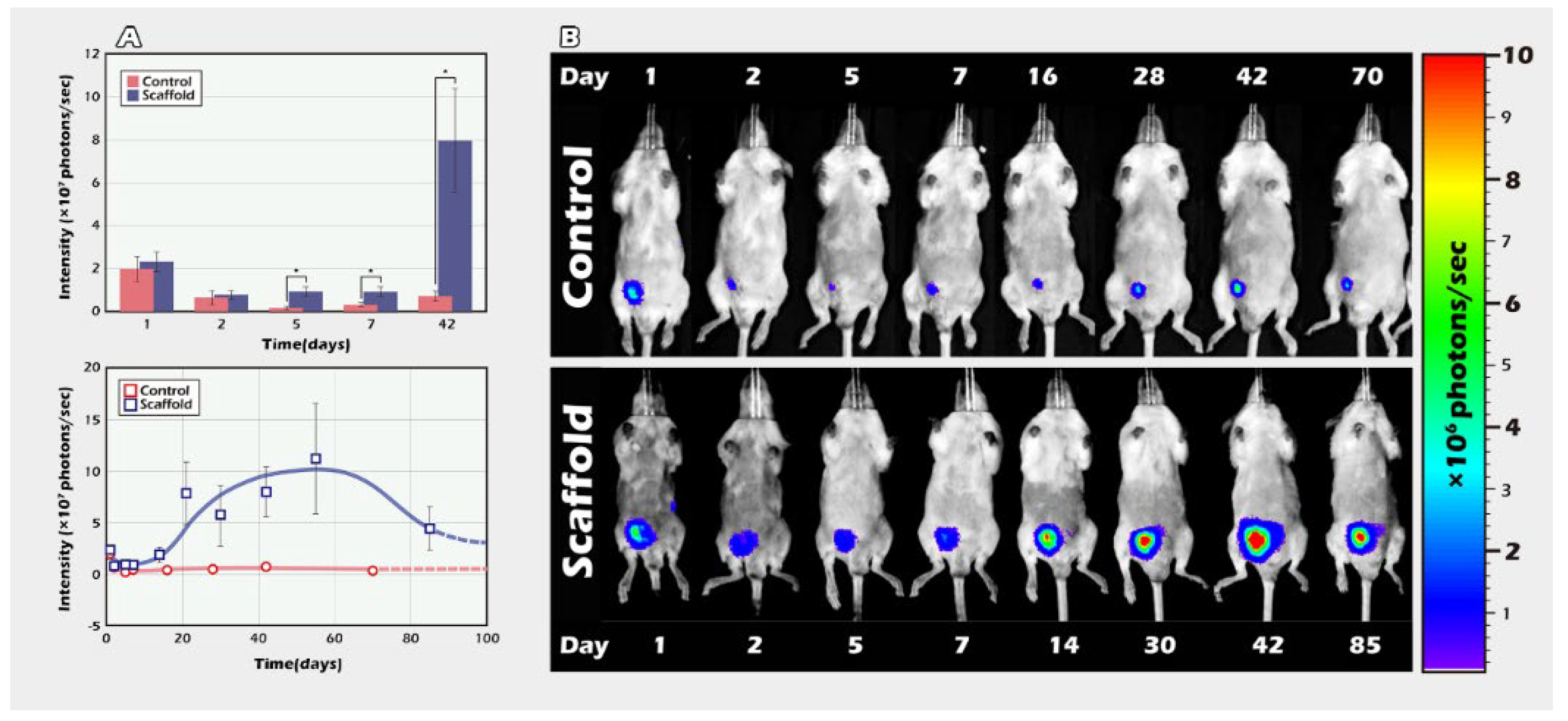
2.2. Long-Term In Vivo Tracking of ITT Spermatogenesis in Age-Matched Donors and Recipients with/without Scaffold Bioengineering
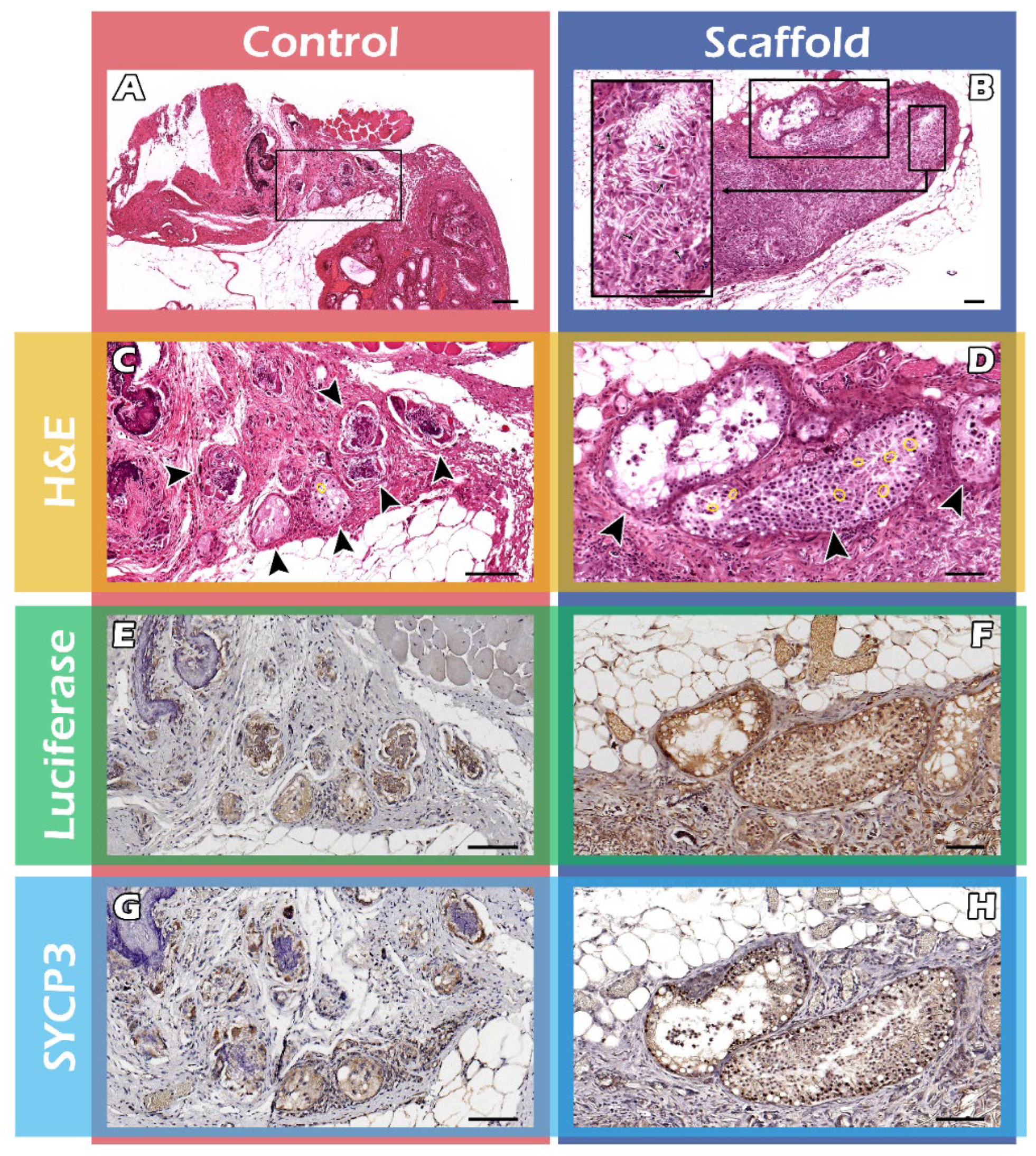
2.3. Histological Assays of Spermatogenesis of the ITT Graft
3. Discussion
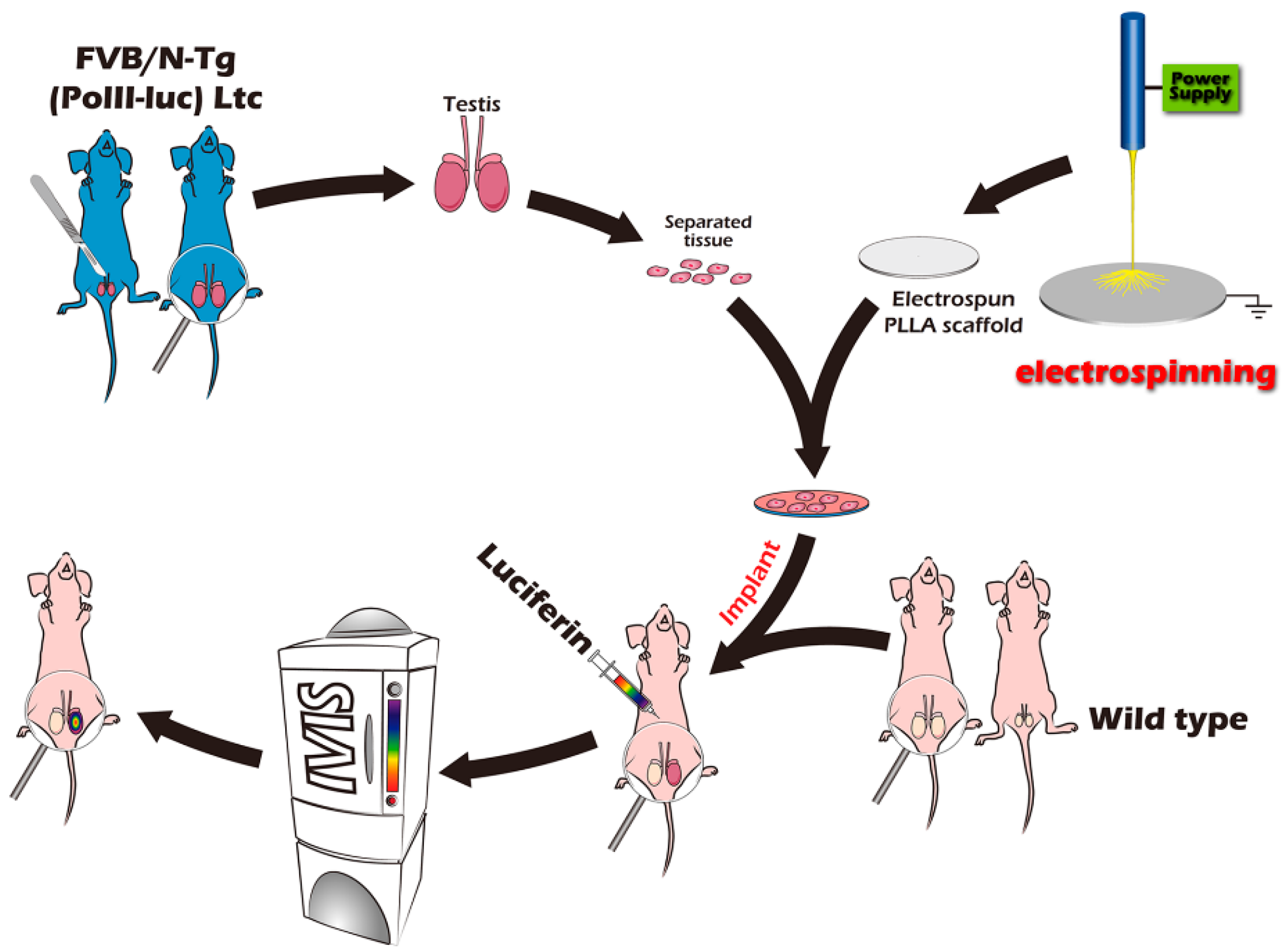
4. Materials and Methods
4.1. In Vivo Study
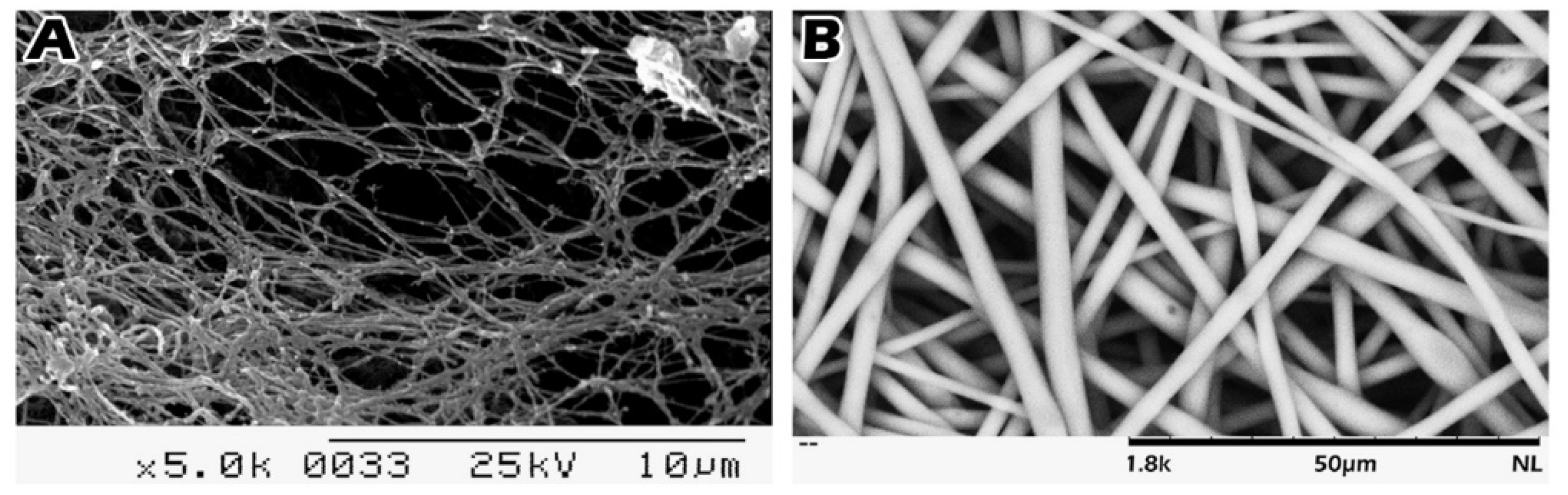
4.2. Scaffold Preparation and Loading of Immature Seminiferous Tubules on the Scaffold
4.3. Scanning Electron Microscopy (SEM)
4.4. In Vivo Bioluminescence Imaging
4.5. Part I Study: BLI Tracking of the Optimal Graft Sites
4.6. Part II Study: BLI Tracking of the ITT Grafts Engineered from PLLA Scaffold
4.7. Histology and Immunohistochemistry (IHC) Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moss, J.L.; Choi, A.W.; Fitzgerald Keeter, M.K.; Brannigan, R.E. Male adolescent fertility preservation. Fertil. Steril. 2016, 105, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Onofre, J.; Baert, Y.; Faes, K.; Goossens, E. Cryopreservation of testicular tissue or testicular cell suspensions: A pivotal step in fertility preservation. Hum. Reprod. Update 2016, 22, 744–761. [Google Scholar] [CrossRef]
- Borgstrom, B.; Fridstrom, M.; Gustafsson, B.; Ljungman, P.; Rodriguez-Wallberg, K.A. A prospective study on the long-term outcome of prepubertal and pubertal boys undergoing testicular biopsy for fertility preservation prior to hematologic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28507. [Google Scholar] [CrossRef]
- Qu, N.; Itoh, M.; Sakabe, K. Effects of Chemotherapy and Radiotherapy on Spermatogenesis: The Role of Testicular Immunology. Int. J. Mol. Sci. 2019, 20, 957. [Google Scholar] [CrossRef]
- Lettieri, G.; D’Agostino, G.; Mele, E.; Cardito, C.; Esposito, R.; Cimino, A.; Giarra, A.; Trifuoggi, M.; Raimondo, S.; Notari, T.; et al. Discovery of the Involvement in DNA Oxidative Damage of Human Sperm Nuclear Basic Proteins of Healthy Young Men Living in Polluted Areas. Int. J. Mol. Sci. 2020, 21, 4189. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, G.; Marra, F.; Moriello, C.; Prisco, M.; Notari, T.; Trifuoggi, M.; Giarra, A.; Bosco, L.; Montano, L.; Piscopo, M. Molecular Alterations in Spermatozoa of a Family Case Living in the Land of Fires. A First Look at Possible Transgenerational Effects of Pollutants. Int. J. Mol. Sci 2020, 21, 6710. [Google Scholar] [CrossRef]
- Clermont, Y. Kinetics of spermatogenesis in mammals. Arch. Anat. Microsc. Morphol. Exp. 1967, 56, 7–60. [Google Scholar] [PubMed]
- Shinohara, T.; Inoue, K.; Ogonuki, N.; Kanatsu-Shinohara, M.; Miki, H.; Nakata, K.; Kurome, M.; Nagashima, H.; Toyokuni, S.; Kogishi, K.; et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum. Reprod. 2002, 17, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, J.P.; Carlson, C.A.; Lin, K.; Hobbie, W.L.; Wigo, E.; Wu, X.; Brinster, R.L.; Kolon, T.F. An experimental protocol for fertility preservation in prepubertal boys recently diagnosed with cancer: A report of acceptability and safety. Hum. Reprod. 2010, 25, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Petit, S.; Vanabelle, B.; Laurent, P.; Wese, J.F.X.; Donnez, J. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum. Reprod. 2011, 26, 737–747. [Google Scholar] [CrossRef]
- Wu, X.; Goodyear, S.M.; Abramowitz, L.K.; Bartolomei, M.S.; Tobias, J.W.; Avarbock, M.R.; Brinster, R.L. Fertile offspring derived from mouse spermatogonial stem cells cryopreserved for more than 14 years. Hum. Reprod. 2012, 27, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Picton, H.M.; Wyns, C.; Anderson, R.A.; Goossens, E.; Jahnukainen, K.; Kliesch, S.; Mitchell, R.T.; Pennings, G.; Rives, N.; Tournaye, H.; et al. A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum. Reprod. 2015, 30, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Luo, Y.; Li, Y.; Fu, C.; Chen, J.; Wang, Y. Design of bioinspired polymeric materials based on poly(D,L-lactic acid) modifications towards improving its cytocompatibility. J. Biomed. Mater. Res. A 2008, 84, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.F.; Yang, C.P.; Tai, C.C.; Tseng, H. Gene Expression and Behavior Analysis of PC12 Cells Grown on Biodegradable Nano-fibrous Membranes. Curr. Nanosci. 2011, 7, 886–892. [Google Scholar] [CrossRef]
- Eslahi, N.; Hadjighassem, M.R.; Joghataei, M.T.; Mirzapour, T.; Bakhtiyari, M.; Shakeri, M.; Pirhajati, V.; Shirinbayan, P.; Koruji, M. The effects of poly L-lactic acid nanofiber scaffold on mouse spermatogonial stem cell culture. Int. J. Nanomed. 2013, 8, 4563–4576. [Google Scholar]
- Hall, P.A.; Watt, F.M. Stem cells: The generation and maintenance of cellular diversity. Development 1989, 106, 619–633. [Google Scholar] [CrossRef]
- Giudice, M.G.; de Michele, F.; Poels, J.; Vermeulen, M.; Wyns, C. Update on fertility restoration from prepubertal spermatogonial stem cells: How far are we from clinical practice? Stem Cell Res. 2017, 21, 171–177. [Google Scholar] [CrossRef]
- Chen, C.H.; Wang, C.W.; Hsu, M.I.; Huang, Y.H.; Lai, W.F.; Tzeng, C.R. Bioluminescence imaging as a tool to evaluate germ cells in vitro and transplantation in vivo as fertility preservation of prepubertal male mice. Fertil. Steril. 2012, 97, 1192–1198. [Google Scholar] [CrossRef]
- Chen, C.H.; Yeh, Y.C.; Wu, G.J.; Huang, Y.H.; Lai, W.F.T.; Liu, J.Y.; Tzeng, C.R. Tracking the rejection and survival of mouse ovarian iso- and allografts in vivo with bioluminescent imaging. Reproduction 2010, 140, 105–112. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yeh, Y.C.; Tzeng, C.R.; Shang, W.J.; Liu, J.Y.; Chen, C.H. Evaluating the effects of immunosuppression by in-vivo bioluminescence imaging after allotransplantation of ovarian grafts. Reprod. Biomed. Online 2011, 22, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Tan, S.J.; Tzeng, C.R. In vivo fate mapping of cryopreserved murine ovarian grafts. J. Ovarian Res. 2014, 7, 81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, Q.R.; Wang, L.F.; Xia, S.S.; Zhang, Y.M.; Xu, J.N.; Li, H.; Ding, Y.Z. Role of interleukin-17A in early graft rejection after orthotopic lung transplantation in mice. J. Thorac. Dis. 2016, 8, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319. [Google Scholar] [CrossRef]
- Close, D.M.; Xu, T.; Sayler, G.S.; Ripp, S. In vivo bioluminescent imaging (BLI): Noninvasive visualization and interrogation of biological processes in living animals. Sensors 2011, 11, 180–206. [Google Scholar] [CrossRef]
- Luetjens, C.M.; Stukenborg, J.B.; Nieschlag, E.; Simoni, M.; Wistuba, J. Complete spermatogenesis in orthotopic but not in ectopic transplants of autologously grafted marmoset testicular tissue. Endocrinology 2008, 149, 1736–1747. [Google Scholar] [CrossRef]
- Yu, J.; Cai, Z.M.; Wan, H.J.; Zhang, F.T.; Ye, J.; Fang, J.Z.; Gui, Y.T.; Ye, J.X. Development of neonatal mouse and fetal human testicular tissue as ectopic grafts in immunodeficient mice. Asian J. Androl. 2006, 8, 393–403. [Google Scholar] [CrossRef]
- Goossens, E.; Geens, M.; De Block, G.; Tournaye, H. Spermatogonial survival in long-term human prepubertal xenografts. Fertil. Steril. 2008, 90, 2019–2022. [Google Scholar] [CrossRef]
- Wyns, C.; Van Langendonckt, A.; Wese, F.X.; Donnez, J.; Curaba, M. Long-term spermatogonial survival in cryopreserved and xenografted immature human testicular tissue. Hum. Reprod. 2008, 23, 2402–2414. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Martinez-Madrid, B.; Van Langendonckt, A.; Francois-Xavier, W.; Donnez, J. Spermatogonial survival after cryopreservation and short-term orthotopic immature human cryptorchid testicular tissue grafting to immunodeficient mice. Hum. Reprod. 2007, 22, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Van Saen, D.; Goossens, E.; Bourgain, C.; Ferster, A.; Tournaye, H. Meiotic activity in orthotopic xenografts derived from human postpubertal testicular tissue. Hum. Reprod. 2011, 26, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Poels, J.; Abou-Ghannam, G.; Herman, S.; Van Langendonckt, A.; Wese, F.X.; Wyns, C. In Search of Better Spermatogonial Preservation by Supplementation of Cryopreserved Human Immature Testicular Tissue Xenografts with N-acetylcysteine and Testosterone. Front Surg. 2014, 1, 47. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; de Avila, J.M.; McLean, D.J. Analysis of gene expression in bovine testis tissue prior to ectopic testis tissue xenografting and during the grafting period. Biol. Reprod. 2007, 76, 1071–1080. [Google Scholar] [CrossRef]
- Caires, K.C.; Schmidt, J.A.; Oliver, A.P.; de Avila, J.; McLean, D.J. Endocrine regulation of the establishment of spermatogenesis in pigs. Reprod Domest Anim. 2008, 43 (Suppl. 2), 280–287. [Google Scholar] [CrossRef]
- Yango, P.; Altman, E.; Smith, J.F.; Klatsky, P.C.; Tran, N.D. Optimizing cryopreservation of human spermatogonial stem cells: Comparing the effectiveness of testicular tissue and single cell suspension cryopreservation. Fertil Steril. 2014, 102, 1491–1498 e1. [Google Scholar] [CrossRef] [PubMed]
- Usas, A.; Huard, J. Muscle-derived stem cells for tissue engineering and regenerative therapy. Biomaterials 2007, 28, 5401–5406. [Google Scholar] [CrossRef]
- Wang, X.; Song, G.; Lou, T. Fabrication and characterization of nano-composite scaffold of PLLA/silane modified hydroxyapatite. Med. Eng. Phys. 2010, 32, 391–397. [Google Scholar] [CrossRef]
- Lee, B.N.; Kim, D.Y.; Kang, H.J.; Kwon, J.S.; Park, Y.H.; Chun, H.J.; Kim, J.H.; Lee, H.B.; Min, B.H.; Kim, M.S. In vivo biofunctionality comparison of different topographic PLLA scaffolds. J. Biomed. Mater. Res. A 2012, 100, 1751–1760. [Google Scholar] [CrossRef]
- Ellis, S.J.; Gomez, N.C.; Levorse, J.; Mertz, A.F.; Ge, Y.; Fuchs, E. Distinct modes of cell competition shape mammalian tissue morphogenesis. Nature 2019, 569, 497–502. [Google Scholar] [CrossRef]
- Yoon, S.D.; Kwon, Y.S.; Lee, K.S. Biodegradation and Biocompatibility of Poly L-lactic Acid Implantable Mesh. Int. Neurourol. J. 2017, 21, S48–S54. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liao, S.; Ngiam, M.; Chan, C.K.; Ramakrishna, S. Degradation behaviors of electrospun resorbable polyester nanofibers. Tissue Eng. Part. B Rev. 2009, 15, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-H.; Tai, C.-C.; Steven Wu, Y.-H.; Hung, S.-H.; Chang, S.-F.; Yang, C.-P.; Tseng, H. Gene expression and behavior analysis of PC12 cells grown on synthetic biodegradable fibrous membranes coated with natural biopolymers. Curr. Nanosci. 2014, 10, 235–242. [Google Scholar] [CrossRef]
- Zinn, K.R.; Chaudhuri, T.R.; Szafran, A.A.; O’Quinn, D.; Weaver, C.; Dugger, K.; Lamar, D.; Kesterson, R.A.; Wang, X.; Frank, S.J. Noninvasive Bioluminescence Imaging in Small Animals. ILAR J. Natl. Res. Counc. Inst. Lab. Anim. Resour. 2008, 49, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Rice, B.W.; Cable, M.D.; Nelson, M.B. In vivo imaging of light-emitting probes. J. Biomed. Opt. 2001, 6, 432–440. [Google Scholar] [CrossRef]
- Hueng, D.Y.; Sytwu, H.K.; Huang, S.M.; Chang, C.; Ma, H.I. Isolation and characterization of tumor stem-like cells from human meningiomas. J. Neurooncol. 2011, 104, 45–53. [Google Scholar] [CrossRef]
- Wu, C.C.; Lu, K.C.; Lin, G.J.; Hsieh, H.Y.; Chu, P.; Lin, S.H.; Sytwu, H.K. Melatonin enhances endogenous heme oxygenase-1 and represses immune responses to ameliorate experimental murine membranous nephropathy. J. Pineal Res. 2012, 52, 460–469. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, S.S.; Chen, S.J.; Lin, Y.C.; Chu, C.C.; Huang, H.H.; Chang, F.W.; Yu, M.H.; Lin, S.H.; Wu, G.J.; et al. OSR1 and SPAK cooperatively modulate Sertoli cell support of mouse spermatogenesis. Sci. Rep. 2016, 6, 37205. [Google Scholar] [CrossRef][Green Version]
- Wyns, C.; Kanbar, M.; Giudice, M.G.; Poels, J. Fertility preservation for prepubertal boys: Lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Update 2021, 27, 433–459. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, H.; Liu, Y.-L.; Lu, B.-J.; Chen, C.-H. Immature Testicular Tissue Engineered from Weaned Mice to Adults for Prepubertal Fertility Preservation—An In Vivo Translational Study. Int. J. Mol. Sci. 2022, 23, 2042. https://doi.org/10.3390/ijms23042042
Tseng H, Liu Y-L, Lu B-J, Chen C-H. Immature Testicular Tissue Engineered from Weaned Mice to Adults for Prepubertal Fertility Preservation—An In Vivo Translational Study. International Journal of Molecular Sciences. 2022; 23(4):2042. https://doi.org/10.3390/ijms23042042
Chicago/Turabian StyleTseng, How, Yung-Liang Liu, Buo-Jia Lu, and Chi-Huang Chen. 2022. "Immature Testicular Tissue Engineered from Weaned Mice to Adults for Prepubertal Fertility Preservation—An In Vivo Translational Study" International Journal of Molecular Sciences 23, no. 4: 2042. https://doi.org/10.3390/ijms23042042
APA StyleTseng, H., Liu, Y.-L., Lu, B.-J., & Chen, C.-H. (2022). Immature Testicular Tissue Engineered from Weaned Mice to Adults for Prepubertal Fertility Preservation—An In Vivo Translational Study. International Journal of Molecular Sciences, 23(4), 2042. https://doi.org/10.3390/ijms23042042





