Sodium Energetic Cycle in the Natronophilic Bacterium Thioalkalivibrio versutus
Abstract
:1. Introduction
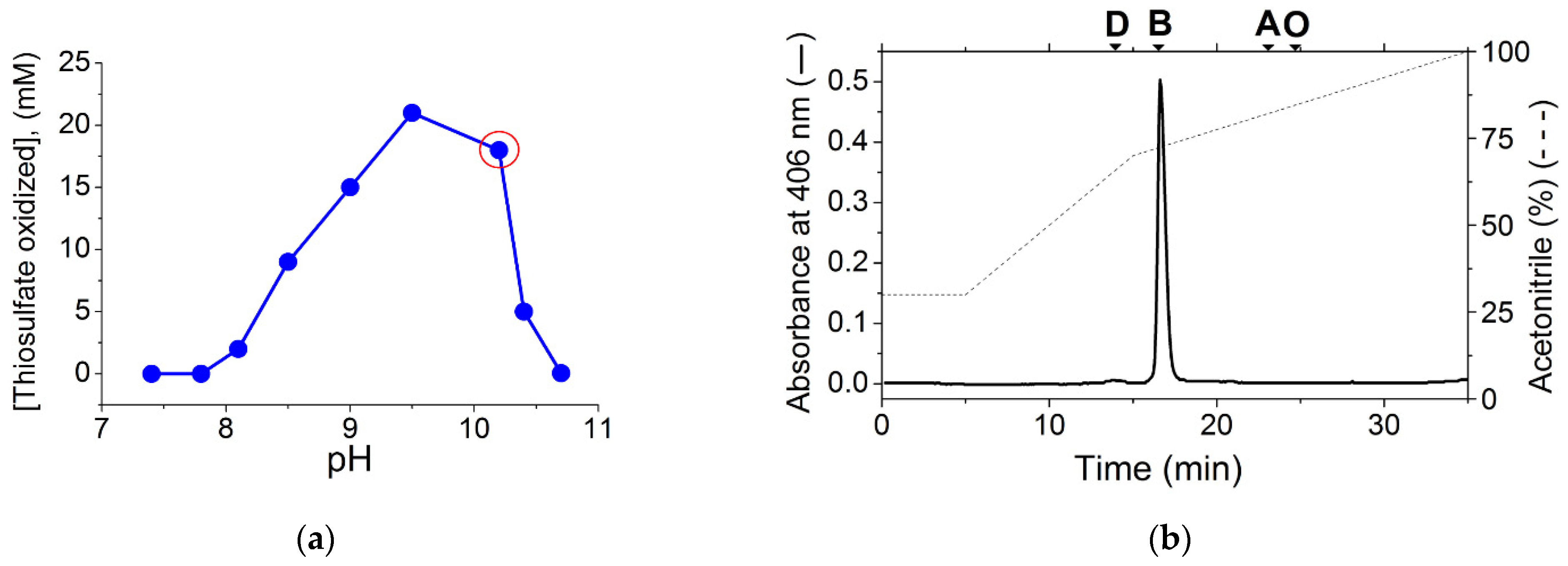
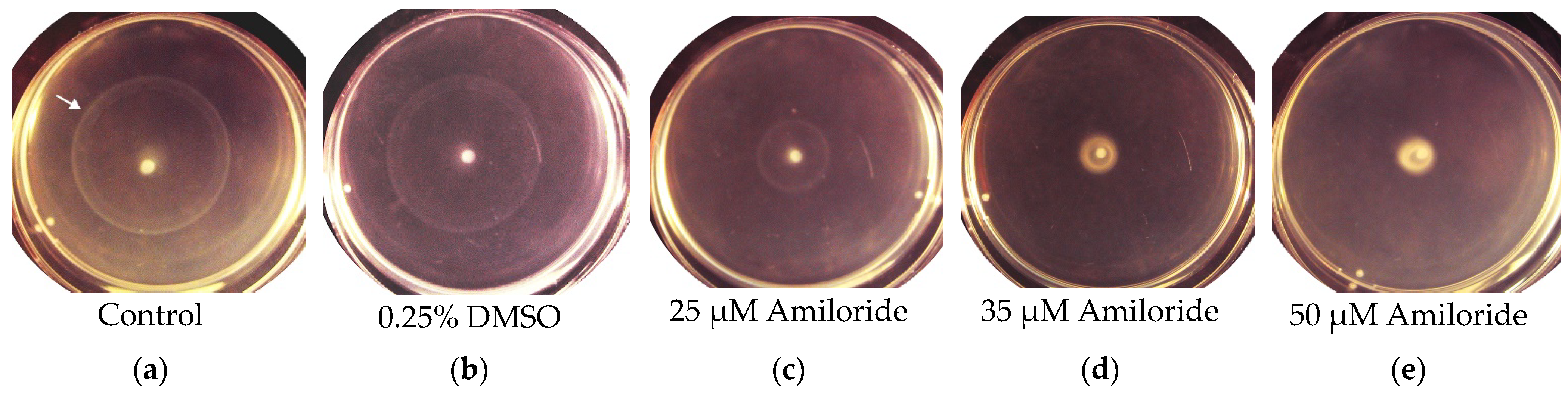
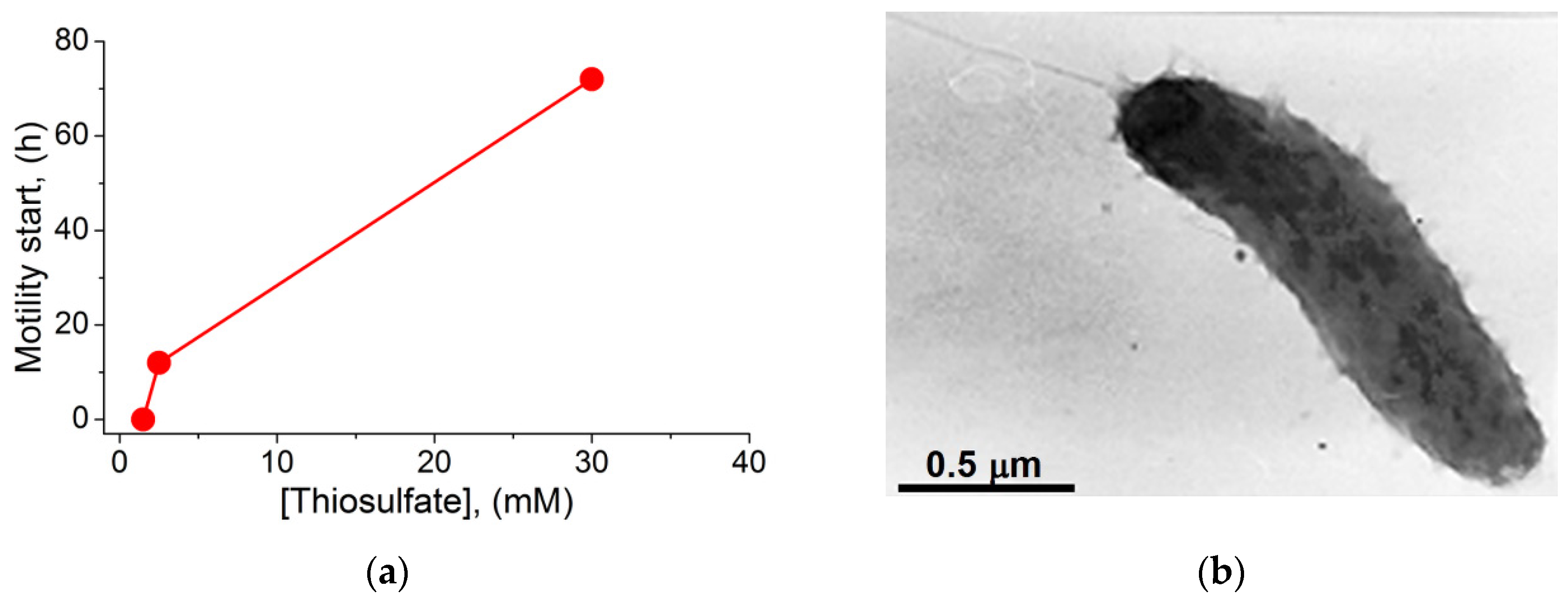
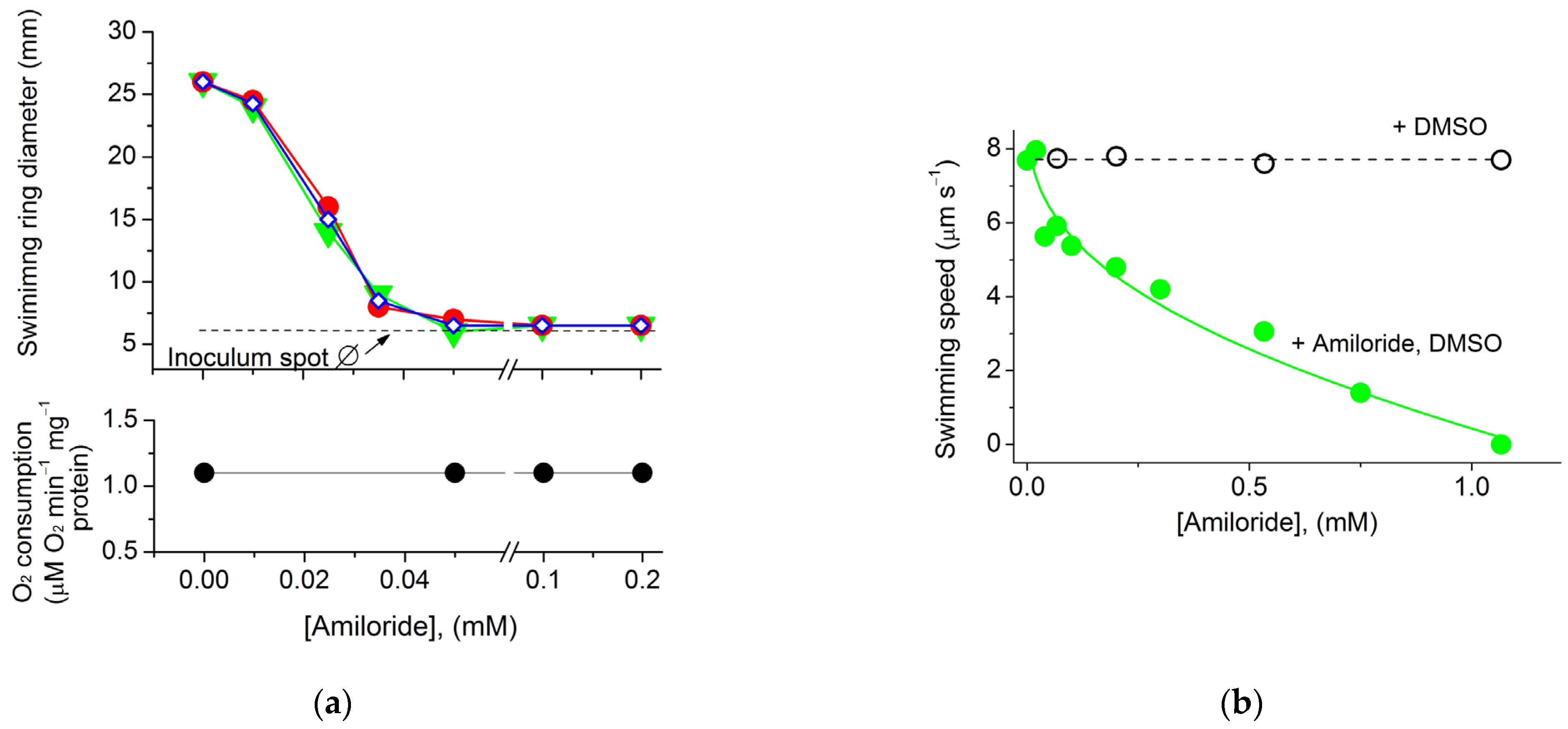
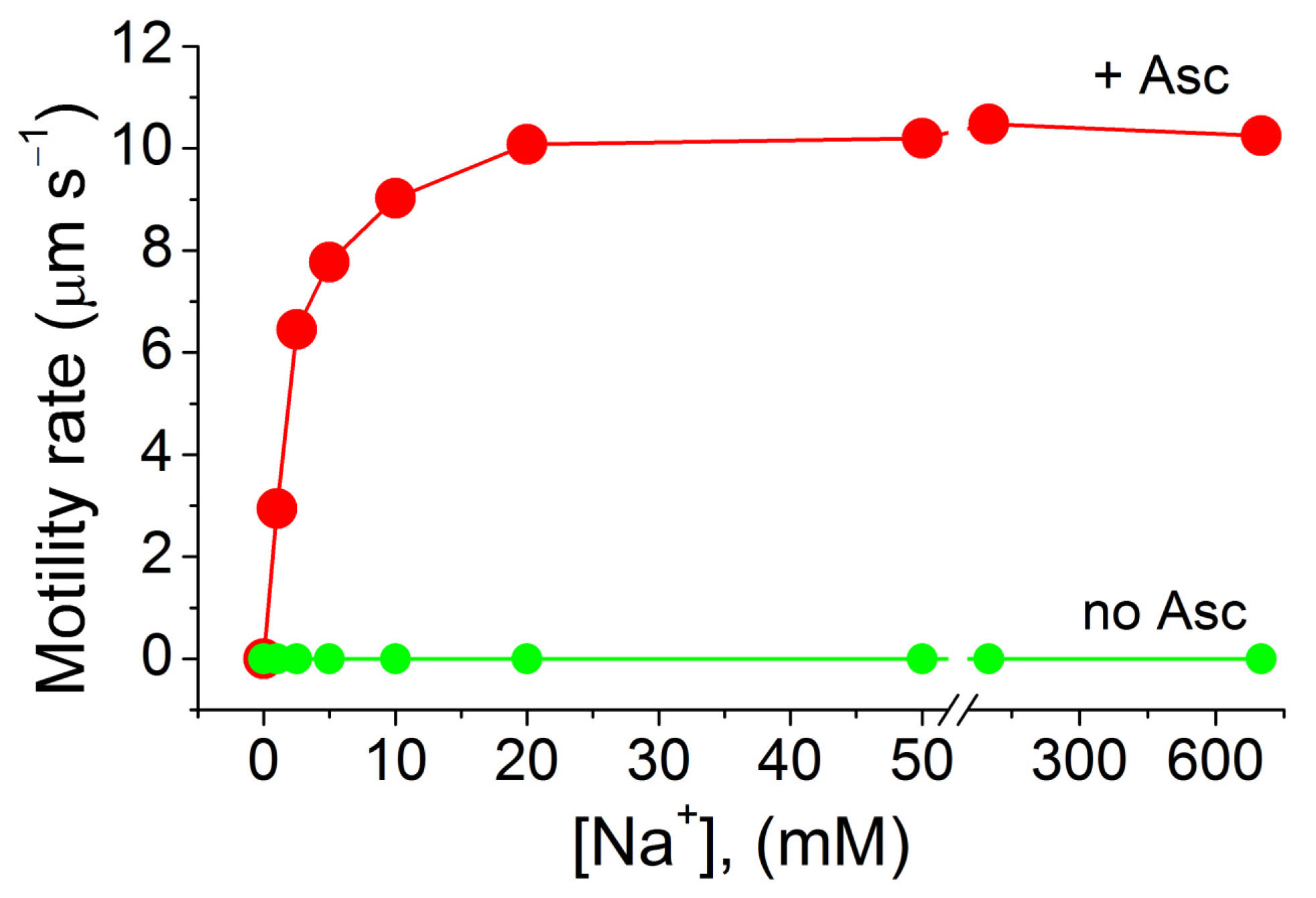
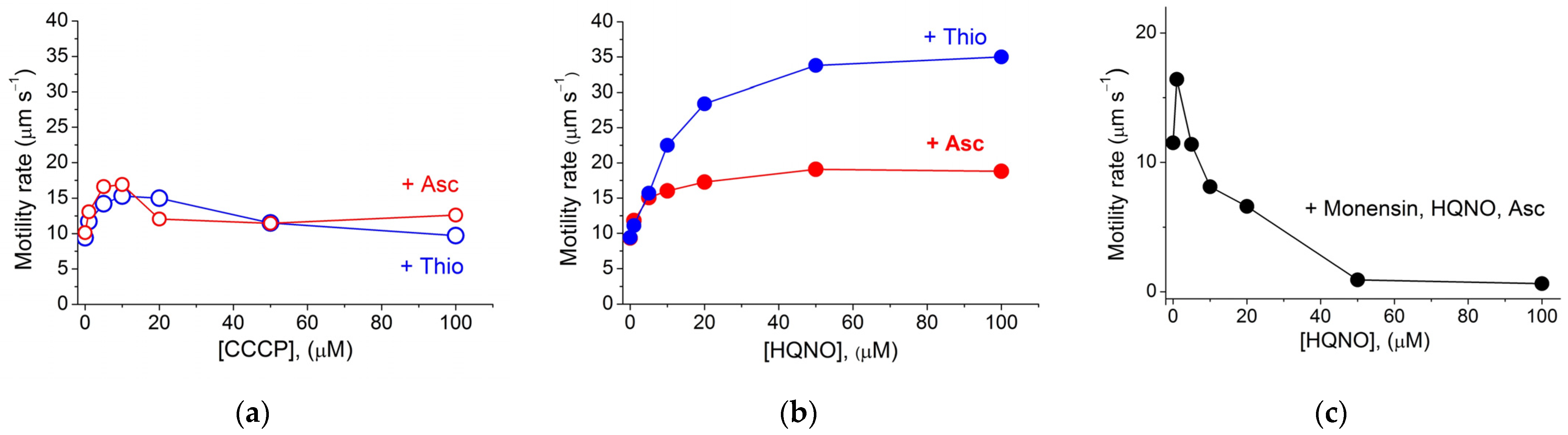
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Bacteria Growth Conditions for the Experiments
3.3. Analysis of Hemes
3.4. Selection of Motile Cells
3.5. Respiratory Activity
3.6. Evaluation of Swimming Speed in a Liquid Medium
3.7. Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Merino, N.; Aronson, H.S.; Bojanova, D.P.; Feyhl-Buska, J.; Wong, M.L.; Zhang, S.; Giovannelli, D. Living at the extremes: Extremophiles and the limits of life in a planetary context. Front. Microbiol. 2019, 10, 780. [Google Scholar] [CrossRef] [Green Version]
- Mehda, S.; Muñoz-Martín, M.Á.; Oustani, M.; Hamdi-Aïssa, B.; Perona, E.; Mateo, P. Lithic cyanobacterial communities in the polyextreme Sahara Desert: Implications for the search for the limits of life. Environ. Microbiol. 2021. [Google Scholar] [CrossRef]
- González, J.M.; Kato, C.; Horikoshi, K. Thermococcus peptonophilus sp. nov., a fast-growing, extremely thermophilic archaebacterium isolated from deep-sea hydrothermal vents. Arch. Microbiol. 1995, 164, 159–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stan-Lotter, H.; Fendrihan, S. Halophilic archaea: Life with desiccation, radiation and oligotrophy over geological times. Life 2015, 5, 1487–1496. [Google Scholar] [CrossRef]
- Gavrilov, S.; Podosokorskaya, O.; Alexeev, D.; Merkel, A.; Khomyakova, M.; Muntyan, M.; Altukhov, I.; Butenko, I.; Bonch-Osmolovskaya, E.; Govorun, V.; et al. Respiratory pathways reconstructed by multi-omics analysis in Melioribacter roseus, residing in a deep thermal aquifer of the West-Siberian megabasin. Front. Microbiol. 2017, 8, 1228. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, D.Y.; Muntyan, M.S.; Toshchakov, S.V.; Korzhenkov, A.; Kublanov, I.V. Phenotypic and genomic properties of a novel deep-lineage haloalkaliphilic member of the phylum Balneolaeota from soda lakes possessing Na+-translocating proteorhodopsin. Front. Microbiol. 2018, 9, 2672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daebeler, A.; Kitzinger, K.; Koch, H.; Herbold, C.W.; Steinfeder, M.; Schwarz, J.; Zechmeister, T.; Karst, S.M.; Albertsen, M.; Nielsen, P.H.; et al. Exploring the upper pH limits of nitrite oxidation: Diversity, ecophysiology, and adaptive traits of haloalkalitolerant Nitrospira. ISME J. 2020, 14, 2967–2979. [Google Scholar] [CrossRef]
- Calisto, F.; Sousa, F.M.; Sena, F.V.; Refojo, P.N.; Pereira, M.M. Mechanisms of Energy Transduction by Charge Translocating Membrane Proteins. Chem. Rev. 2021, 121, 1804–1844. [Google Scholar] [CrossRef]
- Kell, D.B. A protet-based, protonic charge transfer model of energy coupling in oxidative and photosynthetic phosphorylation. Adv. Microb. Physiol. 2021, 78, 1–177. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X.; Madsen, A.M.; Zervas, A.; Nielsen, T.K.; Andrei, A.S.; Lund-Hansen, L.C.; Liu, Y.; Hansen, L.H. Potential rhodopsin- and bacteriochlorophyll-based dual phototrophy in a high Arctic glacier. mBio 2020, 11, e02641-20. [Google Scholar] [CrossRef]
- Xue, Q.; Zhao, D.; Zhang, S.; Zhou, H.; Zuo, Z.; Zhou, J.; Li, M.; Xiang, H. Highly integrated adaptive mechanisms in Spiribacter halalkaliphilus, a bacterium abundant in Chinese soda-saline lakes. Environ. Microbiol. 2021, 23, 6463–6482. [Google Scholar] [CrossRef] [PubMed]
- Mevada, V.A.; Beladiya, U.H.; Gandhi, H.R.; Mangrola, A.V.; Patel, R.K. Alkalophiles: Environmental Distribution, Taxonomy, Physiology, Bioenergetics, Survival Mechanism, and Enzymes. In Physiology, Genomics, and Biotechnological Applications of Extremophiles; Gunjal, A.B., Thombre, R., Parray, J.A., Eds.; IGI Global: Hershey, PA, USA, 2022; pp. 35–64. [Google Scholar] [CrossRef]
- Adams, M.W.; Perler, F.B.; Kelly, R.M. Extremozymes: Expanding the limits of biocatalysis. Biotechnology 1995, 13, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Bankar, A.; Patil, S.; Shinde, M.; Kowligi, B. Potential of microbial extremophiles for biotechnological applications: An overview. In Microbial Extremozymes. Novel Sources and Industrial Applications; Kuddus, M., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 89–110. [Google Scholar] [CrossRef]
- Dumorné, K.; Córdova, D.C.; Astorga-Eló, M.; Renganathan, P. Extremozymes: A potential source for industrial applications. J. Microbiol. Biotechnol. 2017, 27, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Banciu, H.L.; Muntyan, M.S. Adaptive strategies in the double-extremophilic prokaryotes inhabiting soda lakes. Curr. Opin. Microbiol. 2015, 25, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Gorlenko, V.M.; Buryukhaev, S.P.; Matyugina, E.B.; Borzenko, S.V.; Namsaraev, Z.B.; Bryantseva, I.A.; Boldareva, E.N.; Sorokin, D.Y.; Namsaraev, B.B. Microbial communities of the stratified soda Lake Doroninskoe (Transbaikal region). Microbiology 2010, 79, 390–401. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, H.; Dong, H.; Wu, G.; Hou, W.; Zhao, W.; Sun, Y.; Lai, Z. Abundance and diversity of sulfur-oxidizing bacteria along a salinity gradient in four Qinghai-Tibetan lakes, China. Geomicrobiol. J. 2013, 30, 851–860. [Google Scholar] [CrossRef]
- Zakharyuk, A.G.; Kozyreva, L.P.; Khijniak, T.V.; Namsaraev, B.B.; Shcherbakova, V.A. Desulfonatronum zhilinae sp. nov., a novel haloalkaliphilic sulfate-reducing bacterium from soda Lake Alginskoe, Trans-Baikal Region, Russia. Extremophiles 2015, 19, 673–680. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Banciu, H.L.; Muyzer, G. Functional microbiology of soda lakes. Curr. Opin. Microbiol. 2015, 25, 88–96. [Google Scholar] [CrossRef]
- Burganskaya, E.I.; Bryantseva, I.A.; Gaisin, V.A.; Grouzdev, D.S.; Rysina, M.S.; Barkhutova, D.D.; Baslerov, R.V.; Gorlenko, V.M.; Kuznetsov, B.B. Benthic phototrophic community from Kiran soda lake, south-eastern Siberia. Extremophiles 2018, 22, 211–220. [Google Scholar] [CrossRef]
- Kulkarni, S.; Dhakar, K.; Joshi, A. Alkaliphiles: Diversity and bioprospection. In Microbial Diversity in the Genomic Era; Academic Press: Cambridge, MA, USA, 2019; pp. 239–263. [Google Scholar] [CrossRef]
- Lavrentyeva, E.V.; Erdyneeva, E.B.; Banzaraktsaeva, T.G.; Kotsyurbenko, O.R.; Baturina, O.A.; Khakhinov, V.V.; Kozyreva, L.P. Prokaryotic diversity in the biotopes of the gudzhirganskoe Saline Lake (Barguzin Valley, Russia). Microbiology 2020, 89, 359–368. [Google Scholar] [CrossRef]
- Chakraborty, J.; Rajput, V.; Sapkale, V.; Kamble, S.; Dharne, M. Spatio-temporal resolution of taxonomic and functional microbiome of Lonar soda lake of India reveals metabolic potential for bioremediation. Chemosphere 2021, 264, 128574. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Banciu, H.; Robertson, L.A.; Kuenen, J.G.; Muntyan, M.S.; Muyzer, G. Halophilic and haloalkaliphilic sulfur-oxidizing bacteria. In The Prokaryotes: Prokaryotic Physiology and Biochemistry; Rosenberg, E., DeLong, E., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 529–554. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Lysenko, A.M.; Mityushina, L.L.; Tourova, T.P.; Jones, B.E.; Rainey, F.A.; Robertson, L.A.; Kuenen, G.J. Thioalkalimicrobium aerophilum gen. nov., sp. nov. and Thioalkalimicrobium sibericum sp. nov., and Thioalkalivibrio versutus gen. nov., sp. nov., Thioalkalivibrio nitratis sp. nov., novel and Thioalkalivibrio denitrificancs sp. nov., novel obligately alkaliphilic and obligately chemolithoautotrophic sulfur-oxidizing bacteria from soda lakes. Int. J. Syst. Evol. Microbiol. 2001, 51, 565–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grischuk, Y.V.; Muntyan, M.S.; Popova, I.V.; Sorokin, D.Y. Ion transport coupled to terminal oxidase functioning in the extremely alkaliphilic halotolerant bacterium Thioalkalivibrio. Biochemistry 2003, 68, 385–390. [Google Scholar] [CrossRef]
- Muntyan, M.S.; Cherepanov, D.A.; Malinen, A.M.; Bloch, D.A.; Sorokin, D.Y.; Severina, I.I.; Ivashina, T.V.; Lahti, R.; Muyzer, G.; Skulachev, V.P. Cytochrome cbb3 of Thioalkalivibrio is a Na+-pumping cytochrome oxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 7695–7700. [Google Scholar] [CrossRef] [Green Version]
- Skulachev, V.P. Sodium bioenergetics. Trends Biochem. Sci. 1984, 9, 483–485. [Google Scholar] [CrossRef]
- Skulachev, V.P. Membrane-linked energy transductions. Bioenergetic functions of sodium: H+ is not unique as a coupling ion. Eur. J. Biochem. 1985, 151, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. The laws of cell energetics. Eur. J. Biochem. 1992, 208, 203–209. [Google Scholar] [CrossRef]
- Muntyan, M.S.; Morozov, D.A.; Klishin, S.S.; Khitrin, N.V.; Kolomijtseva, G.Y. Evaluation of the electrical potential on the membrane of the extremely alkaliphilic bacterium Thioalkalivibrio. Biochemistry 2012, 77, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Guffanti, A.A.; Krulwich, T.A. Oxidative phosphorylation by ADP + P(i)-loaded membrane vesicles of alkaliphilic Bacillus firmus OF4. J. Biol. Chem. 1994, 269, 21576–21582. [Google Scholar] [CrossRef]
- Slonczewski, J.L.; Fujisawa, M.; Dopson, M.; Krulwich, T.A. Cytoplasmic pH measurement and homeostasis in bacteria and archaea. Adv. Microb. Physiol. 2009, 55, 1–317. [Google Scholar] [CrossRef]
- Skulachev, V.P. Energetics of Biological Membranes; Severin, S.E., Ed.; Nauka: Moscow, Russia, 1989; 564p. [Google Scholar]
- Muntyan, M.S.; Morozov, D.A.; Leonova, Y.F.; Ovchinnikova, T.V. Identification of Na+-pumping Cytochrome Oxidase in the Membranes of Extremely Alkaliphilic Thioalkalivibrio Bacteria. Biochemistry 2020, 85, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Muyzer, G.; Sorokin, D.Y.; Mavromatis, K.; Lapidus, A.; Foster, B.; Sun, H.; Ivanova, N.; Pati, A.; D’haeseleer, P.; Woyke, T.; et al. Complete genome sequence of Thioalkalivibrio sp. K90mix. Stand. Genomic. Sci. 2011, 5, 341–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muntyan, M.S.; Morozov, D.A. Motility of natronophilic bacteria Thioalkalivibrio versutus. Nor. J. Dev. Int. Sci. 2016, 1, 17–19. [Google Scholar]
- Banciu, H.L.; Sorokin, D.Y.; Tourova, T.P.; Galinski, E.A.; Muntyan, M.S.; Kuenen, J.G.; Muyzer, G. Influence of salts and pH on growth and activity of a novel facultatively alkaliphilic, extremely salt-tolerant, obligately chemolithoautotrophic sufur-oxidizing Gammaproteobacterium Thioalkalibacter halophilus gen. nov., sp. nov. from South-Western Siberian soda lakes. Extremophiles 2008, 12, 391–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokin, D.Y.; Muntyan, M.S.; Panteleeva, A.N.; Muyzer, G. Thioalkalivibrio sulfidiphilus sp. nov., a haloalkaliphilic, sulfur-oxidizing gammaproteobacterium from alkaline habitats. Int. J. Syst. Evol. Microbiol. 2012, 62, 1884–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labelle, E.F.; Woodard, P.L.; Cragoe, E.J. The interaction of amiloride analogues with the Na+/H+ exchanger in kidney medulla microsomes. Biochim. Biophys. Acta 1984, 778, 129–138. [Google Scholar] [CrossRef]
- Sugiyama, S.; Cragoe, E.J.; Imae, Y. Amiloride, a specific inhibitor for the Na+-driven flagellar motors of alkalophilic Bacillus. J. Biol. Chem. 1988, 263, 8215–8219. [Google Scholar] [CrossRef]
- Atsumi, T.; Sugiyama, S.; Cragoe, E.J.; Imae, Y. Specific inhibition of the Na+-driven flagellar motors of alkalophilic Bacillus strains by the amiloride analog phenamil. J. Bacteriol. 1990, 172, 1634–1639. [Google Scholar] [CrossRef] [Green Version]
- Atsumi, T.; McCartert, L.; Imae, Y. Polar and lateral flagellar motors of marine Vibrio are driven by different ion-motive forces. Nature 1992, 355, 182–184. [Google Scholar] [CrossRef]
- Imae, Y.; Atsumi, T. Na+-driven bacterial flagellar motors. J. Bioenerg. Biomembr. 1989, 21, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Yamamoto, K.; Kawagishi, I.; Homma, M. The polar flagellar motor of Vibrio cholerae is driven by an Na+ motive force. J. Bacteriol. 1999, 181, 1927–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atsumi, T.; Maekawa, Y.; Tokuda, H.; Imae, Y. Amiloride at pH 7.0 inhibits the Na+-driven flagellar motors of Vibrio alginolyticus but allows cell growth. FEBS Lett. 1992, 314, 114–116. [Google Scholar] [CrossRef] [Green Version]
- Kawagishi, I.; Maekawa, Y.; Atsumi, T.; Homma, M.; Imae, Y. Isolation of the polar and lateral flagellum-defective mutants in Vibrio alginolyticus and identification of their flagellar driving energy sources. J. Bacteriol. 1995, 177, 5158–5160. [Google Scholar] [CrossRef] [Green Version]
- Muntyan, M.S.; Popova, I.V.; Bloch, D.A.; Skripnikova, E.V.; Ustiyan, V.S. Energetics of alkalophilic representatives of the genus Bacillus. Biochemistry 2005, 70, 137–142. [Google Scholar] [CrossRef]
- Rupprecht, A.; Sokolenko, E.A.; Beck, V.; Ninnemann, O.; Jaburek, M.; Trimbuch, T.; Klishin, S.S.; Jezek, P.; Skulachev, V.P.; Pohl, E.E. Role of the transmembrane potential in the membrane proton leak. Biophys. J. 2010, 98, 1503–1511. [Google Scholar] [CrossRef] [Green Version]
- Dimroth, P. Primary sodium ion translocating enzymes. Biochim. Biophys. Acta 1997, 1318, 11–51. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, H.; Unemoto, T. Na+ is translocated at NADH: Quinone oxidoreductase segment in the respiratory chain of Vibrio alginolyticus. J. Biol. Chem. 1984, 259, 7785–7790. [Google Scholar] [CrossRef]
- Laubinger, W.; Dimroth, P. Characterization of the ATP synthase of Propionigenium modestum as a primary sodium pump. Biochemistry 1988, 27, 7531–7537. [Google Scholar] [CrossRef]
- Reidlinger, J.; Müller, V. Purification of ATP synthase from Acetobacterium woodii and identification as a Na+-translocating F1FO-type enzyme. Eur. J. Biochem. 1994, 223, 275–283. [Google Scholar] [CrossRef]
- Murata, T.; Takase, K.; Yamato, I.; Igarashi, K.; Kakinuma, Y. Purification and reconstitution of Na+-translocating vacuolar ATPase from Enterococcus hirae. J. Biol. Chem. 1997, 272, 24885–24890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balnokin, Y.V.; Popova, L.G.; Andreev, I.M. Electrogenicity of the Na+-ATPase from the marine microalga Tetraselmis (Platymonas) viridis and associated H+ countertransport. FEBS Lett. 1999, 462, 402–406. [Google Scholar] [CrossRef] [Green Version]
- Popova, L.G.; Kornilova, A.G.; Shumkova, G.A.; Andreev, I.M.; Balnokin, Y.V. Na+-transporting ATPase in the plasma membrane of halotolerant microalga Dunaliella maritima operates as a Na+ uniporter. Russ. J. Plant Physiol. 2006, 53, 474–480. [Google Scholar] [CrossRef]
- Inoue, K.; Ono, H.; Abe-Yoshizumi, R.; Yoshizawa, S.; Ito, H.; Kogure, K.; Kandori, H. A light-driven sodium ion pump in marine bacteria. Nat. Commun. 2013, 4, 110. [Google Scholar] [CrossRef] [Green Version]
- Mamedov, M.D.; Mamedov, A.M.; Bertsova, Y.V.; Bogachev, A.V. A single mutation converts bacterial Na+-transporting rhodopsin into an H+ transporter. FEBS Lett. 2016, 590, 2827–2835. [Google Scholar] [CrossRef]
- Chernyak, B.V.; Dibrov, P.A.; Glagolev, A.N.; Sherman, M.Y.; Skulachev, V.P. A novel type of energetics in a marine alkali-tolerant bacterium: ΔpNa-driven motility and sodium cycle. FEBS Lett. 1983, 164, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.P.; Chambers, L.A.; Trudinger, P.A. Cyanolysis and spectrophotometric estimation of trithionate in mixture with thiosulphate and tetrathionate. Anal. Chem. 1969, 41, 898–901. [Google Scholar] [CrossRef]
- Muntyan, M.S.; Skripnikova, E.V. Two types of terminal oxidase in alkalotolerant Bacillus FTU. Biochim. Biophys. Acta 1993, 1143, 142–146. [Google Scholar] [CrossRef]
- Sone, N.; Fujiwara, Y. Effects of Aeration during Growth of Bacillus stearothermophilus on Proton Pumping Activity and Change of Terminal Oxidases. J. Biochem. 1991, 110, 1016–1021. [Google Scholar] [CrossRef]
- Muntyan, M.S.; Ustiyan, V.S.; Viryasov, M.B.; Skulachev, V.P. Terminal Oxidases of the bb- and caa3-Types in Bacillus sp. FTU. Biochem. Biophys. Res. Commun. 1995, 207, 55–61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntyan, M.S.; Viryasov, M.B.; Sorokin, D.Y.; Skulachev, V.P. Sodium Energetic Cycle in the Natronophilic Bacterium Thioalkalivibrio versutus. Int. J. Mol. Sci. 2022, 23, 1965. https://doi.org/10.3390/ijms23041965
Muntyan MS, Viryasov MB, Sorokin DY, Skulachev VP. Sodium Energetic Cycle in the Natronophilic Bacterium Thioalkalivibrio versutus. International Journal of Molecular Sciences. 2022; 23(4):1965. https://doi.org/10.3390/ijms23041965
Chicago/Turabian StyleMuntyan, Maria S., Mikhail B. Viryasov, Dimitry Y. Sorokin, and Vladimir P. Skulachev. 2022. "Sodium Energetic Cycle in the Natronophilic Bacterium Thioalkalivibrio versutus" International Journal of Molecular Sciences 23, no. 4: 1965. https://doi.org/10.3390/ijms23041965
APA StyleMuntyan, M. S., Viryasov, M. B., Sorokin, D. Y., & Skulachev, V. P. (2022). Sodium Energetic Cycle in the Natronophilic Bacterium Thioalkalivibrio versutus. International Journal of Molecular Sciences, 23(4), 1965. https://doi.org/10.3390/ijms23041965






