Membrane Vesicles for Nanoencapsulated Sulforaphane Increased Their Anti-Inflammatory Role on an In Vitro Human Macrophage Model
Abstract
:1. Introduction
2. Results
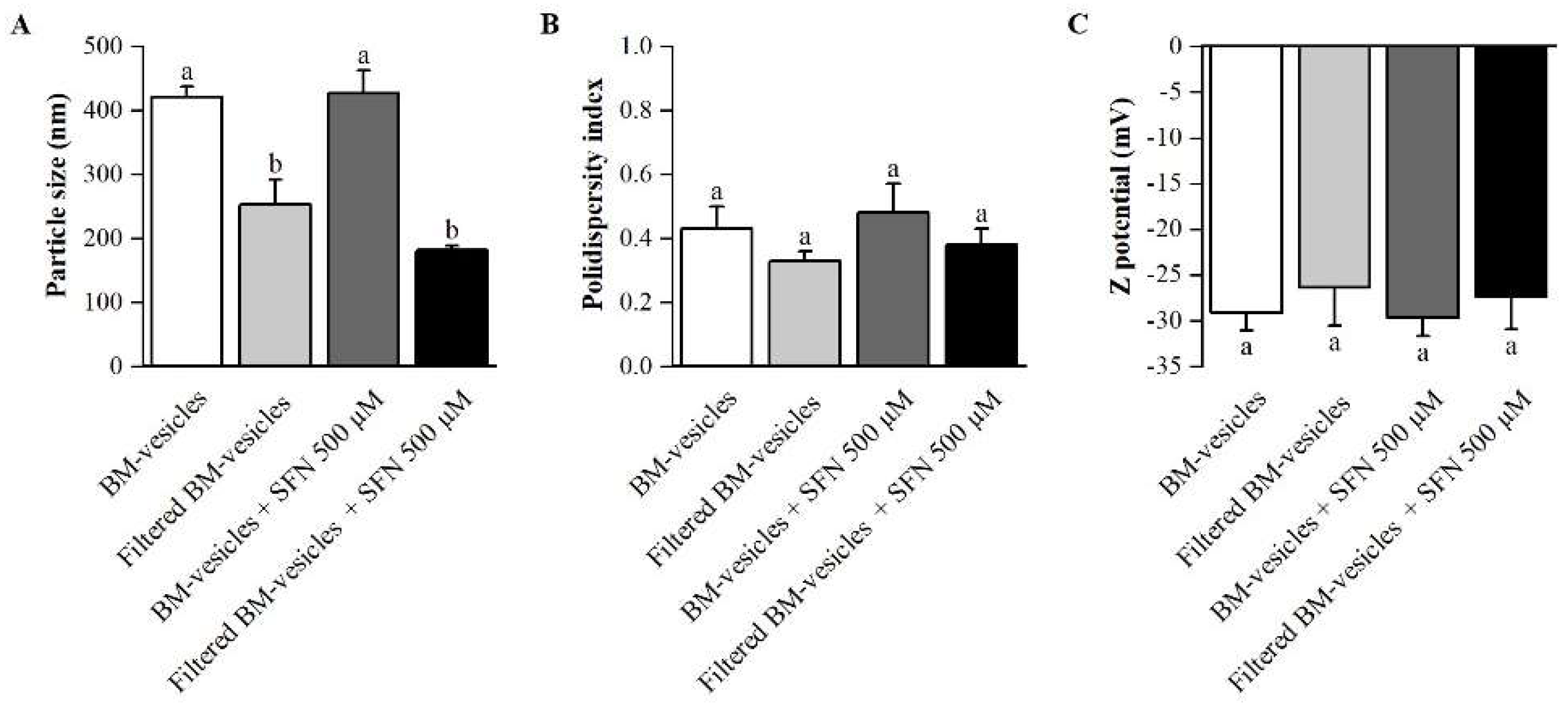
2.1. Formulation Development
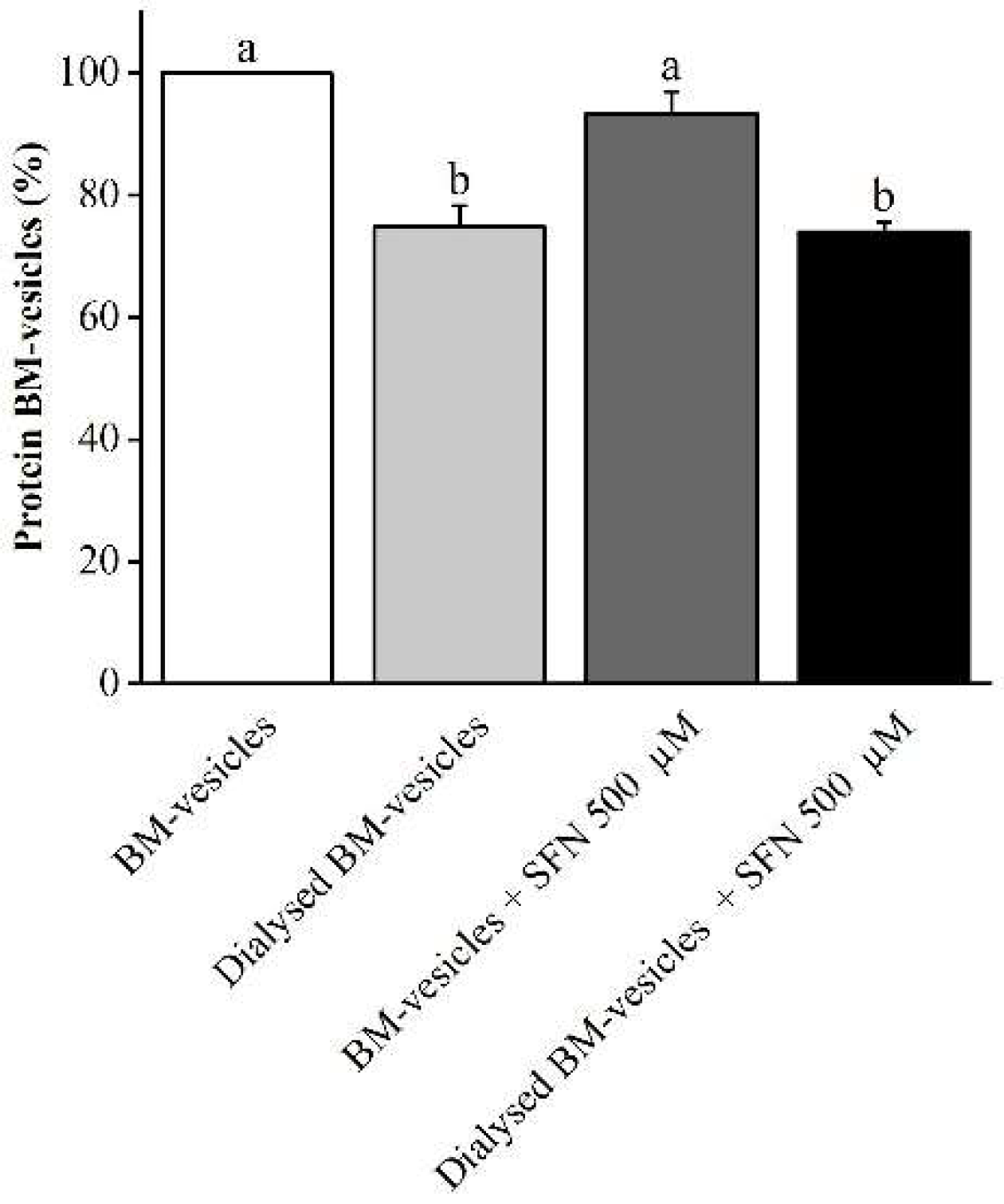
2.2. Drug Encapsulation Studies
2.3. SFN Quantification in Unloaded and SFN-Loaded BM-Vesicles
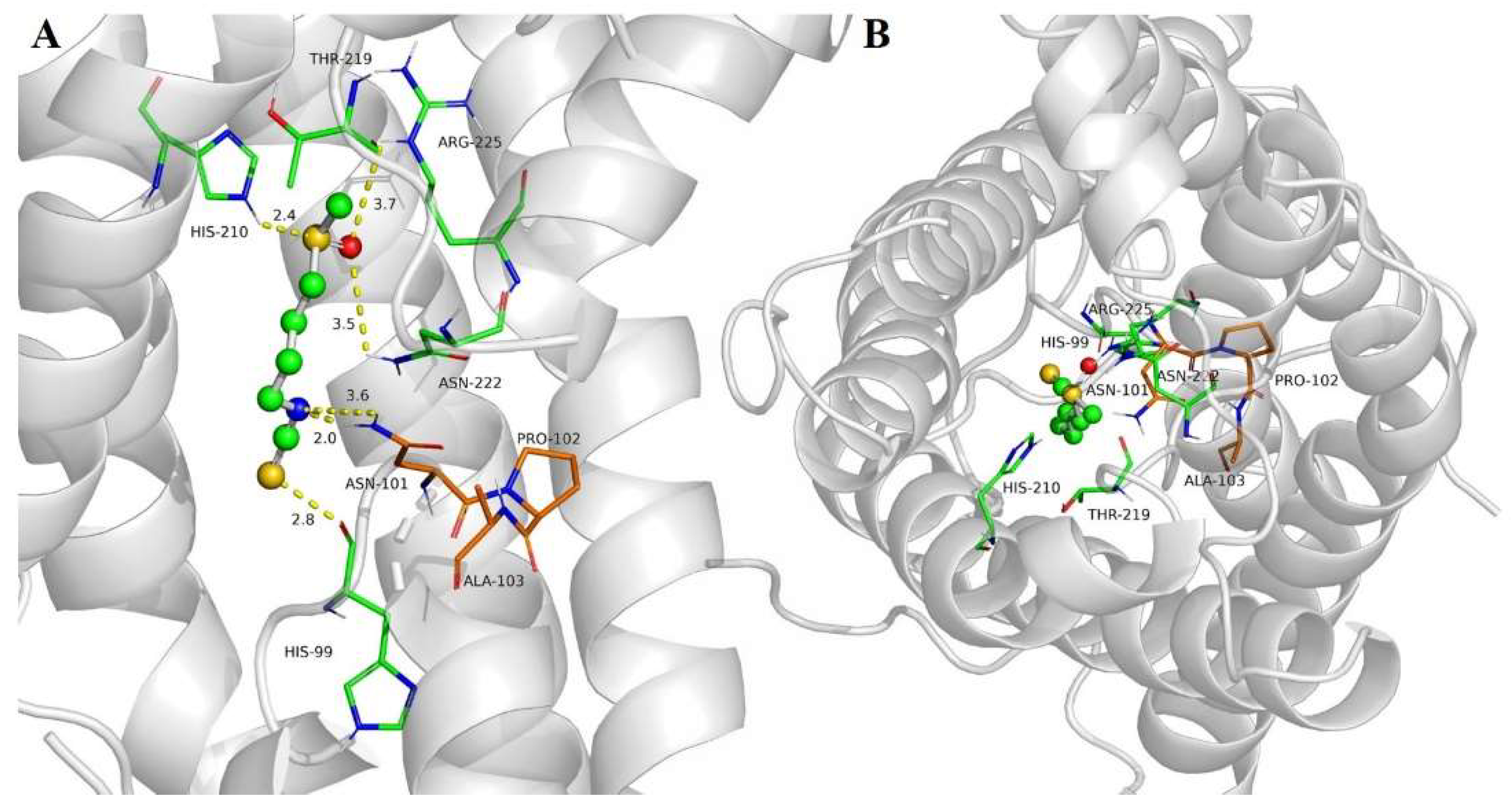
2.4. SFN Interaction with SoPIP2;1 Aquaporin
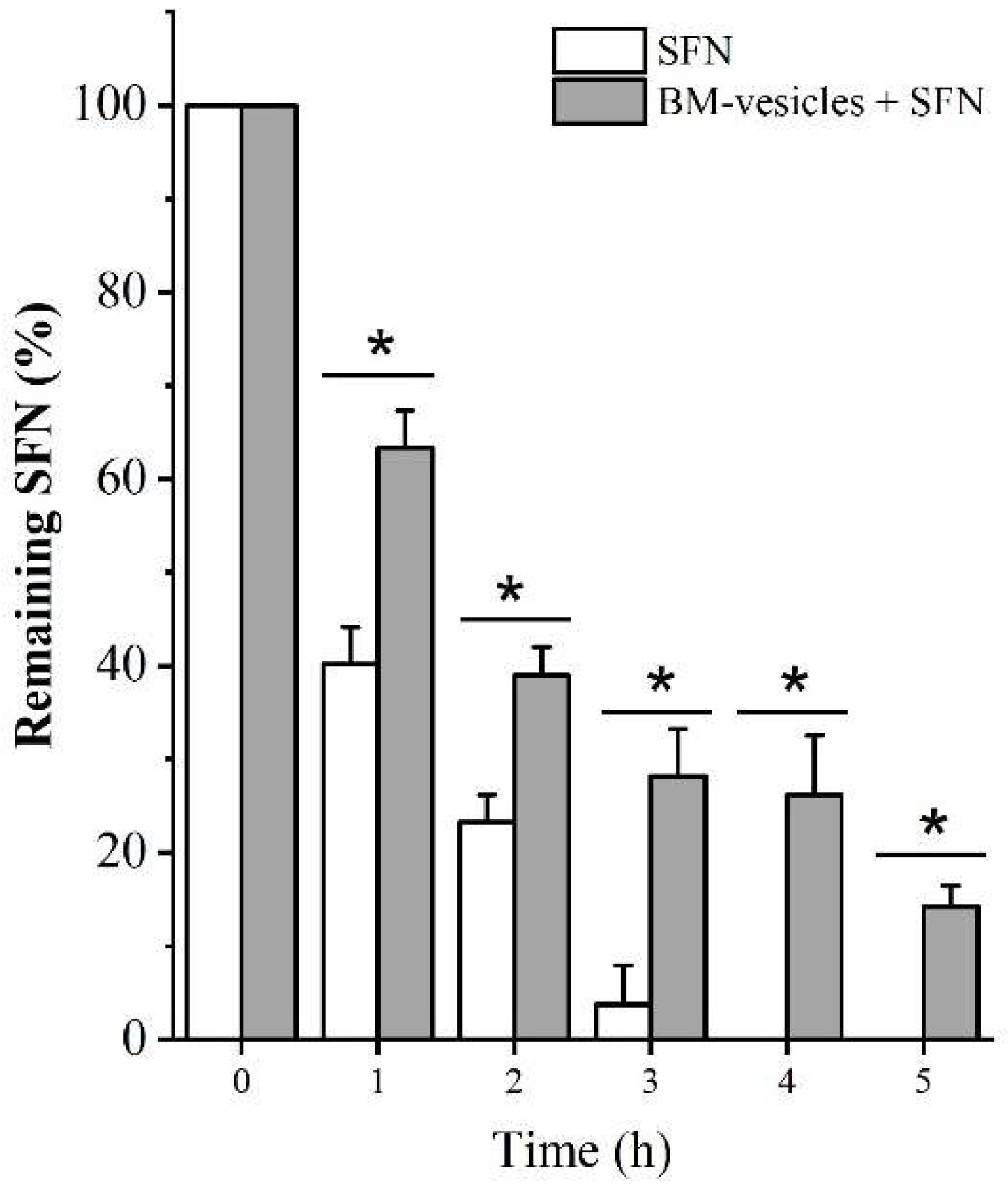
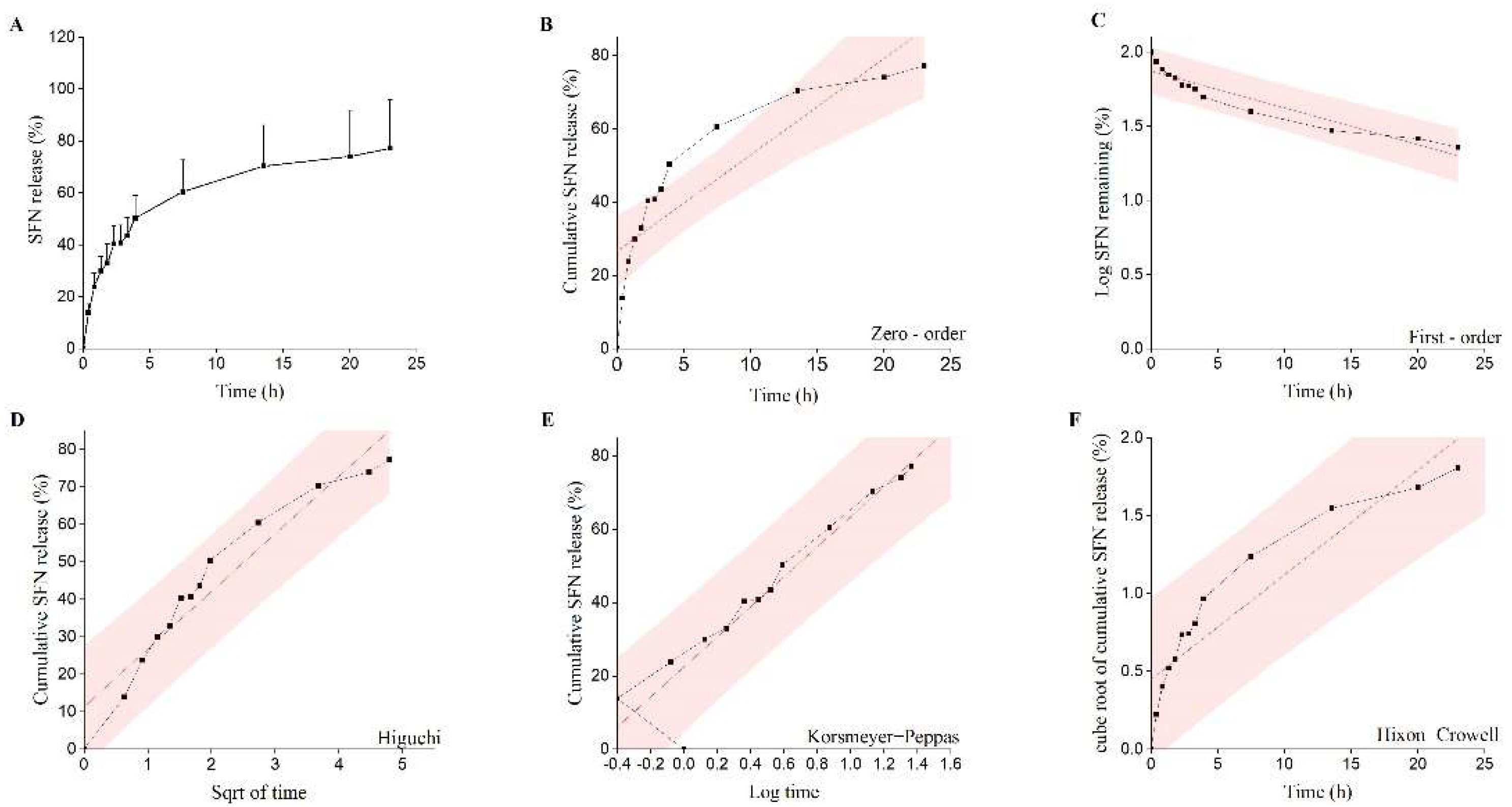
2.5. In Vitro Release Studies
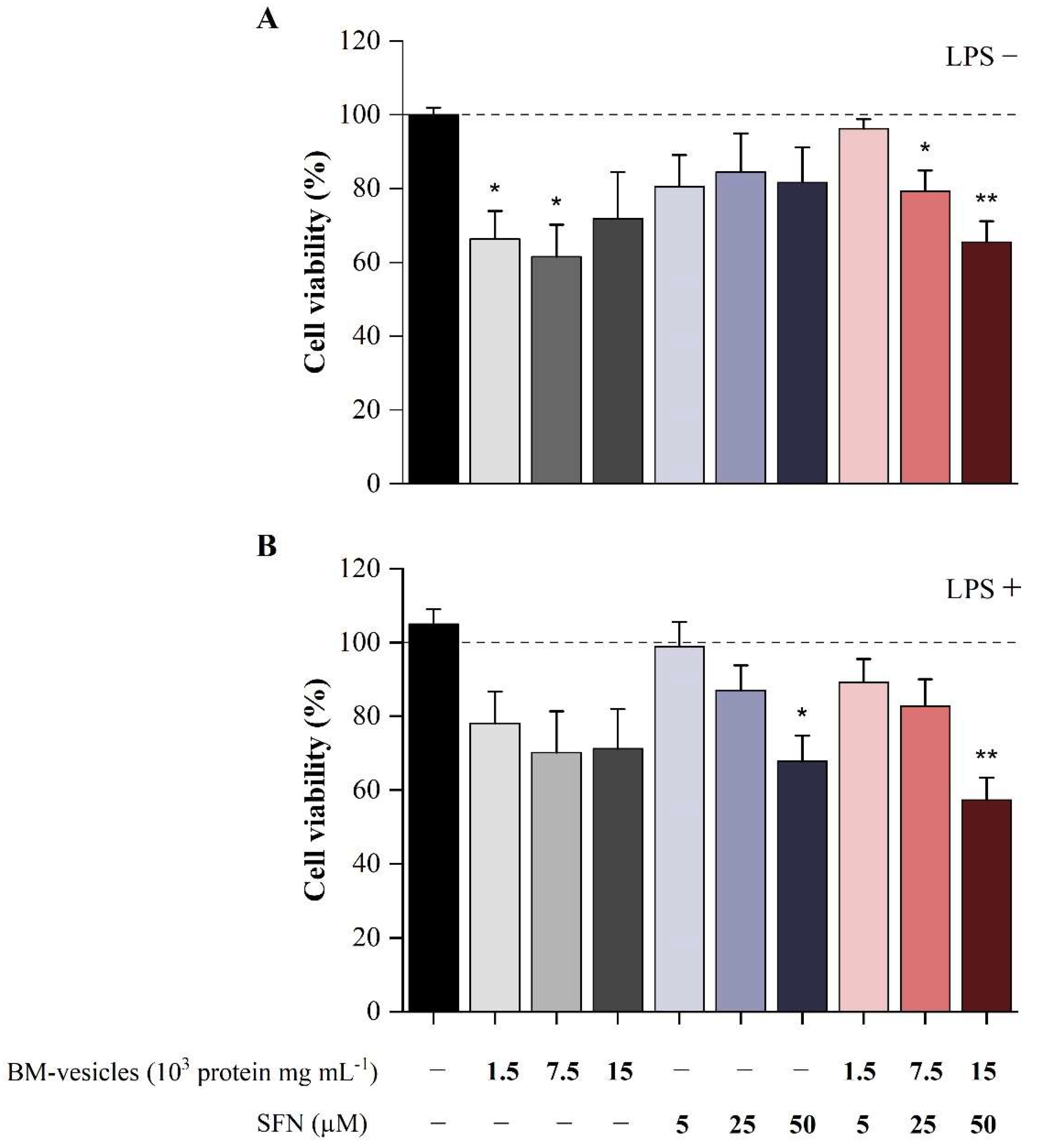
2.6. Effect of BM-Vesicles and SFN on Cell Viability
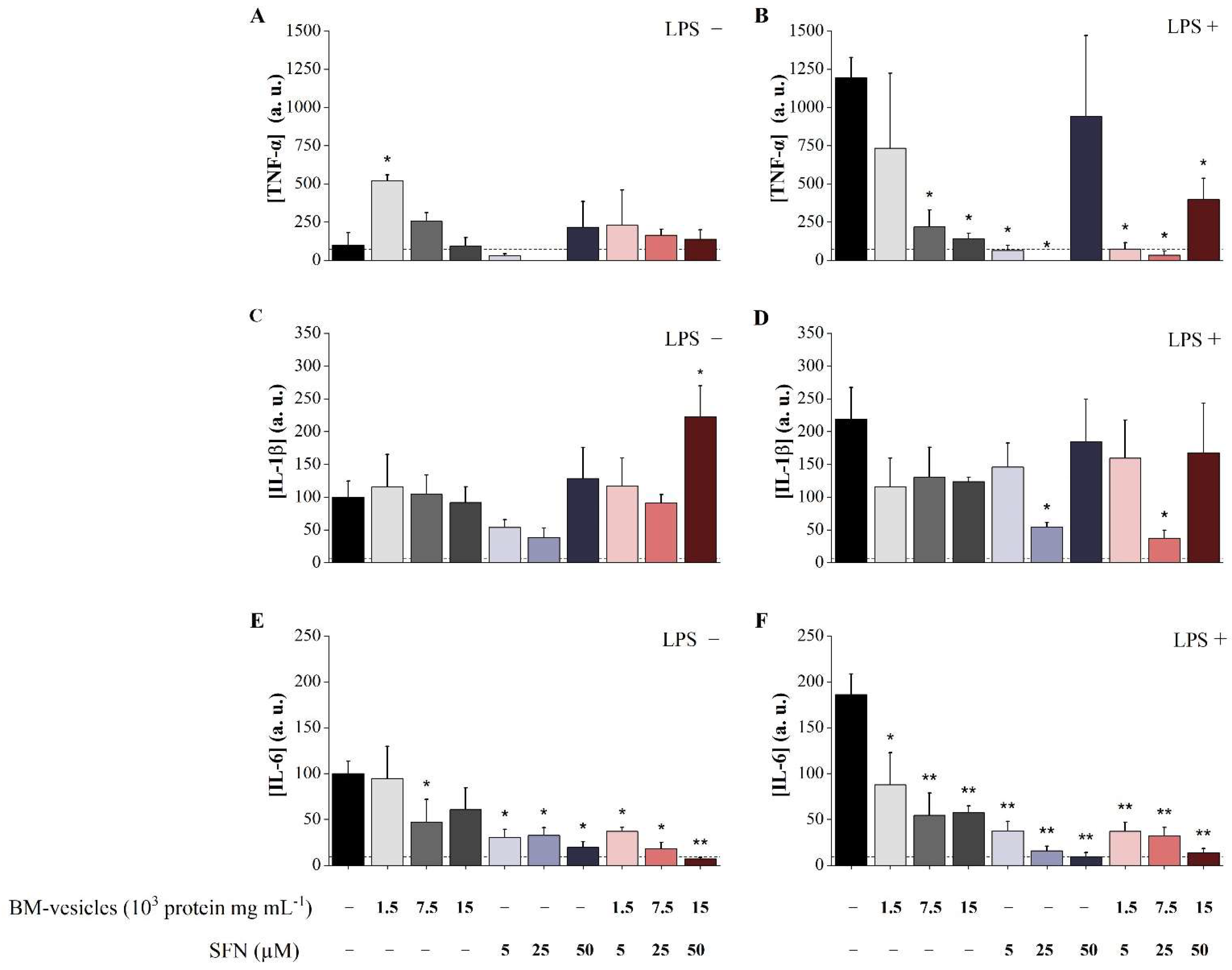
2.7. Analysis of Anti-Inflammatory Potential of Unloaded BM-Vesicles, SFN and SFN-Loaded BM-Vesicles
3. Discussion
4. Materials and Methods
4.1. Development of SFN-Loaded BM-Vesicles
4.1.1. Plant Culture
4.1.2. BM-Vesicles Isolation and SFN Loading
4.2. Physicochemical Characterization
4.2.1. Determination of Entrapment Efficiency of SFN
4.2.2. Particle Size, Zeta Potential and Polydispersity Index Analysis of BM-Vesicles and SFN-Loaded BM-Vesicles
4.2.3. In Vitro Drug Release Study
4.3. Sulforaphane–Aquaporin Binding Studies
4.4. Cell Culture Assays
4.4.1. Preparation of the Compounds to Be Tested in Cell Culture
- Free SFN: (a) 5 µM, (b) 25 µM and (c) 50 µM.
- BM-vesicles: (a) 0.0005 protein mg mL−1, (b) 0.0025 protein mg mL−1 and (c) 0.005 protein mg mL−1.
- SFN-loaded BM-vesicles: (a) SFN 5 µM + BM-vesicles 0.0005 protein mg mL−1, (b) SFN 25 µM + BM-vesicles 0.0025 protein mg mL−1 and (c) SFN 50 µM + BM-vesicles 0.005 protein mg mL−1.
4.4.2. Cell Culture and Differentiation
4.4.3. Cell Stimulation with LPS and Treatment Application
4.4.4. Cell Viability Assay
4.4.5. Cytokine Production Assays
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couzin-Frankel, J. Inflammation bares a dark side. Science 2010, 330, 1621. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Kotas, M.E.; Medzhitov, R. Homeostasis, Inflammation, and Disease Susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [Green Version]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Fine, M. Quantifying the impact of NSAID-associated adverse events. Am. J. Manag. Care 2013, 19, s267–s272. [Google Scholar] [PubMed]
- Martínez-Esparza, M.; Tristán-Manzano, M.; Ruiz-Alcaraz, A.J.; García-Peñarrubia, P. Inflammatory status in human hepatic cirrhosis. World J. Gastroenterol. 2015, 21, 11522–11541. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, T.B.; Xavier, R.J.; Schreiber, S.L.; Shamji, A.F. Small-molecule control of cytokine function: New opportunities for treating immune disorders. Curr. Opin. Chem. Biol. 2014, 23, 23–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapia-Abellán, A.; Ruiz-Alcaraz, A.J.; Hernández-Caselles, T.; Such, J.; Francés, R.; García-Peñarrubia, P.; Martínez-Esparza, M. Role of MAP Kinases and PI3K-Akt on the cytokine inflammatory profile of peritoneal macrophages from the ascites of cirrhotic patients. Liver Int. 2013, 33, 552–560. [Google Scholar] [CrossRef]
- Tapia-Abellán, A.; Ruiz-Alcaraz, A.J.; Antón, G.; Miras-López, M.; Francés, R.; Such, J.; Martínez-Esparza, M.; García-Peñarrubia, P. Regulatory role of PI3K-protein kinase B on the release of interleukin-1β in peritoneal macrophages from the ascites of cirrhotic patients. Clin. Exp. Immunol. 2014, 178, 525–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- García-Ibañez, P.; Yepes-Molina, L.; Ruiz-Alcaraz, A.J.; Martínez-Esparza, M.; Moreno, D.A.; Carvajal, M.; García-Peñarrubia, P. Brassica bioactives could ameliorate the chronic inflammatory condition of endometriosis. Int. J. Mol. Sci. 2020, 21, 9397. [Google Scholar] [CrossRef]
- Mi ekus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef] [PubMed]
- Román, J.; Castillo, A.; Cottet, L.; Mahn, A. Kinetic and structural study of broccoli myrosinase and its interaction with different glucosinolates. Food Chem. 2018, 254, 87–94. [Google Scholar] [CrossRef] [PubMed]
- López-Chillón, M.T.; Carazo-Díaz, C.; Prieto-Merino, D.; Zafrilla, P.; Moreno, D.A.; Villaño, D. Effects of long-term consumption of broccoli sprouts on inflammatory markers in overweight subjects. Clin. Nutr. 2018, 38, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Kao, C.L.; Liu, C.M. The cancer prevention, anti-inflammatory and anti-oxidation of bioactive phytochemicals targeting the TLR4 signaling pathway. Int. J. Mol. Sci. 2018, 19, 2729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [Green Version]
- Eren, E.; Tufekci, K.U.; Isci, K.B.; Tastan, B.; Genc, K.; Genc, S. Sulforaphane inhibits lipopolysaccharide-induced inflammation, cytotoxicity, oxidative stress, and miR-155 expression and switches to Mox phenotype through activating extracellular signal-regulated kinase 1/2-nuclear factor erythroid 2-related factor 2/an. Front. Immunol. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer activity of sulforaphane: The epigenetic mechanisms and the Nrf2 signaling pathway. Oxid. Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef] [PubMed]
- Bayat Mokhtari, R.; Baluch, N.; Homayouni, T.S.; Morgatskaya, E.; Kumar, S.; Kazemi, P.; Yeger, H. The role of Sulforaphane in cancer chemoprevention and health benefits: A mini-review. J. Cell Commun. Signal. 2018, 12, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.J.; Dickinson, S.E.; Karlage, K.L.; Bowden, G.T.; Myrdal, P.B. Stability of sulforaphane for topical formulation. Drug Dev. Ind. Pharm. 2014, 40, 494–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, V.; Bustos, R.; Mahn, A. Insights about stabilization of sulforaphane through microencapsulation. Heliyon 2019, 5, e02951. [Google Scholar] [CrossRef] [PubMed]
- Do, D.P.; Pai, S.B.; Rizvi, S.A.A.; D’Souza, M.J. Development of sulforaphane-encapsulated microspheres for cancer epigenetic therapy. Int. J. Pharm. 2010, 386, 114–121. [Google Scholar] [CrossRef]
- Cristiano, M.C.; Froiio, F.; Spaccapelo, R.; Mancuso, A.; Nisticò, S.P.; Udongo, B.P.; Fresta, M.; Paolino, D. Sulforaphane-loaded ultradeformable vesicles as a potential natural nanomedicine for the treatment of skin cancer diseases. Pharmaceutics 2020, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Sahoo, A.K.; Konkimalla, V.B.; Pal, A.; Si, S.C. Naringin in combination with isothiocyanates as liposomal formulations potentiates the anti-inflammatory activity in different acute and chronic animal models of rheumatoid arthritis. ACS Omega 2020, 5, 28319–28332. [Google Scholar] [CrossRef]
- Fattal, E.; Hillaireau, H.; Mura, S.; Nicolas, J.; Tsapis, N. Targeted delivery using biodegradable polymeric nanoparticles. In Fundamentals and Applications of Controlled Release Drug Delivery; Springer: Boston, MA, USA, 2012; pp. 255–288. ISBN 9781461408819. [Google Scholar]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 3702518. [Google Scholar] [CrossRef]
- Martínez Ballesta, M.C.; García-Gomez, P.; Yepes-Molina, L.; Guarnizo, A.L.; Teruel, J.A.; Carvajal, M. Plasma membrane aquaporins mediates vesicle stability in broccoli. PLoS ONE 2018, 13, e0192422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, M.; Zhao, X.; Xing, H.; Xun, Z.; Yang, T.; Cai, C.; Wang, D.; Ding, P. Liposome-chaperoned cell-free synthesis for the design of proteoliposomes: Implications for therapeutic delivery. Acta Biomater. 2018, 76, 1–20. [Google Scholar] [CrossRef]
- Martínez Ballesta, M.C.; Pérez-Sánchez, H.; Moreno, D.A.; Carvajal, M. Plant plasma membrane aquaporins in natural vesicles as potential stabilizers and carriers of glucosinolates. Colloids Surf. B Biointerfaces 2016, 143, 318–326. [Google Scholar] [CrossRef]
- Seneviratne, R.; Khan, S.; Moscrop, E.; Rappolt, M.; Muench, S.P.; Jeuken, L.J.C.; Beales, P.A. A reconstitution method for integral membrane proteins in hybrid lipid-polymer vesicles for enhanced functional durability. Methods 2018, 147, 142–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.B.; Wang, B.; Zhang, L.; et al. Grape exosome-like nanoparticles induce intestinal stem cells and protect mice from DSS-induced colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yepes-Molina, L.; Hernández, J.A.; Carvajal, M. Nanoencapsulation of Pomegranate Extract to Increase Stability and Potential Dermatological Protection. Pharmaceutics 2021, 13, 271. [Google Scholar] [CrossRef]
- Yepes-Molina, L.; Martínez-Ballesta, M.C.; Carvajal, M. Plant plasma membrane vesicles interaction with keratinocytes reveals their potential as carriers. J. Adv. Res. 2020, 23, 101–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Zhang, W.; Tang, Q.; Zhou, Y.; Li, Y.; Rong, T.; Wang, H.; Chen, Y. Isolated cell-bound membrane vesicles (CBMVs) as a novel class of drug nanocarriers. J. Nanobiotechnology 2020, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Yepes-Molina, L.; Carvajal, M. Nanoencapsulation of sulforaphane in broccoli membrane vesicles and their in vitro antiproliferative activity. Pharm. Biol. 2021, 59, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Garcia-Ibañez, P.; Carvajal, M. The use of biovesicles to improve the efficiency of Zn foliar fertilization. Colloids Surf. B Biointerfaces 2019, 173, 899–905. [Google Scholar] [CrossRef]
- Rios, J.J.; Yepes-Molina, L.; Martinez-Alonso, A.; Carvajal, M. Nanobiofertilization as a novel technology for highly efficient foliar application of Fe and B in almond trees. R. Soc. Open Sci. 2020, 7, 200905. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ibañez, P.; Roses, C.; Agudelo, A.; Milagro, F.I.; Barceló, A.M.; Viadel, B.; Nieto, J.A.; Moreno, D.A.; Carvajal, M. The influence of red cabbage extract nanoencapsulated with brassica plasma membrane vesicles on the gut microbiome of obese volunteers. Foods 2021, 10, 1038. [Google Scholar] [CrossRef]
- Chalbi, N.; Martínez-Ballesta, M.C.; Youssef, N.B.; Carvajal, M. Intrinsic stability of Brassicaceae plasma membrane in relation to changes in proteins and lipids as a response to salinity. J. Plant Physiol. 2015, 175, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The 1,2-Benzenedithiole-Based Cyclocondensation Assay: A Valuable Tool for the Measurement of Chemopreventive Isothiocyanates. Crit. Rev. Food Sci. Nutr. 2012, 52, 525–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.; He, Y.; Liang, J.; Cheng, Z.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.; Wang, J. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef]
- Vallar, S.; Houivet, D.; El Fallah, J.; Kervadec, D.; Haussonne, J.M. Oxide slurries stability and powders dispersion: Optimization with zeta potential and rheological measurements. J. Eur. Ceram. Soc. 1999, 19, 1017–1021. [Google Scholar] [CrossRef]
- Kohli, A.K.; Alpar, H.O. Potential use of nanoparticles for transcutaneous vaccine delivery: Effect of particle size and charge. Int. J. Pharm. 2004, 275, 13–17. [Google Scholar] [CrossRef]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of positively and negatively charged liposomes on skin permeation of drugs. J. Drug Target. 2001, 9, 49–59. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef]
- Wacker, M.G. Challenges in the drug release testing of next-generation nanomedicines—What do we know? Mater. Today Proc. 2017, 2, 214–217. [Google Scholar] [CrossRef]
- Woo, B.H.; Kostanski, J.W.; Gebrekidan, S.; Dani, B.A.; Thanoo, B.C.; DeLuca, P.P. Preparation, characterization and in vivo evaluation of 120-day poly(D,L-lactide) leuprolide microspheres. J. Control. Release 2001, 75, 307–315. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Haghiralsadat, F.; Amoabediny, G.; Helder, M.N.; Naderinezhad, S.; Sheikhha, M.H.; Forouzanfar, T.; Zandieh-Doulabi, B. A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 169–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaili, Z.; Bayrami, S.; Dorkoosh, F.A.; Akbari Javar, H.; Seyedjafari, E.; Zargarian, S.S.; Haddadi-Asl, V. Development and characterization of electrosprayed nanoparticles for encapsulation of Curcumin. J. Biomed. Mater. Res. Part A 2018, 106, 285–292. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. Multipronged, strategic delivery of paclitaxel-topotecan using engineered liposomes to ovarian cancer. Drug Dev. Ind. Pharm. 2016, 42, 136–149. [Google Scholar] [CrossRef]
- Lapenda, T.L.S.; Morais, W.A.; Almeida, F.J.F.; Ferraz, M.S.; Lira, M.C.B.; Santos, N.P.S.; MacIel, M.A.M.; Santos-Magalhães, N.S. Encapsulation of trans-dehydrocrotonin in liposomes: An enhancement of the antitumor activity. J. Biomed. Nanotechnol. 2013, 9, 499–510. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.X.; Luo, Z.G.; Tamer, T.M. Co-encapsulation of Vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef]
- Tyerman, S.D.; McGaughey, S.A.; Qiu, J.; Yool, A.J.; Byrt, C.S. Adaptable and Multifunctional Ion-Conducting Aquaporins. Annu. Rev. Plant Biol. 2021, 72, 703–736. [Google Scholar] [CrossRef]
- Abassi, P.; Abassi, F.; Yari, F.; Hashemi, M.; Nafisi, S. Study on the interaction of sulforaphane with human and bovine serum albumins. J. Photochem. Photobiol. B Biol. 2013, 122, 61–67. [Google Scholar] [CrossRef]
- Hung, Y.L.; Suzuki, K. The pattern recognition receptors and lipopolysaccharides (LPS)-induced systemic inflammation. Int. J. Res. Stud. Med. Health Sci. 2017, 2, 1–7. [Google Scholar]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [Green Version]
- Zariwala, M.G.; Bendre, H.; Markiv, A.; Farnaud, S.; Renshaw, D.; Taylor, K.M.G.; Somavarapu, S. Hydrophobically modified chitosan nanoliposomes for intestinal drug delivery. Int. J. Nanomed. 2018, 13, 5837–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varypataki, E.M.; van der Maaden, K.; Bouwstra, J.; Ossendorp, F.; Jiskoot, W. Cationic Liposomes Loaded with a Synthetic Long Peptide and Poly(I:C): A Defined Adjuvanted Vaccine for Induction of Antigen-Specific T Cell Cytotoxicity. AAPS J. 2015, 17, 216–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear Factor κB Is a Molecular Target for Sulforaphane-mediated Anti-inflammatory Mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, J.E.; Park, Z.Y.; Kim, N.D.; Lee, J.Y. Sulforaphane inhibits the engagement of LPS with TLR4/MD2 complex by preferential binding to Cys133 in MD2. Biochem. Biophys. Res. Commun. 2013, 434, 600–605. [Google Scholar] [CrossRef]
- Verma, J.N.; Wassef, N.M.; Wirtz, R.A.; Atkinson, C.T.; Aikawa, M.; Loomis, L.D.; Alving, C.R. Phagocytosis of liposomes by macrophages: Intracellular fate of liposomal malaria antigen. Biochim. Biophys. Acta 1991, 1066, 229–238. [Google Scholar] [CrossRef]
- Solomon, D.; Gupta, N.; Mulla, N.S.; Shukla, S.; Guerrero, Y.A.; Gupta, V. Role of In Vitro Release Methods in Liposomal Formulation Development: Challenges and Regulatory Perspective. AAPS J. 2017, 19, 1669–1681. [Google Scholar] [CrossRef]
- Schroeter, A.; Engelbrecht, T.; Neubert, R.H.H.; Goebel, A.S.B. New nanosized technologies for dermal and transdermal drug delivery. A review. J. Biomed. Nanotechnol. 2010, 6, 511–528. [Google Scholar] [CrossRef]
- Chaudhary, H.; Kohli, K.; Kumar, V. A novel nano-carrier transdermal gel against inflammation. Int. J. Pharm. 2014, 465, 175–186. [Google Scholar] [CrossRef]
- Xi, H.; Cun, D.; Xiang, R.; Guan, Y.; Zhang, Y.; Li, Y.; Fang, L. Intra-articular drug delivery from an optimized topical patch containing teriflunomide and lornoxicam for rheumatoid arthritis treatment: Does the topical patch really enhance a local treatment? J. Control. Release 2013, 169, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Boakye, C.H.A.; Patel, K.; Doddapaneni, R.; Bagde, A.; Marepally, S.; Singh, M. Novel amphiphilic lipid augments the co-delivery of erlotinib and IL36 siRNA into the skin for psoriasis treatment. J. Control. Release 2017, 246, 120–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ong, S.G.M.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem substance and compound databases. Nucleic Acids Res. 2016, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Frick, A.; Järvå, M.; Ekvall, M.; Uzdavinys, P.; Nyblom, M.; Törnroth-Horsefield, S. Mercury increases water permeability of a plant aquaporin through a non-cysteine-related mechanism. Biochem. J. 2013, 454, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [Green Version]
- Sanner, M.F. Python: A programming language for software integration and development. J. Mol. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A Semiempirical Free Energy Force Field with Charge-Based Desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System, Version 2.3; Schrödinger LLC: New York, NY, USA, 2020. [Google Scholar]
- Islam, S.U.; Lee, J.H.; Shehzad, A.; Ahn, E.M.; Lee, Y.M.; Lee, Y.S. Decursinol angelate inhibits LPS-induced macrophage polarization through modulation of the NFκB and MAPK signaling pathways. Molecules 2018, 23, 1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
| ITCs (µM) | |||
|---|---|---|---|
| Pre-dialysis | Post-dialysis | ||
| BM-vesicles | 98.22 ± 6.79 | 91.45 ± 10.28 | n.s. |
| BM-vesicles + SFN 500 µM | 495.59 ± 10.51 | 285.81 ± 13.95 | * |
| ITCs (µmol mg protein−1) | |||
| Pre-dialysis | Post-dialysis | ||
| BM-vesicles | 0.67 ± 0.04 | 0.79 ± 0.09 | n.s. |
| BM-vesicles + SFN 500 µM | 3.19 ± 0.07 | 2.46 ± 0.12 | * |
| Release Model | Equation | R2 | η |
|---|---|---|---|
| Zero-order model | Mt/M∞ = K0t+ C | 0.746 | |
| First-order model | ln(1 − Mt/M∞) = K1t + C | 0.727 | - |
| Higuchi model | Mt/M∞ = KHt1/2 + C | 0.847 | - |
| Hixon−Crowell model | (1 − Mt/M∞)1/3 = KSt + C | 0.601 | - |
| Korsmeyer−Peppas model | Mt/M∞ = KKtη | 0.976 | 0.33 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yepes-Molina, L.; Pérez-Jiménez, M.I.; Martínez-Esparza, M.; Teruel, J.A.; Ruiz-Alcaraz, A.J.; García-Peñarrubia, P.; Carvajal, M. Membrane Vesicles for Nanoencapsulated Sulforaphane Increased Their Anti-Inflammatory Role on an In Vitro Human Macrophage Model. Int. J. Mol. Sci. 2022, 23, 1940. https://doi.org/10.3390/ijms23041940
Yepes-Molina L, Pérez-Jiménez MI, Martínez-Esparza M, Teruel JA, Ruiz-Alcaraz AJ, García-Peñarrubia P, Carvajal M. Membrane Vesicles for Nanoencapsulated Sulforaphane Increased Their Anti-Inflammatory Role on an In Vitro Human Macrophage Model. International Journal of Molecular Sciences. 2022; 23(4):1940. https://doi.org/10.3390/ijms23041940
Chicago/Turabian StyleYepes-Molina, Lucía, María Isabel Pérez-Jiménez, María Martínez-Esparza, José A. Teruel, Antonio J. Ruiz-Alcaraz, Pilar García-Peñarrubia, and Micaela Carvajal. 2022. "Membrane Vesicles for Nanoencapsulated Sulforaphane Increased Their Anti-Inflammatory Role on an In Vitro Human Macrophage Model" International Journal of Molecular Sciences 23, no. 4: 1940. https://doi.org/10.3390/ijms23041940
APA StyleYepes-Molina, L., Pérez-Jiménez, M. I., Martínez-Esparza, M., Teruel, J. A., Ruiz-Alcaraz, A. J., García-Peñarrubia, P., & Carvajal, M. (2022). Membrane Vesicles for Nanoencapsulated Sulforaphane Increased Their Anti-Inflammatory Role on an In Vitro Human Macrophage Model. International Journal of Molecular Sciences, 23(4), 1940. https://doi.org/10.3390/ijms23041940








