Male Fertility under Environmental Stress: Do Polyamines Act as Pollen Tube Growth Protectants?
Abstract
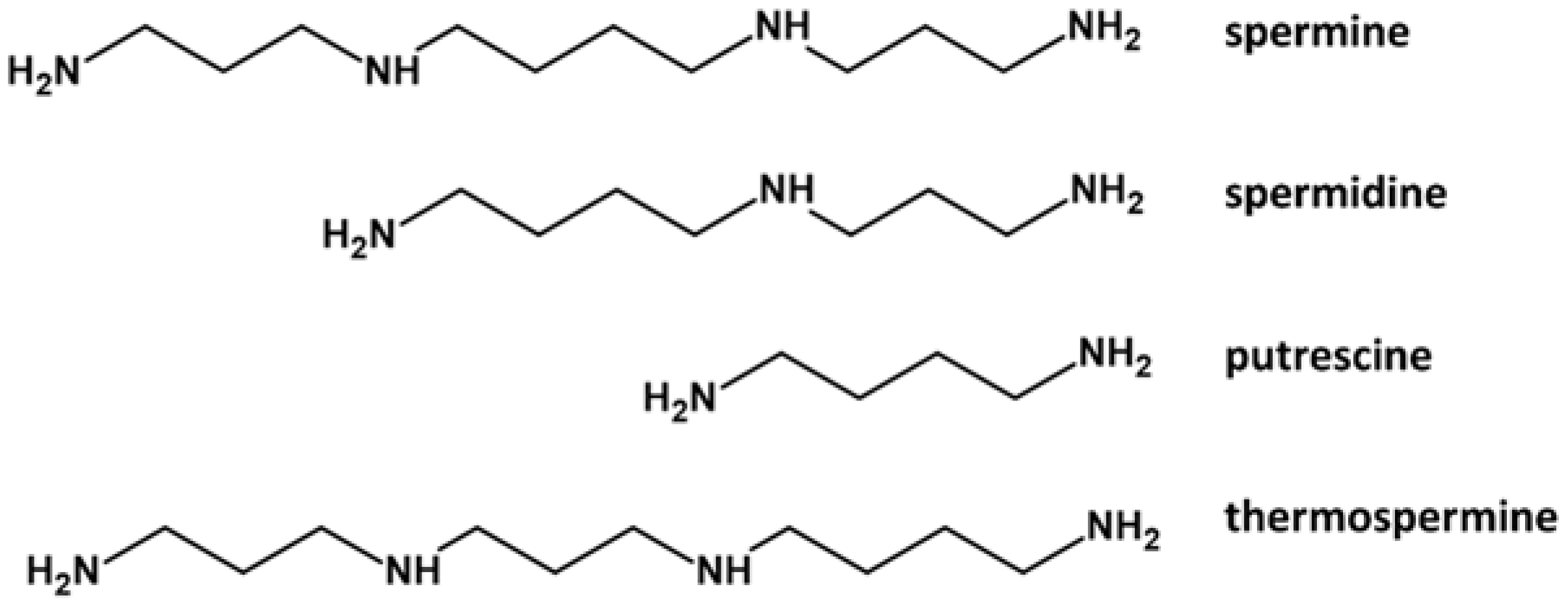
1. Polyamines in Pollen Development
2. Effects of Environmental Stress on Pollen Tube Growth
2.1. Heat Stress
2.2. Heavy Metal-Induced Stress
2.3. Light (UV-B) Stress
2.4. Osmotic Stress
2.5. Nutrient Depletion
2.6. Cold Stress
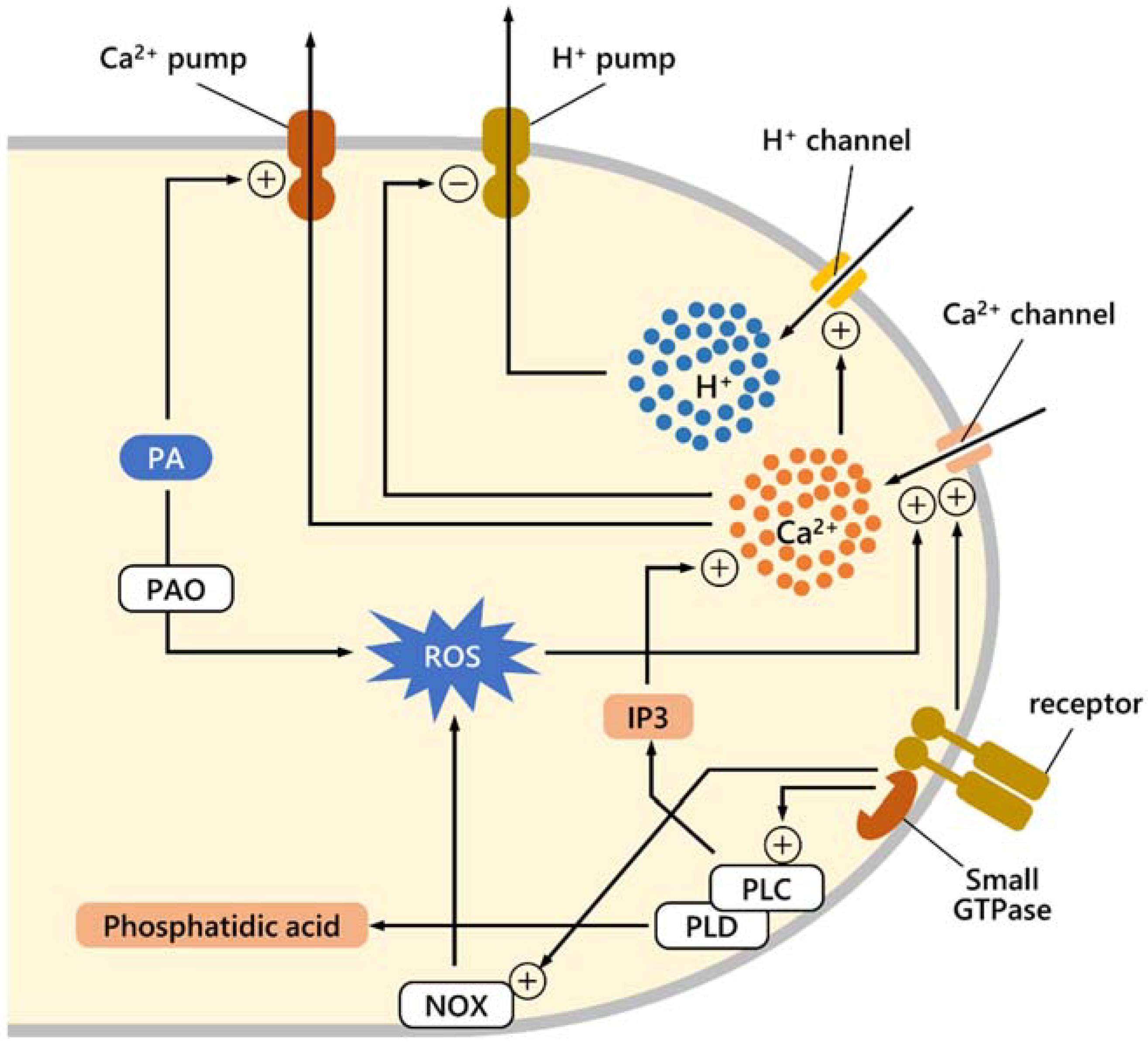
3. Can Polyamines Ameliorate the Damaging Effect of Stress?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pegg, A.E.; Michael, A.J. Spermine synthase. Cell. Mol. life Sci. 2010, 67, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Polyamines in eukaryotes, bacteria, and archaea. J. Biol. Chem. 2016, 291, 14896–14903. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, I.; Cai, G.; Serafini-Fracassini, D.; Del Duca, S. Polyamines in pollen: From microsporogenesis to fertilization. Front. Plant Sci. 2016, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Paupière, M.J.; Müller, F.; Li, H.; Rieu, I.; Tikunov, Y.M.; Visser, R.G.F.F.; Bovy, A.G. Untargeted metabolomic analysis of tomato pollen development and heat stress response. Plant Reprod. 2017, 30, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, B.; Fan, W.; Fan, S.; Xu, Y.; Liu, C.; Lv, T.; Liu, W.; Wu, L.; Xian, L.; et al. Polyamines involved in regulating self-incompatibility in apple. Genes 2021, 12, 1797. [Google Scholar] [CrossRef] [PubMed]
- Antognoni, F.; Agostani, S.; Spinelli, C.; Koskinen, M.; Elo, H.; Bagni, N. Effect of bis (guanylhydrazones) on growth and polyamine uptake in plant cells. J. Plant Growth Regul. 1999, 18, 39–44. [Google Scholar] [CrossRef]
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Pottosin, I.; Shabala, S. Polyamines control of cation transport across plant membranes: Implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 2014, 5, 154. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine Function in Plants: Metabolism, Regulation on Development, and Roles in Abiotic Stress Responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef]
- Gerlin, L.; Baroukh, C.; Genin, S. Polyamines: Double agents in disease and plant immunity. Trends Plant Sci. 2021, 26, 1061–1071. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Marina, M.; Sirera, F.V.; Rambla, J.L.; Gonzalez, M.E.; Blázquez, M.A.; Carbonell, J.; Pieckenstain, F.L.; Ruiz, O.A. Thermospermine catabolism increases Arabidopsis thaliana resistance to Pseudomonas viridiflava. J. Exp. Bot. 2013, 64, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.A.; Grossniklaus, U. She’s the boss: Signaling in pollen tube reception. Curr. Opin. Plant Biol. 2011, 14, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Guo, J.; Li, H.; Yang, Z. Signaling in Pollen Tube Growth: Crosstalk, Feedback, and Missing Links. Mol. Plant 2013, 6, 1053–1064. [Google Scholar] [CrossRef]
- Di Sandro, A.; Del Duca, S.; Verderio, E.; Hargreaves, A.J.; Scarpellini, A.; Cai, G.; Cresti, M.; Faleri, C.; Iorio, R.A.; Hirose, S.; et al. An extracellular transglutaminase is required for apple pollen tube growth. Biochem. J. 2010, 429, 261–271. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, X.; Tian, X.; Zhang, Y.; Li, Y. Organelle movement and apical accumulation of secretory vesicles in pollen tubes of Arabidopsis thaliana depend on class XI myosins. Plant J. 2020, 104, 1685–1697. [Google Scholar] [CrossRef]
- Mollet, J.C.; Leroux, C.; Dardelle, F.; Lehner, A. Cell Wall Composition, Biosynthesis and Remodeling during Pollen Tube Growth. Plants 2013, 2, 107–147. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Chen, C.Y.; Tao, L.Z.; Andreyeva, T.; Twell, D.; Wu, H.-M. Regulation of pollen tube growth by Rac-like GTPases. J. Exp. Bot. 2003, 54, 73–81. [Google Scholar] [CrossRef]
- Potocky, M.; Jones, M.A.; Bezvoda, R.; Smirnoff, N.; Zarsky, V. Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol. 2007, 174, 742–751. [Google Scholar] [CrossRef]
- Heilmann, I.; Ischebeck, T. Male functions and malfunctions: The impact of phosphoinositides on pollen development and pollen tube growth. Plant Reprod. 2016, 29, 3–20. [Google Scholar] [CrossRef]
- Winship, J.L.; Rounds, C.; Hepler, K.P. Perturbation analysis of calcium, alkalinity and secretion during growth of lily pollen tubes. Plants 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Muschietti, J.P.; Wengier, D.L. How many receptor-like kinases are required to operate a pollen tube. Curr. Opin. Plant Biol. 2018, 41, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Çetinbaş-Genç, A.; Conti, V.; Cai, G. Let’s shape again: The concerted molecular action that builds the pollen tube. Plant Reprod. 2022. [Google Scholar] [CrossRef] [PubMed]
- De Storme, N.; Geelen, D. The impact of environmental stress on male reproductive development in plants: Biological processes and molecular mechanisms. Plant Cell Environ. 2014, 37, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mesihovic, A.; Iannacone, R.; Firon, N.; Fragkostefanakis, S. Heat stress regimes for the investigation of pollen thermotolerance in crop plants. Plant Reprod. 2016, 29, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. High temperature susceptibility of sexual reproduction in crop plants. J. Exp. Bot. 2020, 71, 555–568. [Google Scholar] [CrossRef]
- Snider, J.L.; Oosterhuis, D.M.; Loka, D.A.; Kawakami, E.M. High temperature limits in vivo pollen tube growth rates by altering diurnal carbohydrate balance in field-grown Gossypium hirsutum pistils. J. Plant Physiol. 2011, 168, 1168–1175. [Google Scholar] [CrossRef]
- Arshad, M.S.; Farooq, M.; Asch, F.; Krishna, J.S.V.; Prasad, P.V.V.; Siddique, K.H.M. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol. Biochem. 2017, 115, 57–72. [Google Scholar] [CrossRef]
- Seif Barghi, S.; Mostafaii, H.; Peighami, F.; Asghari Zakaria, R.; Farjami Nejhad, R. Response of in Vitro Pollen Germination and Cell Membrane Thermostabilty of Lentil Genotypes to High Temperature. Int. J. Agric. Res. Rev. 2013, 3, 13–20. [Google Scholar]
- Jiang, Y.; Bueckert, R.; Warkentin, T.; Davis, A.R. High Temperature Effects on in vitro Pollen Germination and Seed Set in Field Pea. Can. J. Plant Sci. 2018, 98, 71–80. [Google Scholar] [CrossRef]
- Singh, A.; Antre, S.H.; Ravikumar, R.L.; Kuchanur, P.H.; Lohithaswa, H.C. Genetic evidence of pollen selection mediated phenotypic changes in maize conferring transgenerational heat-stress tolerance. Crop Sci. 2020, 60, 1907–1924. [Google Scholar] [CrossRef]
- Zamir, D.; Gadish, I. Pollen selection for low temperature adaptation in tomato. Theor. Appl. Genet. 1987, 74, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Frankis, R.C.; Grayson, G.K. Heat-shock response in germinating pine pollen. Sex. Plant Reprod. 1990, 3, 195–199. [Google Scholar] [CrossRef]
- Dupuis, I. In vitro pollination: A new tool for analyzing environmental stress. Int. Rev. Cytol. 1992, 140, 391–405. [Google Scholar] [CrossRef]
- Saini, H.S.; Sedgley, M.; Aspinall, D. Effect of heat stress during floral development on pollen tube growth and ovary anatomy in wheat (Triticum aestivum L.). Funct. Plant Biol. 1983, 10, 137–144. [Google Scholar] [CrossRef]
- Schrauwen, J.A.M.; Reijnen, W.H.; Deleeuw, H.C.G.M.; van Herpen, M.M.A. Response of pollen to heat stress. Acta Bot. Neerl. 1986, 35, 321–327. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Perumal, R.; Jagadish, S.V.K.; Ciampitti, I.A.; Welti, R.; Prasad, P.V.V. Sensitivity of sorghum pollen and pistil to high-temperature stress. Plant Cell Environ. 2018, 41, 1065–1082. [Google Scholar] [CrossRef]
- Begcy, K.; Weigert, A.; Egesa, A.O.; Dresselhaus, T. Compared to Australian cultivars, European summer wheat (Triticum aestivum) overreacts when moderate heat stress is applied at the pollen development stage. Agronomy 2018, 8, 99. [Google Scholar] [CrossRef]
- Begcy, K.; Nosenko, T.; Zhou, L.-Z.; Fragner, L.; Weckwerth, W.; Dresselhaus, T. Male sterility in maize after transient heat stress during the tetrad stage of pollen development. Plant Physiol. 2019, 181, 683–700. [Google Scholar] [CrossRef]
- Poidevin, L.; Forment, J.; Unal, D.; Ferrando, A. Transcriptome and translatome changes in germinated pollen under heat stress uncover roles of transporter genes involved in pollen tube growth. Plant Cell Environ. 2021, 44, 2167–2184. [Google Scholar] [CrossRef]
- Parrotta, L.; Faleri, C.; Cresti, M.; Cai, G. Heat stress affects the cytoskeleton and the delivery of sucrose synthase in tobacco pollen tubes. Planta 2016, 243, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa, L.; Matanguihan, J.B.; Murphy, K.M. Effect of high temperature on pollen morphology, plant growth and seed yield in quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2019, 205, 33–45. [Google Scholar] [CrossRef]
- Parrotta, L.; Faleri, C.; Guerriero, G.; Cai, G. Cold stress affects cell wall deposition and growth pattern in tobacco pollen tubes. Plant Sci. 2019, 283, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Wang, R.; Xiang, Y.; An, L.; Cao, S. Heat stress induces actin cytoskeletal reorganization and transcript profiles of vegetative profilins and actin depolymerizing factors (ADFs) in Arabidopsis. Acta Physiol. Plant 2016, 38, 37. [Google Scholar] [CrossRef]
- Muhlemann, J.K.; Younts, T.L.B.; Muday, G.K. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc. Natl. Acad. Sci. USA 2018, 115, E11188–E11197. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Katano, K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front. Plant Sci. 2018, 9, 490. [Google Scholar] [CrossRef]
- Parantainen, A.; Pulkkinen, P. Pollen viability of Scots pine (Pinus sylvestris) in different temperature conditions: High levels of variation among and within latitudes. For. Ecol. Manag. 2002, 167, 149–160. [Google Scholar] [CrossRef]
- Flores-Rentería, L.; Whipple, A.V.; Benally, G.J.; Patterson, A.; Canyon, B.; Gehring, C.A. Higher temperature at lower elevation sites fails to promote acclimation or adaptation to heat stress during pollen germination. Front. Plant Sci. 2018, 9, 536. [Google Scholar] [CrossRef]
- Sawidis, T.; Reiss, H.D. Effects of heavy metals on pollen tube growth and ultrastructure. Protoplasma 1995, 185, 113–122. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Gao, Y.; Lü, W.; Sheng, X. Different heavy metals have various effects on Picea wilsonii pollen germination and tube growth. Plant Signal. Behav. 2015, 10, e989015. [Google Scholar] [CrossRef][Green Version]
- Sawidis, T. Effect of cadmium on pollen germination and tube growth in Lilium longiflorum and Nicotiana tabacum. Protoplasma 2008, 233, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Tuna, A.L.; Burun, B.; Yokas, İ.; Coban, E. The Effects of Heavy Metals on Pollen Germination and Pollen Tube Length in the Tobacco Plant. Turkish J. Biol. 2002, 26, 109–113. [Google Scholar]
- Acharya, S.; Sharma, D.K.; Joshi, H.C.; Chakraborti, M. Effects of some heavy metals on in vitro pollen germination and pollen tube growth of Jatropha curcas. Range Manag. Agrofor. 2011, 32, 52–55. [Google Scholar]
- Munzuroglu, Ö.; Gur, N. The Effects of Heavy Metals on the Pollen Germination and Pollen Tube Growth of Apples (Malus sylvestris Miller cv. Golden). Turkish, J. Biol. 2000, 24, 677–684. [Google Scholar]
- Muradoglu, F.; Beyhan, O.; Sonmez, F. Response to heavy metals on pollen viability, germination and tube growth of some apple cultivars. Fresenius Environ. Bull. 2017, 26, 4456–4461. [Google Scholar]
- Gür, N.; Topdemir, A. Effects of some heavy metals on in vitro pollen germination and tube growth of apricot (Armenica vulgaris Lam.) and cherry (Cerasus avium L.). World Appl. Sci. J. 2008, 4, 195–198. [Google Scholar]
- Breygina, M.; Matveyeva, N.; Polevova, S.; Meychik, N.; Nikolaeva, Y.; Mamaeva, A.; Yermakov, I. Ni 2+ effects on Nicotiana tabacum L. pollen germination and pollen tube growth. Biometals 2012, 25, 1221–1233. [Google Scholar] [CrossRef]
- Sheng, X.; Zhang, S.; Jiang, L.; Li, K.; Gao, Y.; Li, X. Lead stress disrupts the cytoskeleton organization and cell wall construction during Picea wilsonii pollen germination and tube growth. Biol. Trace Elem. Res. 2012, 146, 86–93. [Google Scholar] [CrossRef]
- Schmidt, A.-C.; Mattusch, J.; Reisser, W.; Jung, K.; Kristen, U. Cytotoxic effects of arsenic species. J. Appl. Bot. 2003, 77, 17–20. [Google Scholar]
- Scoccianti, V.; Iacobucci, M.; Speranza, A.; Antognoni, F. Over-accumulation of putrescine induced by cyclohexylamine interferes with chromium accumulation and partially restores pollen tube growth in Actinidia deliciosa. Plant Physiol. Biochem. 2013, 70, 424–432. [Google Scholar] [CrossRef]
- Olasupo, F.O.; Ilori, C.O.; Muyiwa, A.A. Radio-sensitivity of cowpea to ultra-violet radiation by pollen treatment. J. Plant Breed. Crop Sci. 2016, 8, 228–239. [Google Scholar]
- Koti, S.; Reddy, K.R.; Kakani, V.G.; Zhao, D.; Reddy, V.R. Soybean (Glycine max) pollen germination characteristics, flower and pollen morphology in response to enhanced ultraviolet-B radiation. Ann. Bot. 2004, 94, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, J.; Caldwell, M.M.; Flint, S.D.; Durham, S. Susceptibility of pollen to UV-B radiation: An assay of 34 taxa. Am. J. Bot. 1998, 85, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; An, L.; Tan, L.; Hou, Z.; Wang, X. Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 taxa in vitro. Environ. Exp. Bot. 2000, 43, 45–53. [Google Scholar] [CrossRef]
- Chesnokov, Y.V.; Manteuffel, R. Dose effect of UV-B irradiation on pollen tube growth and seed-specific promoter activities in irradiated pollen grains of Nicotiana plumbaginifolia. Sex. Plant Reprod. 2000, 12, 361–364. [Google Scholar] [CrossRef]
- Wang, S.; Xie, B.; Yin, L.; Duan, L.; Li, Z.; Egrinya Eneji, A.; Tsuji, W.; Tsunekawa, A. Increased UV-B radiation affects the viability, reactive oxygen species accumulation and antioxidant enzyme activities in maize (Zea mays L.) pollen. Photochem. Photobiol. 2010, 86, 110–116. [Google Scholar] [CrossRef]
- Koubouris, G.C.; Kavroulakis, N.; Metzidakis, I.T.; Vasilakakis, M.D.; Sofo, A. Ultraviolet-B radiation or heat cause changes in photosynthesis, antioxidant enzyme activities and pollen performance in olive tree. Photosynthetica 2015, 53, 279–287. [Google Scholar] [CrossRef]
- He, J.; Bai, X.; Wang, R.; Cao, B.; She, X. The involvement of nitric oxide in ultraviolet-B-inhibited pollen germination and tube growth of Paulownia tomentosa in vitro. Physiol. Plant 2007, 131, 273–282. [Google Scholar] [CrossRef]
- Navvabpour, S.; Almas, E.; Pakdel, M. The role of reactive oxygen species in UV-B-inhibited pollen germination and tube growth of canola (Brassica napus L.). Iran. J. Genet. Plant Breed. 2021, in press. [Google Scholar]
- Yang, H.; Zhao, Z.; Qiang, W.; An, L.; Xu, S.; Wang, X. Effects of enhanced UV-B radiation on the hormonal content of vegetative and reproductive tissues of two tomato cultivars and their relationships with reproductive characteristics. Plant Growth Regul. 2004, 43, 251–258. [Google Scholar] [CrossRef]
- Yeloff, D.; Blokker, P.; Boelen, P.; Rozema, J. Is pollen morphology of Salix polaris affected by enhanced UV-B irradiation? Results from a field experiment in high arctic tundra. Arct. Antarct. Alp. Res. 2008, 40, 770–774. [Google Scholar] [CrossRef]
- Rozema, J.; Broekman, R.A.; Blokker, P.; Meijkamp, B.B.; de Bakker, N.; van de Staaij, J.; van Beem, A.; Ariese, F.; Kars, S.M. UV-B absorbance and UV-B absorbing compounds (para-coumaric acid) in pollen and sporopollenin: The perspective to track historic UV-B levels. J. Photochem. Photobiol. B Biol. 2001, 62, 108–117. [Google Scholar] [CrossRef]
- Youhnovski, N.; Werner, C.; Hesse, M. N, N′, N ″-Triferuloylspermidine, a new UV absorbing polyamine derivative from pollen of Hippeastrum× hortorum. Z. Naturforsch. C 2001, 56, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yang, Y.-P.; Duan, Y.-W. Pollen sensitivity to ultraviolet-B (UV-B) suggests floral structure evolution in alpine plants. Sci. Rep. 2014, 4, 1–4. [Google Scholar] [CrossRef]
- Ravikumar, R.L.; Patil, B.S.; Salimath, P.M. Drought tolerance in sorghum by pollen selection using osmotic stress. Euphytica 2003, 133, 371–376. [Google Scholar] [CrossRef]
- Zonia, L.; Munnik, T. Osmotically Induced Cell Swelling versus Cell Shrinking Elicits Specific Changes in Phospholipid Signals in Tobacco Pollen Tubes. Plant Physiol. 2004, 134, 813–823. [Google Scholar] [CrossRef]
- Whitley, P.; Hinz, S.; Doughty, J. Arabidopsis FAB1/PIKfyve proteins are essential for development of viable pollen. Plant Physiol. 2009, 151, 1812–1822. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.; Ning, J.; Liu, Y.; Xu, J.; Tian, S.; Zhang, L.; Sun, M.G. NtProRP1, a novel proline-rich protein, is an osmotic stress-responsive factor and specifically functions in pollen tube growth and early embryogenesis in Nicotiana tabacum. Plant Cell Environ. 2014, 37, 499–511. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhang, H.Q.; Pierson, E.S.; Huang, F.Y.; Linskens, H.F.; Hepler, P.K.; Cresti, M. Enforced growth-rate fluctuation causes pectin ring formation in the cell wall of Lilium longiflorum pollen tubes. Planta 1996, 200, 41–49. [Google Scholar] [CrossRef]
- Biagini, G.; Faleri, C.; Cresti, M.; Cai, G. Sucrose concentration in the growth medium affects the cell wall composition of tobacco pollen tubes. Plant Reprod. 2014, 27, 129–144. [Google Scholar] [CrossRef]
- Winship, L.J.; Obermeyer, G.; Geitmann, A.; Hepler, P.K. Under pressure, cell walls set the pace. Trends Plant Sci. 2010, 15, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Winship, L.J.; Obermeyer, G.; Geitmann, A.; Hepler, P.K. Pollen tubes and the physical world. Trends Plant Sci. 2011, 16, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Rounds, C.M.; Hepler, P.K.; Fuller, S.J.; Winship, L.J. Lily Pollen.Pdf. Plant Physiol. 2010, 152, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, L.; McKenna, S.T.; Kunkel, J.G.; Hepler, P.K. NAD(P)H oscillates in pollen tubes and is correlated with tip growth. Plant Physiol. 2006, 142, 1460–1468. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, L.; Faleri, C.; Del Duca, S.; Cai, G. Depletion of sucrose induces changes in the tip growth mechanism of tobacco pollen tubes. Ann. Bot. 2018, 122, 23–43. [Google Scholar] [CrossRef]
- Wang, X.-L.; Takai, T.; Kamijo, S.; Gunawan, H.; Ogawa, H.; Okumura, K. NADPH oxidase activity in allergenic pollen grains of different plant species. Biochem. Biophys. Res. Commun. 2009, 387, 430–434. [Google Scholar] [CrossRef]
- Mandrone, M.; Antognoni, F.; Aloisi, I.; Potente, G.; Poli, F.; Cai, G.; Faleri, C.; Parrotta, L.; Del Duca, S. Compatible and incompatible pollen-styles interaction in Pyrus communis l. Show different transglutaminase features, polyamine pattern and metabolomics profiles. Front. Plant Sci. 2019, 10, 741. [Google Scholar] [CrossRef]
- Paris, R.; Pagliarani, G.; Savazzini, F.; Aloisi, I.; Iorio, R.A.; Tartarini, S.; Ricci, G.; Del Duca, S. Comparative analysis of allergen genes and pro-inflammatory factors in pollen and fruit of apple varieties. Plant Sci. 2017, 264, 57–68. [Google Scholar] [CrossRef]
- Lassig, R.; Gutermuth, T.; Bey, T.D.; Konrad, K.R.; Romeis, T. Pollen tube NAD(P)H oxidases act as a speed control to dampen growth rate oscillations during polarized cell growth. Plant J. 2014, 78, 94–106. [Google Scholar] [CrossRef]
- Potocký, M.; Pejchar, P.; Gutkowska, M.; Jiménez-Quesada, M.J.; Potocká, A.; Alché, J.d.D.; Kost, B.; Žárský, V. NADPH oxidase activity in pollen tubes is affected by calcium ions, signaling phospholipids and Rac/Rop GTPases. J. Plant Physiol. 2012, 169, 1654–1663. [Google Scholar] [CrossRef]
- Gao, Y.B.; Wang, C.L.; Wu, J.Y.; Zhou, H.S.; Jiang, X.T.; Wu, J.; Zhang, S.-L. Low temperature inhibits pollen tube growth by disruption of both tip-localized reactive oxygen species and endocytosis in Pyrus bretschneideri Rehd. Plant Physiol. Biochem. 2014, 74, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Changsong, Z.; Diqiu, Y.U. Analysis of the cold-responsive transcriptome in the mature pollen of Arabidopsis. J. Plant Biol. 2010, 53, 400–416. [Google Scholar] [CrossRef]
- Kazemi-Shahandashti, S.S.; Maali-Amiri, R. Global insights of protein responses to cold stress in plants: Signaling, defence, and degradation. J. Plant Physiol. 2018, 226, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Çetinbaş-Genç, A.; Cai, G.; Del Duca, S. Treatment with spermidine alleviates the effects of concomitantly applied cold stress by modulating Ca2+, pH and ROS homeostasis, actin filament organization and cell wall deposition in pollen tubes of Camellia sinensis. Plant Physiol. Biochem. 2020, 156, 578–590. [Google Scholar] [CrossRef]
- Pan, J.; Wang, W.; Li, D.; Shu, Z.; Ye, X.; Chang, P.; Wang, Y. Gene expression profile indicates involvement of NO in Camellia sinensis pollen tube growth at low temperature. BMC Genom. 2016, 17, 809. [Google Scholar] [CrossRef] [PubMed]
- Benkő, P.; Jee, S.; Kaszler, N.; Fehér, A.; Gémes, K. Polyamines treatment during pollen germination and pollen tube elongation in tobacco modulate reactive oxygen species and nitric oxide homeostasis. J. Plant Physiol. 2020, 244, 153085. [Google Scholar] [CrossRef]
- Nagib, A.; Setsuko, K. Comparative analyses of the proteomes of leaves and flowers at various stages of development reveal organ-specific functional differentiation of proteins in soybean. Proteomics 2009, 9, 4889–4907. [Google Scholar]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Shao, J.; Mattoo, A.K. Genomic analysis of the polyamine biosynthesis pathway in duckweed Spirodela polyrhiza L.: Presence of the arginine decarboxylase pathway, absence of the ornithine decarboxylase pathway, and response to abiotic stresses. Planta 2021, 254, 1–17. [Google Scholar] [CrossRef]
- Gupta, K.; Gupta, B.; Ghosh, B.; Sengupta, D.N. Spermidine and abscisic acid-mediated phosphorylation of a cytoplasmic protein from rice root in response to salinity stress. Acta Physiol. Plant 2012, 34, 29–40. [Google Scholar] [CrossRef]
- Roy, M.; Ghosh, B. Polyamines, both common and uncommon, under heat stress in rice (Oryza sativa) callus. Physiol. Plant 1996, 98, 196–200. [Google Scholar] [CrossRef]
- Amini, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.-S.; López-Gómez, M.; Sadeghzadeh, B.; Sobhani-Najafabadi, A.; Kariman, K. Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J. Plant Physiol. 2021, 258, 153387. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Cuin, T.A.; Pottosin, I. Polyamines prevent NaCl-induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 2007, 581, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Drechsel, O.; Heyer, A.G.; Hincha, D.K.; Zuther, E. Changes in free polyamine levels, expression of polyamine biosynthesis genes, and performance of rice cultivars under salt stress: A comparison with responses to drought. Front. Plant Sci. 2014, 5, 182. [Google Scholar] [CrossRef]
- Liu, K.; Fu, H.; Bei, Q.; Luan, S. Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 2000, 124, 1315–1326. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Pan, X.; Jiang, Q.; Xi, Z. Exogenous putrescine alleviates drought stress by altering reactive oxygen species scavenging and biosynthesis of polyamines in the seedlings of Cabernet Sauvignon. Front. Plant Sci. 2021, 12, 2881. [Google Scholar] [CrossRef]
- Sengupta, A.; Chakraborty, M.; Saha, J.; Gupta, B.; Gupta, K. Polyamines: Osmoprotectants in Plant Abiotic Stress Adaptation. In Osmolytes and Plants Acclimation to Changing Environment: Emerging Omics Technologies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 97–127. [Google Scholar] [CrossRef]
- Papenfus, H.B.; Kulkarni, M.G.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Effect of a commercial seaweed extract (Kelpak®) and polyamines on nutrient-deprived (N, P and K) okra seedlings. Sci. Hortic. 2013, 151, 142–146. [Google Scholar] [CrossRef]
- Nada, K.; Iwatani, E.; Doi, T.; Tachibana, S. Effect of putrescine pretreatment to roots on growth and lactate metabolism in the root of tomato (Lycopersicon esculentum Mill.) under root-zone hypoxia. J. Jpn. Soc. Hortic. Sci. 2004, 73, 337–339. [Google Scholar] [CrossRef]
- Pandolfi, C.; Pottosin, I.; Cuin, T.; Mancuso, S.; Shabala, S. Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant Cell Physiol. 2010, 51, 422–434. [Google Scholar] [CrossRef]
- Capell, T.; Bassie, L.; Christou, P. Modulation of the polyamine biosynthetic pathway in transgenic rice confers tolerance to drought stress. Proc. Natl. Acad. Sci. USA 2004, 101, 9909–9914. [Google Scholar] [CrossRef]
- Waie, B.; Rajam, M.V. Effect of increased polyamine biosynthesis on stress responses in transgenic tobacco by introduction of human S-adenosylmethionine gene. Plant Sci. 2003, 164, 727–734. [Google Scholar] [CrossRef]
- Roy, M.; Wu, R. Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. 2002, 163, 987–992. [Google Scholar] [CrossRef]
- Cheng, L.; Zou, Y.; Ding, S.; Zhang, J.; Yu, X.; Cao, J.; Lu, G. Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J. Integr. Plant Biol. 2009, 51, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Miyazaki, A.; Takahashi, T.; Michael, A.; Kusano, T. The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett. 2006, 580, 6783–6788. [Google Scholar] [CrossRef] [PubMed]
- Choubey, A.; Rajam, M.V. RNAi-mediated silencing of spermidine synthase gene results in reduced reproductive potential in tobacco. Physiol. Mol. Biol. Plants 2018, 24, 1069–1081. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Takahashi, T.; Michael, A.J.; Kusano, T. A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem. Biophys. Res. Commun. 2007, 352, 486–490. [Google Scholar] [CrossRef]
- Seo, S.Y.; Kim, Y.J.; Park, K.Y. Increasing polyamine contents enhances the stress tolerance via reinforcement of antioxidative properties. Front. Plant Sci. 2019, 10, 1331. [Google Scholar] [CrossRef]
- Wu, J.; Shang, Z.; Wu, J.; Jiang, X.; Moschou, P.N.; Sun, W.; Roubelakis-Angelakis, K.A.; Zhang, S. Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+-permeable channels and pollen tube growth. Plant J. 2010, 63, 1042–1053. [Google Scholar] [CrossRef]
- Aloisi, I.; Cai, G.; Tumiatti, V.; Minarini, A.; Del Duca, S. Natural polyamines and synthetic analogs modify the growth and the morphology of Pyrus communis pollen tubes affecting ROS levels and causing cell death. Plant Sci. 2015, 239, 92–105. [Google Scholar] [CrossRef]
- Do, T.H.T.; Choi, H.; Palmgren, M.; Martinoia, E.; Hwang, J.-U.; Lee, Y. Arabidopsis ABCG28 is required for the apical accumulation of reactive oxygen species in growing pollen tubes. Proc. Natl. Acad. Sci. USA 2019, 116, 12540–12549. [Google Scholar] [CrossRef]
- Aloisi, I.; Cai, G.; Faleri, C.; Navazio, L.; Serafini-Fracassini, D.; Del Duca, S. Spermine regulates pollen tube growth by modulating Ca2+-dependent actin organization and cell wall structure. Front. Plant Sci. 2017, 8, 1–20. [Google Scholar] [CrossRef]
- Pottosin, I.; Velarde-Buendia, A.M.; Bose, J.; Fuglsang, A.T.; Shabala, S. Polyamines cause plasma membrane depolarization, activate Ca2+-, and modulate H+-ATPase pump activity in pea roots. J. Exp.Bot. 2014, 65, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhang, S.; Lin, Y.; Zhang, J.; Li, L.; Chen, H.; Zhang, Q. Phospholipase Dδ regulates pollen tube growth by modulating actin cytoskeleton organization in Arabidopsis. Plant Signal. Behav. 2021, 16, 1915610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhou, X.-M.; Zhao, L.-L.; Cheung, A.Y.; Sun, M.-X. Autophagy-mediated compartmental cytoplasmic deletion is essential for tobacco pollen germination and male fertility. Autophagy 2020, 16, 2180–2192. [Google Scholar] [CrossRef]
- Dowd, P.E.; Coursol, S.; Skirpan, A.L.; Kao, T.H.; Gilroy, S. Petunia phospholipase C1 is involved in pollen tube growth. Plant Cell 2006, 18, 1438–1453. [Google Scholar] [CrossRef] [PubMed]
- Pleskot, R.; Pejchar, P.; Bezvoda, R.; Lichtscheidl, I.K.; Wolters-Arts, M.; Marc, J.; Žárský, V.; Potocký, M. Turnover of phosphatidic acid through distinct signaling pathways affects multiple aspects of pollen tube growth in tobacco. Front. Plant Sci. 2012, 3, 54. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Nada, K.; Tachibana, S. Ameliorative effect of polyamines on the high temperature inhibition of in vitro pollen germination in tomato (Lycopersicon esculentum Mill.). Sci. Hortic. 1999, 80, 203–212. [Google Scholar] [CrossRef]
- Wolukau, J.N.; Zhang, S.; Xu, G.; Chen, D. The effect of temperature, polyamines and polyamine synthesis inhibitor on in vitro pollen germination and pollen tube growth of Prunus mume. Sci. Hortic. 2004, 99, 289–299. [Google Scholar] [CrossRef]
- Sorkheh, K.; Shiran, B.; Rouhi, V.; Khodambashi, M.; Wolukau, J.N.; Ercisli, S. Response of in vitro pollen germination and pollen tube growth of almond (Prunus dulcis Mill.) to temperature, polyamines and polyamine synthesis inhibitor. Biochem. Syst. Ecol. 2011, 39, 749–757. [Google Scholar] [CrossRef]
- Song, J.; Nada, K.; Tachibana, S. Suppression of S-adenosylmethionine decarboxylase activity is a major cause for high-temperature inhibition of pollen germination and tube growth in tomato (Lycopersicon esculentum Mill.). Plant Cell Physiol. 2002, 43, 619–627. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Fang, J.; Guo, Z.; Lu, S. Down-regulation of S-adenosylmethionine decarboxylase genes results in reduced plant length, pollen viability, and abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2014, 116, 311–322. [Google Scholar] [CrossRef]
- Aloisi, I.; Cai, G.; Serafini-Fracassini, D.; Del Duca, S. Transglutaminase as polyamine mediator in plant growth and differentiation. Amino Acids 2016, 48, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.; Serafini-Fracassini, D.; Del Duca, S. Regulation of pollen tube growth by transglutaminase. Plants 2013, 2, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Martin-Tanguy, J.; Cabanne, F.; Perdrizet, E.; Martin, C. The distribution of hydroxycinnamic acid amides in flowering plants. Phytochemistry 1978, 17, 1927–1928. [Google Scholar] [CrossRef]
- Grienenberger, E.; Besseau, S.; Geoffroy, P.; Debayle, D.; Heintz, D.; Lapierre, C.; Pollet, B.; Heitz, T.; Legrand, M. A BAHD acyltransferase is expressed in the tapetum of Arabidopsis anthers and is involved in the synthesis of hydroxycinnamoyl spermidines. Plant J. 2009, 58, 246–259. [Google Scholar] [CrossRef]
- Hafidh, S.; Fíla, J.; Honys, D. Male gametophyte development and function in angiosperms: A general concept. Plant Reprod. 2016, 29, 31–51. [Google Scholar] [CrossRef]
- Lin, S.; Mullin, C.A. Lipid, polyamide, and flavonol phagostimulants for adult western corn rootworm from sunflower (Helianthus annuus L.) pollen. J. Agric. Food Chem. 1999, 47, 1223–1229. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisi, I.; Piccini, C.; Cai, G.; Del Duca, S. Male Fertility under Environmental Stress: Do Polyamines Act as Pollen Tube Growth Protectants? Int. J. Mol. Sci. 2022, 23, 1874. https://doi.org/10.3390/ijms23031874
Aloisi I, Piccini C, Cai G, Del Duca S. Male Fertility under Environmental Stress: Do Polyamines Act as Pollen Tube Growth Protectants? International Journal of Molecular Sciences. 2022; 23(3):1874. https://doi.org/10.3390/ijms23031874
Chicago/Turabian StyleAloisi, Iris, Chiara Piccini, Giampiero Cai, and Stefano Del Duca. 2022. "Male Fertility under Environmental Stress: Do Polyamines Act as Pollen Tube Growth Protectants?" International Journal of Molecular Sciences 23, no. 3: 1874. https://doi.org/10.3390/ijms23031874
APA StyleAloisi, I., Piccini, C., Cai, G., & Del Duca, S. (2022). Male Fertility under Environmental Stress: Do Polyamines Act as Pollen Tube Growth Protectants? International Journal of Molecular Sciences, 23(3), 1874. https://doi.org/10.3390/ijms23031874






