Identification of a Negative Regulator for Salt Tolerance at Seedling Stage via a Genome-Wide Association Study of Thai Rice Populations
Abstract
:1. Introduction
2. Results
2.1. Phenotypic Variation in Local Thai Rice Seedlings under Salinity Stress
2.2. Genome-Wide Association between Phenotypic Traits under Salt Stress and SNPs
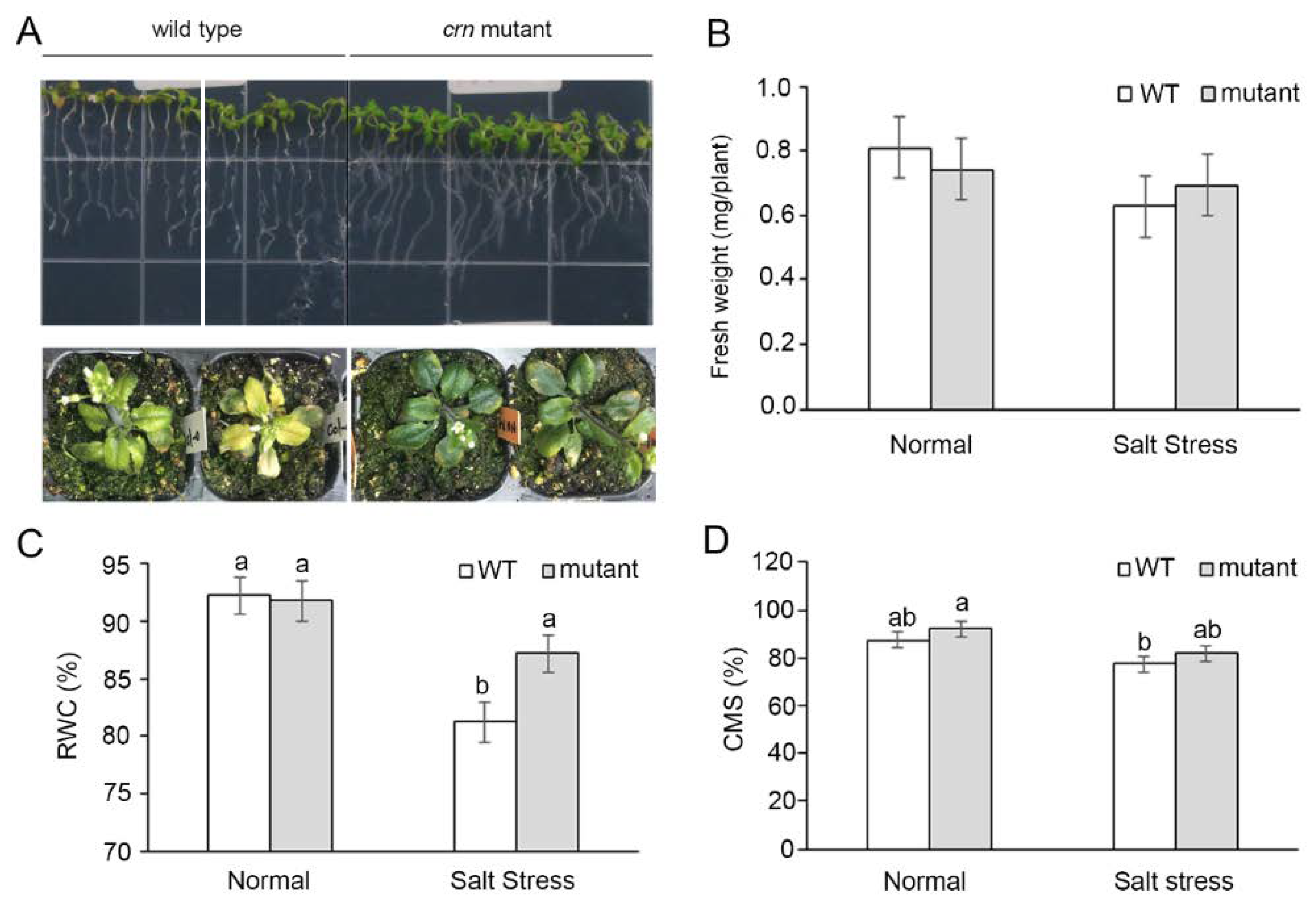
2.3. Characterization of a Gene Putative Associated with Salt Tolerance Using an Arabidopsis Mutant
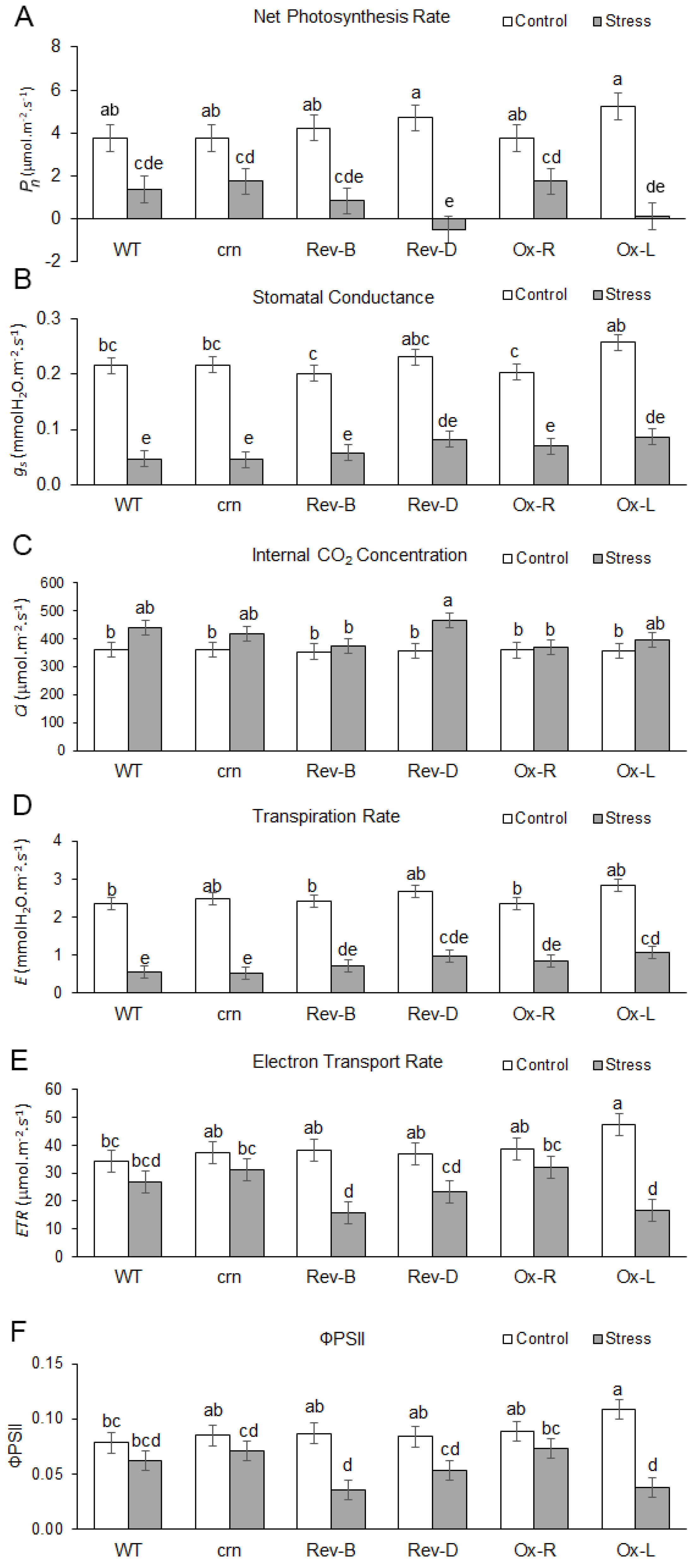
2.4. Ectopic Expression of the OsCRN Gene in crn Mutant and WT Increased the Susceptibility to Salt Stress
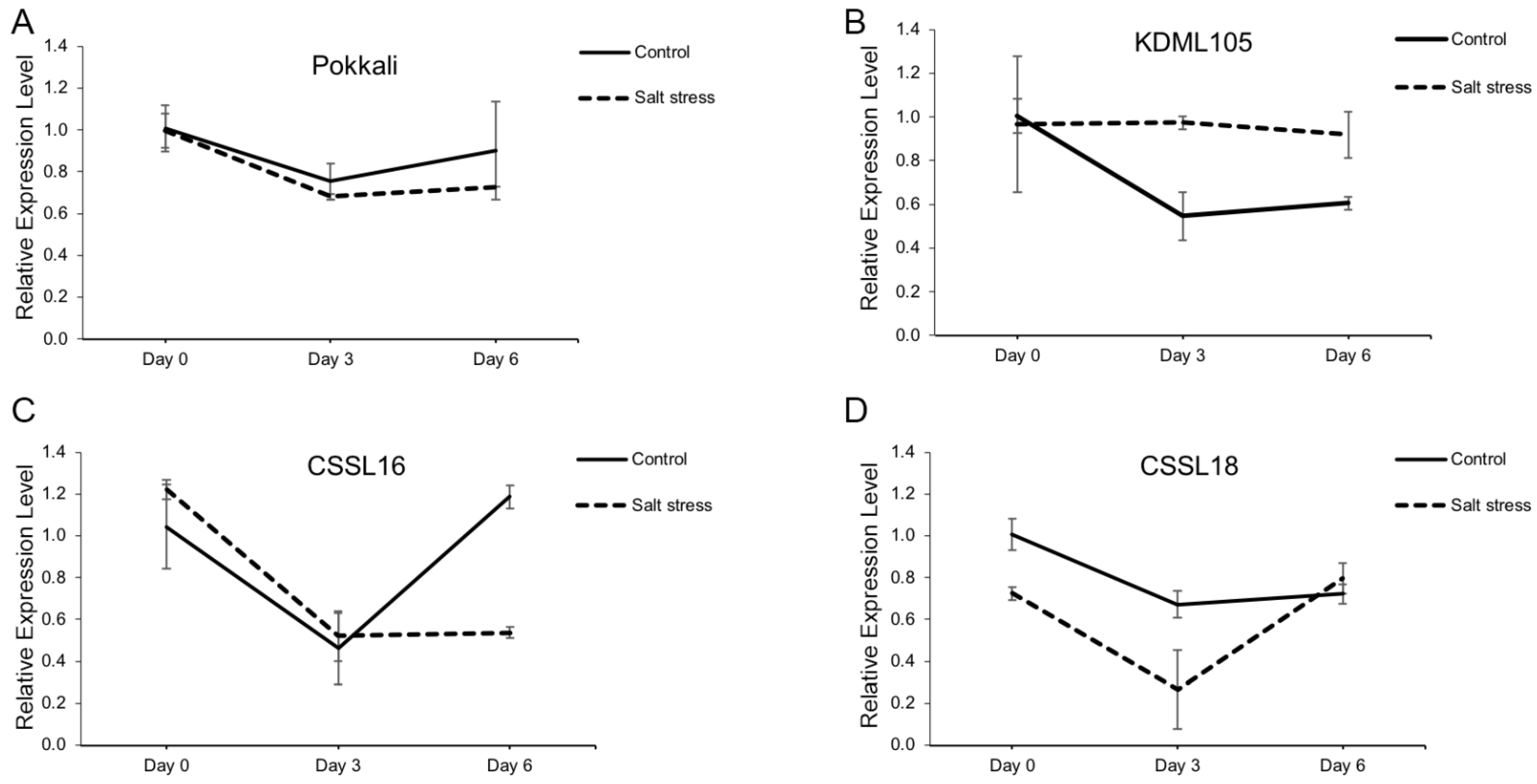
2.5. The Salt-Tolerant Rice Varieties Lowered OsCRN Gene Expression under Salt Stress Condition
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Phenotypic Data Collection
4.2. Genome-Wide Association Analysis
4.3. Validation of Salt Responses in Arabidopsis
4.3.1. Phenotypic Comparison between crn Mutant and Arabidopsis WT
4.3.2. Photosynthesis Performance Comparison of Wild Type (WT), crn Mutant, Complemented Lines, Rev-B and Rev-D, and Ectopic Expression Lines, Ox-R and Ox-L
4.4. Vector Construction and Transformation
4.5. Agrobacterium Transformation by Freeze Thaw Method
4.6. Construction of Transgenic Arabidopsis and Screening of Homozygous T3 Plants
4.7. Gene Expression Analysis of OsCRN under Salt Stress Condition in Rice
4.7.1. Plant Materials and Growth Conditions
4.7.2. RNA Extraction and Gene Expression Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Papademetriou, M.K. Rice production in the Asia-Pacific region: Issues and perspectives. In Bridging the Rice Yield Gap in the Asia-Pacific Region; Papademetriou, M.K., Dent, F.J., Herath, E.M., Eds.; RAP PUBLICATION: Bangkok, Thailand, 2000; Volume 220, pp. 4–25. [Google Scholar]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 18. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Understanding salinity responses and adopting ‘omics-based’ approaches to generate salinity tolerant cultivars of rice. Front. Plant Sci. 2015, 6, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2019, 21, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Feng, F.; Lian, X.; Teng, X.; Wei, H.; Yu, H.; Xie, W.; Yan, M.; Fan, P.; Li, Y. Genome-wide association study (GWAS) of mesocotyl elongation based on re-sequencing approach in rice. BMC Plant Biol. 2015, 15, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Sang, T.; Zhao, Q.; Feng, Q.; Zhao, Y.; Li, C.; Zhu, C.; Lu, T.; Zhang, Z.; Li, M. Genome-wide association studies of 14 agronomic traits in rice landraces. Nat. Genet. 2010, 42, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhao, Y.; Li, C.; Wang, A.; Zhao, Q.; Li, W.; Guo, Y.; Deng, L.; Zhu, C.; Fan, D. Genome-wide association study of flowering time and grain yield traits in a worldwide collection of rice germplasm. Nat. Genet. 2012, 44, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhang, C.; Zhang, S.; Qiang, Y.; Zhang, Y.; Zhong, H.; Li, Y. Uncovering the novel QTLs and candidate genes of salt tolerance in rice with linkage mapping, RTM-GWAS, and RNA-seq. Rice 2021, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Nayyeripasand, L.; Garoosi, G.A.; Ahmadikhah, A. Genome-wide association study (GWAS) to identify salt-tolerance QTLs carrying novel candidate genes in rice during early vegetative stage. Rice 2021, 14, 9. [Google Scholar] [CrossRef]
- Lar, S.M.; Seo, J.; Jang, S.G.; Zhang, H.; Lee, A.R.; Cao, F.Y.; Lee, J.H.; Kim, N.E.; Lee, Y.; Park, Y.J. Genome-wide association study for detecting salt-tolerance loci and candidate genes in rice. Agriculture 2021, 11, 1174. [Google Scholar] [CrossRef]
- Le, T.D.; Gathignol, F.; Vu, H.T.; Nguyen, K.L.; Tran, L.H.; Vu, H.T.T.; Dinh, T.X.; Lazennec, F.; Pham, X.H.; Véry, A.A. Genome-wide association mapping of salinity tolerance at the seedling stage in a panel of vietnamese landraces reveals new valuable qtls for salinity stress tolerance breeding in rice. Plants 2021, 10, 1088. [Google Scholar] [CrossRef]
- Yadav, A.K.; Kumar, A.; Grover, N.; Ellur, R.K.; Bollinedi, H.; Krishnan, S.G.; Bhowmick, P.K.; Vinod, K.K.; Nagarajan, M.; Singh, A.K. Genome-wide association study reveals marker–trait associations for early vegetative stage salinity tolerance in rice. Plants 2021, 10, 559. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, X.; Zhao, Y.; Khan, N.U.; Zhao, Z.; Zhang, Y.; Wen, X.; Tang, F.; Wang, F.; Li, Z. Genetic basis and identification of candidate genes for salt tolerance in rice by GWAS. Sci. Rep. 2020, 10, 9958. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, N.; Jia, T.; Leach, L.; Cockram, J.; Waugh, R.; Ramsay, L.; Thomas, B.; Luo, Z. Genome-wide association mapping of agronomic and morphologic traits in highly structured populations of barley cultivars. Theor. Appl. Genet. 2012, 124, 233–246. [Google Scholar] [CrossRef]
- Cockram, J.; White, J.; Zuluaga, D.L.; Smith, D.; Comadran, J.; Macaulay, M.; Luo, Z.; Kearsey, M.J.; Werner, P.; Harrap, D. Genome-wide association mapping to candidate polymorphism resolution in the unsequenced barley genome. PNAS 2010, 107, 21611–21616. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, L.; Yao, J.; Zheng, Y.; Zhao, C. Association mapping of six agronomic traits on chromosome 4A of wheat (Triticum aestivum L.). Mol. Plant Breed. 2010, 1. [Google Scholar] [CrossRef]
- Cavanagh, C.R.; Chao, S.; Wang, S.; Huang, B.E.; Stephen, S.; Kiani, S.; Forrest, K.; Saintenac, C.; Brown-Guedira, G.L.; Akhunova, A. Genome-wide comparative diversity uncovers multiple targets of selection for improvement in hexaploid wheat landraces and cultivars. PNAS 2013, 110, 8057–8062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shehzad, T.; Iwata, H.; Okuno, K. Genome-wide association mapping of quantitative traits in sorghum (Sorghum bicolor (L.) Moench) by using multiple models. Breed. Sci. 2009, 59, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Simko, I.; Pechenick, D.A.; McHale, L.K.; Truco, M.J.; Ochoa, O.E.; Michelmore, R.W.; Scheffler, B.E. Association mapping and marker-assisted selection of the lettuce dieback resistance gene Tvr1. BMC Plant Biol. 2009, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Warburton, M.; Crouch, J. Association mapping for enhancing maize (Zea mays L.) genetic improvement. Crop Sci. 2011, 51, 433–449. [Google Scholar] [CrossRef]
- Li, H.; Peng, Z.; Yang, X.; Wang, W.; Fu, J.; Wang, J.; Han, Y.; Chai, Y.; Guo, T.; Yang, N. Genome-wide association study dissects the genetic architecture of oil biosynthesis in maize kernels. Nat. Genet. 2013, 45, 43–50. [Google Scholar] [CrossRef]
- Atwell, S.; Huang, Y.S.; Vilhjálmsson, B.J.; Willems, G.; Horton, M.; Li, Y.; Meng, D.; Platt, A.; Tarone, A.M.; Hu, T.T. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 2010, 465, 627–631. [Google Scholar] [CrossRef]
- Aesomnuk, W.; Ruengphayak, S.; Ruanjaichon, V.; Sreewongchai, T.; Malumpong, C.; Vanavichit, A.; Toojinda, T.; Wanchana, S.; Arikit, S. Estimation of the genetic diversity and population structure of Thailand’s rice landraces using SNP markers. Agronomy 2021, 11, 995. [Google Scholar] [CrossRef]
- Lekklar, C.; Pongpanich, M.; Suriya-Arunroj, D.; Chinpongpanich, A.; Tsai, H.; Comai, L.; Chadchawan, S.; Buaboocha, T. Genome-wide association study for salinity tolerance at the flowering stage in a panel of rice accessions from Thailand. BMC Genom. 2019, 20, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, M.; Brown, I.; Adams, S.; Knight, M.; Ainslie, A.; Mansfield, J. The RPM1 plant disease resistance gene facilitates a rapid and sustained increase in cytosolic calcium that is necessary for the oxidative burst and hypersensitive cell death. Plant J. 2000, 23, 441–450. [Google Scholar] [CrossRef]
- Feng, J.; Li, J.; Gao, Z.; Lu, Y.; Yu, J.; Zheng, Q.; Yan, S.; Zhang, W.; He, H.; Ma, L. SKIP confers osmotic tolerance during salt stress by controlling alternative gene splicing in Arabidopsis. Mol. Plant 2015, 8, 1038–1052. [Google Scholar] [CrossRef] [Green Version]
- Chutimanukul, P.; Kositsup, B.; Plaimas, K.; Buaboocha, T.; Siangliw, M.; Toojinda, T.; Comai, L.; Chadchawan, S. Photosynthetic responses and identification of salt tolerance genes in a chromosome segment substitution line of ‘Khao Dawk Mali 105′ rice. Environ. Exp. Bot. 2018, 155, 497–508. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Kositsup, B.; Plaimas, K.; Buaboocha, T.; Siangliw, M.; Toojinda, T.; Comai, L.; Chadchawan, S. Data in support of photosynthetic responses in a chromosome segment substitution line of ‘Khao Dawk Mali 105′rice at seedling stage. Data Brief 2018, 21, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Chutimanukul, P.; Kositsup, B.; Plaimas, K.; Siangliw, M.; Toojinda, T. Effect of salt stress on antioxidant enzyme activity and hydrogen peroxide content in chromosome segment substitution line of ‘Khao Dawk Mali 105′rice. ANRES 2019, 53, 465–471. [Google Scholar]
- Khrueasan, N.; Chutimanukul, P.; Plaimas, K.; Buaboocha, T.; Siangliw, M.; Toojinda, T.; Comai, L.; Chadchawan, S. Comparison between the transcriptomes of ‘KDML105’rice and a salt-tolerant chromosome segment substitution line. Genes 2019, 10, 742. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Guo, Q.; Meng, S.; Zhang, X.; Xu, Z.; Guo, W.; Shen, X. Genome-wide association analysis reveals genetic variations and candidate genes associated with salt tolerance related traits in Gossypium hirsutum. BMC Genom. 2021, 22, 26. [Google Scholar] [CrossRef]
- Li, H.; Yue, H.; Li, L.; Liu, Y.; Zhang, H.; Wang, J.; Jiang, X. Seed biostimulant Bacillus sp. MGW9 improves the salt tolerance of maize during seed germination. AMB Express 2021, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Huqe, M.A.S.; Haque, M.S.; Sagar, A.; Uddin, M.N.; Hossain, M.A.; Hossain, A.; Rahman, M.M.; Wang, X.; Al-Ashkar, I.; Ueda, A. Characterization of maize hybrids (Zea mays L.) for detecting salt tolerance based on morpho-physiological characteristics, ion accumulation and genetic variability at early vegetative stage. Plants 2021, 10, 2549. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Basit, A.; Ara, N.; Ullah, I. Morpho-physiological responses of tomato genotypes under saline conditions. Gesunde Pflanz 2021, 73, 541–553. [Google Scholar] [CrossRef]
- Quan, X.; Liang, X.; Li, H.; Xie, C.; He, W.; Qin, Y. Identification and characterization of wheat germplasm for salt tolerance. Plants 2021, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Hannachi, S.; Werbrouck, S.; Bahrini, I.; Abdelgadir, A.; Siddiqui, H.A.; Van Labeke, M.C. Obtaining salt stress-tolerant eggplant somaclonal variants from in vitro selection. Plants 2021, 10, 2539. [Google Scholar] [CrossRef]
- Farooq, S.; Azam, F. The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J. Plant Physiol. 2006, 163, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Ahamadi, J.; Fotokian, M.H. Identification and mapping of quantitative trait loci associated with salinity tolerance in rice (Oryza sativa) using ssr markers. Iran. J. Biotechnol. 2011, 9, 1. [Google Scholar]
- Shiu, S.H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.H.; Mayer, K.F.; Li, W.H. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Chaudhuri, S.; Yang, L.; Chen, Y.; Poovaiah, B.W. Calcium/calmodulin up-regulates a cytoplasmic receptor-like kinase in plants. J. Biol. Chem. 2004, 279, 42552–42559. [Google Scholar] [CrossRef] [Green Version]
- Vij, S.; Giri, J.; Dansana, P.K.; Kapoor, S.; Tyagi, A.K. The receptor-like cytoplasmic kinase (OsRLCK) gene family in rice: Organization, phylogenetic relationship, and expression during development and stress. Mol. Plant 2008, 1, 732–750. [Google Scholar] [CrossRef]
- Sonsungsan, P.; Chantanakool, P.; Suratanee, A.; Buaboocha, T.; Comai, L.; Chadchawan, S.; Plaimas, K. Identification of key genes in ‘Luang Pratahn’, thai salt-tolerant rice, based on time-course data and weighted co-expression networks. Front. Plant Sci. 2021, 12, 744654. [Google Scholar] [CrossRef] [PubMed]
- Chintakovid, N.; Maipoka, M.; Phaonakrop, N.; Mickelbart, M.V.; Roytrakul, S.; Chadchawan, S. Proteomic analysis of drought-responsive proteins in rice reveals photosynthesis-related adaptations to drought stress. Acta Physiol. Plant. 2017, 39, 240. [Google Scholar] [CrossRef]
- Kumar, A. Development and characterization of rice genotypes for water use effciency and drought resistance. Doctoral Dissertation, University of Arkansas, Fayetteville, AR, USA, 2017. [Google Scholar]
- Khrueasan, N.; Siangliw, M.; Toojinda, T.; Imyim, A.; Buaboocha, T.; Chadchawan, S. Physiological mechanisms of the seedling stage salt tolerance of near isogenic rice lines with the’KDML105’genetic background. Int. J. Agric. Biol. 2020, 23, 927–934. [Google Scholar]
- Chutimanukul, P.; Saputro, T.B.; Mahaprom, P.; Plaimas, K.; Comai, L.; Buaboocha, T.; Siangliw, M.; Toojinda, T.; Chadchawan, S. Combining genome and gene co-expression network analyses for the identification of genes potentially regulating salt tolerance in rice. Front. Plant Sci. 2021, 12, 704549. [Google Scholar] [CrossRef]
- Pieczynski, M.; Marczewski, W.; Hennig, J.; Dolata, J.; Bielewicz, D.; Piontek, P.; Wyrzykowska, A.; Krusiewicz, D.; Strzelczyk-Zyta, D.; Konopka-Postupolska, D. Down-regulation of CBP 80 gene expression as a strategy to engineer a drought-tolerant potato. Plant Biotechnol. J. 2013, 11, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Blum, A.; Ebercon, A. Cell membrane stability as a measure of drought and heat tolerance in wheat. Crop Sci. 1981, 21, 43–47. [Google Scholar] [CrossRef]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Stephens, M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat. Methods 2014, 11, 407–409. [Google Scholar] [CrossRef]
- Turner, S.D. qqman: An R package for visualizing GWAS results using QQ and manhattan plots. Biorxiv 2014, 005165. [Google Scholar]
- Alan, R. The spectral determination of chlorophyll a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Plant Physiol. 1994, 144, 307–313. [Google Scholar]
- Froger, A.; Hall, J.E. Transformation of plasmid DNA into E. coli using the heat shock method. J. Vis. Exp. 2007, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Höfgen, R.; Willmitzer, L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988, 16, 9877. [Google Scholar] [CrossRef] [Green Version]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, S.J.; Mott, E.K.; Parsley, K.; Aspinall, S.; Gray, J.C.; Cottage, A. A rapid and robust method of identifying transformed Arabidopsis thaliana seedlings following floral dip transformation. Plant Methods 2006, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vajrabhaya, M.; Vajrabhaya, T. Somaclonal variation for salt tolerance in rice. In Rice; Springer: Berlin/Heidelberg, Germany, 1991; pp. 368–382. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
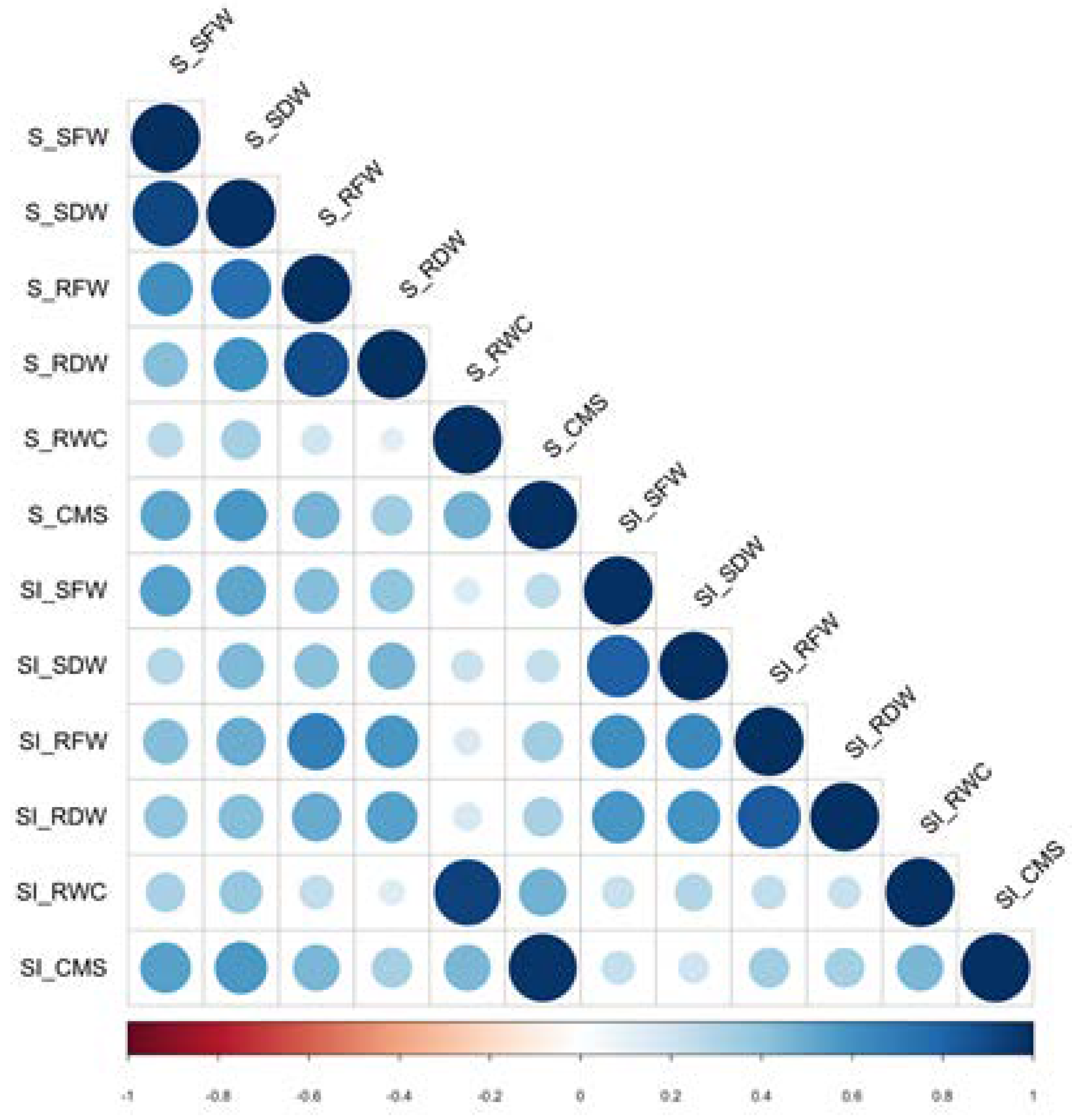
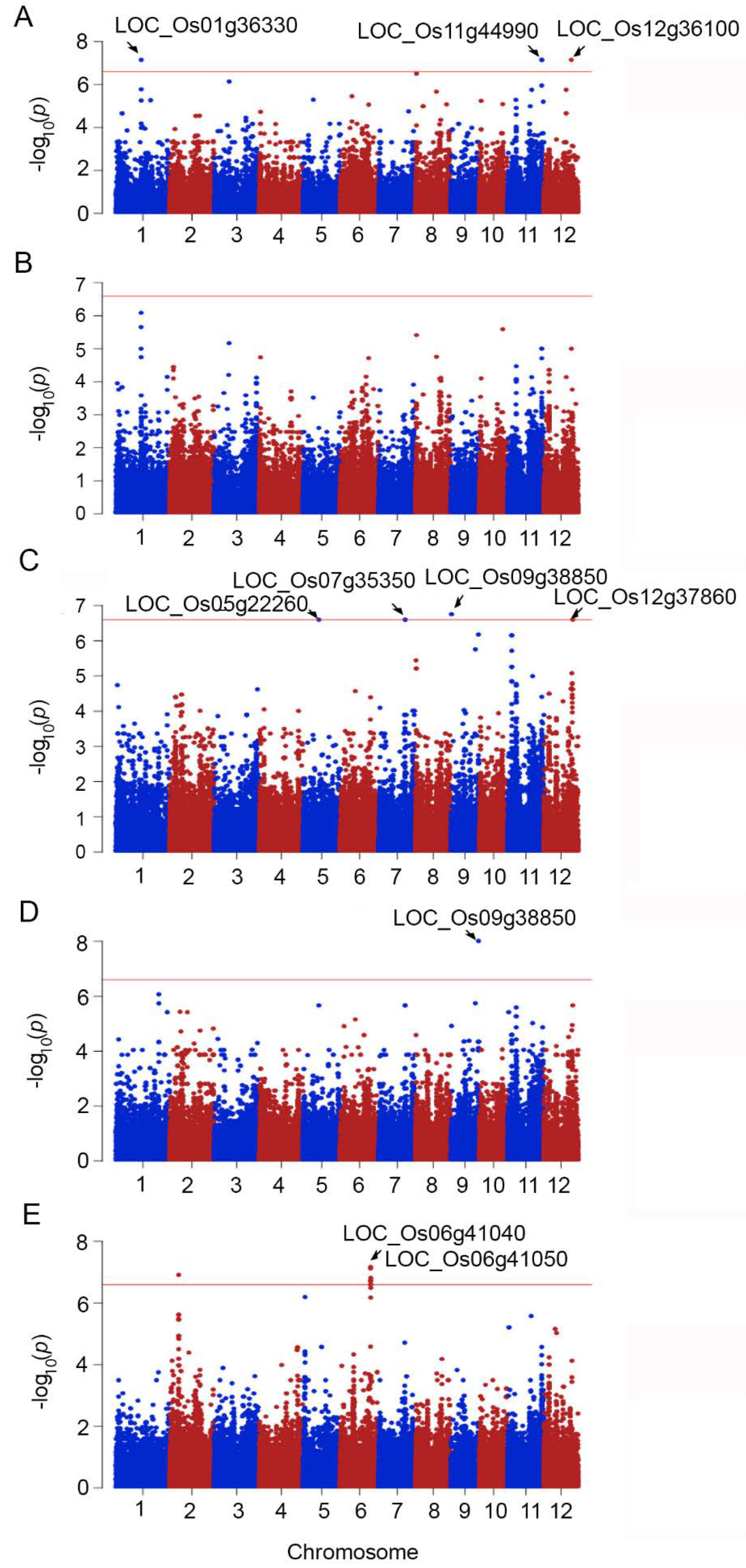
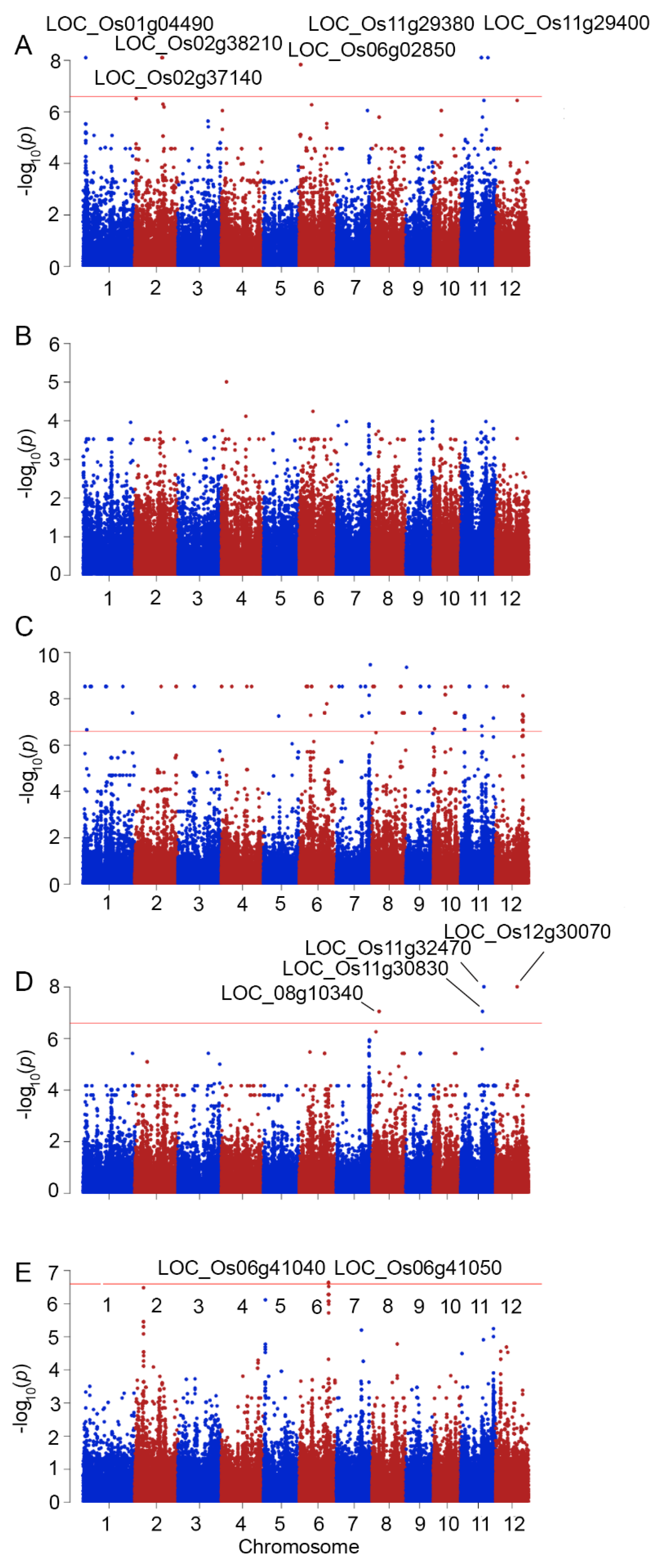
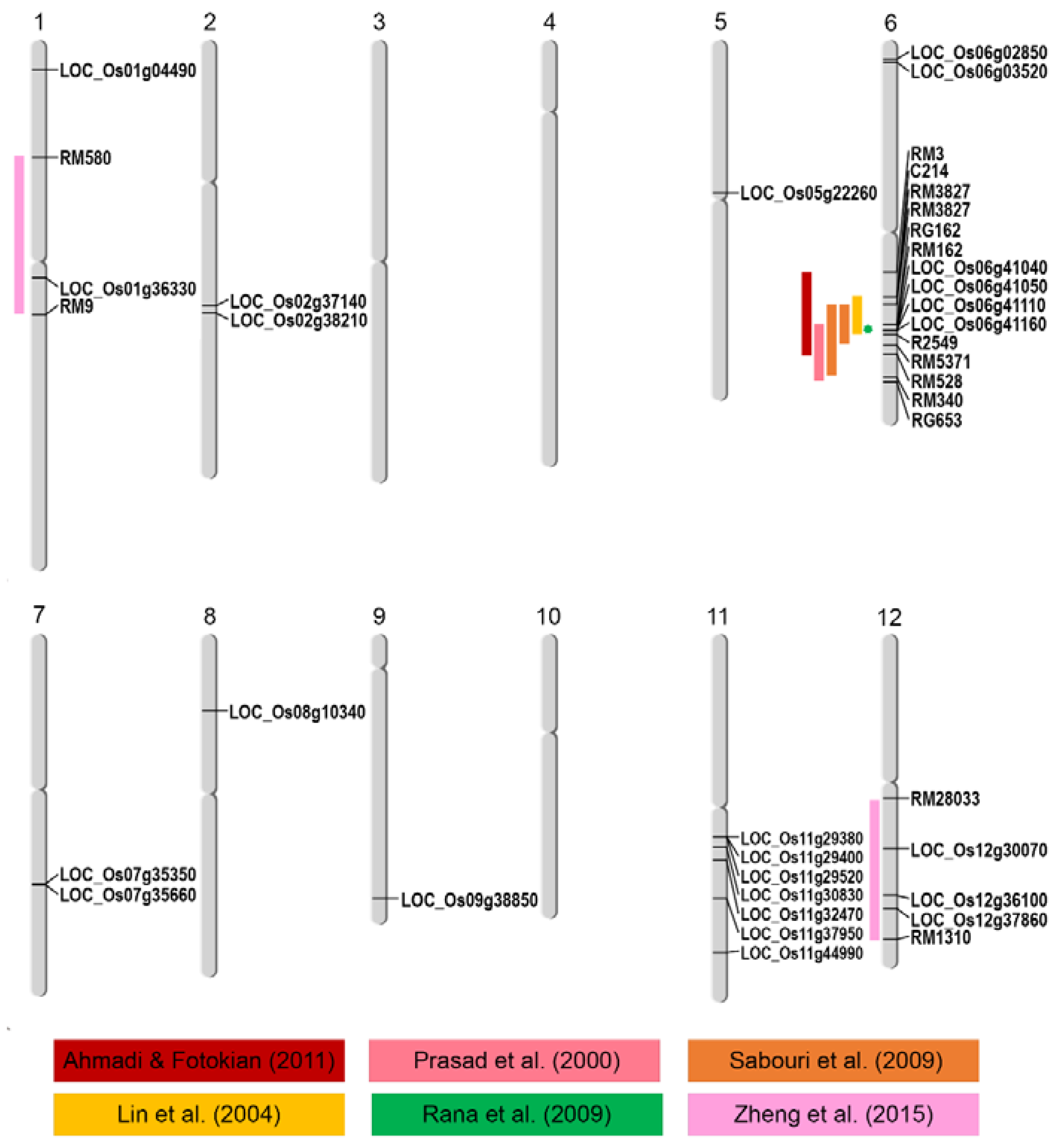

| Traits | Normal | Salt Stress | Stability Index | % Change |
|---|---|---|---|---|
| SFW (g/plant) | 1.07 ± 0.47 | 0.80 ± 0.36 | 0.77 ± 0.22 | −23.43 ± 21.90 |
| SDW (g/plant) | 0.15 ± 0.06 | 0.11 ± 0.04 | 0.73 ± 0.20 | −45.38 ± 19.77 |
| RFW (g/plant) | 0.91 ± 0.27 | 0.48 ± 0.18 | 0.55 ± 0.20 | −27.42 ± 19.67 |
| RDW (g/plant) | 0.11 ± 0.04 | 0.06 ± 0.02 | 0.53 ± 0.19 | −46.55 ± 19.24 |
| RWC (%) | 90.48 ± 4.22 | 83.91 ± 10.48 | 0.93 ± 0.12 | −7.13 ± 11.95 |
| CMS (%) | 94.96 ± 2.29 | 78.40 ± 10.55 | 0.83 ± 0.11 | −17.39 ± 11.40 |
| Trait | SNPs Position | p_Wald | Locus | Gene Name | Description | |
|---|---|---|---|---|---|---|
| 1 | SI_SFW | 1999390 | 7.86 × 10−9 | LOC_Os01g04490 | Ser/Thr protein kinase | protein modification process, kinase activity |
| 2 | S_SFW | 20146031 | 7.08 × 10−8 | LOC_Os01g36330 | expressed protein | - |
| 3 | SI_SFW | 22455127 | 7.86 × 10−9 | LOC_Os02g37140 | expressed protein | - |
| 4 | SI_SFW | 23113356 | 7.86 × 10−9 | LOC_Os02g38210 | elongation factor Tu | translation factor activity and hydrolase activity |
| 5 | S_RFW | 12601958 | 2.52 × 10−7 | LOC_Os05g22260 | crooked neck, putative | nucleic acid metabolic process, binding |
| 6 | SI_SFW | 1048462 | 1.46 × 10−8 | LOC_Os06g02850 | expressed protein | - |
| 1048463 | 1.46 × 10−8 | |||||
| 7 | SI_SFW | 1361687 | 1.46 × 10−8 | LOC_Os06g03520 | DUF581 domain-containing protein | - |
| 8 | S_CMS | 24510573 | 7.54 × 10−8 | LOC_Os06g41040 | pentatricopeptide | - |
| SI_CMS | 24510573 | 2.3 × 10−7 | ||||
| 9 | S_CMS | 24516977 | 6.75 × 10−8 | LOC_Os06g41050 | expressed protein | response to abiotic stimulus, DNA metabolic process, cell cycle, reproduction |
| S_CMS | 24517076 | 2.51 × 10−7 | ||||
| SI_CMS | 24516977 | 2.4 × 10−7 | ||||
| 10 | S_CMS | 24577287 | 2.51 × 10−7 | LOC_Os06g41110 | tubulin binding cofactor C | involve in the folding and assembly of α- and β-tubulin monomers |
| 11 | S_CMS | 24628013 | 2.51 × 10−7 | LOC_Os06g41160 | expressed protein | carbohydrate metabolic process, metabolic process |
| 24628494 | 2.51 × 10−7 | |||||
| 12 | S_RFW | 21151187 | 2.52 × 10−7 | LOC_Os07g35350 | glucan endo-1,3-beta-glucosidase precursor | carbohydrate metabolic process, hydrolase activity |
| 13 | S_RFW | 21365153 | 2.52 × 10−7 | LOC_Os07g35660 | DUF26 kinases | kinase activity, protein modification process |
| 14 | SI_RDW | 6056897 | 8.8 × 10−8 | LOC_Os08g10340 | OsFBX278–F-box domain-containing protein | - |
| 6056962 | 8.8 × 10−8 | |||||
| 15 | RDW | 22317650 | 9.82 × 10−9 | LOC_Os09g38850 | OsWAK91–OsWAK receptor-like protein kinase | kinase activity, protein binding |
| 16 | SI_SFW | 17049126 | 7.86 × 10−9 | LOC_Os11g29380 | MCM2–Putative minichromosome maintenance MCM complex subunit 2 | multicellular organismal development and embryo development |
| 17 | SI_SFW | 17065481 | 7.86 × 10−9 | LOC_Os11g29400 | 6-phosphogluconate dehydrogenase | catalytic activity |
| 18 | SI_SFW | 17130888 | 7.86 × 10−9 | LOC_Os11g29520 | NBS-LRR disease resistance protein | protein binding, response to stress |
| 19 | SI_RDW | 17953322 | 8.8 × 10−8 | LOC_Os11g30830 | expressed protein | transferase activity, response to endogenous stimulus |
| 20 | SI_RDW | 19167004 | 9.7 × 10−9 | LOC_Os11g32470 | NEF1 | response to water deprivation |
| 21 | SI_SFW | 22502331 | 7.86 × 10−9 | LOC_Os11g37950 | WIP3–Wound-induced protein precursor | protein and carbohydrate binding, response to abiotic and biotic stress |
| 22 | SFW | 27245040 | 7.08 × 10−9 | LOC_Os11g44990 | NB-ARC domain-containing protein | protein binding, response to stress |
| 27245051 | 7.08 × 10−8 | |||||
| 23 | SI_RDW | 18009787 | 9.7 × 10−9 | LOC_Os12g30070 | disease resistance protein RPM1 | trigger plants defense systems against biotic stress |
| 24 | SFW | 22127633 | 7.08 × 10−8 | LOC_Os12g36100 | kinesin-4 | nucleotide binding, motor activity |
| 25 | RFW | 23267837 | 2.52 × 10−7 | LOC_Os12g37860 | expressed protein | regulation of gene expression, epigenetic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojonna, T.; Suttiyut, T.; Khunpolwattana, N.; Pongpanich, M.; Suriya-arunroj, D.; Comai, L.; Buaboocha, T.; Chadchawan, S. Identification of a Negative Regulator for Salt Tolerance at Seedling Stage via a Genome-Wide Association Study of Thai Rice Populations. Int. J. Mol. Sci. 2022, 23, 1842. https://doi.org/10.3390/ijms23031842
Kojonna T, Suttiyut T, Khunpolwattana N, Pongpanich M, Suriya-arunroj D, Comai L, Buaboocha T, Chadchawan S. Identification of a Negative Regulator for Salt Tolerance at Seedling Stage via a Genome-Wide Association Study of Thai Rice Populations. International Journal of Molecular Sciences. 2022; 23(3):1842. https://doi.org/10.3390/ijms23031842
Chicago/Turabian StyleKojonna, Thammaporn, Thiti Suttiyut, Nopphakhun Khunpolwattana, Monnat Pongpanich, Duangjai Suriya-arunroj, Luca Comai, Teerapong Buaboocha, and Supachitra Chadchawan. 2022. "Identification of a Negative Regulator for Salt Tolerance at Seedling Stage via a Genome-Wide Association Study of Thai Rice Populations" International Journal of Molecular Sciences 23, no. 3: 1842. https://doi.org/10.3390/ijms23031842
APA StyleKojonna, T., Suttiyut, T., Khunpolwattana, N., Pongpanich, M., Suriya-arunroj, D., Comai, L., Buaboocha, T., & Chadchawan, S. (2022). Identification of a Negative Regulator for Salt Tolerance at Seedling Stage via a Genome-Wide Association Study of Thai Rice Populations. International Journal of Molecular Sciences, 23(3), 1842. https://doi.org/10.3390/ijms23031842






