Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice
Abstract
1. Introduction
2. Results
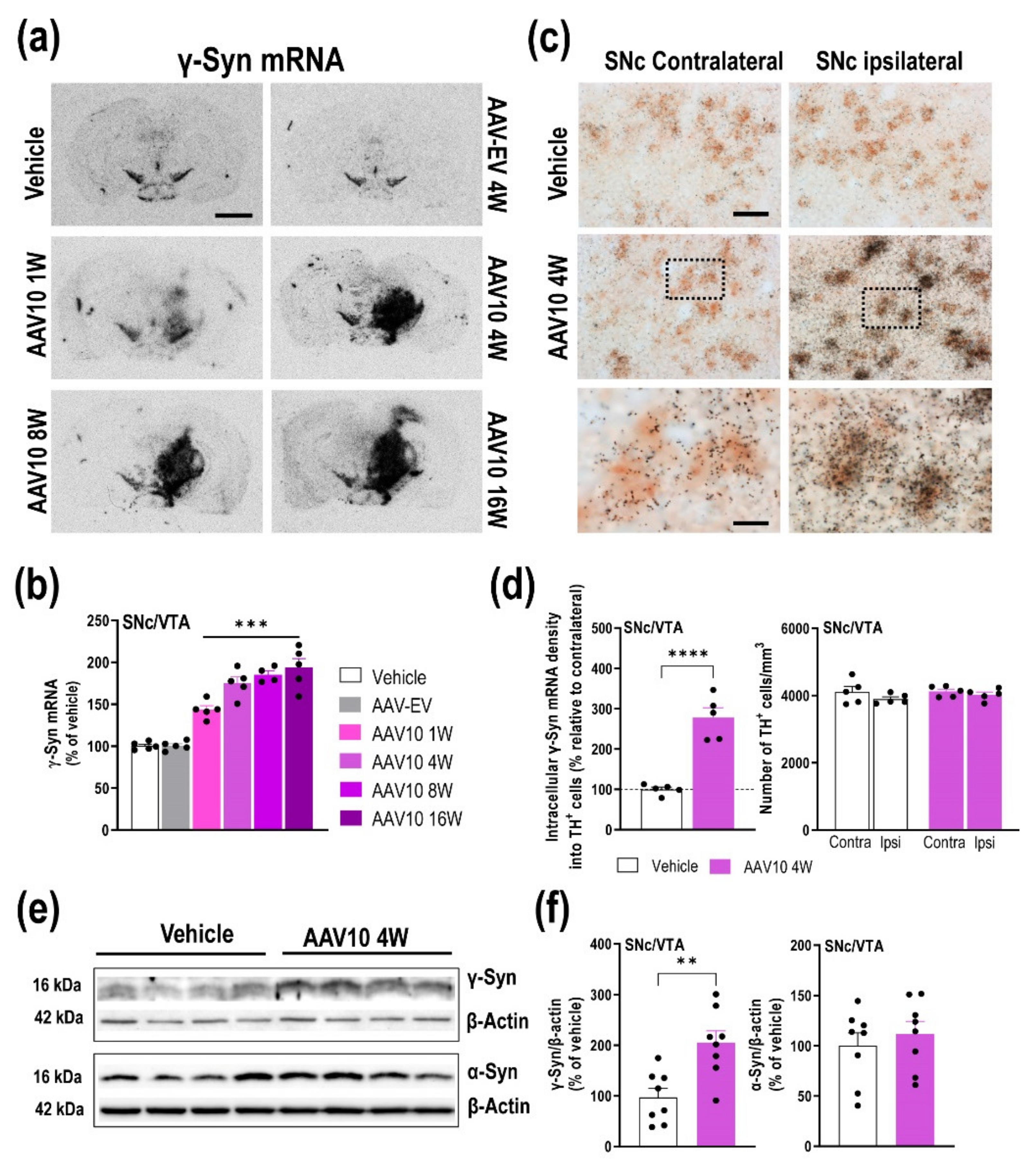
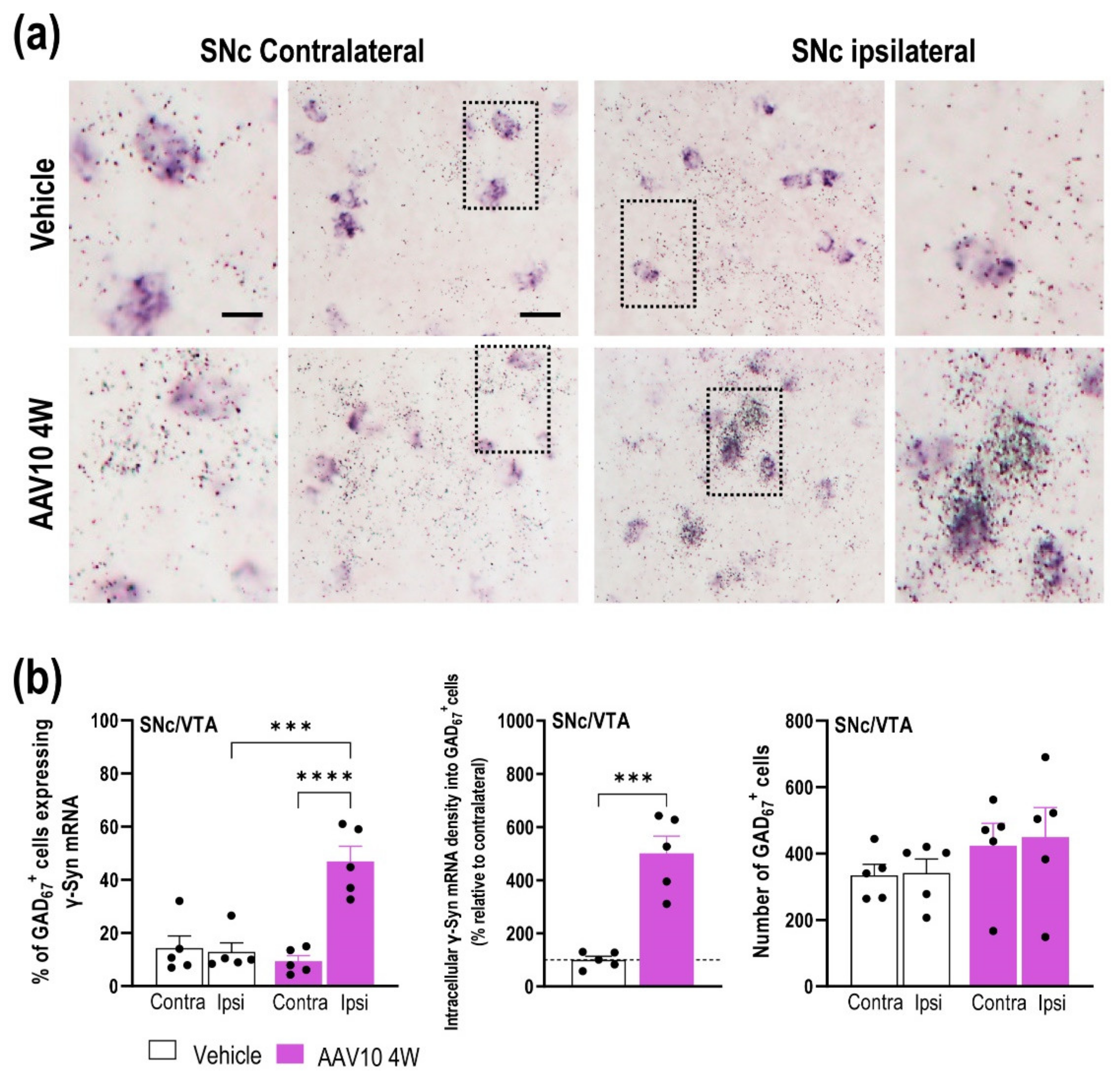
2.1. Mouse Model Characterization with γ-Synuclein (γ-Syn) Overexpression in Midbrain Dopamine (DA) Neurons
2.2. Overexpression of γ-Syn in Midbrain DA Neurons Triggers Deficiencies in Forebrain DA Neurotransmission
2.3. Progressive Motor and Cognitive Impairments in Mice Overexpression of γ-Syn in Midbrain DA Neurons
2.4. In Vivo γ-Syn Knockdown Selectively in Midbrain DA Neurons Induced by IN-ASO
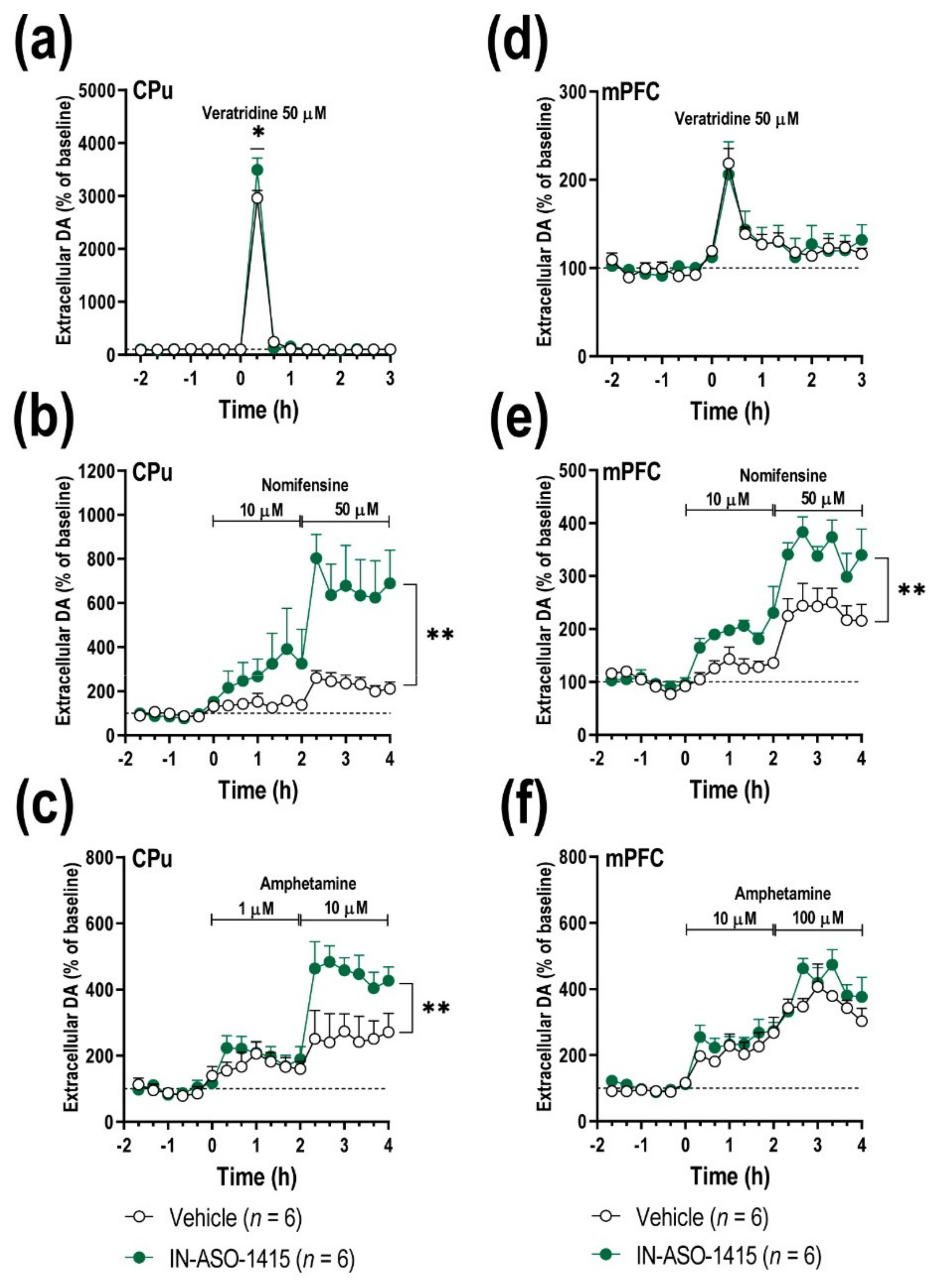
2.5. IN-ASO-Induced γ-Syn Knockdown Enhances Forebrain DA Neurotransmission
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Mouse Model Overexpressing Murine γ-Syn in DA Neurons
4.3. Conjugated Antisense Oligonucleotides
4.4. In Situ Hybridization
4.5. Western Blot
4.6. In Vivo Microdialysis
4.7. Behavioral Testing
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- George, J.M. The synucleins. Genome Biol. 2002, 3, REVIEWS3002. [Google Scholar]
- Li, J.Y.; Henning Jensen, P.; Dahlström, A. Differential localization of alpha-, beta- and gamma-synucleins in the rat CNS. Neuroscience 2002, 113, 463–478. [Google Scholar] [CrossRef]
- Winham, C.L.; Le, T.; Jellison, E.R.; Silver, A.C.; Levesque, A.A.; Koob, A.O. γ-Synuclein induces human cortical astrocyte proliferation and subsequent BDNF expression and release. Neuroscience 2019, 410, 41–54. [Google Scholar] [CrossRef]
- Abeliovich, A.; Schmitz, Y.; Fariñas, I.; Choi-Lundberg, D.; Ho, W.H.; Castillo, P.E.; Shinsky, N.; Verdugo, J.M.; Armanini, M.; Ryan, A.; et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 2000, 25, 239–252. [Google Scholar] [CrossRef]
- Chandra, S.; Chen, X.; Rizo, J.; Jahn, R.; Südhof, T.C. A broken alpha helix in folded alpha-synuclein. J. Biol. Chem. 2003, 278, 15313–15318. [Google Scholar] [CrossRef] [PubMed]
- Burré, J.; Sharma, M.; Tsetsenis, T.; Buchman, V.; Etherton, M.R.; Südhof, T.C. Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 2010, 329, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Nemani, V.M.; Lu, W.; Berge, V.; Nakamura, K.; Onoa, B.; Lee, M.K.; Chaudhry, F.A.; Nicoll, R.A.; Edwards, R.H. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 2010, 65, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kouadio, A.; Cartledge, D.; Godwin, A.K. Role of gamma-synuclein in microtubule regulation. Exp. Cell Res. 2011, 317, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Oaks, A.W.; Sidhu, A. Parallel mechanisms for direct and indirect membrane protein trafficking by synucleins. Commun. Integr. Biol. 2013, 6, e26794. [Google Scholar] [CrossRef]
- Galvin, J.E.; Uryu, K.; Lee, V.M.; Trojanowski, J.Q. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc. Natl. Acad. Sci. USA 1999, 96, 13450–13455. [Google Scholar] [CrossRef] [PubMed]
- Mukaetova-Ladinska, E.B.; Milne, J.; Andras, A.; Abdel-All, Z.; Cerejeira, J.; Greally, E.; Robson, J.; Jaros, E.; Perry, R.; McKeith, I.G.; et al. Alpha- and gamma-synuclein proteins are present in cerebrospinal fluid and are increased in aged subjects with neurodegenerative and vascular changes. Dement. Geriatr. Cogn. Disord. 2008, 26, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Surgucheva, I.; Newell, K.L.; Burns, J.; Surguchov, A. New α- and γ-synuclein immunopathological lesions in human brain. Acta Neuropathol. Commun. 2014, 2, 132. [Google Scholar] [CrossRef]
- Oeckl, P.; Metzger, F.; Nagl, M.; von Arnim, C.A.; Halbgebauer, S.; Steinacker, P.; Ludolph, A.C.; Otto, M. Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer’s and Creutzfeldt-Jakob disease, but no alteration in synucleinopathies. Mol. Cell Proteomics 2016, 15, 3126–3138. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the a-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. Alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef] [PubMed]
- Singleton, A.B.; Farrer, M.; Johnson, J.; Singleton, A.; Hague, S.; Kachergus, J.; Hulihan, M.; Peuralinna, T.; Dutra, A.; Nussbaum, R.; et al. Alpha-Synuclein locus triplication causes Parkinson’s disease. Science 2003, 302, 841. [Google Scholar] [CrossRef]
- Pals, P.; Lincoln, S.; Manning, J.; Heckman, M.; Skipper, L.; Hulihan, M.; Van den Broeck, M.; De Pooter, T.; Cras, P.; Crook, J.; et al. Alpha-Synuclein promoter confers susceptibility to Parkinson’s disease. Ann. Neurol. 2004, 56, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Jakes, R.; Spillantini, M.G. The synucleinopathies: Twenty years on. J. Parkinsons Dis. 2017, 7, S53–S71. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. Parkinson’s disease and other alpha-synucleinopathies. Clin. Chem. Lab. Med. 2001, 39, 308–312. [Google Scholar] [CrossRef]
- Ji, H.; Liu, Y.E.; Jia, T.; Wang, M.; Liu, J.; Xiao, G.; Joseph, B.K.; Rosen, C.; Shi, Y.E. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997, 57, 759–764. [Google Scholar]
- Alarcón-Arís, D.; Recasens, A.; Galofré, M.; Carballo-Carbajal, I.; Zacchi, N.; Ruiz-Bronchal, E.; Pavia-Collado, R.; Chica, R.; Ferrés-Coy, A.; Santos, M.; et al. Selective α-synuclein knockdown in monoamine neurons by intranasal oligonucleotide delivery: Potential therapy for Parkinson’s disease. Mol. Ther. 2018, 26, 550–567. [Google Scholar] [CrossRef] [PubMed]
- Oaks, A.W.; Sidhu, A. Synuclein modulation of monoamine transporters. FEBS Lett. 2011, 585, 1001–1006. [Google Scholar] [CrossRef]
- Wersinger, C.; Sidhu, A. Attenuation of dopamine transporter activity by alpha-synuclein. Neurosci. Lett. 2003, 340, 189–192. [Google Scholar] [CrossRef]
- Wersinger, C.; Sidhu, A. Disruption of the interaction of alpha-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry 2005, 44, 13612–13624. [Google Scholar] [CrossRef]
- Boyer, F.; Dreyer, J.L. The role of gamma-synuclein in cocaine induced behaviour in rats. Eur. J. Neurosci. 2008, 27, 2938–2951. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, C.; Prou, D.; Vernier, P.; Sidhu, A. Modulation of dopamine transporter function by alpha-synuclein is altered by impairment of cell adhesion and by induction of oxidative stress. FASEB J. 2003, 17, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Senior, S.L.; Ninkina, N.; Deacon, R.; Bannerman, D.; Buchman, V.L.; Cragg, S.J.; Wade-Martins, R. Increased striatal dopamine release and hyperdopaminergic-like behaviour in mice lacking both alpha-synuclein and gamma-synuclein. Eur. J. Neurosci. 2008, 27, 947–957. [Google Scholar] [CrossRef]
- Anwar, S.; Peters, O.; Millership, S.; Ninkina, N.; Doig, N.; Connor-Robson, N.; Threlfell, S.; Kooner, G.; Deacon, R.M.; Bannerman, D.M.; et al. Functional alterations to the nigrostriatal system in mice lacking all three members of the synuclein family. J. Neurosci. 2011, 31, 7264–7274. [Google Scholar] [CrossRef] [PubMed]
- Kokhan, V.S.; Van’kin, G.I.; Bachurin, S.O.; Shamakina, I.Y. Differential involvement of the gamma-synuclein in cognitive abilities on the model of knockout mice. BMC Neurosci. 2013, 14, 53. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Kokhan, T.Y.G.; Samsonova, A.N.; Fisenko, V.P.; Ustyugov, A.A.; Aliev, G. The dopaminergic dysfunction and altered working memory performance of aging mice lacking gamma-synuclein gene. CNS Neurol. Disord. Drug Targets 2018, 17, 604–607. [Google Scholar] [CrossRef]
- Alarcón-Arís, D.; Pavia-Collado, R.; Miquel-Rio, L.; Coppola-Segovia, V.; Ferrés-Coy, A.; Ruiz-Bronchal, E.; Galofré, M.; Paz, V.; Campa, L.; Revilla, R.; et al. Anti-α-synuclein ASO delivered to monoamine neurons prevents α-synuclein accumulation in a Parkinson’s disease-like mouse model and in monkeys. EBioMedicine 2020, 59, 102944. [Google Scholar] [CrossRef]
- Brown, M.W.; Aggleton, J.P. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001, 2, 51–61. [Google Scholar] [CrossRef]
- Winters, B.D.; Forwood, S.E.; Cowell, R.A.; Saksida, L.M.; Bussey, T.J. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: Heterogeneity of function within the temporal lobe. J. Neurosci. 2004, 24, 5901–5908. [Google Scholar] [CrossRef]
- Pavia-Collado, R.; Cóppola-Segovia, V.; Miquel-Rio, L.; Alarcón-Aris, D.; Rodríguez-Aller, R.; Torres-López, M.; Paz, V.; Ruiz-Bronchal, E.; Campa, L.; Artigas, F.; et al. Intracerebral administration of a ligand-ASO conjugate selectively reduces α-synuclein accumulation in monoamine neurons of double mutant human A30P*A53T*α-synuclein transgenic mice. Int. J. Mol. Sci. 2021, 22, 2939. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, K.; Pieschl, R.; Strong, T.; Molski, T.; Mattson, G.; Lodge, N.J.; Li, Y.W. Ex vivo assessment of binding site occupancy of monoamine reuptake inhibitors: Methodology and biological significance. Neuropharmacology 2008, 55, 63–70. [Google Scholar] [CrossRef]
- Ferrés-Coy, A.; Galofré, M.; Pilar-Cuéllar, F.; Vidal, R.; Paz, V.; Ruiz-Bronchal, E.; Campa, L.; Pazos, A.; Caso, J.R.; Leza, J.C.; et al. Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration. Mol. Psychiatry 2016, 21, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzi, A.; Manashirov, S.; Chen, A.; Artigas, F. Oligonucleotides as therapeutic tools for brain disorders: Focus on major depressive disorder and Parkinson’s disease. Pharmacol. Ther. 2021, 227, 107873. [Google Scholar] [CrossRef] [PubMed]
- Taymans, J.M.; Vandenberghe, L.H.; Haute, C.V.; Thiry, I.; Deroose, C.M.; Mortelmans, L.; Wilson, J.M.; Debyser, Z.; Baekelandt, V. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum. Gene Ther. 2007, 18, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Fajardo-Serrano, A.; Rico, A.J.; Roda, E.; Honrubia, A.; Arrieta, S.; Ariznabarreta, G.; Chocarro, J.; Lorenzo-Ramos, E.; Pejenaute, A.; Vázquez, A.; et al. Adeno-associated viral vectors as versatile tools for Parkinson’s research, both for disease modeling purposes and for therapeutic uses. Int. J. Mol. Sci. 2021, 22, 6389. [Google Scholar] [CrossRef]
- Gao, G.; Zhong, L.; Danos, O. Exploiting natural diversity of AAV for the design of vectors with novel properties. Methods Mol. Biol. 2011, 807, 93–118. [Google Scholar] [PubMed]
- Tanguy, Y.; Biferi, M.G.; Besse, A.; Astord, S.; Cohen-Tannoudji, M.; Marais, T.; Barkats, M. Systemic AAVrh10 provides higher transgene expression than AAV9 in the brain and the spinal cord of neonatal mice. Front. Mol. Neurosci. 2015, 8, 36. [Google Scholar] [CrossRef]
- Bey, K.; Deniaud, J.; Dubreil, L.; Joussemet, B.; Cristini, J.; Ciron, C.; Hordeaux, J.; Le Boulc’h, M.; Marche, K.; Maquigneau, M.; et al. Intra-CSF AAV9 and AAVrh10 administration in nonhuman primates: Promising routes and vectors for which neurological diseases? Mol. Ther. Methods Clin. Dev. 2020, 17, 771–784. [Google Scholar] [CrossRef]
- Haery, L.; Deverman, B.E.; Matho, K.S.; Cetin, A.; Woodard, K.; Cepko, C.; Guerin, K.I.; Rego, M.A.; Ersing, I.; Bachle, S.M.; et al. Adeno-associated virus technologies and methods for targeted neuronal manipulation. Front. Neuroanat. 2019, 13, 93. [Google Scholar] [CrossRef]
- Thomsen, D.R.; Stenberg, R.M.; Goins, W.F.; Stinski, M.F. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 1984, 81, 659–663. [Google Scholar] [CrossRef]
- Bäck, S.; Dossat, A.; Parkkinen, I.; Koivula, P.; Airavaara, M.; Richie, C.T.; Chen, Y.H.; Wang, Y.; Harvey, B.K. Neuronal activation stimulates cytomegalovirus promoter-driven transgene expression. Mol. Ther. Methods Clin. Dev. 2019, 14, 180–188. [Google Scholar] [CrossRef]
- Van Bockstaele, E.J.; Pickel, V.M. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995, 682, 215–221. [Google Scholar] [CrossRef]
- Carr, D.B.; Sesack, S.R. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse 2000, 38, 114–123. [Google Scholar] [CrossRef]
- Korotkova, T.M.; Ponomarenko, A.A.; Brown, R.E.; Haas, H.L. Functional diversity of ventral midbrain dopamine and GABAergic neurons. Mol. Neurobiol. 2004, 29, 243–259. [Google Scholar] [CrossRef]
- Nair-Roberts, R.G.; Chatelain-Badie, S.D.; Benson, E.; White-Cooper, H.; Bolam, J.P.; Ungless, M.A. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 2008, 152, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.E.; Zaborszky, L. Vglut2 afferents to the medial prefrontal and primary somatosensory cortices: A combined retrograde tracing in situ hybridization. J. Comp. Neurol. 2005, 483, 351–373. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Kawasaki, A.; Sakata-Haga, H.; Fukui, Y.; Kawano, H.; Nogami, H.; Hisano, S. Particular subpopulations of midbrain and hypothalamic dopamine neurons express vesicular glutamate transporter 2 in the rat brain. J. Comp. Neurol. 2006, 498, 581–592. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Sheen, W.; Morales, M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur. J. Neurosci. 2007, 25, 106–118. [Google Scholar] [CrossRef]
- Javitch, J.; Strittmatter, S.; Snyder, S. Differential visualization of dopamine and norepinephrine uptake sites in rat brain using [3H]mazindol autoradiography. J. Neurosci. 1985, 5, 1513–1521. [Google Scholar] [CrossRef]
- Nirenberg, M.J.; Chan, J.; Vaughan, R.A.; Uhl, G.R.; Kuhar, M.J.; Pickel, V.M. Immunogold localization of the dopamine transporter: An ultrastructural study of the rat ventral tegmental area. J. Neurosci. 1997, 17, 5255–5262. [Google Scholar] [CrossRef]
- Wu, N.; Joshi, P.R.; Cepeda, C.; Masliah, E.; Levine, M.S. Alpha-synuclein overexpression in mice alters synaptic communication in the corticostriatal pathway. J. Neurosci. Res. 2010, 88, 1764–1776. [Google Scholar] [CrossRef]
- Larsen, K.E.; Schmitz, Y.; Troyer, M.D.; Mosharov, E.; Dietrich, P.; Quazi, A.Z.; Savalle, M.; Nemani, V.; Chaudhry, F.A.; Edwards, R.H.; et al. Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis. J. Neurosci. 2006, 26, 11915–11922. [Google Scholar] [CrossRef] [PubMed]
- Gureviciene, I.; Gurevicius, K.; Tanila, H. Role of alpha-synuclein in synaptic glutamate release. Neurobiol. Dis. 2007, 28, 83–89. [Google Scholar] [CrossRef]
- Lautenschläger, J.; Kaminski, C.F.; Kaminski Schierle, G.S. α-Synuclein—Regulator of Exocytosis, Endocytosis, or Both? Trends Cell Biol. 2017, 27, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ninan, I.; Antonova, I.; Battaglia, F.; Trinchese, F.; Narasanna, A.; Kolodilov, N.; Dauer, W.; Hawkins, R.D.; Arancio, O. alpha-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004, 23, 4506–4516. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.B.; Hatami, A.; David, H.; Masliah, E.; Roberts, K.; Evans, C.E.; Levine, M.S. Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein. Neuroscience 2009, 159, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Martín, E.D.; González-García, C.; Milán, M.; Fariñas, I.; Ceña, V. Stressor-related impairment of synaptic transmission in hippocampal slices from alpha-synuclein knockout mice. Eur. J. Neurosci. 2004, 20, 3085–3091. [Google Scholar] [CrossRef] [PubMed]
- Cabin, D.E.; Shimazu, K.; Murphy, D.; Cole, N.B.; Gottschalk, W.; McIlwain, K.L.; Orrison, B.; Chen, A.; Ellis, C.E.; Paylor, R.; et al. Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein. J. Neurosci. 2002, 22, 8797–8807. [Google Scholar] [CrossRef]
- Chandra, S.; Fornai, F.; Kwon, H.B.; Yazdani, U.; Atasoy, D.; Liu, X.; Hammer, R.E.; Battaglia, G.; German, D.C.; Castillo, P.E.; et al. Double-knockout mice for alpha- and beta-synucleins: Effect on synaptic functions. Proc. Natl. Acad. Sci. USA 2004, 101, 14966–14971. [Google Scholar] [CrossRef] [PubMed]
- Yavich, L.; Tanila, H.; Vepsäläinen, S.; Jäkälä, P. Role of alpha-synuclein in presynaptic dopamine recruitment. J. Neurosci. 2004, 24, 11165–11170. [Google Scholar] [CrossRef]
- Chadchankar, H.; Ihalainen, J.; Tanila, H.; Yavich, L. Decreased reuptake of dopamine in the dorsal striatum in the absence of a-synuclein. Brain Res. 2011, 1382, 37–44. [Google Scholar] [CrossRef]
- Surguchov, A. Molecular and cellular biology of synucleins. Int. Rev. Cell Mol. Biol. 2008, 270, 225–317. [Google Scholar]
- Marshall, J.F.; O’Dell, S.J.; Navarrete, R.; Rosenstein, A.J. Dopamine high-affinity transport site topography in rat brain: Major differences between dorsal and ventral striatum. Neuroscience 1990, 37, 11–21. [Google Scholar] [CrossRef]
- Sesack, S.R.; Hawrylak, V.A.; Matus, C.; Guido, M.A.; Levey, A.I. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J. Neurosci. 1998, 18, 2697–2708. [Google Scholar] [CrossRef]
- Miner, L.H.; Schroeter, S.; Blakely, R.D.; Sesack, S.R. Ultrastructural localization of the norepinephrine transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to probable dopamine terminals. J. Comp. Neurol. 2003, 466, 478–494. [Google Scholar] [CrossRef] [PubMed]
- Raiteri, M.; del Carmine, R.; Bertollini, A.; Levi, G. Effect of sympathomimetic amines on the synaptosomal transport of noradrenaline, dopamine and 5-hydroxytryptamine. Eur. J. Pharmacol. 1977, 41, 133–143. [Google Scholar] [CrossRef]
- Carboni, E.; Silvagni, A.; Vacca, C.; Di Chiara, G. Cumulative effect of norepinephrine and dopamine carrier blockade on extracellular dopamine increase in the nucleus accumbens shell, bed nucleus of stria terminalis and prefrontal cortex. J. Neurochem. 2006, 96, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Masana, M.; Bortolozzi, A.; Artigas, F. Selective enhancement of mesocortical dopaminergic transmission by noradrenergic drugs: Therapeutic opportunities in schizophrenia. Int. J. Neuropsychopharmacol. 2011, 14, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Devoto, P.; Flore, G.; Pani, L.; Gessa, G.L. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol. Psychiatry 2001, 6, 657–664. [Google Scholar] [CrossRef]
- Masana, M.; Castañé, A.; Santana, N.; Bortolozzi, A.; Artigas, F. Noradrenergic antidepressants increase cortical dopamine: Potential use in augmentation strategies. Neuropharmacology 2012, 63, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 2008; pp. 1–256. [Google Scholar]
- Santana, N.; Artigas, F. Expression of serotonin2C receptors in pyramidal and GABAergic neurons of rat prefrontal cortex: A comparison with striatum. Cereb. Cortex 2017, 27, 3125–3139. [Google Scholar]
- Zhao, T.T.; Shin, K.S.; Kim, K.S.; Park, H.J.; Kim, H.J.; Lee, K.E.; Lee, M.K. Effects of (-)-sesamin on motor and memory deficits in an MPTP-lesioned mouse model of Parkinson’s disease treated with l-DOPA. Neuroscience 2016, 339, 644–654. [Google Scholar] [CrossRef]



| Mice | Experimental Conditions | Baseline DA | |
|---|---|---|---|
| CPu | mPFC | ||
| Vehicle 4 weeks | aCSF aCSF + DMSO | 8.1 ± 2.3 15.4 ± 2.6 | 18.2 ± 1.4 25.4 ± 2.9 |
| AAV10 4 weeks | aCSF aCSF + DMSO | 9.8 ± 1.1 12.8 ± 1.9 | 21.6 ± 3.3 20.2 ± 2.1 |
| Vehicle 16 weeks | aCSF aCSF + DMSO | 7.2 ± 1.4 7.4 ± 1.5 | 16.7 ± 2.8 26.2 ± 3.1 |
| AAV10 16 weeks | aCSF aCSF + DMSO | 7.4 ± 1.9 7.5 ± 1.3 | 13.6 ± 1.9 23.5 ± 0.6 |
| Vehicle 3 days | aCSF aCSF + DMSO | 16.2 ± 1.18 20.1 ± 1.9 | 19.2 ± 1.9 14.1 ± 0.7 |
| IN-ASO-1415 3 days | aCSF aCSF + DMSO | 17.6 ± 1.8 21.3 ± 4.1 | 20.1 ± 3.7 15.2 ± 1.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavia-Collado, R.; Rodríguez-Aller, R.; Alarcón-Arís, D.; Miquel-Rio, L.; Ruiz-Bronchal, E.; Paz, V.; Campa, L.; Galofré, M.; Sgambato, V.; Bortolozzi, A. Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice. Int. J. Mol. Sci. 2022, 23, 1807. https://doi.org/10.3390/ijms23031807
Pavia-Collado R, Rodríguez-Aller R, Alarcón-Arís D, Miquel-Rio L, Ruiz-Bronchal E, Paz V, Campa L, Galofré M, Sgambato V, Bortolozzi A. Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice. International Journal of Molecular Sciences. 2022; 23(3):1807. https://doi.org/10.3390/ijms23031807
Chicago/Turabian StylePavia-Collado, Rubén, Raquel Rodríguez-Aller, Diana Alarcón-Arís, Lluís Miquel-Rio, Esther Ruiz-Bronchal, Verónica Paz, Leticia Campa, Mireia Galofré, Véronique Sgambato, and Analia Bortolozzi. 2022. "Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice" International Journal of Molecular Sciences 23, no. 3: 1807. https://doi.org/10.3390/ijms23031807
APA StylePavia-Collado, R., Rodríguez-Aller, R., Alarcón-Arís, D., Miquel-Rio, L., Ruiz-Bronchal, E., Paz, V., Campa, L., Galofré, M., Sgambato, V., & Bortolozzi, A. (2022). Up and Down γ-Synuclein Transcription in Dopamine Neurons Translates into Changes in Dopamine Neurotransmission and Behavioral Performance in Mice. International Journal of Molecular Sciences, 23(3), 1807. https://doi.org/10.3390/ijms23031807







