Lipid Droplet Accumulation Promotes RPE Dysfunction
Abstract
:1. Introduction
2. Results
2.1. Lipid Droplets Were Observed in Aged Mice RPE
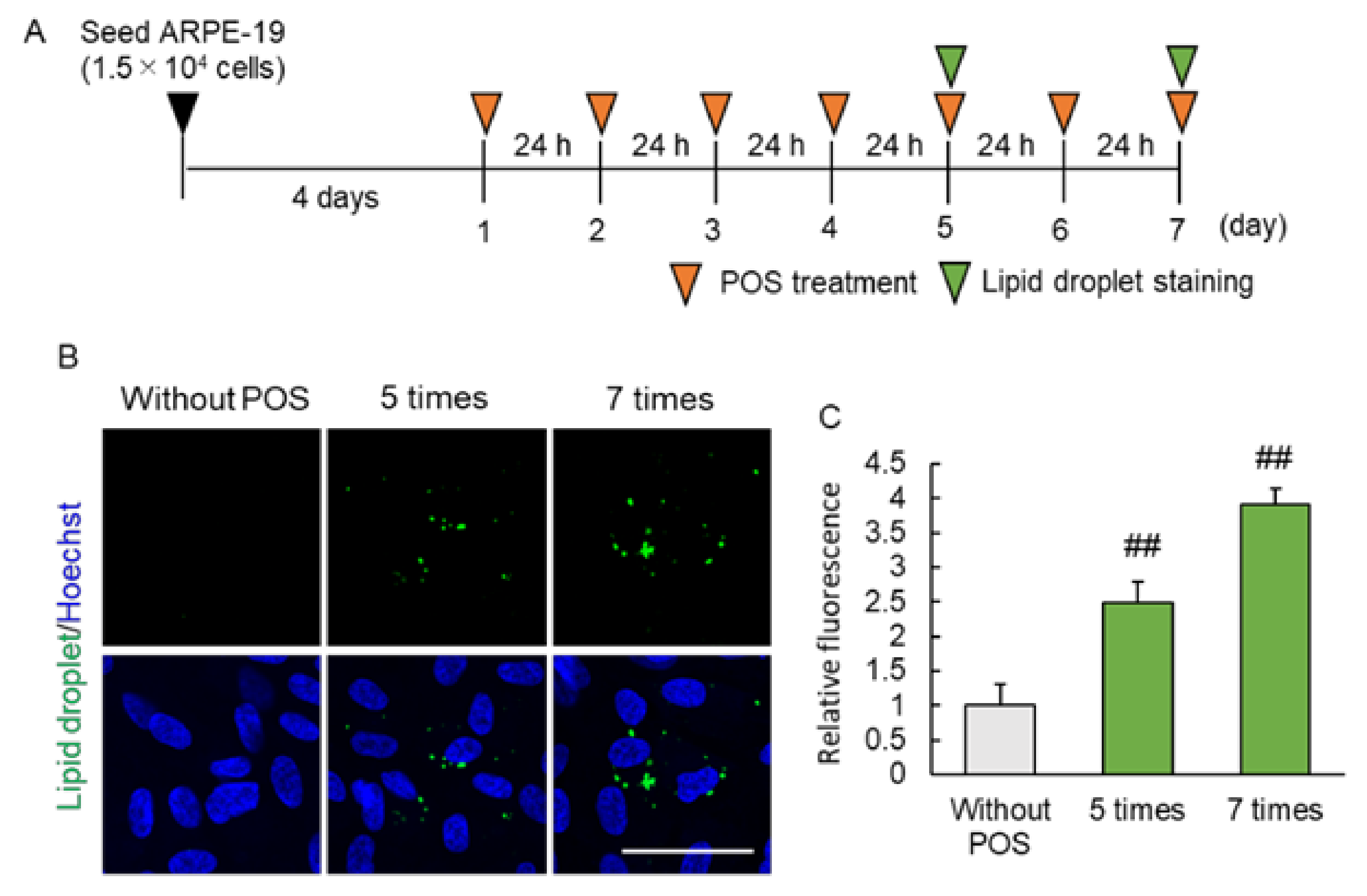
2.2. Continuous Photoreceptor Outer Segment (POS) Phagocytosis Caused Intracellular Lipid Droplet Accumulation
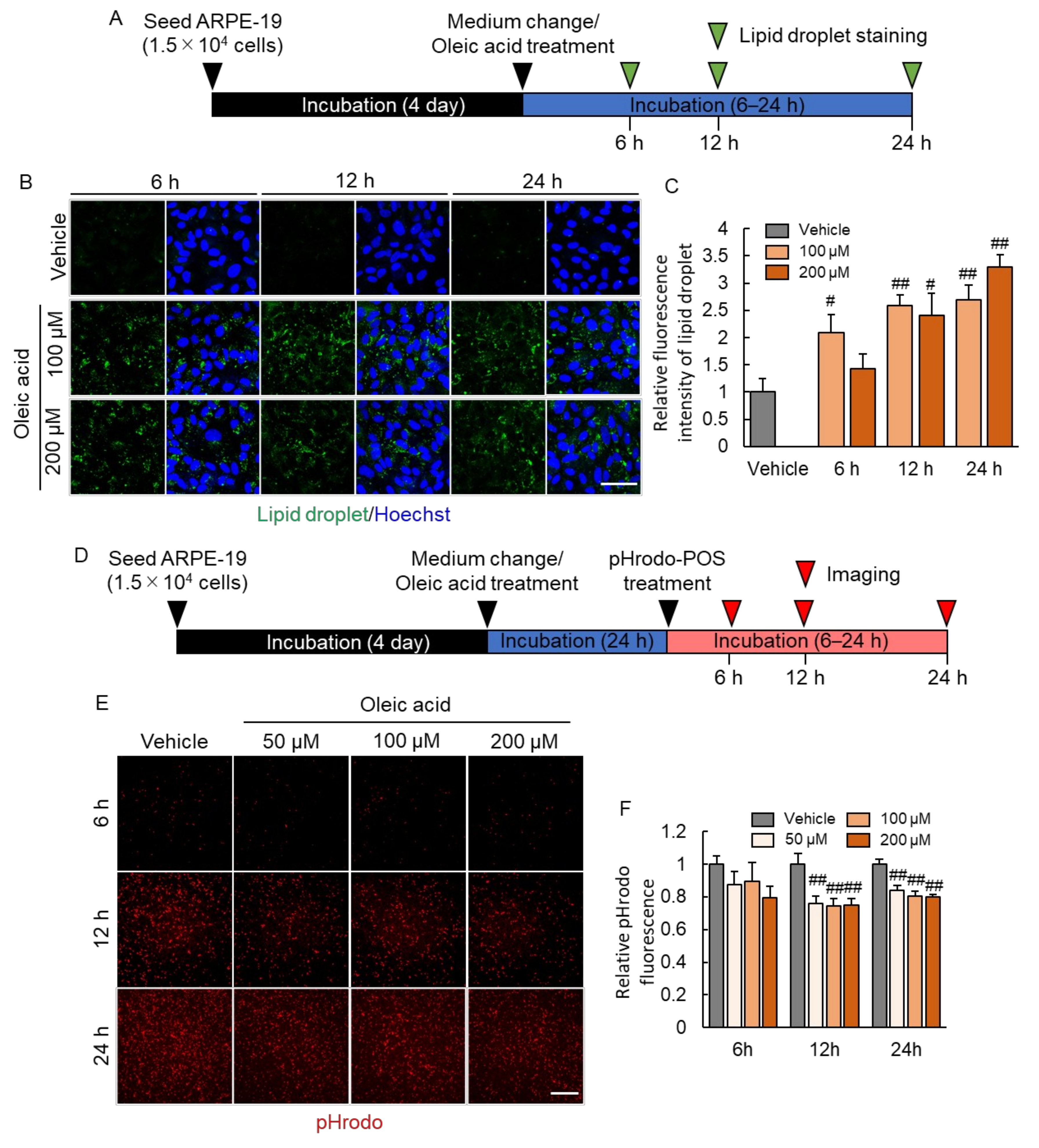
2.3. Lipid Droplet Accumulation Suppressed Phagocytosis of RPE
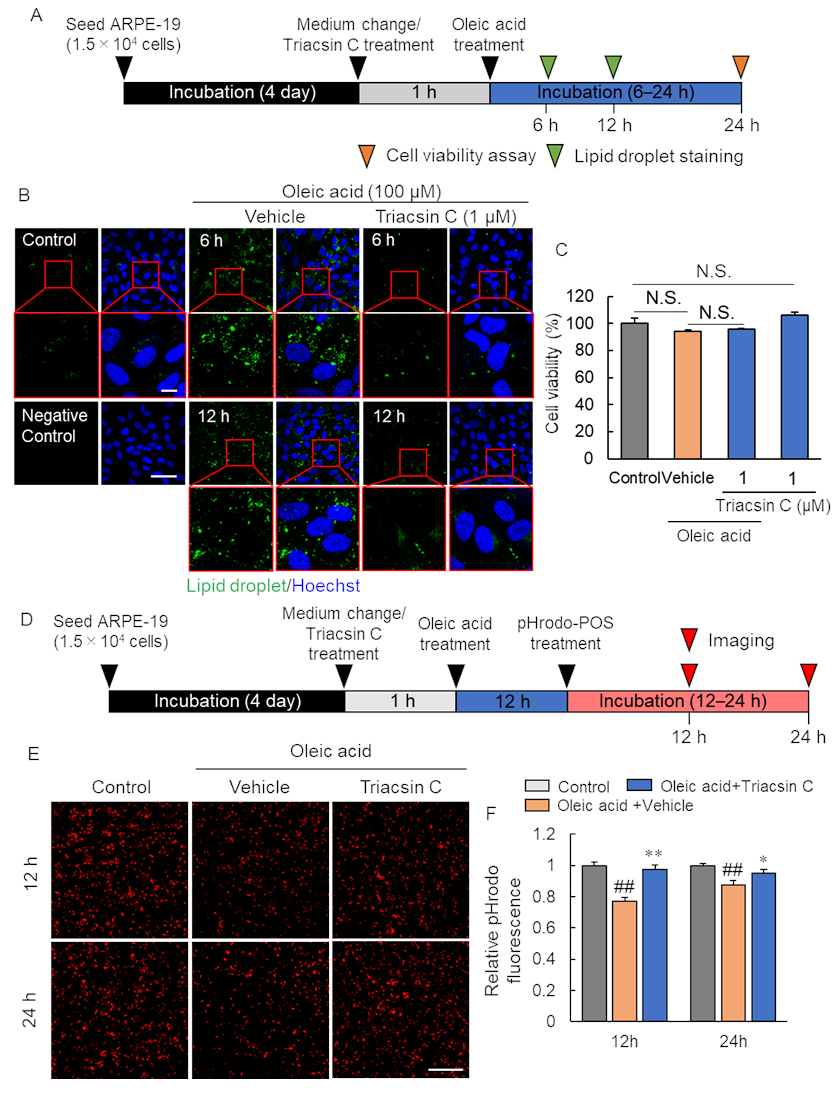
2.4. Pharmacological Inhibition of Lipid Droplet Accumulation Improved Phagocytosis of RPE
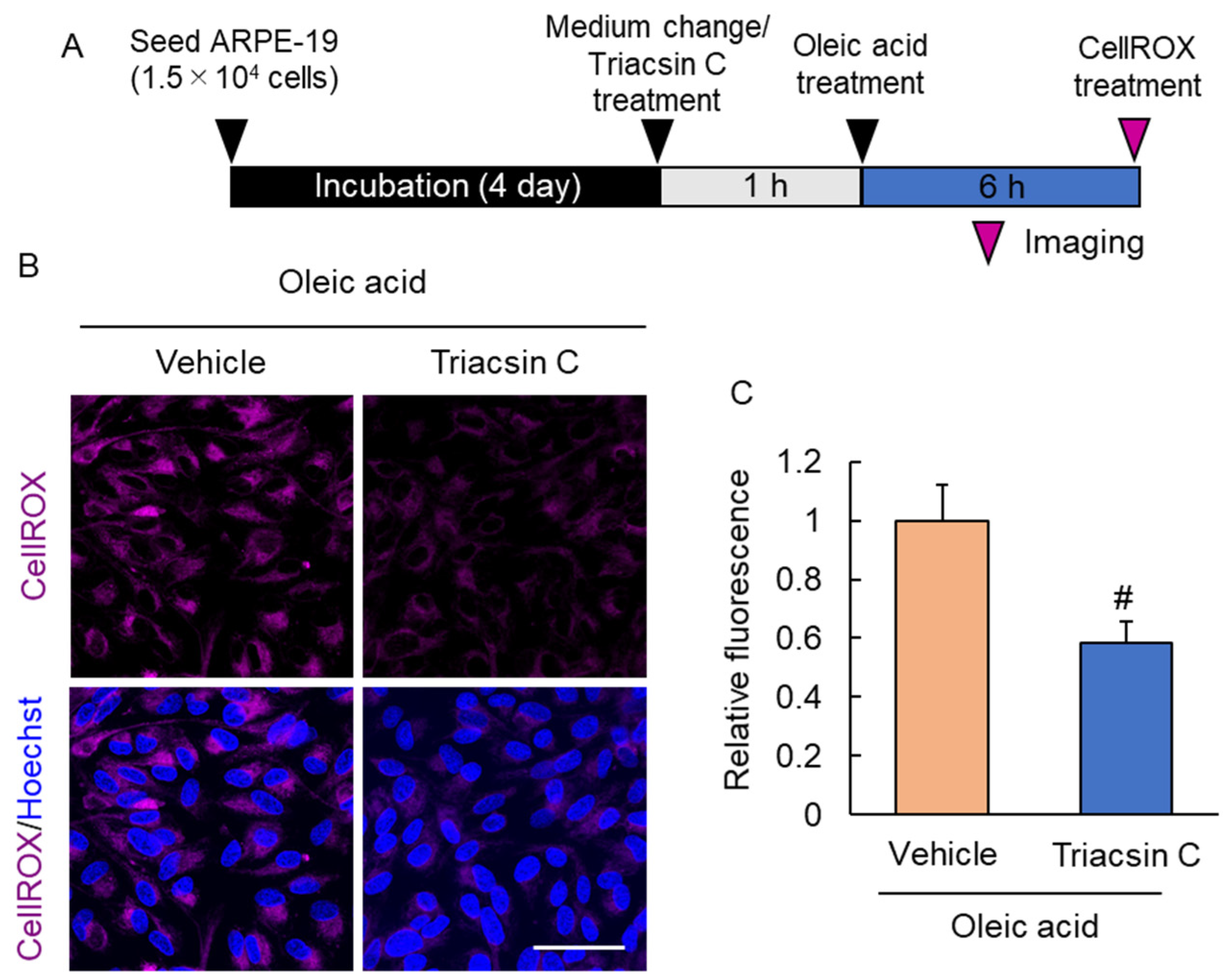
2.5. Suppression of Lipid Droplet Accumulation Inhibited Reactive Oxygen Species Production
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Western Blotting Assay In Vivo
4.3. Transmission Electron Microscope (TEM) Image
4.4. Cell Culture
4.5. POS Isolation from Porcine Eye and Treatment of the ARPE-19 Cell
4.6. POS Labeling by pHrodo™ Indicator
4.7. Lipid Droplet Staining
4.8. Oleic Acid-Induced Lipid Droplet Accumulation Model In Vitro
4.9. Cell Counting Kit-8 (CCK-8) Assay
4.10. Phagocytosis Assay Using pHrodo-POS
4.11. Intracellular Reactive Oxygen Species (ROS) Detection
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-Y.; Zhao, H.; Martinez, J.; Doggett, T.A.; Kolesnikov, A.V.; Tang, P.H.; Ablonczy, Z.; Chan, C.-C.; Zhou, Z.; Green, D.R.; et al. Noncanonical Autophagy Promotes the Visual Cycle. Cell 2013, 154, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apte, R.S. Targeting Tissue Lipids in Age-Related Macular Degeneration. eBioMedicine 2016, 5, 26–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Jia, L.; Khan, N.; Lin, C.; Mitter, S.K.; Boulton, M.E.; Dunaief, J.L.; Klionsky, D.J.; Guan, J.-L.; Thompson, D.A.; et al. Deletion of Autophagy Inducer RB1CC1 Results in Degeneration of the Retinal Pigment Epithelium. Autophagy 2015, 11, 939–953. [Google Scholar] [CrossRef] [Green Version]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.; Ding, J.; et al. Dysregulated Autophagy in the RPE Is Associated with Increased Susceptibility to Oxidative Stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golestaneh, N.; Chu, Y.; Xiao, Y.-Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional Autophagy in RPE, a Contributing Factor in Age-Related Macular Degeneration. Cell Death Dis. 2018, 8, e2537. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Du, N.; Tso, M.O.; Neufeld, A.H. Autophagy and Exomoses in the Aged Retinal Pigment Epithelium: Possible Relevance to Drusen Formation and Age-Related Macular Degeneration. PLoS ONE 2009, 4, e4160. [Google Scholar] [CrossRef] [Green Version]
- Sarks, J.P.; Sarks, S.H.; Killingsworth, M.C. Evolution of Geographic Atrophy of the Retinal Pigment Epithelium. Eye 1988, 2, 552–577. [Google Scholar] [CrossRef]
- Inana, G.; Murat, C.; An, W.; Yao, X.; Harris, I.R.; Cao, J. RPE Phagocytic Function Declines in Age-Related Macular Degeneration and Is Rescued by Human Umbilical Tissue Derived Cells. J. Transl. Med. 2018, 16, 63. [Google Scholar] [CrossRef] [Green Version]
- SanGiovanni, J.P.; Chew, E.Y. The Role of Omega-3 Long-Chain Polyunsaturated Fatty Acids in Health and Disease of the Retina. Prog. Retin. Eye Res. 2005, 24, 87–138. [Google Scholar] [CrossRef]
- Ye, F.; Kaneko, H.; Hayashi, Y.; Takayama, K.; Hwang, S.-J.; Nishizawa, Y.; Kimoto, R.; Nagasaka, Y.; Tsunekawa, T.; Matsuura, T.; et al. Malondialdehyde Induces Autophagy Dysfunction and VEGF Secretion in the Retinal Pigment Epithelium in Age-Related Macular Degeneration. Free Radic. Biol. Med. 2016, 94, 121–134. [Google Scholar] [CrossRef]
- Holz, F.G.; Bellman, C.; Staudt, S.; Schütt, F.; Völcker, H.E. Fundus Autofluorescence and Development of Geographic Atrophy in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1051–1056. [Google Scholar] [CrossRef]
- Zanzottera, E.C.; Ach, T.; Huisingh, C.; Messinger, J.D.; Spaide, R.F.; Curcio, C.A. Visualizing Retinal Pigment Epithelium Phenotypes in the Transition to Geographic Atrophy in Age-Related Macular Degeneration. Retina 2016, 36 (Suppl. 1), S12–S25. [Google Scholar] [CrossRef] [PubMed]
- Holz, F.G.; Strauss, E.C.; Schmitz-Valckenberg, S.; van Lookeren Campagne, M. Geographic Atrophy: Clinical Features and Potential Therapeutic Approaches. Ophthalmology 2014, 121, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Ohsaki, Y.; Cheng, J.; Suzuki, M.; Shinohara, Y. Lipid Droplets: A Classic Organelle with New Outfits. Histochem. Cell Biol. 2008, 130, 263–279. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, M.; Okada, S.; Nakagomi, A.; Moriya, J.; Shimizu, I.; Nojima, A.; Yoshida, Y.; Ichimiya, H.; Kamimura, N.; Kobayashi, Y.; et al. Inhibition of Endothelial P53 Improves Metabolic Abnormalities Related to Dietary Obesity. Cell Rep. 2014, 7, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy Regulates Lipid Metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- Marschallinger, J.; Iram, T.; Zardeneta, M.; Lee, S.E.; Lehallier, B.; Haney, M.S.; Pluvinage, J.V.; Mathur, V.; Hahn, O.; Morgens, D.W.; et al. Lipid-Droplet-Accumulating Microglia Represent a Dysfunctional and Proinflammatory State in the Aging Brain. Nat. Neurosci. 2020, 23, 194–208. [Google Scholar] [CrossRef]
- Sztalryd, C.; Brasaemle, D.L. The Perilipin Family of Lipid Droplet Proteins: Gatekeepers of Intracellular Lipolysis. Biochim. Biophys. Acta 2017, 1862, 1221–1232. [Google Scholar] [CrossRef]
- Strauss, O. The Retinal Pigment Epithelium in Visual Function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Murphy, D.J. The Biogenesis and Functions of Lipid Bodies in Animals, Plants and Microorganisms. Prog. Lipid Res. 2001, 40, 325–438. [Google Scholar] [CrossRef]
- Handa, J.T.; Bowes Rickman, C.; Dick, A.D.; Gorin, M.B.; Miller, J.W.; Toth, C.A.; Ueffing, M.; Zarbin, M.; Farrer, L.A. A Systems Biology Approach towards Understanding and Treating Non-Neovascular Age-Related Macular Degeneration. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yako, T.; Nakamura, M.; Otsu, W.; Nakamura, S.; Shimazawa, M.; Hara, H. Mitochondria Dynamics in the Aged Mice Eye and the Role in the RPE Phagocytosis. Exp. Eye Res. 2021, 213, 108800. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Ho, J.A. Autophagic Regulation of Retinal Pigment Epithelium Homeostasis. J. Pigment. Disord. 2015, 2, 227. [Google Scholar] [CrossRef]
- Tsuiki, E.; Fujita, A.; Ohsaki, Y.; Cheng, J.; Irie, T.; Yoshikawa, K.; Senoo, H.; Mishima, K.; Kitaoka, T.; Fujimoto, T. All-Trans-Retinol Generated by Rhodopsin Photobleaching Induces Rapid Recruitment of TIP47 to Lipid Droplets in the Retinal Pigment Epithelium. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2858–2867. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Anderson, R.E. Chemistry and Metabolism of Lipids in the Vertebrate Retina. Prog. Lipid Res. 1983, 22, 79–131. [Google Scholar] [CrossRef]
- Daemen, F.J. Vertebrate Rod Outer Segment Membranes. Biochim. Biophys. Acta 1973, 300, 255–288. [Google Scholar] [CrossRef]
- Acar, N.; Berdeaux, O.; Grégoire, S.; Cabaret, S.; Martine, L.; Gain, P.; Thuret, G.; Creuzot-Garcher, C.P.; Bron, A.M.; Bretillon, L. Lipid Composition of the Human Eye: Are Red Blood Cells a Good Mirror of Retinal and Optic Nerve Fatty Acids? PLoS ONE 2012, 7, e35102. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Kern, T.S.; Hellström, A.; Smith, L.E.H. Fatty Acid Oxidation and Photoreceptor Metabolic Needs. J. Lipid Res. 2021, 62, 100035. [Google Scholar] [CrossRef] [Green Version]
- Childs, B.G.; Baker, D.J.; Wijshake, T.; Conover, C.A.; Campisi, J.; van Deursen, J.M. Senescent Intimal Foam Cells Are Deleterious at All Stages of Atherosclerosis. Science 2016, 354, 472–477. [Google Scholar] [CrossRef]
- den Brok, M.H.; Raaijmakers, T.K.; Collado-Camps, E.; Adema, G.J. Lipid Droplets as Immune Modulators in Myeloid Cells. Trends Immunol. 2018, 39, 380–392. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. Risk Factors Associated with Age-Related Macular Degeneration. A Case-Control Study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study Report Number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of Mitochondrial Dysfunction and Their Impact on Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, F.; Heid, I.M.; Weber, B.H.F. Recombinant Haplotypes Narrow the ARMS2/HTRA1 Association Signal for Age-Related Macular Degeneration. Genetics 2017, 205, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, M.; Curcio, C.A. Esterified Cholesterol Is Highly Localized to Bruch’s Membrane, as Revealed by Lipid Histochemistry in Wholemounts of Human Choroid. J. Histochem. Cytochem. 2009, 57, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and Heterophagy Dysregulation Leads to Retinal Pigment Epithelium Dysfunction and Development of Age-Related Macular Degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and Its Role in Age-Related Macular Degeneration. Cell Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [Green Version]
- Brandstetter, C.; Mohr, L.K.M.; Latz, E.; Holz, F.G.; Krohne, T.U. Light Induces NLRP3 Inflammasome Activation in Retinal Pigment Epithelial Cells via Lipofuscin-Mediated Photooxidative Damage. J. Mol. Med. 2015, 93, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Ng, K.-P.; Gugiu, B.; Renganathan, K.; Davies, M.W.; Gu, X.; Crabb, J.S.; Kim, S.R.; Różanowska, M.B.; Bonilha, V.L.; Rayborn, M.E.; et al. Retinal Pigment Epithelium Lipofuscin Proteomics. Mol. Cell. Proteom. 2008, 7, 1397–1405. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Banerjee, K.; Lehmann, G.L.; Almeida, D.; Hajjar, K.A.; Benedicto, I.; Jiang, Z.; Radu, R.A.; Thompson, D.H.; Rodriguez-Boulan, E.; et al. Lipofuscin Causes Atypical Necroptosis through Lysosomal Membrane Permeabilization. Proc. Natl. Acad. Sci. USA 2021, 118, e2100122118. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Baron, M.; Bouhlel, M.A.; Vanhoutte, J.; Copin, C.; Sebti, Y.; Derudas, B.; Mayi, T.; Bories, G.; Tailleux, A.; et al. Human Atherosclerotic Plaque Alternative Macrophages Display Low Cholesterol Handling but High Phagocytosis Because of Distinct Activities of the PPARγ and LXRα Pathways. Circ. Res. 2011, 108, 985–995. [Google Scholar] [CrossRef]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Murase, H.; Tsuruma, K.; Shimazawa, M.; Hara, H. TUDCA Promotes Phagocytosis by Retinal Pigment Epithelium via MerTK Activation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2511–2518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, Y.; Kuse, Y.; Inoue, Y.; Nakamura, S.; Hara, H.; Shimazawa, M. Transient Acceleration of Autophagic Degradation by Pharmacological Nrf2 Activation Is Important for Retinal Pigment Epithelium Cell Survival. Redox. Biol. 2018, 19, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Homma, T.; Kurahashi, T.; Kang, E.S.; Fujii, J. Oxidative Stress Triggers Lipid Droplet Accumulation in Primary Cultured Hepatocytes by Activating Fatty Acid Synthesis. Biochem. Biophys. Res. Commun. 2015, 464, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, K.; Sandoval, H.; Yamamoto, S.; Jaiswal, M.; Sanz, E.; Li, Z.; Hui, J.; Graham, B.H.; Quintana, A.; et al. Glial Lipid Droplets and ROS Induced by Mitochondrial Defects Promote Neurodegeneration. Cell 2015, 160, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Lam, T.; Harmancey, R.; Vasquez, H.; Gilbert, B.; Patel, N.; Hariharan, V.; Lee, A.; Covey, M.; Taegtmeyer, H. Reversal of Intramyocellular Lipid Accumulation by Lipophagy and a P62-Mediated Pathway. Cell Death Discov. 2016, 2, 16061. [Google Scholar] [CrossRef] [Green Version]
- Loving, B.A.; Tang, M.; Neal, M.C.; Gorkhali, S.; Murphy, R.; Eckel, R.H.; Bruce, K.D. Lipoprotein Lipase Regulates Microglial Lipid Droplet Accumulation. Cells 2021, 10, 198. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Green, D.R. Autophagy and Phagocytosis Converge for Better Vision. Autophagy 2014, 10, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Intartaglia, D.; Giamundo, G.; Conte, I. Autophagy in the Retinal Pigment Epithelium: A New Vision and Future Challenges. FEBS J. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Murase, H.; Tsuruma, K.; Kuse, Y.; Shimazawa, M.; Hara, H. Progranulin Increases Phagocytosis by Retinal Pigment Epithelial Cells in Culture. J. Neurosci. Res. 2017, 95, 2500–2510. [Google Scholar] [CrossRef] [PubMed]
- Orban, T.; Palczewska, G.; Palczewski, K. Retinyl Ester Storage Particles (Retinosomes) from the Retinal Pigmented Epithelium Resemble Lipid Droplets in Other Tissues. J. Biol. Chem. 2011, 286, 17248–17258. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yako, T.; Otsu, W.; Nakamura, S.; Shimazawa, M.; Hara, H. Lipid Droplet Accumulation Promotes RPE Dysfunction. Int. J. Mol. Sci. 2022, 23, 1790. https://doi.org/10.3390/ijms23031790
Yako T, Otsu W, Nakamura S, Shimazawa M, Hara H. Lipid Droplet Accumulation Promotes RPE Dysfunction. International Journal of Molecular Sciences. 2022; 23(3):1790. https://doi.org/10.3390/ijms23031790
Chicago/Turabian StyleYako, Tomohiro, Wataru Otsu, Shinsuke Nakamura, Masamitsu Shimazawa, and Hideaki Hara. 2022. "Lipid Droplet Accumulation Promotes RPE Dysfunction" International Journal of Molecular Sciences 23, no. 3: 1790. https://doi.org/10.3390/ijms23031790
APA StyleYako, T., Otsu, W., Nakamura, S., Shimazawa, M., & Hara, H. (2022). Lipid Droplet Accumulation Promotes RPE Dysfunction. International Journal of Molecular Sciences, 23(3), 1790. https://doi.org/10.3390/ijms23031790





