COMT Val/Met and Psychopathic Traits in Children and Adolescents: A Systematic Review and New Evidence of a Developmental Trajectory toward Psychopathy
Abstract
1. Introduction
1.1. Psychopathy
1.2. Psychopathic Traits in Youth
1.3. Psychopathic Traits and Genetics
1.3.1. Catechol-O-Methyltransferase (COMT)
1.3.2. Psychopathic Traits and COMT
2. Materials and Methods
2.1. Systematic Review—Data Selection
2.2. New Evidence—Original Data
2.2.1. Participants
2.2.2. Measures
2.2.3. Genotyping
2.2.4. Analyses
3. Results
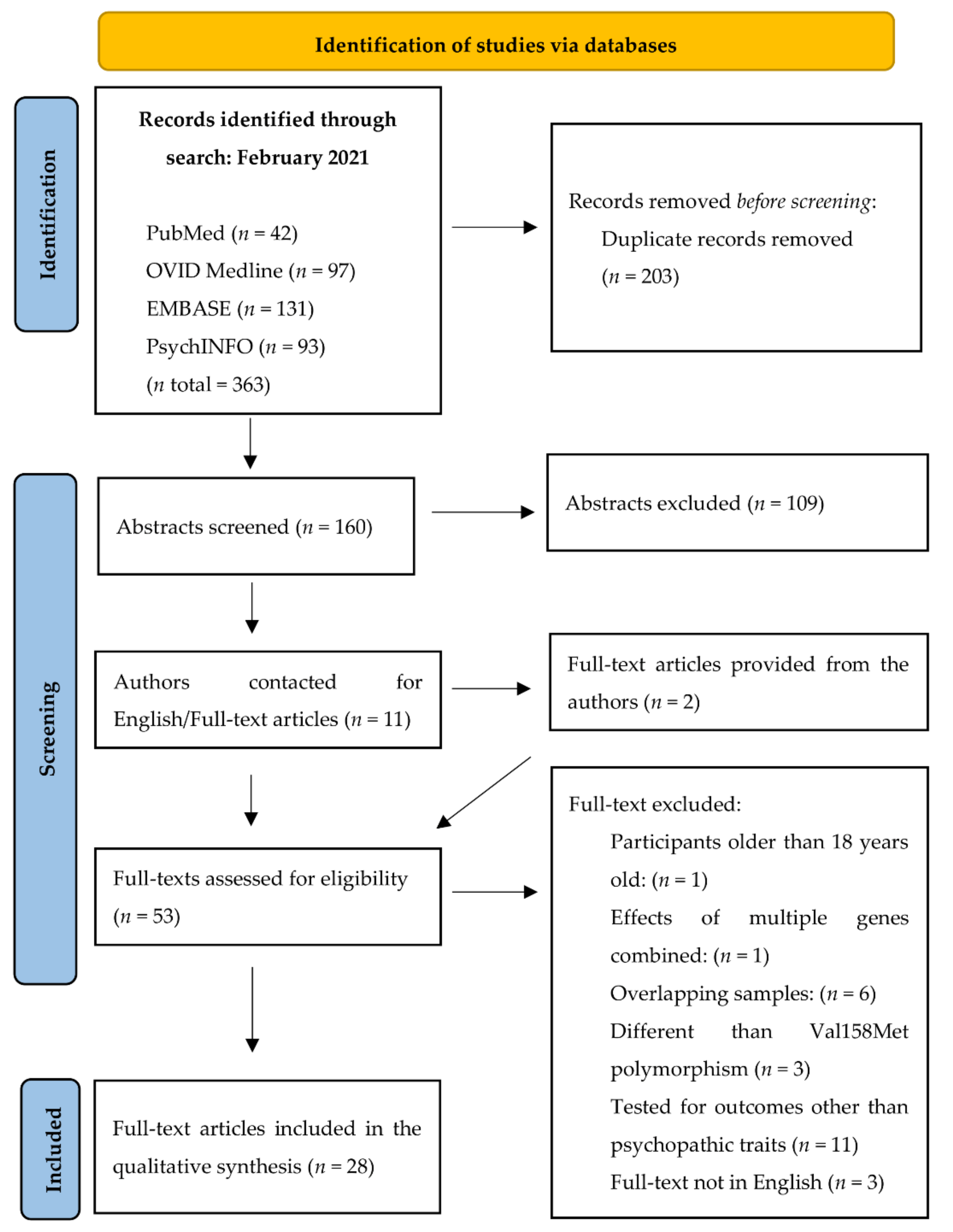
3.1. Search Results and Data Extraction
3.2. COMT in Psychopathic Traits—Systematic Review
3.2.1. Environmental Factors
3.2.2. Sex
3.2.3. Diagnosis of ADHD and/or Externalizing Disorders with Aggression
3.2.4. Age
3.2.5. Other Factors
Ethnicity
Autism Spectrum Disorder and 22q11.2 Deletion Syndrome
3.3. Original Data
4. Discussion
4.1. COMT and Psychopathic Traits
4.2. COMT, Dopamine and Development
4.3. Clinical Implications
4.4. Limitations
4.5. Future Directions in the Field
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blair, R.J.; Peschardt, K.S.; Budhani, S.; Mitchell, D.G.; Pine, D.S. The development of psychopathy. J. Child Psychol. Psychiatry 2006, 47, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Patrick, C.J. (Ed.) Handbook of Psychopathy; Guilford Publications: New York, NY, USA, 2018. [Google Scholar]
- Schroeder, M.L.; Schroeder, K.G.; Hare, R.D. Generalizability of a checklist for assessment of psychopathy. J. Consult. Clin. Psychol. 1983, 51, 511. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M.; Cacciola, J.S.; Alterman, A.I.; McKay, J.R.; Cook, T.G. The 2-year test-retest reliability of the Psychopathy Checklist—Revised in methadone patients. Assessment 1999, 6, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Frick, P.J.; Kimonis, E.R.; Dandreaux, D.M.; Farell, J.M. The 4 year stability of psychopathic traits in non-referred youth. Behav. Sci. Law 2003, 21, 713–736. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.R.; Rijo, D.; Salekin, R.T. Psychopathic traits in children and youth: The state-of-the-art after 30 years of research. Aggress. Violent Behav. 2020, 55, 101454. [Google Scholar] [CrossRef]
- Barry, C.T.; Frick, P.J.; DeShazo, T.M.; McCoy, M.; Ellis, M.; Loney, B.R. The importance of callous–unemotional traits for extending the concept of psychopathy to children. J. Abnorm. Psychol. 2000, 109, 335. [Google Scholar] [CrossRef]
- Salekin, R.T.; Frick, P.J. Psychopathy in children and adolescents: The need for a developmental perspective. J. Abnorm. Child Psychol. 2005, 33, 403–409. [Google Scholar] [CrossRef]
- Baskin-Sommers, A.R.; Baskin, D. Psychopathic traits mediate the relationship between exposure to violence and violent juvenile offending. J. Psychopathol. Behav. Assess. 2016, 38, 341–349. [Google Scholar] [CrossRef]
- Blair, R.J. The neurobiology of psychopathic traits in youths. Nat. Rev. Neurosci. 2013, 14, 786–799. [Google Scholar] [CrossRef]
- Blair, R.J.; Leibenluft, E.; Pine, D.S. Conduct disorder and callous–unemotional traits in youth. N. Engl. J. Med. 2014, 371, 2207–2216. [Google Scholar] [CrossRef]
- Viding, E.; McCrory, E.J. Why should we care about measuring callous–unemotional traits in children? Br. J. Psychiatry 2012, 200, 177–178. [Google Scholar] [CrossRef]
- Baskin-Sommers, A.R.; Waller, R.; Fish, A.M.; Hyde, L.W. Callous-unemotional traits trajectories interact with earlier conduct problems and executive control to predict violence and substance use among high risk male adolescents. J. Abnorm. Child Psychol. 2015, 43, 1529–1541. [Google Scholar] [CrossRef]
- Viding, E.; McCrory, E.J. Understanding the development of psychopathy: Progress and challenges. Psychol. Med. 2018, 48, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Frick, P.J.; Ray, J.V.; Thornton, L.C.; Kahn, R.E. Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychol. Bull. 2014, 140, 1–57. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.D.; Loeber, R.; Lahey, B.B.; Rathouz, P.J. Developmental transitions among affective and behavioral disorders in adolescent boys. J. Child Psychol. Psychiatry 2005, 46, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Akutagava-Martins, G.C.; Salatino-Oliveira, A.; Kieling, C.; Genro, J.P.; Polanczyk, G.V.; Anselmi, L.; Menezes, A.M.; Gonçalves, H.; Wehrmeister, F.C.; Barros, F.C.; et al. COMT and DAT1 genes are associated with hyperactivity and inattention traits in the 1993 Pelotas Birth Cohort: Evidence of sex-specific combined effect. J. Psychiatry Neurosci. 2016, 41, 405. [Google Scholar] [CrossRef] [PubMed]
- Colins, O.F.; Andershed, H. Childhood and adolescent psychopathy. In Routledge International Handbook of Psychopathy and Crime; Delisi, M., Ed.; Routledge: Abingdon, UK, 2018; pp. 166–184. [Google Scholar]
- da Silva, D.R.; Vagos, P.; Rijo, D. Conceptualizing psychopathic traits from an evolutionary-based perspective: An empirical study in a community sample of boys and girls. Curr. Psychol. 2019, 40, 3931–3943. [Google Scholar] [CrossRef]
- Frick, P.J.; Viding, E. Antisocial behavior from a developmental psychopathology perspective. Dev. Psychopathol. 2009, 21, 1111–1131. [Google Scholar] [CrossRef]
- Blonigen, D.M.; Hicks, B.M.; Krueger, R.F.; Patrick, C.J.; Iacono, W.G. Continuity and change in psychopathic traits as measured via normal-range personality: A longitudinal-biometric study. J. Abnorm. Psychol. 2006, 115, 85. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, N.M.; Rijsdijk, F.V.; McCrory, E.J.; Viding, E. Etiology of different developmental trajectories of callous-unemotional traits. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 656–664. [Google Scholar]
- Forsman, M.; Lichtenstein, P.; Andershed, H.; Larsson, H. Genetic effects explain the stability of psychopathic personality from mid-to late adolescence. J. Abnorm. Psychol. 2008, 117, 606–617. [Google Scholar] [CrossRef]
- Blonigen, D.M.; Hicks, B.M.; Krueger, R.F.; Patrick, C.J.; Iacono, W.G. Psychopathic personality traits: Heritability and genetic overlap with internalizing and externalizing psychopathology. Psychol. Med. 2005, 35, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Zai, C.C.; Ehtesham, S.; Choi, E.; Nowrouzi, B.; De Luca, V.; Stankovich, L.; Davidge, K.; Freeman, N.; King, N.; Kennedy, J.L.; et al. Dopaminergic system genes in childhood aggression: Possible role for DRD2. World J. Biol. Psychiatry 2012, 13, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Barnes, J.C. Two dopamine receptor genes (DRD2 and DRD4) predict psychopathic personality traits in a sample of American adults. J. Crim. Justice 2013, 41, 188–195. [Google Scholar] [CrossRef]
- Kant, T.; Koyama, E.; Zai, C.C.; Beitchman, J.H.; Kennedy, J.L. Association of the MAOA-uVNTR polymorphism with psychopathic traits may change from childhood to adolescence. Eur. Arch. Psychiatry Clin. Neurosci. 2022. [Google Scholar] [CrossRef]
- Fowler, T.; Langley, K.; Rice, F.; van den Bree, M.B.M.; Ross, K.; Wilkinson, L.S.; Owen, M.J.; O’Donovan, M.C.; Thapar, A. Psychopathy trait scores in adolescents with childhood ADHD: The contribution of genotypes affecting MAOA, 5HTT and COMT activity. Psychiatr. Genet. 2009, 19, 312–319. [Google Scholar] [CrossRef]
- Grossman, M.H.; Emanuel, B.S.; Budarf, M.L. Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1→q11.2. Genomics 1992, 12, 822–825. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Deater-Deckard, K.; Zhang, W. Interacting effect of Catechol-O-Methyltransferase (COMT) and Monoamine Oxidase A (MAOA) Gene Polymorphisms, and stressful life events on aggressive behavior in Chinese Male adolescents. Front. Psychol. 2018, 9, 1079. [Google Scholar] [CrossRef]
- Hong, J.; Shu-Leong, H.; Tao, X.; Lap-Ping, Y. Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport 1998, 9, 2861–2864. [Google Scholar] [CrossRef]
- Iofrida, C.; Palumbo, S.; Pellegrini, S. Molecular genetics and antisocial behavior: Where do we stand? Exp. Biol. Med. 2014, 239, 1514–1523. [Google Scholar] [CrossRef]
- Goldman, D.; Weinberger, D.R.; Malhotra, A.K.; Goldberg, T.E. The role of COMT Val158Met in cognition. Biol. Psychiatry 2009, 65, e1–e2. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lipska, B.K.; Halim, N.; Ma, Q.D.; Matsumoto, M.; Melhem, S.; Kolachana, B.S.; Hyde, T.M.; Herman, M.M.; Apud, J.; et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): Effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 2004, 75, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Lotta, T.; Vidgren, J.; Tilgmann, C.; Ulmanen, I.; Melen, K.; Julkunen, I.; Taskinen, J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995, 34, 4202–4210. [Google Scholar] [CrossRef]
- Mariz, C.; Cruz, O.S.; Moreira, D. The influence of environmental and genetic factors on the development of psychopathy: A systematic review. Aggress. Violent Behav. 2022, 62, 101715. [Google Scholar] [CrossRef]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Benning, S.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat. Neurosci. 2010, 13, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Gogos, J.A.; Morgan, M.; Luine, V.; Santha, M.; Ogawa, S.; Pfaff, D.; Karayiorgou, M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc. Natl. Acad. Sci. USA 1998, 95, 9991–9996. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, M.D.; Harder, V.S.; Althoff, R.R.; Rettew, D.C.; Ehli, E.A.; Lengyel-Nelson, T.; Davies, G.E.; Ayer, L.; Sulman, J.; Stanger, C.; et al. COMT Val158Met genotype as a risk factor for problem behaviors in youth. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 841–849. [Google Scholar] [CrossRef]
- Bearden, C.E.; Jawad, A.F.; Lynch, D.R.; Monterossso, J.R.; Sokol, S.; McDonald-McGinn, D.M.; Saitta, S.C.; Harris, S.E.; Moss, E.; Wang, P.P.; et al. Effects of COMT genotype on behavioral symptomatology in the 22q11.2 Deletion Syndrome. Child Neuropsychol. 2005, 11, 109–117. [Google Scholar] [CrossRef]
- Nobile, M.; Rusconi, M.; Bellina, M.; Marino, C.; Giorda, R.; Carlet, O.; Vanzin, L.; Molteni, M.; Battaglia, M. COMT Val158Met polymorphism and socioeconomic status interact to predict attention deficit/hyperactivity problems in children aged 10–14. Eur. Child Adolesc. Psychiatry 2010, 19, 549–557. [Google Scholar] [CrossRef]
- Waller, R.; Trentacosta, C.J.; Shaw, D.S.; Neiderhiser, J.M.; Ganiban, J.M.; Reiss, D.; Leve, L.D.; Hyde, L.W. Heritable temperament pathways to early callous–unemotional behavior. Br. J. Psychiatry 2016, 209, 475–482. [Google Scholar] [CrossRef]
- Hyde, L.W.; Waller, R.; Trentacosta, C.J.; Shaw, D.S.; Neiderhiser, J.M.; Ganiban, J.M.; Reiss, D.; Leve, L.D. Heritable and nonheritable pathways to early callous-unemotional behaviors. Am. J. Psychiatry 2016, 173, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Pritchett, L.; Løhaugen, G.; Kogachi, S.; Fukaya, E.; Hernandez, A.; Jiang, C.; Alicata, D.; Ernst, T.; Chang, L. Sex-differences in cognition and behaviors in children with family history of substance use disorders. Drug Alcohol Depend. 2015, 146, e173. [Google Scholar] [CrossRef]
- White, T.P.; Loth, E.; Rubia, K.; Krabbendam, L.; Whelan, R.; Banaschewski, T.; Barker, G.J.; Bokde, A.L.; Büchel, C.; Conrod, P.; et al. Sex differences in COMT polymorphism effects on prefrontal inhibitory control in adolescence. Neuropsychopharmacology 2014, 39, 2560–2569. [Google Scholar] [CrossRef]
- Caspi, A.; Langley, K.; Milne, B.; Moffitt, T.E.; O’Donovan, M.; Owen, M.J.; Polo Tomas, M.; Poulton, R.; Rutter, M.; Taylor, A.; et al. A replicated molecular genetic basis for subtyping antisocial behavior in children with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2008, 65, 203–210. [Google Scholar] [CrossRef]
- Tunbridge, E.M.; Weickert, C.S.; Kleinman, J.E.; Herman, M.M.; Chen, J.; Kolachana, B.S.; Harrison, P.J.; Weinberger, D.R. Catechol-O-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb. Cortex 2007, 17, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.T.; Boettiger, C.A. Age modulates the effect of COMT genotype on delay discounting behavior. Psychopharmacology 2012, 222, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.M.; Huemer, J.; Rabl, U.; Boubela, R.N.; Kalcher, K.; Berger, A.; Banaschewski, T.; Barker, G.; Bokde, A.; Büchel, C.; et al. Oppositional COMT Val158Met effects on resting state functional connectivity in adolescents and adults. Brain Struct. Funct. 2016, 221, 103–114. [Google Scholar] [CrossRef]
- Dumontheil, I.; Roggeman, C.; Ziermans, T.; Peyrard-Janvid, M.; Matsson, H.; Kere, J.; Klingberg, T. Influence of the COMT genotype on working memory and brain activity changes during development. Biol. Psychiatry 2011, 70, 222–229. [Google Scholar] [CrossRef]
- Sinclair, D.; Purves-Tyson, T.D.; Allen, K.M.; Weickert, C.S. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology 2014, 231, 1581–1599. [Google Scholar] [CrossRef]
- Farrell, A.D.; Sullivan, T.N.; Esposito, L.E.; Meyer, A.L.; Valois, R.F. A latent growth curve analysis of the structure of aggression, drug use, and delinquent behaviors and their interrelations over time in urban and rural adolescents. J. Res. Adolesc. 2005, 15, 179–204. [Google Scholar] [CrossRef]
- Rothmond, D.A.; Weickert, C.S.; Webster, M.J. Developmental changes in human dopamine neurotransmission: Cortical receptors and terminators. BMC Neurosci. 2012, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Zai, C.C.; Nowrouzi, B.; Beitchman, J.H.; Kennedy, J.L. Study of the catechol-O-methyltransferase (COMT) gene with high aggression in children. Aggress. Behav. 2013, 39, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Calati, R.; Porcelli, S.; Giegling, I.; Hartmann, A.M.; Möller, H.J.; De Ronchi, D.; Serretti, A.; Rujescu, D. Catechol-O-methyltransferase gene modulation on suicidal behavior and personality traits: Review, meta-analysis and association study. J. Psychiatr. Res. 2011, 45, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Joo, Y.; Kim, B.; Chung, S.; Kim, H.L.; Lee, I.; Choi, B.; Kim, C.; Song, K. Association of Ala72Ser polymorphism with COMT enzyme activity and the risk of schizophrenia in Koreans. Hum. Genet. 2005, 116, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Giakoumaki, S.G.; Roussos, P.; Bitsios, P. Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology 2008, 33, 3058–3068. [Google Scholar] [CrossRef]
- Ficks, C.A.; Waldman, I.D. Candidate genes for aggression and antisocial behavior: A meta-analysis of association studies of the 5HTTLPR and MAOA-uVNTR. Behav. Genet. 2014, 44, 427–444. [Google Scholar] [CrossRef]
- Qayyum, A.; Zai, C.C.; Hirata, Y.; Tiwari, A.K.; Cheema, S.; Nowrouzi, B.; Beitchman, J.H.; Kennedy, L. The role of the catechol-O-methyltransferase (COMT) GeneVal158Met in aggressive behavior, a review of genetic studies. Curr. Neuropharmacol. 2015, 13, 802–814. [Google Scholar] [CrossRef]
- Abraham, E.; Scott, M.A.; Blair, C. Catechol-O-methyltransferase Val158Met Genotype and Early-Life Family Adversity Interactively Affect Attention-Deficit Hyperactivity Symptoms Across Childhood. Front. Genet. 2020, 11, 724. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Beitchman, J.H.; Mik, H.M.; Ehtesham, S.; Douglas, L.; Kennedy, J.L. MAOA and persistent, pervasive childhood aggression. Mol. Psychiatry 2004, 9, 546–547. [Google Scholar] [CrossRef]
- Achenbach, T.M. The Child Behavior Checklist and related instruments. In The Use of Psychological Testing for Treatment Planning and Outcomes Assessment; Maruish, M.E., Ed.; Lawrence Erlbaum Associates Publishers: Mahwah, NJ, USA, 1999. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Zhang, W.; Cao, C.; Wang, M.; Ji, L.; Cao, Y. Monoamine Oxidase A (MAOA) and Catechol-O-Methyltransferase (COMT) Gene Polymorphisms Interact with Maternal Parenting in Association with Adolescent Reactive Aggression but not Proactive Aggression: Evidence of Differential Susceptibility. J. Youth Adolesc. 2016, 45, 812–829. [Google Scholar] [CrossRef] [PubMed]
- Derringer, J. A simple correction for non-independent tests. psyArXiv 2018. Available online: https://psyarxiv.com/f2tyw/ (accessed on 22 October 2021).
- Hygen, B.W.; Belsky, J.; Stenseng, F.; Lydersen, S.; Guzey, I.C.; Wichstrøm, L. Child exposure to serious life events, COMT, and aggression: Testing differential susceptibility theory. Dev. Psychol. 2015, 51, 1098. [Google Scholar] [CrossRef] [PubMed]
- Nederhof, E.; Belsky, J.; Ormel, J.; Oldehinkel, A.J. Effects of divorce on Dutch boys’ and girls’ externalizing behavior in Gene x Environment perspective: Diathesis stress or differential susceptibility in the Dutch Tracking Adolescents’ Individual Lives Survey study? Dev. Psychopathol. 2012, 24, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, C.W.; Hankin, B.L.; Jenness, J.L.; Young, J.F.; Smolen, A. Observed positive parenting behaviors and youth genotype: Evidence for gene-environment correlations and moderation by parent personality traits. Dev. Psychopathol. 2013, 25, 175–191. [Google Scholar] [CrossRef][Green Version]
- Davies, P.T.; Pearson, J.K.; Cicchetti, D.; Martin, M.J.; Cummings, E.M. Emotional insecurity as a mediator of the moderating role of dopamine genes in the association between interparental conflict and youth externalizing problems. Dev. Psychopathol. 2019, 31, 1111–1126. [Google Scholar] [CrossRef]
- Brennan, P.A.; Hammen, C.; Sylvers, P.; Bor, W.; Najman, J.; Lind, P.; Montgomery, G.; Smith, A.K. Interactions between the COMT Val108/158Met polymorphism and maternal prenatal smoking predict aggressive behavior outcomes. Biol. Psychol. 2011, 87, 99–105. [Google Scholar] [CrossRef]
- Salatino-Oliveira, A.; Murray, J.; Kieling, C.; Genro, J.P.; Polanczyk, G.; Anselmi, L.; Wehrmeister, F.; de Barros, F.C.; Menezes, A.M.; Rohde, L.A.; et al. COMT and prenatal maternal smoking in associations with conduct problems and crime: The Pelotas 1993 birth cohort study. Sci. Rep. 2016, 6, 29900. [Google Scholar] [CrossRef]
- Thapar, A.; Langley, K.; Fowler, T.; Rice, F.; Turic, D.; Whittinger, N.; Aggleton, J.; Van den Bree, M.; Owen, M.; O’Donovan, M. Catechol O-methyltransferase gene variant and birth weight predict early-onset antisocial behavior in children with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2005, 62, 1275–1278. [Google Scholar] [CrossRef][Green Version]
- Sengupta, S.M.; Grizenko, N.; Schmitz, N.; Schwartz, G.; Ben Amor, L.; Bellingham, J.; de Guzman, R.; Polotskaia, A.; Ter Stepanian, M.; Thakur, G.; et al. COMT Val108/158Met gene variant, birth weight, and conduct disorder in children with ADHD. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 1363–1369. [Google Scholar] [CrossRef]
- Pálmason, H.; Moser, D.; Sigmund, J.; Vogler, C.; Hänig, S.; Schneider, A.; Seitz, C.; Marcus, A.; Meyer, J.; Freitag, C.M. Attention-deficit/hyperactivity disorder phenotype is influenced by a functional catechol-O-methyltransferase variant. J. Neural Transm. 2010, 117, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.-J.; Liu, J.; Wang, Y.-F.; Yang, L.; Guan, L.-L.; Faraone, S.V. Attention Deficit Hyperactivity Disorder comorbid oppositional defiant disorder and its predominately inattentive type: Evidence for an association with COMT but not MAOA in a Chinese sample. Behav. Brain Funct. 2009, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Paloyelis, Y.; Asherson, P.; Mehta, M.A.; Faraone, S.V.; Kuntsi, J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology 2010, 35, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Nikolac Perkovic, M.; Kiive, E.; Nedic Erjavec, G.; Veidebaum, T.; Curkovic, M.; Dodig-Curkovic, K.; Muck-Seler, D.; Harro, J.; Pivac, N. The association between the catechol-O-methyltransferase Val108/158Met polymorphism and hyperactive-impulsive and inattentive symptoms in youth. Psychopharmacology 2013, 230, 69–76. [Google Scholar] [CrossRef]
- DeYoung, C.G.; Getchell, M.; Koposov, R.A.; Yrigollen, C.M.; Haeffel, G.J.; af Klinteberg, B.; Oreland, L.; Ruchkin, V.V.; Pakstis, A.J.; Grigorenko, E.L. Variation in the catechol-O-methyltransferase Val 158 Met polymorphism associated with conduct disorder and ADHD symptoms, among adolescent male delinquents. Psychiatr. Genet. 2010, 20, 20–24. [Google Scholar] [CrossRef]
- Mills, S.; Langley, K.; Van den Bree, M.; Street, E.; Turic, D.; Owen, M.J.; O’Donovan, M.C.; Thapar, A. No evidence of association between Catechol-O-Methyltransferase (COMT) Val158Met genotype and performance on neuropsychological tasks in children with ADHA: A case-control study. BMC Psychiatry 2004, 4, 15. [Google Scholar] [CrossRef]
- Salatino-Oliveira, A.; Genro, J.P.; Guimarães, A.P.; Chazan, R.; Zeni, C.; Schmitz, M.; Polanczyk, G.; Roman, T.; Rohde, L.A.; Hutz, M.H. Cathechol-O-methyltransferase Val(158)Met polymorphism is associated with disruptive behavior disorders among children and adolescents with ADHD. J. Neural Transm. 2012, 119, 729–733. [Google Scholar] [CrossRef]
- Karam, R.A.; Rezk, N.A.; Abdelrahman, H.M.; Hassan, T.H.; Mohammad, D.; Hashim, H.M.; Fattah, N.R. Catechol-O-methyltransferase Val158Met polymorphism and hyperactivity symptoms in Egyptian children with autism spectrum disorder. Res. Dev. Disabil. 2013, 34, 2092–2097. [Google Scholar] [CrossRef]
- Amstadter, A.B.; Macpherson, L.; Wang, F.; Banducci, A.N.; Reynolds, E.K.; Potenza, M.N.; Gelernter, J.; Lejuez, C.W. The relationship between risk-taking propensity and the COMT Val(158)Met polymorphism among early adolescents as a function of sex. J. Psychiatr. Res. 2012, 46, 940–945. [Google Scholar] [CrossRef]
- Langley, K.; Heron, J.; O’Donovan, M.C.; Owen, M.J.; Thapar, A. Genotype link with extreme antisocial behavior: The contribution of cognitive pathways. Arch. Gen. Psychiatry 2010, 67, 1317–1323. [Google Scholar] [CrossRef][Green Version]
- Park, Y.; Waldman, I.D. Influence of the COMT val108/158met polymorphism on continuous performance task indices. Neuropsychologia 2014, 61, 45–55. [Google Scholar] [CrossRef] [PubMed]
- van Goozen, S.H.M.; Langley, K.; Northover, C.; Hubble, K.; Rubia, K.; Schepman, K.; O’Donovan, M.C.; Thapar, A. Identifying mechanisms that underlie links between COMT genotype and aggression in male adolescents with ADHD. J. Child Psychol. Psychiatry 2016, 57, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Lindenberg, A.; Weinberger, D.R. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat. Rev. Neurosci. 2006, 7, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Buckholtz, J.W.; Treadway, M.T.; Cowan, R.L.; Woodward, N.D.; Li, R.; Ansari, M.S.; Baldwin, R.M.; Schwartzman, A.N.; Shelby, E.S.; Smith, C.E.; et al. Dopaminergic network differences in human impulsivity. Science 2010, 329, 532. [Google Scholar] [CrossRef]
- Arnsten, A.F. Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. 1997, 11, 151–162. [Google Scholar] [CrossRef]
- da Silva, D.R.; Rijo, D.; Salekin, R.T. Child and adolescent psychopathy: A state-of-the-art reflection on the construct and etiological theories. J. Crim. Justice 2012, 40, 269–277. [Google Scholar] [CrossRef]
- da Silva, D.R.; Rijo, D.; Salekin, R.T. The evolutionary roots of psychopathy. Aggress. Violent Behav. 2015, 21, 85–96. [Google Scholar] [CrossRef]
- Caldwell, M.F.; McCormick, D.; Wolfe, J.; Umstead, D. Treatment-related changes in psychopathy features and behavior in adolescent offenders. Crim. Justice Behav. 2012, 39, 144–155. [Google Scholar] [CrossRef]
- Colins, O.F.; Andershed, H.; Salekin, R.T.; Fanti, K.A. Comparing different approaches for subtyping children with conduct problems: Callous-unemotional traits only versus the multidimensional psychopathy construct. J. Psychopathol. Behav. Assess. 2018, 40, 6–15. [Google Scholar] [CrossRef]
- Romeo, R.; Knapp, M.; Scott, S. Economic cost of severe antisocial behaviour in children—And who pays it. Br. J. Psychiatry 2006, 188, 547–553. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Nichols, T.; Callicott, J.H.; Ding, J.; Kolachana, B.; Buckholtz, J.; Mattay, V.S.; Egan, M.; Weinberger, D.R. Impact of complex genetic variation in COMT on human brain function. Mol. Psychiatry 2006, 11, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, M.; Bijttebier, P.; Claes, S.; Colpin, H.; Goossens, L.; Hankin, B.; Van Den Noortgate, W.; Verschueren, K.; Young, J.; Van Leeuwen, K. Parenting, Effortful Control, and Adolescents’ Externalizing Problem Behavior: Moderation by Dopaminergic Genes. J. Youth Adolesc. 2020, 49, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Tuvblad, C.; Narusyte, J.; Comasco, E.; Andershed, H.; Andershed, A.K.; Colins, O.F.; Fanti, K.A.; Nilsson, K.W. Physical and verbal aggressive behavior and COMT genotype: Sensitivity to the environment. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2016, 171, 708–718. [Google Scholar] [CrossRef]
- Tadić, A.; Victor, A.; Başkaya, Ö.; von Cube, R.; Hoch, J.; Kouti, I.; Anicker, N.J.; Höppner, W.; Lieb, K.; Dahmen, N. Interaction between gene variants of the serotonin transporter promoter region (5-HTTLPR) and catechol O-methyltransferase (COMT) in borderline personality disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150B, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Feil, R.; Fraga, M.F. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2012, 13, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Norrholm, S.D.; Jovanovic, T.; Smith, A.; Binder, E.B.; Klengel, T.; Conneely, K.; Mercer, K.B.; Davis, J.S.; Kerley, K.; Winkler, J.A.; et al. Differential genetic and epigenetic regulation of catechol-O-methyltransferase is associated with impaired fear inhibition in posttraumatic stress disorder. Front. Behav. Neurosci. 2013, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Zito, J.M.; Safer, D.J.; Sai, D.; Gardner, J.F.; Thomas, D.; Coombes, P.; Dubowski, M.; Mendez-Lewis, M. Psychotropic medication patterns among youth in foster care. Pediatrics 2008, 121, e157–e163. [Google Scholar] [CrossRef] [PubMed]
|
Authors& Year of Publication | N | Age | Sample Characteristics: Clinical | Ethnicity |
Sex F: |
Sex M: | Behavioral AT | Environmental Factors | Environmental AT | Key Findings | Effect Estimates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abraham et al. (2020) | 1292 | From birth to 11 years old | - | African American (56%) and White (44%) | 50.1% | 49.9% | Teachers: DSM-IV hyperactivity/impulsivity, and Inattention ADHD symptom severity | SES risk | Family income-to-needs ratio, household density, neighborhood safety, maternal education, a consistent partnership of a spouse/partner living in the home, maximum work hours of primary or secondary caregiver per week, and job prestige | Non-significant main effects of Val158Met and SES risk on hyperactivity/impulsivity in early and middle childhood. Significant Val158Met x SES risk on hyperactivity/impulsivity and inattention symptoms. Met carriers > Val/Val. | p = 0.108 (Val158Met—early) p = 0.323 (Val158Met—middle) p = 0.025 (Val158Met x SES—early) ⁺ p = 0.018 (Val158Met x SES—middle) ⁺ |
| Davies et al. (2019) | 279 | From 13 to 16 years old | - | White (73%), African American (17%), multiracial (8%), other races (2%) | 51.0% | 49.0% | Parents: CBCL, Teachers: SDQ, Teacher’s Rating Scale of Child Actual Behavior, Adolescents: SDQ | Interparental conflict | Parents: interparental interaction task: verbal aggression, negativity and conflict, coerciveness, support, problem-solving communication, positive affect, negative escalation, cohesiveness Adolescents: security in the interparental subsystem | Non-significant interparental conflict x Val158Met in predicting externalizing symptoms. | p = 0.41 |
| Salatino-Oliveira et al. (2016) | 4095 | From 11 to 15 to 18/19 years old | - | 63.7% White | 51.1% | 48.9% | Age 11 and 15: SDQ for child and maternal mental health; age 18/19: self-report questionnaire about criminal behavior in the last 12 months | Prenatal maternal smoking | Perinatal assessment questionnaires | Non-significant effects of Val158Met on conduct scores and crime rate. Non-significant interaction between Val158Met x prenatal maternal smoking on conduct scores and crime rate *. | p = 0.932 (Val158Met—age 11) p = 0.472 (Val158Met—age 15) p = 0.282 (Val158Me t—age 18) p = 0.196 (Val158Met x smoking—age 18) |
| van Goozen et al. (2016) | 194 | 10–17 years old (mean age: 13.95) | DSM-IV ADHD or ICD-10 Hyperkinetic Disorder | - | - | 100.0% | DAWBA, SDQ, executive functioning: Wisconsin Card Sorting Task and Go/No-Go Task, cognitive and affective empathy through video-watching, fear conditioning using skin conduct response | - | - | Significantly reduced response inhibition, set shifting abilities, fear empathy and autonomic responsiveness to conditioned aversive stimulus in Val carriers < Met/Met. | p = 0.02 (Response inhib.) ⁺ p = 0.01 (shifting ability) ⁺⁺ p = 0.04 (fear empathy) ⁺ p = 0.001 (responsiveness to CS) ⁺⁺⁺ |
| Zhang et al. (2016) | 1399 | 12–13 years old (mean age: 12.32) | - | 100% Chinese Han | 47.2% | 52.8% | PRQ | Maternal parenting | CRPR | Significant Val158Met x positive parenting on reactive aggression: Met carriers + positive parenting > Val/Val + positive parenting. Non-significant interaction between Val158Met x parenting on proactive aggression. | p < 0.01 (Val158Met x (+) parenting—reactive) ⁺⁺ p > 0.05 (Val158Met x (-) parenting—reactive) p > 0.05 (Val158Met x parenting—proactive) |
| Hygen et al. (2015) | 704 | 4–5.58 years old (mean age: 4.56) | - | 95.5% Norwegian | 49.6% | 50.4% | TRF | SLE | PAPA | Non-significant main effect of Val158Met on aggression. Significant Val158Met x SLE interaction on aggressive behaviors. Val/Val + SLE > Met carriers + SLE. Val/Val—SLE < Met carriers—SLE. | p = 0.78 (Val158Met) p = 0.02 (Val158Met x SLE (with SLE)) ⁺ p = 0.03 (Val158Met x SLE (without SLE)) ⁺ |
| Park & Waldman (2014) | Clinically-referred: 224 Twin sample: 156 | Mean age: 12.2 | Clinically-referred for assessment of criteria for an externalizing disorder | 80% European-American, 6% African American, 0.1% Hispanic 6.7% Other, %7.2 Missing | 46.0% | 53.9% | The A-X Continuous Performance Task | - | - | Significant main effect of Val158Met on commission error variability: Val/Val > Met carriers, representing impulsivity. Significant main effect of Val158Met on Signal Detection Theory indices (variability in sensitivity): Val carriers > Met/Met. Significant interaction Val158Met x clinical status on commission error variability. | p = 0.038 (Val158Met- commission errors) ⁺ p = 0.014 (Val158Met—SDT) ⁺ p = 0.010 (Val158Met x clinical status—commission errors) ⁺ |
| Hirata et al. (2013) | 144 | 6–16 years old (mean age: 10.8) | Clinically-referred for behavioral problems and persistent aggression & healthy adult controls | 77.6% European Caucasian, 5.6% African Canadian, 16.7% mixed ancestry | 27.8% | 72.2% | CBCL, TRF, PSD | - | - | Non-significant effects of Val158Met polymorphism on callous-unemotional scores for the full sample. Significant association between Val158Met and CU scores in the European subgroup. | p = 0.173 (Val158Met—CU) p = 0.030 (Val158Met Europe—CU) ⁺ |
| Oppenheimer et al. (2013) | 263 | 9–15 years old (mean age: 12.03) | - | 70% Caucasian, 7% African American, 6% Latino, 4% Asian/Pacific Islander, 13% other/mixed ethnicity | 56.0% | 44.0% | Videotaped psychosocial stressor challenge: Youth negative affect during stressor task | Parenting behaviors | Videotaped parent–child discussion: warmth and responsiveness Big Five Inventory for parent personality | Significant association between Val158Met and child anger: Met/Met > Val/Val. Emotionally stable and extraverted parents had higher positive parenting to children with Val/Val genotype. | p < 0.01 (Val158Met—anger) ⁺⁺ p = 0.02 (Val158Met—Parent warmth) ⁺ |
| Nikolac Perkovic et al. (2013) | 807 | Median age: 10 and 15 | - | 100% Caucasian | - | 100.0% | Teacher-report version of the SNAP-IV DSM-IV for ADHD | - | - | Significant difference between Val158Met genotype frequencies in youth with vs. without ADHD symptoms: Met/Met > Val carriers. Higher hyperactive-impulsive and inattentive scores in Met/Met > Val carriers. | p = 0.003 (Val158Met frequency) ⁺⁺ p = 0.008 (Val158Met—hyperactive-impulsive) ⁺⁺ p = 0.001 (Val158Met—inattentive) ⁺⁺⁺ |
| Karam et al. (2013) | Case: 80 Control: 100 | 3–12 years old (mean age: 9) | ASD | 100% Egyptian | 17.0% | 83.0% | CARS, CPRS-R:L | - | - | Significant association between Val158Met and hyperactivity scores: Val/Val > Met carriers. | p = 0.006; p = 0.03 (Val158Met—hyperactivity) ⁺ |
| Amstadter et al. (2012) | 223 | 9–13 years old (mean age: 11.3) | - | 50% European American, 34.2% African American, 2.7% Latino, and 13.1% other (mixed ethnicity) | 44.4% | 55.6% | BART-Y | - | - | Significant correlation between Val158Met and BART performance in girls: Met carriers > Val/Val risk-taking behaviors. | p < 0.001 (Val158Met—BART in girls) ⁺⁺⁺ p = 0.47 (Val158Met—BART in boys) |
| Salatino-Oliveira et al. (2012) | 516 | Mean age: 10.55 | ADHD | 77.5% European-Brazilian | 23.0% | 77.0% | K-SADS-E, clinical evaluation of ADHD and comorbid conditions using DSM-IV criteria | - | - | Significant association between Val158Met and disruptive behavior disorders: Val/Val genotype was more frequent in children with ADHD comorbid with DBD (ODD and CD). | p = 0.016 ⁺ |
| Nederhof et al. (2012) | 1134 | Mean age: 11.09 and 16.13 | - | 100% Dutch ancestry | 52.0% | 48.0% | YSR | Parental separation | Before age 11: TRAILS Family History Interview Between age 11 and 16: Event History Calendar | Significant Val158Met genotype x parental separation on externalizing problems: Met carriers + separated parents > Val/Val + separated parents. Met carriers + parents together ≈ Val/Val + parents together. | p = 0.03 (Val158Met x parents separate—externalize) ⁺ p = 0.29 (Val158Met x parents together—externalize) |
| Brennan et al. (2011) | 470 | 15 and 20 years old | - | 92% Caucasian ethnicity | 57.0% | 43.0% | Age 15 mother: CBCL, age 15 teacher: TRF, age 15 youth: YSR | Maternal smoking during pregnancy | Questionnaire (yes/no) | Significant Val158Met x maternal smoking during pregnancy interaction on aggressive behaviors at age 15: Val/Val + mothers who smoked >>. Val158Met genotype alone did not significantly predict aggressive behaviors. ** | p < 0.05 (Val158Met x maternal smoking—aggression)⁺ |
| Langley et al. (2010) | 4365 | 7.5 years old (mean age: 7.65) and 8 years old (mean age: 8.59) | - | 100% European origin | 49.0% | 51.0% | DAWBA, DSM-IV CD symptoms, Test of Everyday Attention for Children battery, Skuse Social Cognition Scale | - | - | Significant Val158Met x ADHD on antisocial behaviors (CD symptoms). Val/Val genotype + ADHD > Met carriers + ADHD. | p < 0.001 ⁺⁺⁺ |
| Nobile et al. (2010) | 618 | 10–14 years old (mean age: 12.1) | - | >95% Caucasian and of Italian ancestry | 48.6% | 51.4% | CBCL for DSM-oriented scales: ADHD problems, ODD, and CD | Socioeconomic status | Parental employment: Hollingshead 9-point scale | Significant Val158Met x SES on attention deficit/hyperactivity problems: Val/Val + low SES >> Non-significant effects of Val158Met genotype alone on behavior. | p = 0.004 (Val158Met x SES—ADHD) ⁺⁺ p = 0.420 (Val158Met—behavior) |
| Palmason et al. (2010) | 166 | 6–13 years old (mean age: 9.7) | Clinically-referred for ADHD | - | 15.7% | 84.3% | Kinder-DIPS, DCL-HKS | Birth weight | Semi-structured, detailed interview with parents | Significant association between Val158Met and increased ADHD symptom severity: Met carriers > Val/Val. Non-significant Val158Met x birth weight effect on ADHD and CD. | p = 0.005 (Val158Met—ADHD) ⁺⁺ p = 0.697 (Val158Met x birth weight—ADHD/CD) |
| DeYoung et al. (2010) | 174 | Mean age: 16.23 | Male adolescent inmates, incarcerated for at least 6 months | 98% Russian ancestry | - | 100.0% | K-SADS-PL | - | - | Significant association between Val158Met genotype and CD/ADHD. Diagnosis and symptoms of CD: Val/Val > Met carriers. ADHD symptoms: Met/Met > Val carriers. | p < 0.01 (Val158Met—CD diagnosis and symptoms) ⁺⁺ p < 0.05 (Val158Met—ADHD symptoms) ⁺ |
| Paloyelis et al. (2010) | Case: 36 Control: 32 | 11–20 years old (mean age: 15.42) | ADHD | 100% Caucasian | - | 100.0% | Hypothetical delay discounting task, real-time delay discounting task, BIS-11A, Revised Conners’ Parent Rating Scales | - | - | Significant association between Val158Met genotype and impulsivity independent of ADHD diagnosis: Met/Met > Val carriers. | p < 0.05 ⁺ |
| Albaugh et al. (2010) | 149 | 6–18 years old (mean age: 10.93) | - | - | 41.6% | 58.4% | Mother rated CBCL: Aggressive Behavior scale & Attention Problems scale | - | - | Significant association between Val158Met genotype and aggressive behaviors (including direct and relational aggression): Met carriers > Val/Val. Non-significant association between Val/Val genotype and attention problems. | p = 0.016 (Val158Met—aggression) ⁺ p = 0.062 (Val158Met—attention) |
| Fowler et al. (2009) | 147 | 12–19 years old (mean age: 14.5) | ADHD | 100% UK White origin | 7.5% | 92.5% | Parent version CAPA, Child version of the CAPA, Child ADHD Teacher Telephone Interview, PCL-YV | - | - | Significant association between Val158Met genotype and emotional dysfunction psychopathy scores: Val/Val > Met carriers. | p = 0.02 ⁺ |
| Qian et al. (2009) | 171 | 6–17.5 years old (mean age: 10.3) | ADHD | 100% Chinese Han | - | 100.0% | Parents: CDIS Teachers: Rutter’s Scale | - | - | Significant association between Val158Met genotype frequencies and ADHD with co-morbid ODD: Val/Val more frequent in ADHD + ODD than ADHD alone (Met more frequent). | p = 0.019 (Val158Met—ADHD + ODD) ⁺ |
| Caspi et al. (2008) | 241 (a) 2232 (b) 1037 (c) | Clinical sample (a): 5–14 years old (mean age: 9.25) Birth cohort studies: 5 and 7 years old (b); 11.13 and 15 years old (c) | 100% ADHD (a) 8% ADHD (b) 6% ADHD (c)) | 100% UK White origin 100% England and Wales 100% New Zealander | 11% (a) | 89% (a) | CAPA(a) Child ADHD Teacher Telephone Interview(a) mother and teacher report on criteria for ADHD specified by DSM-IV(b) CBCL(b) Diagnostic Interview Schedule for Children–Child Version(c) Adolescents followed to adulthood(c) | Significant association between Val158Met genotype and total number of CD symptoms, aggression and antisocial behavior: ADHD+ Val/Val > ADHD + Met carriers (a, b, c) ((a) is also reported in Thapar et al., 2005). Children without ADHD, there was no significant association between Val158Met genotype and aggression/antisocial behavior (b, c). | p = 0.05 (Val158Met + ADHD—CD symptoms) ⁺ p = 0.04 (Val158Met + ADHD—aggression) ⁺ p = 0.03 (Val158Met + ADHD—antisocial) ⁺ p = 0.38; 0.37 (Val158Met—aggression; antisocial (no ADHD)) | ||
| Sengupta et al. (2006) | 191 | 6–12 years old (mean age: 9) | ADHD | 90.1% White, 4.2% Black, 1.6% Aboriginal, 3.6% Half-white, 5% Half-Asian | 12.6% | 87.4% | Parents: DISC-IV, Teachers: Conners Global Index-Teacher version questionnaire | Birth weight | Mother’s report | Non-significant main effects and interaction effect of Val158Met x birth weight on CD symptom scores. | p = 0.72 (Val158Met—CD) p = 0.71 (Val158Met x birth weight—CD) |
| Thapar et al. (2005) | 240 | 5–14 years old (mean age: 9.25) | ADHD | 100% UK White origin | 11.3% | 88.8% | CAPA–parent version: DSM-IV CD symptom score | Birth weight | Mother’s report | Significant main effect of Val158Met genotype and CD symptoms: Val/Val > Met carriers. Significant Val158Met x birth weight interaction on CD symptoms: Val/Val + low birth weight >> | p = 0.002 (Val158Met—CD) ⁺⁺ p = 0.006 (Val158Met x birth weight—CD) ⁺⁺ |
| Bearden et al. (2005) | 38 | Mean age: 10.9 | 22q11.2 Deletion Syndrome | 92% Caucasian ethnicity | 61.0% | 39.0% | CBCL | - | - | Significant association between Val158Met genotype and CBCL ratings (total/internalizing/externalizing problems scales, clinically behavioral problems): Val carriers > Met/Met. | p ≤ 0.01 = (Val158Met—total/ internalizing problems) ⁺⁺ p ≤ 0.05 = (Val158Met—externalizing problems, clinical behav. problems) ⁺ |
| Mills et al. (2004) | 124 | 6–16 years old (mean age: 9.2) | Meeting ICD-10 criteria for Hyperkinetic Disorder or DSM-III-R/IV criteria for ADHD | 100% British Caucasian | 8.0% | 92.0% | Parents: CAPA, Teachers: CHATTI MFFT, CPT | - | - | Non-significant association between the Val158Met genotype and neurocognitive performance (impulsiveness and response inhibition). | p > 0.05 |
| Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|
| Below Age 13 (n = 81) | Above Age 13 (n = 59) | Below Age 13 (n = 70) | Above Age 13 (n = 80) | |||||
| Val Carriers (n = 58) | Met/Met (n = 23) | Val Carriers (n = 47) | Met/Met (n = 12) | Val Carriers (n = 52) | Met/Met (n = 18) | Val Carriers (n = 58) | Met/Met (n = 22) | |
| Mean Age (S.D) | 10.24 (1.95) | 10.03 (2.28) | 14.87 (1.58) | 14.24 (1.04) | 10.54 (1.82) | 9.84 (2.12) | 14.80 (1.26) | 15.07 (1.45) |
| Mean CBCL Aggressive Behavior (S.D) | 65.67 (16.16) | 72.52 (16.18) | 71.74 (13.83) | 61.91 (12.31) * | 60.9 (12.71) | 61.63 (15.77) | 71.12 (15.22) | 66.45 (15.04) |
| Mean CBCL Attention Deficit / Hyperactivity (S.D) | 59.07 (11.58) | 63.77 (8.70) | 64.32 (7.64) | 58.43 (9.88) | 57.91 (9.37) | 59.5 (12.45) * | 67.67 (9.87) | 63.31 (11.53) |
| Mean CBCL Oppositional Defiant Problems (S.D) | 61.32 (12.46) | 65.92 (11.62) | 67.77 (10.12) | 61.13 (8.70) | 58.67 (9.3) | 61.2 (13.35) | 67.75 (10.60) | 66.23 (12.19) |
| Mean CBCL Conduct Problems (S.D) | 61.46 (13.75) | 71.07 (13.80) * | 70.09 (11.98) | 62.28 (10.96) | 57.97 (10.97) | 59.1 (12.44) | 74.33 (13.53) | 69.69 (13.78) |
| Mean PSD Narcissism Subscale (S.D) | 0.60 (0.49) | 0.81 (0.63) | 0.94 (0.52) | 0.64 (0.44) | 0.47 (0.4) | 0.31 (0.31) | 0.89 (0.65) | 0.63 (0.60) |
| Mean PSD Impulsivity Subscale (S.D) | 0.87 (0.64) | 1.25 (0.48) * | 1.29 (0.58) | 0.82 (0.67) * | 0.68 (0.57) | 0.77 (0.55) | 1.22 (0.69) | 1.03 (0.68) |
| Mean PSD Callous-Unemotional Traits Subscale (S.D) | 0.89(0.42) | 0.89(0.52) | 1.09 (0.37) | 0.78 (0.24) ** | 0.79 (0.37) | 0.93 (0.43) | 0.93 (0.49) | 0.86 (0.43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kant, T.; Koyama, E.; Zai, C.C.; Beitchman, J.H.; Kennedy, J.L. COMT Val/Met and Psychopathic Traits in Children and Adolescents: A Systematic Review and New Evidence of a Developmental Trajectory toward Psychopathy. Int. J. Mol. Sci. 2022, 23, 1782. https://doi.org/10.3390/ijms23031782
Kant T, Koyama E, Zai CC, Beitchman JH, Kennedy JL. COMT Val/Met and Psychopathic Traits in Children and Adolescents: A Systematic Review and New Evidence of a Developmental Trajectory toward Psychopathy. International Journal of Molecular Sciences. 2022; 23(3):1782. https://doi.org/10.3390/ijms23031782
Chicago/Turabian StyleKant, Tuana, Emiko Koyama, Clement C. Zai, Joseph H. Beitchman, and James L. Kennedy. 2022. "COMT Val/Met and Psychopathic Traits in Children and Adolescents: A Systematic Review and New Evidence of a Developmental Trajectory toward Psychopathy" International Journal of Molecular Sciences 23, no. 3: 1782. https://doi.org/10.3390/ijms23031782
APA StyleKant, T., Koyama, E., Zai, C. C., Beitchman, J. H., & Kennedy, J. L. (2022). COMT Val/Met and Psychopathic Traits in Children and Adolescents: A Systematic Review and New Evidence of a Developmental Trajectory toward Psychopathy. International Journal of Molecular Sciences, 23(3), 1782. https://doi.org/10.3390/ijms23031782






