Catalytic Sulfation of Betulin with Sulfamic Acid: Experiment and DFT Calculation
Abstract
:1. Introduction
2. Results
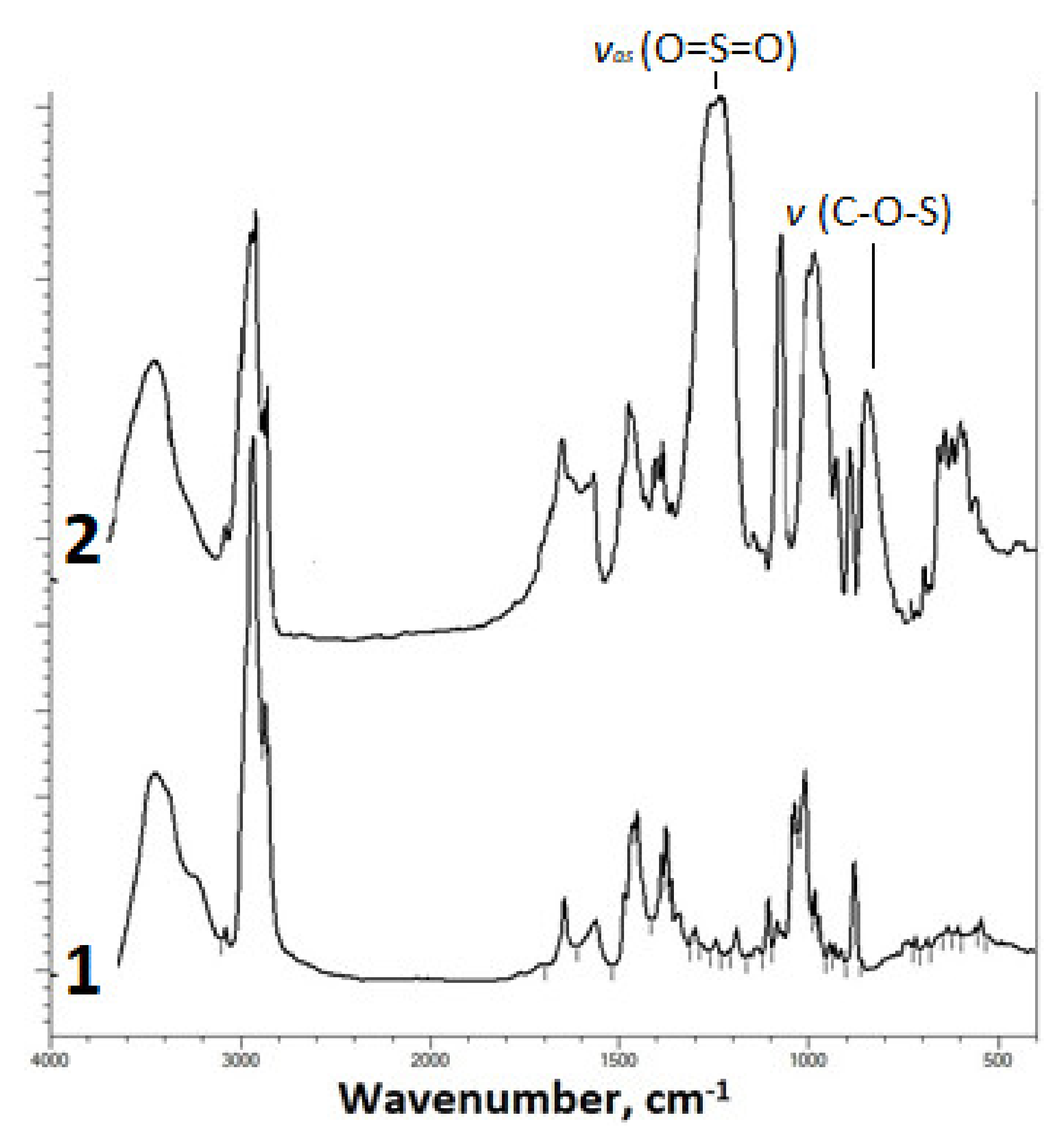
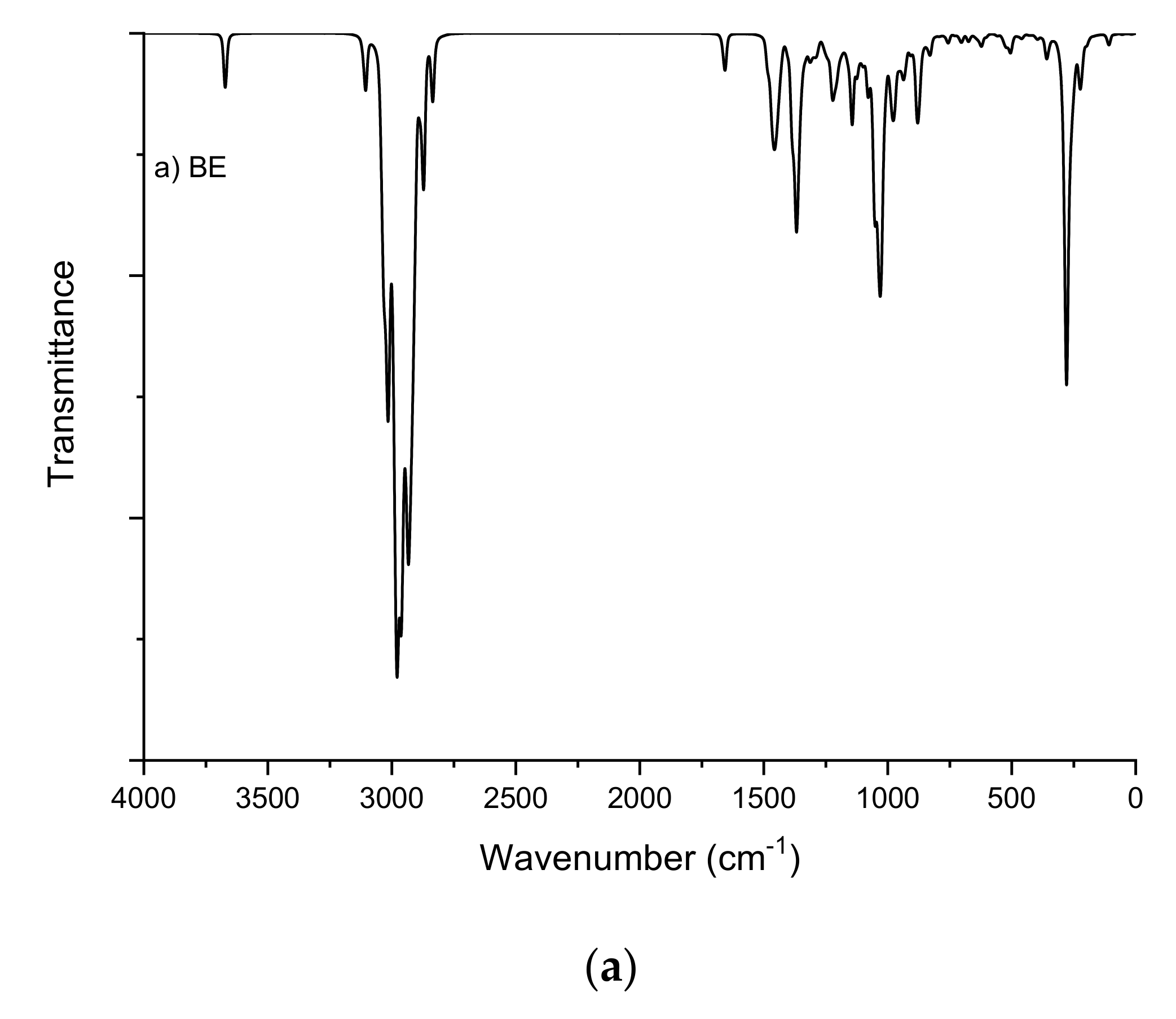
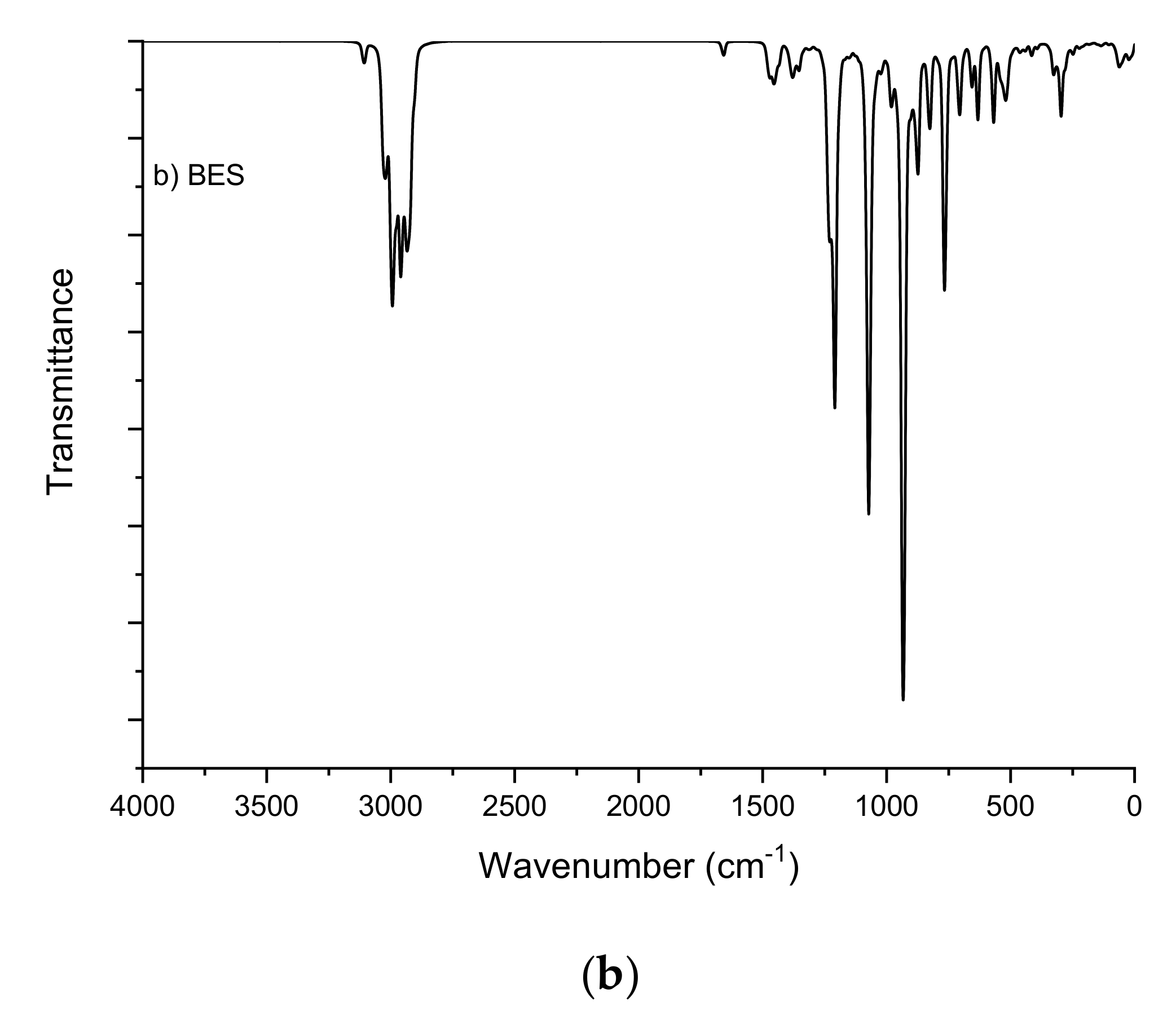
2.1. Fourier-Transform Infrared Spectroscopy Study
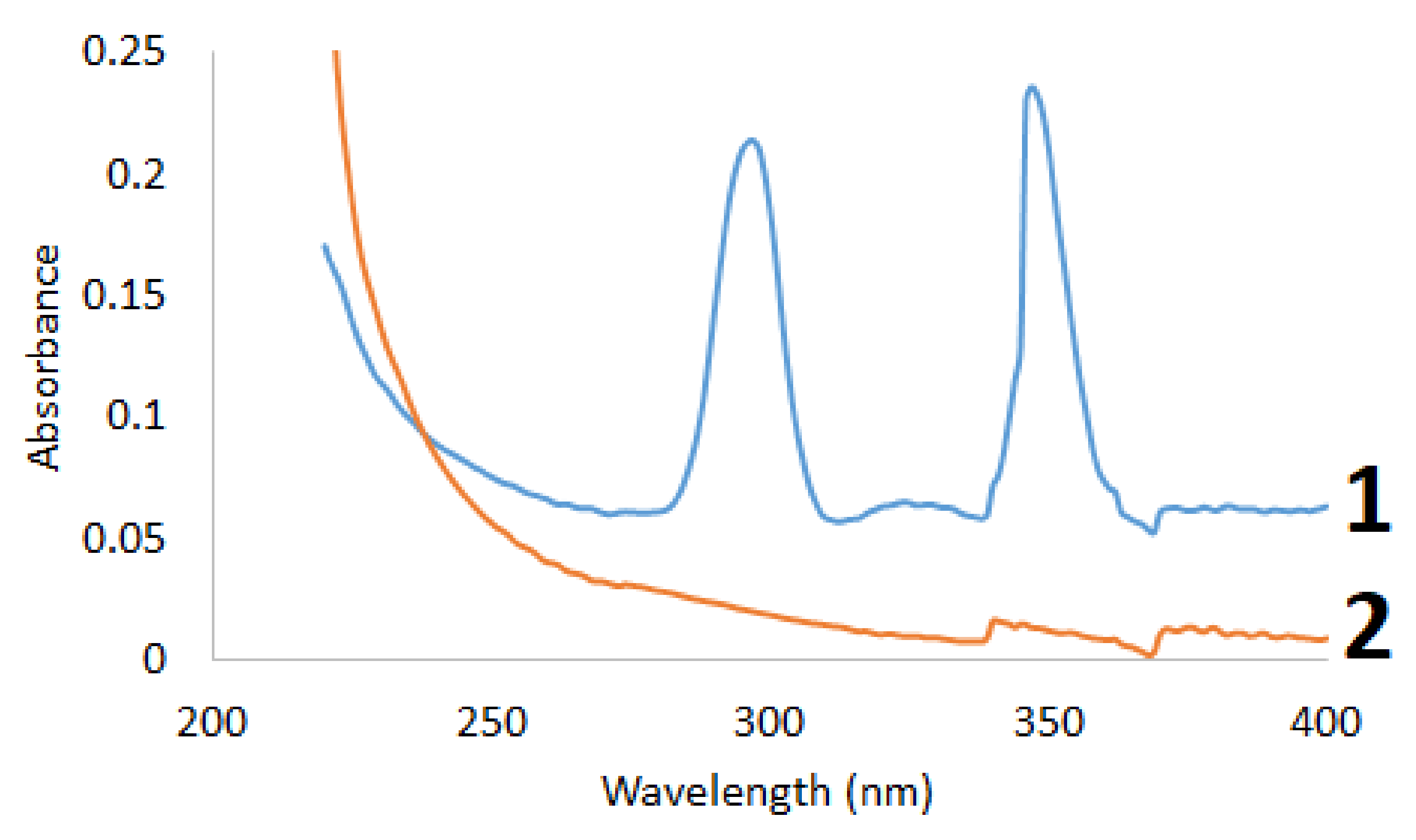
2.2. Ultraviolet—Visible Spectroscopy Study
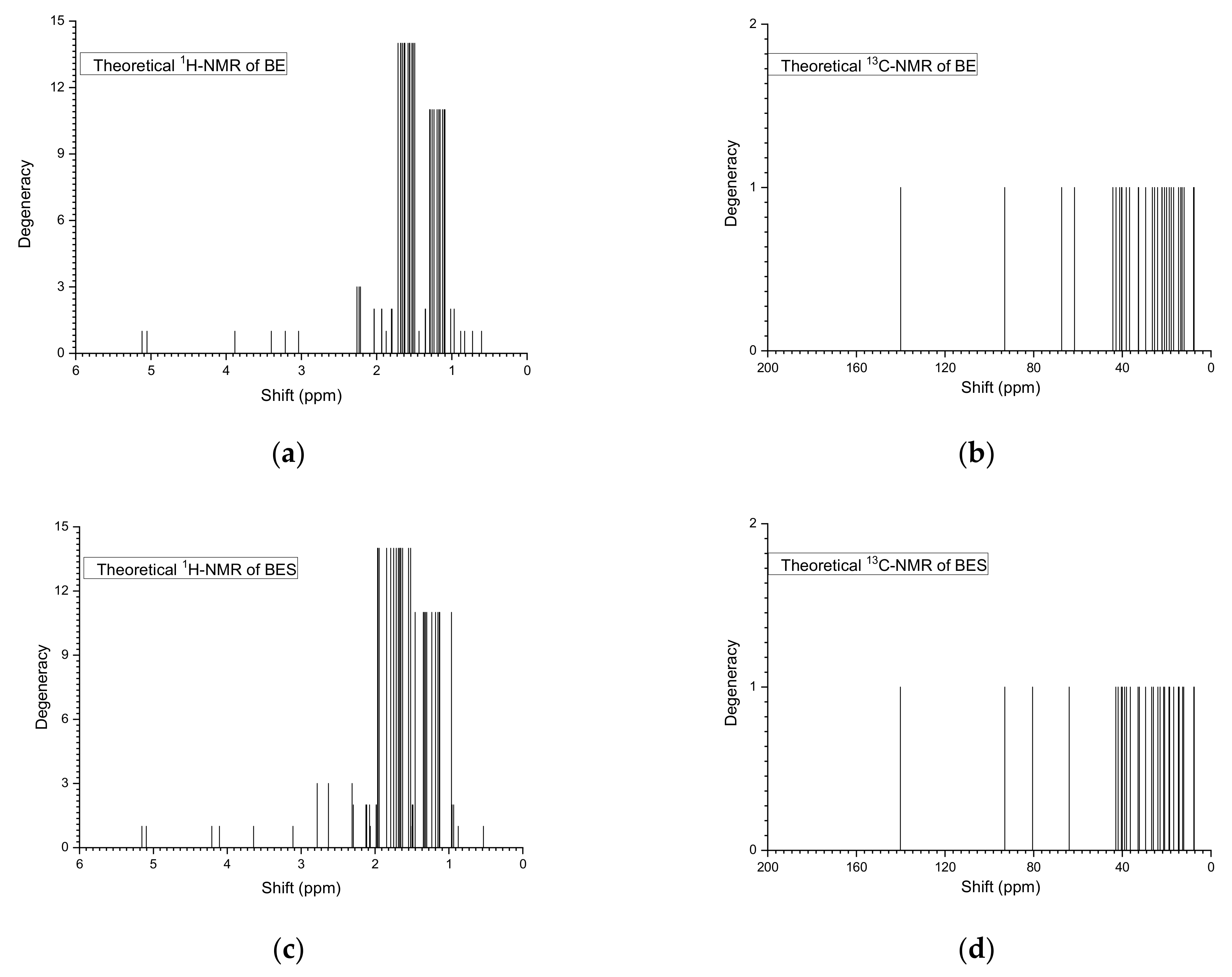
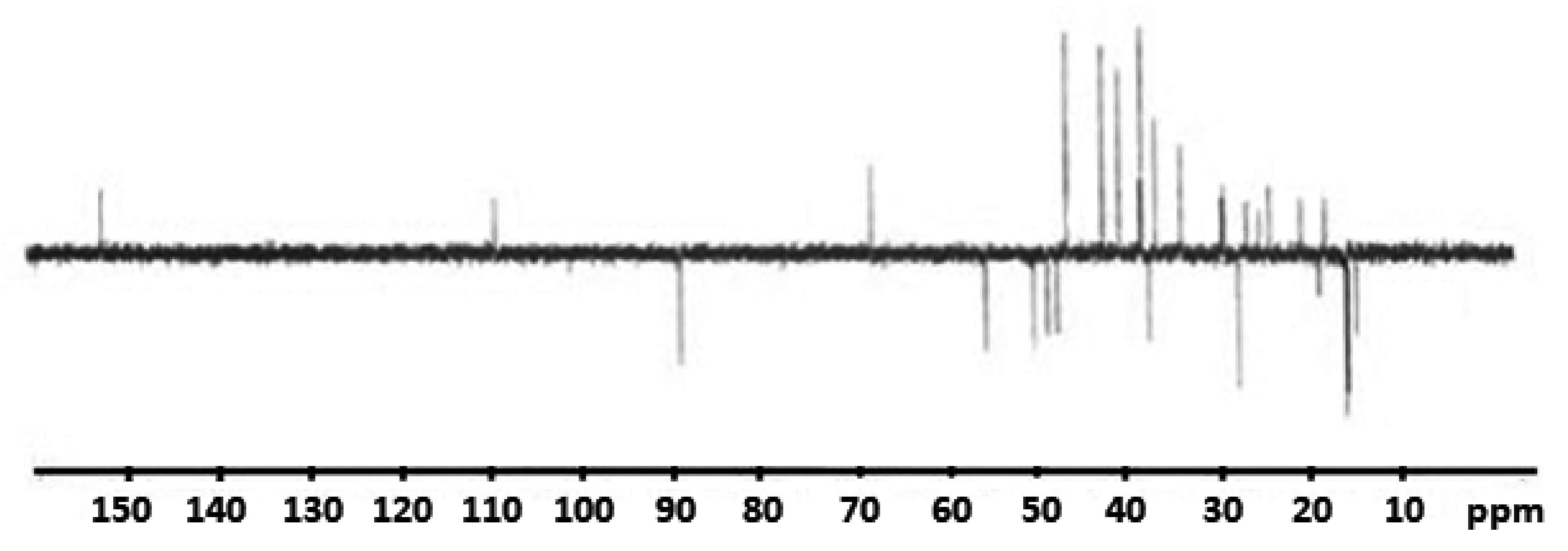
2.3. Nuclear Magnetic Resonance Study
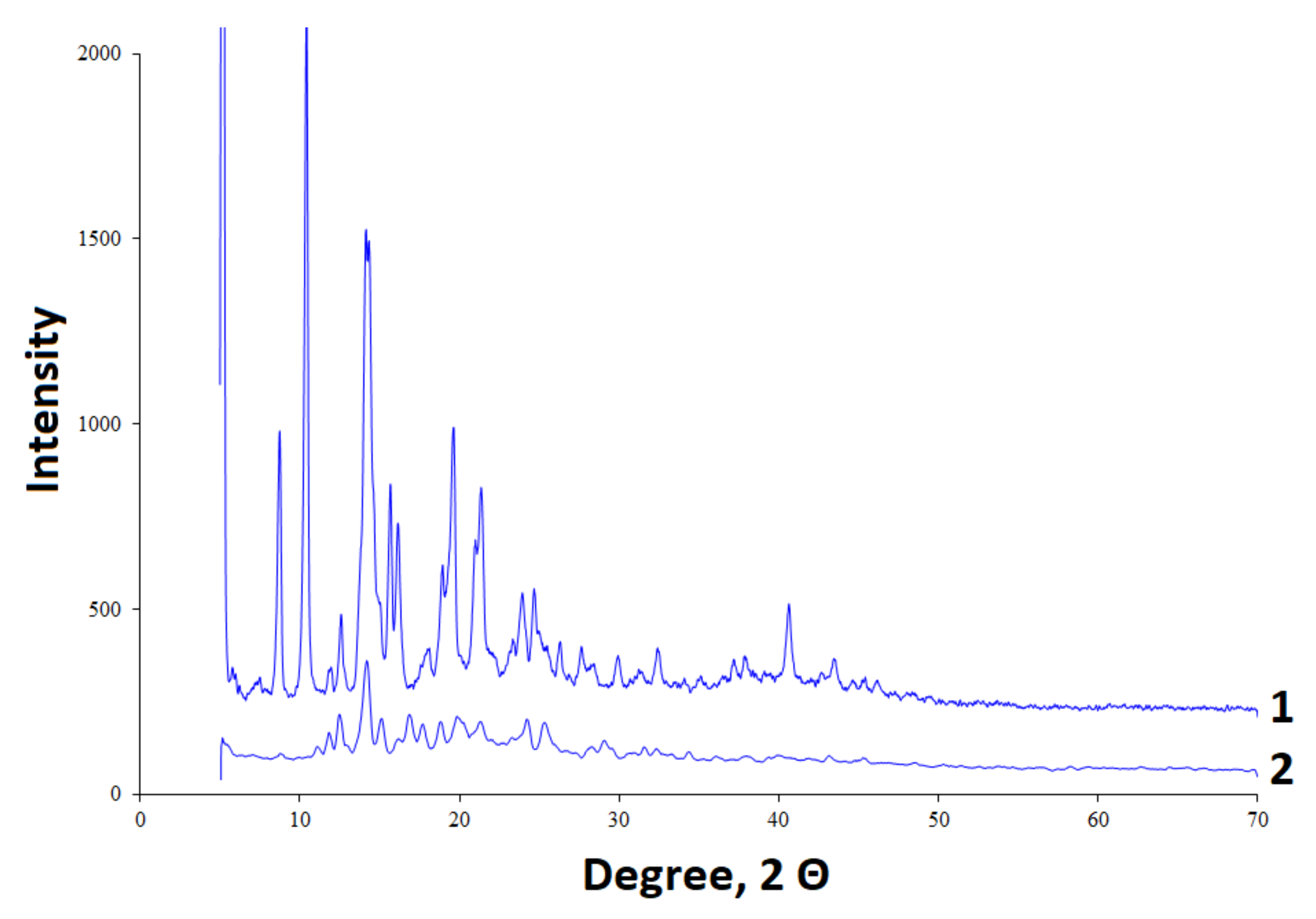
2.4. X-Ray Diffraction Study
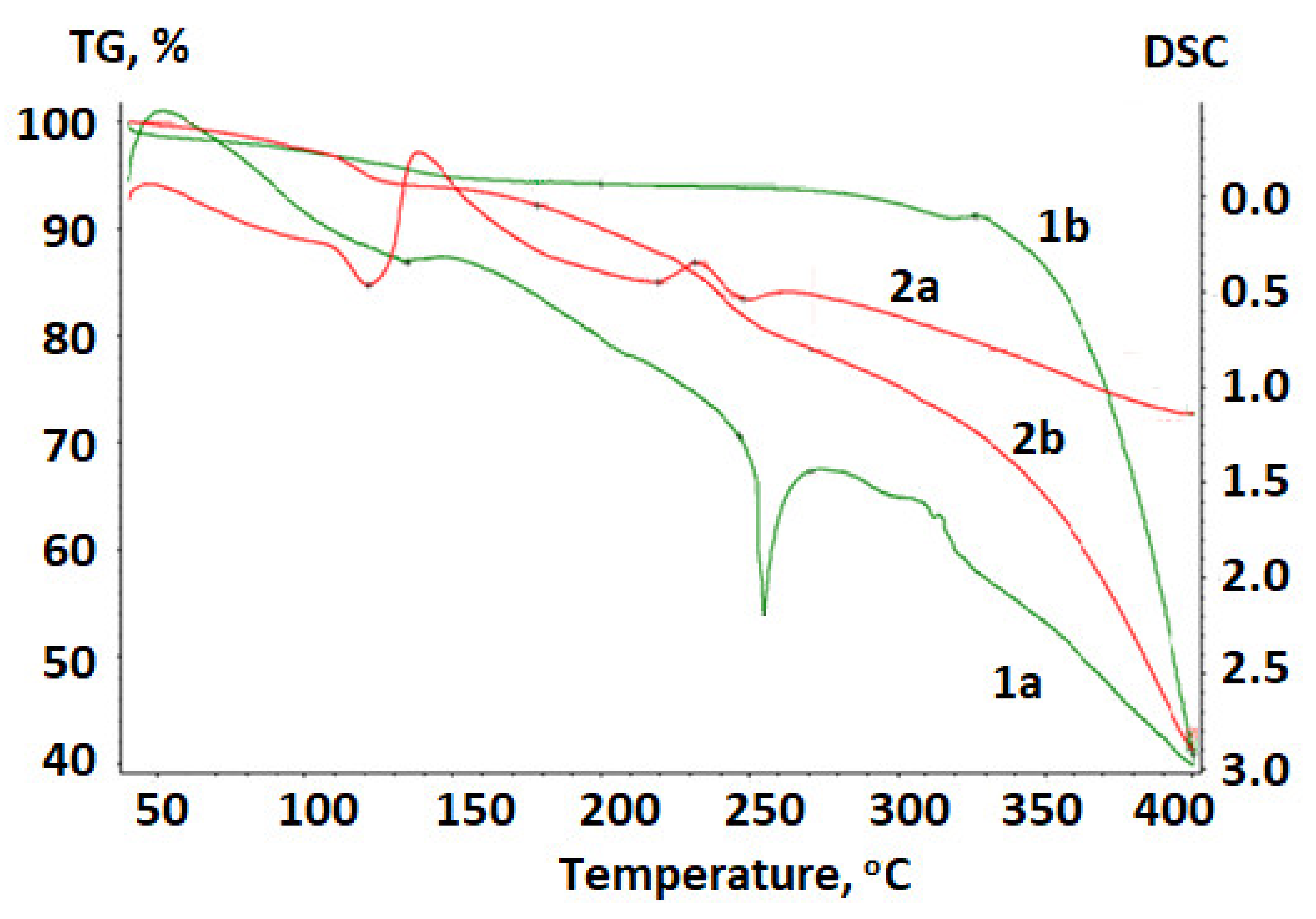
2.5. Thermal Analysis
2.6. Acidity Constants
2.7. Theoretical Calculations
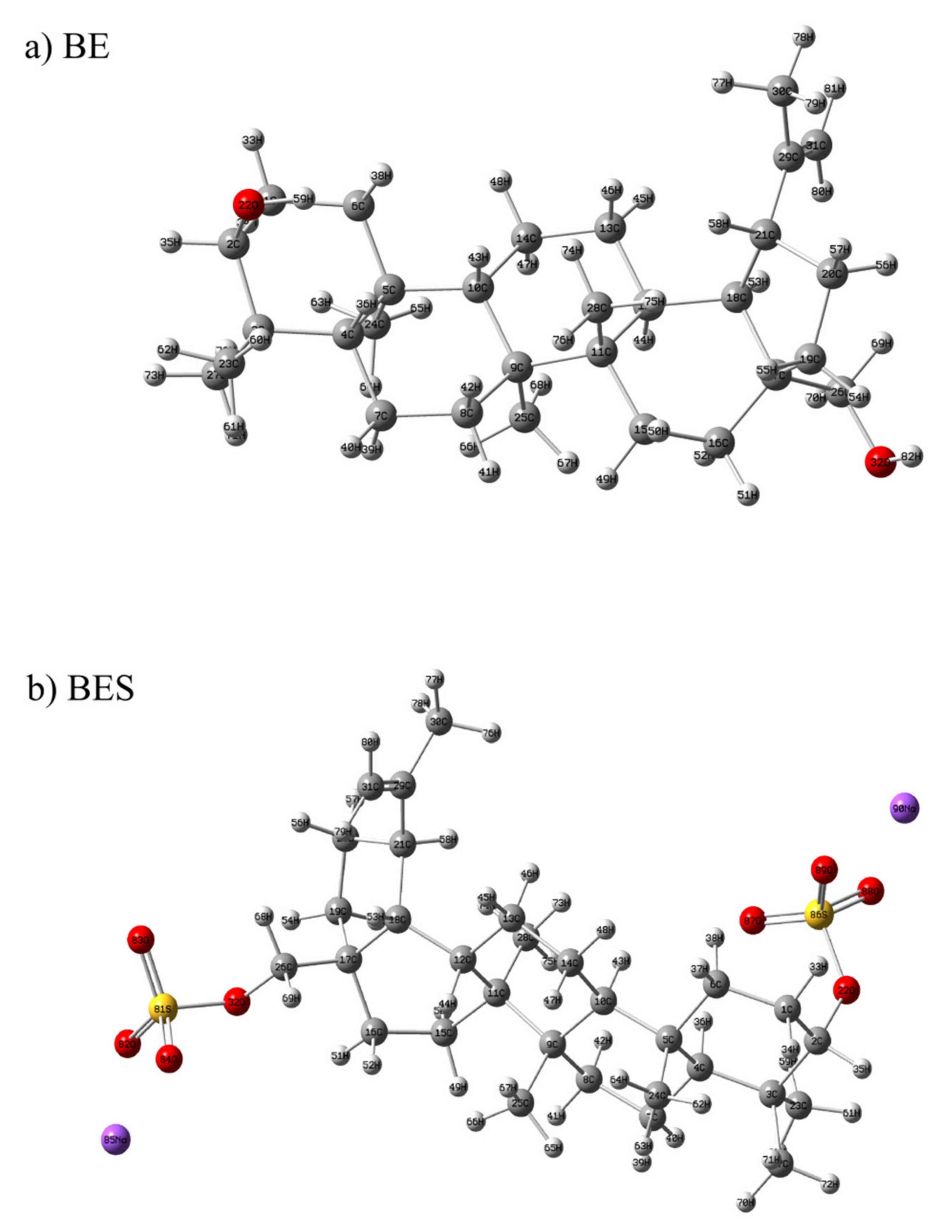
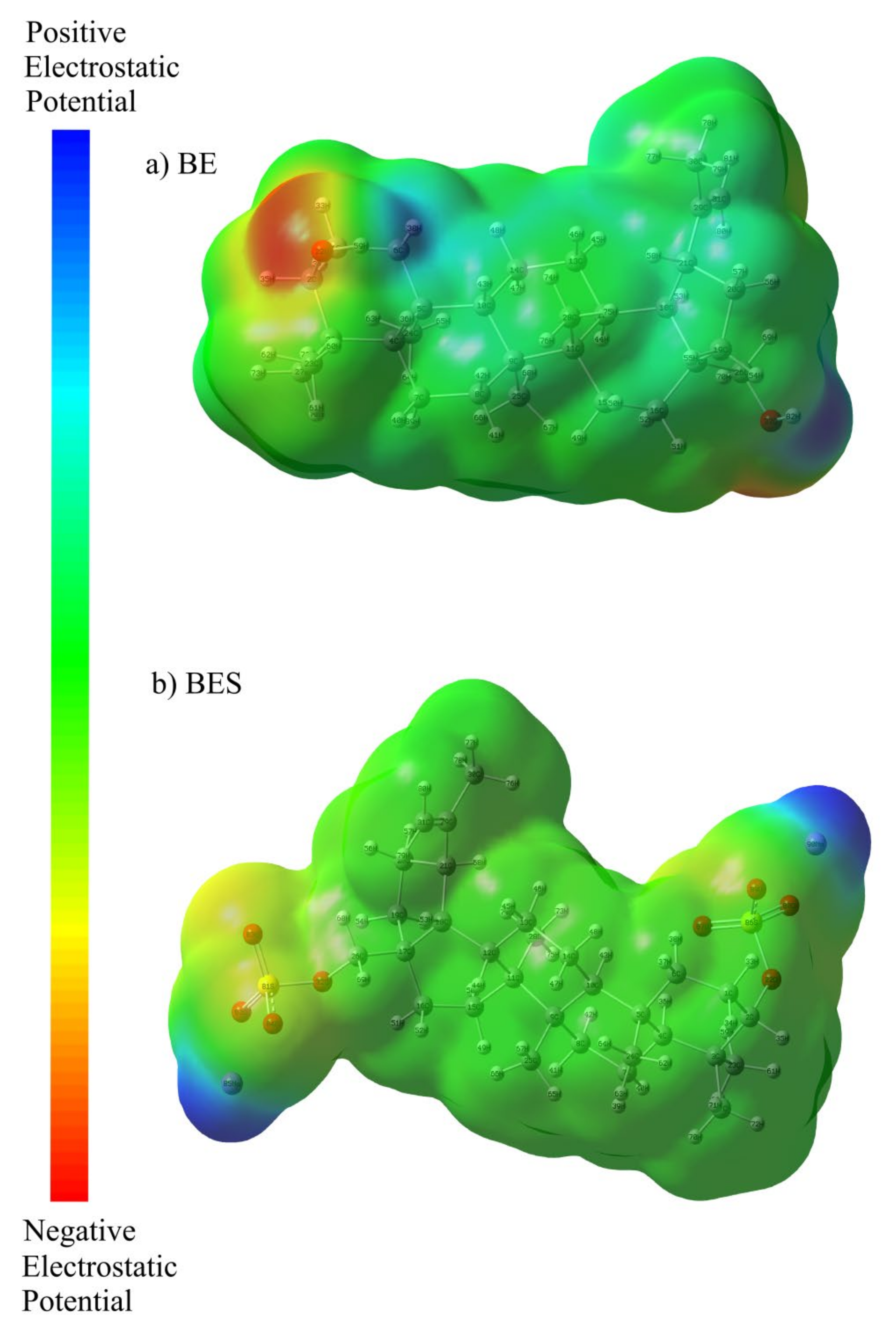
2.7.1. Optimized Geometry and MEP Analysis of Betulin and Betulin Disulfate
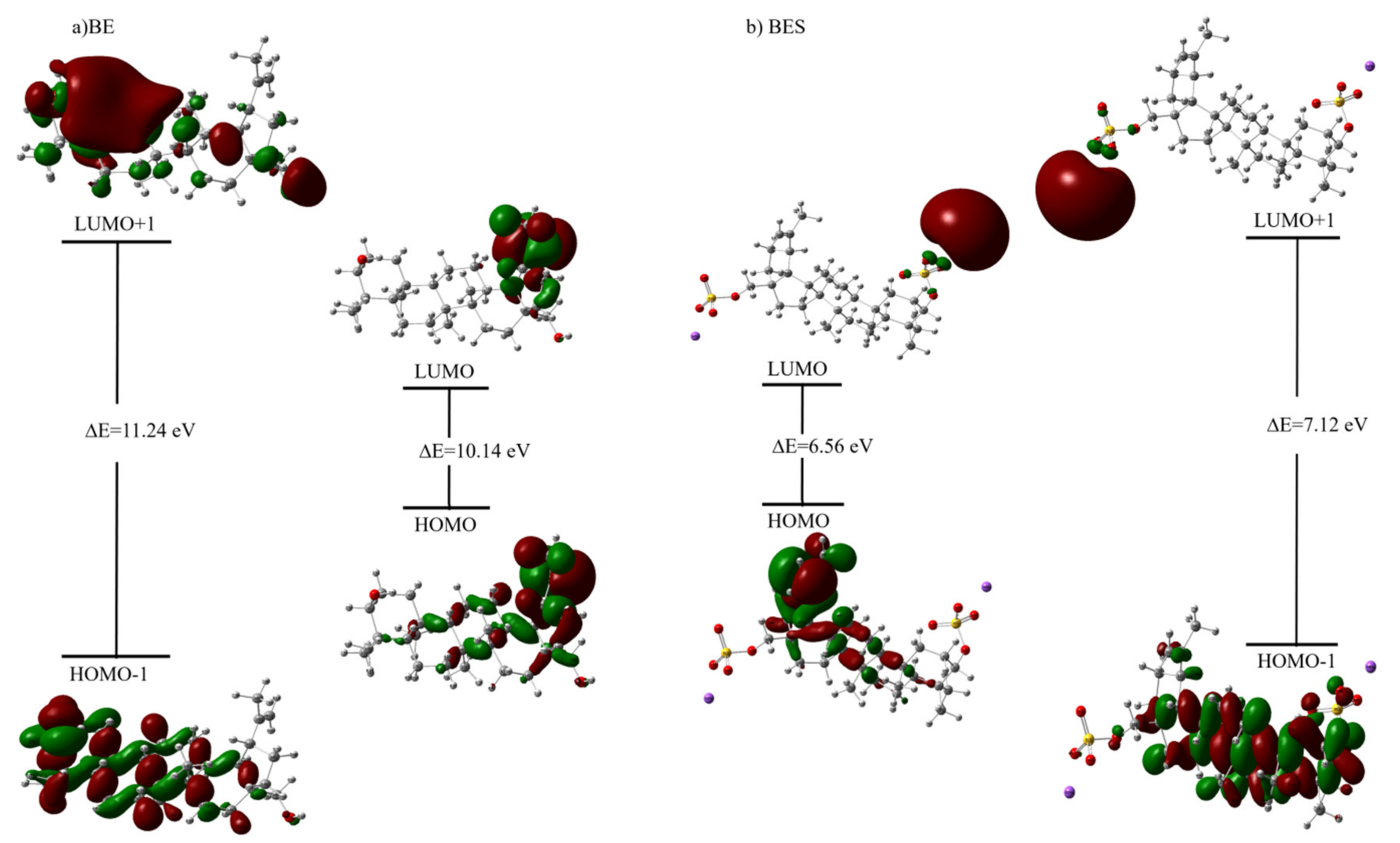
2.7.2. HOMO—LUMO Analysis and Calculated Electronic Properties
2.7.3. Mulliken Atomic Charges
2.7.4. Spectroscopic Analysis
2.7.5. O–H Vibration
2.7.6. C–H Vibration
2.7.7. C=C Vibration
2.7.8. O–S Vibration
3. Material and Methods
3.1. Sulfation of Betulin
3.2. Methods for Physicochemical Analysis
3.3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kazachenko, A.S.; Miroshnikova, A.V.; Tarabanko, V.E.; Skripnikov, A.M.; Malyar, Y.N.; Borovkova, V.S.; Sychev, V.V.; Taran, O.P. Thermal Conversion of Flax Shives in Sub- and Supercritical Ethanol in the Presence of Ru/C Catalyst. Catalysts 2021, 11, 970. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Baryshnikov, S.V.; Miroshnikova, A.V.; Kazachenko, A.S.; Malyar, Y.N.; Skripnikov, A.M.; Taran, O.P. Fractionation of Birch Wood by Integrating Alkaline-Acid Treatments and Hydrogenation in Ethanol over a Bifunctional Ruthenium Catalyst. Catalysts 2021, 11, 1362. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tarabanko, V.E.; Miroshnikova, A.V.; Sychev, V.V.; Skripnikov, A.M.; Malyar, Y.N.; Mikhlin, Y.L.; Baryshnikov, S.V.; Taran, O.P. Reductive Catalytic Fractionation of Flax Shive over Ru/C Catalysts. Catalysts 2021, 11, 42. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef] [Green Version]
- Borovkova, V.S.; Malyar, Y.N.; Sudakova, I.G.; Chudina, A.I.; Skripnikov, A.M.; Fetisova, O.Y.; Kazachenko, A.S.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; et al. Molecular Characteristics and Antioxidant Activity of Spruce (Picea abies) Hemicelluloses Isolated by Catalytic Oxidative Delignification. Molecules 2022, 27, 266. [Google Scholar] [CrossRef]
- Lachowicz, H.; Wróblewska, H.; Sajdak, M.; Komorowicz, M.; Wojtan, R. The chemical composition of silver birch (Betula pendula Roth.) wood in Poland depending on forest stand location and forest habitat type. Cellulose 2019, 26, 3047–3067. [Google Scholar] [CrossRef] [Green Version]
- Mononen, K.; Jääskeläinen, A.-S.; Alvila, L.; Pakkanen, T.T.; Vuorinen, T. Chemical changes in silver birch (Betula pendula Roth) wood caused by hydrogen peroxide bleaching and monitored by color measurement (CIELab) and UV-Vis, FTIR and UVRR spectroscopy. Holzforschung 2005, 59, 381–388. [Google Scholar] [CrossRef]
- Hiltunen, E.; Mononen, K.; Alvila, L.; Pakkanen, T.T. Discolouration of birch wood: Analysis of extractives from discoloured surface of vacuum-dried European white birch (Betula pubescens) board. Wood Sci. Technol. 2007, 42, 103. [Google Scholar] [CrossRef]
- Ashour, M.; Wink, M.; Gershenzon, J. Biochemistry of Terpenoids: Monoterpenes, Sesquiterpenes and Diterpenes. In Annual Plant Reviews Volume 40: Biochemistry of Plant Secondary Metabolism; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 258–303. [Google Scholar] [CrossRef]
- Tolstikova, T.G.; Sorokina, I.V.; Tolstikov, G.A.; Tolstikov, A.G.; Flekhter, O.B. Biological activity and pharmacological prospects of lupane terpenoids: I. natural lupane derivatives. Russ. J. Bioorg. Chem. 2006, 32, 37–49. [Google Scholar] [CrossRef]
- Šiman, P.; Filipová, A.; Tichá, A.; Niang, M.; Bezrouk, A.; Havelek, R. Effective Method of Purification of Betulin from Birch Bark: The Importance of Its Purity for Scientific and Medicinal Use. PLoS ONE 2016, 11, e0154933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef] [Green Version]
- Svetlana, A.K.; Tatyana, P.S.; Mikhail, A.M.; Yury, N.M.; Anna, S.K.; Valeri, A.D.; Boris, N.K. Preparation and Antitumor Activity of Betulin Dipropionate and its Composites: A minireview. Biointerface Res. Appl. Chem. 2022, 12, 6873–6894. [Google Scholar]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Dastghaib, S.; Ahmadi, M.; Mehrbod, P.; Khadem, F.; Behrouj, H.; Aghanoori, M.-R.; Machaj, F.; Ghamsari, M.; Rosik, J.; et al. Betulin and its derivatives as novel compounds with different pharmacological effects. Biotechnol. Adv. 2020, 38, 107409. [Google Scholar] [CrossRef] [PubMed]
- Ressmann, A.K.; Strassl, K.; Gaertner, P.; Zhao, B.; Greiner, L.; Bica, K. New aspects for biomass processing with ionic liquids: Towards the isolation of pharmaceutically active betulin. Green Chem. 2012, 14, 940–944. [Google Scholar] [CrossRef]
- Myszka, H.; Grzywacz, D.; Zdrowowicz, M.; Spisz, P.; Butowska, K.; Rak, J.; Piosik, J.; Jaśkiewicz, M.; Kamysz, W.; Liberek, B. Design, synthesis and biological evaluation of betulin-3-yl 2-amino-2-deoxy-β-D-glycopyranosides. Bioorg. Chem. 2020, 96, 103568. [Google Scholar] [CrossRef]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive Review on Betulin as a Potent Anticancer Agent. BioMed Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef] [Green Version]
- Heidary Navid, M.; Laszczyk-Lauer, M.N.; Reichling, J.; Schnitzler, P. Pentacyclic triterpenes in birch bark extract inhibit early step of herpes simplex virus type 1 replication. Phytomedicine 2014, 21, 1273–1280. [Google Scholar] [CrossRef]
- Shikov, A.N.; Djachuk, G.I.; Sergeev, D.V.; Pozharitskaya, O.N.; Esaulenko, E.V.; Kosman, V.M.; Makarov, V.G. Birch bark extract as therapy for chronic hepatitis C—A pilot study. Phytomedicine 2011, 18, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Dehaen, W.; Mashentseva, A.A.; Seitembetov, T.S. Allobetulin and Its Derivatives: Synthesis and Biological Activity. Molecules 2011, 16, 2443. [Google Scholar] [CrossRef] [PubMed]
- Kaplun, A.; Bezrukov, D.A.; Popenko, V.I.; Shvets, V.I. Spherical amorphous nanoparticles from birch bark triterpenoids-A novel type of submicronic vehicle for drug delivery. Russ. J. Biopharm. 2011, 3, 28–40. [Google Scholar]
- Wang, H.M.; Şoica, C.M.; Wenz, G. A Comparison Investigation on the Solubilization of Betulin and Betulinic Acid in Cyclodextrin Derivatives. Nat. Prod. Commun. 2012, 7, 1934578X1200700304. [Google Scholar] [CrossRef] [Green Version]
- Şoica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin Complex in γ-Cyclodextrin Derivatives: Properties and Antineoplasic Activities in In Vitro and In Vivo Tumor Models. Int. J. Mol. Sci. 2012, 13, 4992. [Google Scholar] [CrossRef] [PubMed]
- Vorobyova, O.; Deryabina, O.; Malygina, D.; Plotnikova, N.; Solovyeva, A.; Belyaeva, K.; Melnikova, N. Betulin-3,28-diphosphate as a Component of Combination Cytostatic Drugs for the Treatment of Ehrlich Ascites Carcinoma In Vitro and In Vivo Experiments. Sci. Pharm. 2018, 86, 17. [Google Scholar] [CrossRef] [Green Version]
- Bureeva, S.; Andia-Pravdivy, J.; Symon, A.; Bichucher, A.; Moskaleva, V.; Popenko, V.; Shpak, A.; Shvets, V.; Kozlov, L.; Kaplun, A. Selective inhibition of the interaction of C1q with immunoglobulins and the classical pathway of complement activation by steroids and triterpenoids sulfates. Bioorg. Med. Chem. 2007, 15, 3489–3498. [Google Scholar] [CrossRef]
- Grishkovets, V.I. Synthesis of triterpenoid sulfates using the SO3—dimethyl sulfoxide complex. Chem. Nat. Compd. 1999, 35, 73–74. [Google Scholar] [CrossRef]
- Levdanskii, V.A.; Levdanskii, A.V.; Kuznetsov, B.N. Sulfation of Betulin by Sulfamic Acid in DMF and Dioxane. Chem. Nat. Compd. 2014, 50, 1029–1031. [Google Scholar] [CrossRef]
- Dzhil’bert, E.E. Sulfonation of Organic Compounds; Himiya: Moscow, Russia, 1969; p. 415. (In Russian) [Google Scholar]
- Caputo, H.E.; Straub, J.E.; Grinstaff, M.W. Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem. Soc. Rev. 2019, 48, 2338–2365. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Desai, U.R. Chemical Sulfation of Small Molecules—Advances and Challenges. Tetrahedron 2010, 66, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spillane, W.; Malaubier, J.-B. Sulfamic Acid and Its N- and O-Substituted Derivatives. Chem. Rev. 2014, 114, 2507–2586. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Vasilieva, N.Y.; Borovkova, V.S.; Fetisova, O.Y.; Issaoui, N.; Malyar, Y.N.; Elsuf’ev, E.V.; Karacharov, A.A.; Skripnikov, A.M.; Miroshnikova, A.V.; et al. Food Xanthan Polysaccharide Sulfation Process with Sulfamic Acid. Foods 2021, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.; Malyar, Y.; Vasilyeva, N.; Fetisova, O.; Chudina, A.; Sudakova, I.; Antonov, A.; Borovkova, V.; Kuznetsova, S. Isolation and sulfation of galactoglucomannan from larch wood (Larix sibirica). Wood Sci. Technol. 2021, 55, 1091–1107. [Google Scholar] [CrossRef]
- Kazachenko, A.; Vasilyeva, N.; Malyar, Y.; Kazachenko, A. Mathematical Optimization, the Effect of the Catalyst and Solvent on the Process of Starch Sulfation with Sulfamic Acid. In Lecture Notes in Networks and Systems; Springer International Publishing AG: Cham, Switzerland, 2021; pp. 275–282. [Google Scholar] [CrossRef]
- Malyar, Y.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Borovkova, V.S.; Skripnikov, A.M.; Miroshnikova, A.V.; Zimonin, D.V.; Ionin, V.A.; Kazachenko, A.S.; Issaoui, N. Modification of Arabinogalactan Isolated from Larix sibirica Ledeb. into Sulfated Derivatives with the Controlled Molecular Weights. Molecules 2021, 26, 5364. [Google Scholar] [CrossRef]
- Kazachenko, A.; Malyar, Y.; Vasilyeva, N.; Borovkova, V.; Issaoui, N. Optimization of guar gum galactomannan sulfation process with sulfamic acid. Biomass Convers. Biorefin. 2021, 1–10. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Malyar, Y.N.; Issaoui, N.; Vasilieva, N.Y.; Karacharov, A.A. Synthesis optimization, DFT and physicochemical study of chitosan sulfates. J. Mol. Struct. 2021, 1245, 131083. [Google Scholar] [CrossRef]
- Kazachenko, A.; Akman, F.; Medimagh, M.; Issaoui, N.; Vasilieva, N.; Malyar, Y.N.; Sudakova, I.G.; Karacharov, A.; Miroshnikova, A.; Al-Dossary, O.M. Sulfation of Diethylaminoethyl-Cellulose: QTAIM Topological Analysis and Experimental and DFT Studies of the Properties. ACS Omega 2021, 6, 22603–22615. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Skvortsova, G.P.; Levdansky, V.A.; Lutoshkin, M.A. Development of a method for sulfation of ethanolignin of fir wood using sulfamic acid. J. Sib. Fed. Univ. Ser. Chem. 2018, 11, 122–130. [Google Scholar]
- Kazachenko, A.S.; Malyar, Y.N.; Vasilyeva, N.Y.; Bondarenko, G.N.; Korolkova, I.V.; Antonov, A.V.; Karacharov, A.A.; Fetisova, O.Y.; Skvortsova, G.P. «Green» synthesis and characterization of galactomannan sulfates obtained using sulfamic acid. Biomass Convers. Biorefin. 2020, 1–10. [Google Scholar] [CrossRef]
- Akman, F.; Kazachenko, A.S.; Vasilyeva, N.Y.; Malyar, Y.N. Synthesis and characterization of starch sulfates obtained by the sulfamic acid-urea complex. J. Mol. Struct. 2020, 1208, 127899. [Google Scholar] [CrossRef]
- Levdansky, V.; Vasilyeva, N.; Malyar, Y.; Levdansky, A.; Kondrasenko, A.; Kazachenko, A.; Kuznetsov, B. Sulfation of ethanol lignin of abies wood by sulfamic acid in N,N-dimethylformamide medium. Biomass Convers. Biorefin. 2020, 1–8. [Google Scholar] [CrossRef]
- Kuznetsov, B.N.; Vasilyeva, N.Y.; Kazachenko, A.S.; Levdansky, V.A.; Kondrasenko, A.A.; Malyar, Y.N.; Skvortsova, G.P.; Lutoshkin, M.A. Optimization of the process of abies ethanol lignin sulfation by sulfamic acid–urea mixture in 1,4-dioxane medium. Wood Sci. Technol. 2020, 54, 365–381. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Levdansky, V.A.; Levdansky, A.V.; Kuznetsov, B.N. Mathematical optimization of the process of birch wood xylan sulfation by sulfamic acid in N, N-dimethylformamide medium. Khimiya Rastit. Syr’ya 2021, 2, 87–94. [Google Scholar] [CrossRef]
- Akman, F.; Issaoui, N.; Kazachenko, A. Intermolecular hydrogen bond interactions in the thiourea/water complexes (Thio-(H2O)n) (n = 1, …, 5): X-ray, DFT, NBO, AIM, and RDG analyses. J. Mol. Model. 2020, 26, 161. [Google Scholar] [CrossRef] [PubMed]
- Yokoya, M.; Kimura, S.; Yamanaka, M. Urea Derivatives as Functional Molecules: Supramolecular Capsules, Supramolecular Polymers, Supramolecular Gels, Artificial Hosts, and Catalysts. Chem. Eur. J. 2021, 27, 5601–5614. [Google Scholar] [CrossRef]
- Civera, C.; del Valle, J.C.; Elorza, M.A.; Elorza, B.; Arias, C.; Díaz-Oliva, C.; Catalán, J.; García Blanco, F. Solvatochromism in urea/water and urea-derivative/water solutions. Phys. Chem. Chem. Phys. 2020, 22, 25165–25176. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Akman, F.; Abdelmoulahi, H.; Issaoui, N.; Malyar, Y.N.; Al-Dossary, O.; Wojcik, M.J. Intermolecular hydrogen bonds interactions in water clusters of ammonium sulfamate: FTIR, X-ray diffraction, AIM, DFT, RDG, ELF, NBO analysis. J. Mol. Liq. 2021, 342, 117475. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Ukkola, J.; Liimatainen, H. Direct sulfation of cellulose fibers using a reactive deep eutectic solvent to produce highly charged cellulose nanofibers. Cellulose 2019, 26, 2303–2316. [Google Scholar] [CrossRef] [Green Version]
- Sass, R.L. A neutron diffraction study on the crystal structure of sulfamic acid. Acta Crystallogr. 1960, 13, 320–324. [Google Scholar] [CrossRef] [Green Version]
- Levdansky, A.V.; Vasilyeva, N.Y.; Kondrasenko, A.A.; Levdansky, V.A.; Malyar, Y.N.; Kazachenko, A.S.; Kuznetsov, B.N. Sulfation of arabinogalactan with sulfamic acid under homogeneous conditions in dimethylsulfoxide medium. Wood Sci. Technol. 2021, 55, 1725–1744. [Google Scholar] [CrossRef]
- Malyar, Y.N.; Kazachenko, A.S.; Vasilyeva, N.Y.; Fetisova, O.Y.; Borovkova, V.S.; Miroshnikova, A.V.; Levdansky, A.V.; Skripnikov, A.M. Sulfation of wheat straw soda lignin: Role of solvents and catalysts. Catal. Today 2021, in press. [Google Scholar] [CrossRef]
- Vasilyeva, N.Y.; Kazachenko, A.S.; Malyar, Y.; Kuznetsov, B.N. Sulfation of betulin with chlorosulfonic acid in pyridine. J. Sib. Fed. Univ. Chem. 2020, 13, 447–459. [Google Scholar] [CrossRef]
- Cabassi, F.; Casu, B.; Perlin, A.S. Infrared absorption and raman scattering of sulfate groups of heparin and related glycosaminoglycans in aqueous solution. Carbohydr. Res. 1978, 63, 1–11. [Google Scholar] [CrossRef]
- Krichen, F.; Bougatef, H.; Capitani, F.; Ben Amor, I.; Koubaa, I.; Gargouri, J.; Maccari, F.; Mantovani, V.; Galeotti, F.; Volpi, N.; et al. Purification and structural elucidation of chondroitin sulfate/dermatan sulfate from Atlantic bluefin tuna (Thunnus thynnus) skins and their anticoagulant and ACE inhibitory activities. RSC Adv. 2018, 8, 37965–37975. [Google Scholar] [CrossRef] [Green Version]
- Levdanskii, V.A.; Levdanskii, A.V.; Kuznetsov, B.N. Sulfonation of Betulinic Acid by Sulfamic Acid. Chem. Nat. Compd. 2015, 51, 894–896. [Google Scholar] [CrossRef]
- Drebushchak, T.N.; Mikhailovskaya, A.V.; Drebushchak, V.A.; Mikhailenko, M.A.; Myz’, S.A.; Shakhtshneider, T.P.; Kuznetsova, S.A. Crystalline forms of betulin: Polymorphism or pseudopolymorphism? J. Struct. Chem. 2020, 61, 1260–1266. [Google Scholar] [CrossRef]
- Melnikova, N.B.; Malygina, D.S.; Klabukova, I.N.; Belov, D.V.; Vasin, V.A.; Petrov, P.S.; Knyazev, A.V.; Markin, A.V. Betulin-3,28-diphosphate. Physico-Chemical Properties and In Vitro Biological Activity Experiments. Molecules 2018, 23, 1175. [Google Scholar] [CrossRef] [Green Version]
- Shakhtshneider, T.; Mikhailenko, M.; Drebushchak, V.; Drebushchak, T.; Malyar, Y.; Kuznetsova, S. Effect of ball-milling on the formation of betulin and betulin diacetate composites with polyethylene glycol. Mater. Today Proc. 2019, 12, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Gong, N.; Zhang, L.; Lu, Y.; Du, G. Structural and Computational Study of 4 New Solvatomorphs of Betulin: A Combined X-Ray, Hirshfeld Surface, and Thermal Analysis. J. Pharm. Sci. 2017, 106, 826–834. [Google Scholar] [CrossRef]
- Morales, J.E.T.; Pedroso, M.T.C.; Siguenza, J.C.; Villavicencio, C.B. Evaluation of Reactivity (pKa) of Substituted Aromatic Diamines, Monomers for Polyamides Synthesis, via Nuclear Magnetic Resonance, NMR-1H. Chem. Proc. 2021, 3, 8436. [Google Scholar] [CrossRef]
- Akman, F. Prediction of Chemical Reactivity of Cellulose and Chitosan Based on Density Functional Theory. Cellul. Chem. Technol. 2017, 51, 253–262. [Google Scholar]
- Akman, F.; Kazachenko, A.; Malyar, Y. A density functional theory study of sulfated monolignols: P-Coumaril and coniferyl alcohols. Cellul. Chem. Technol. 2021, 55, 41–54. [Google Scholar] [CrossRef]
- Fazilath Basha, A.; Liakath Ali Khan, F.; Muthu, S.; Raja, M. Computational evaluation on molecular structure (Monomer, Dimer), RDG, ELF, electronic (HOMO-LUMO, MEP) properties, and spectroscopic profiling of 8-Quinolinesulfonamide with molecular docking studies. Comput. Theor. Chem. 2021, 1198, 113169. [Google Scholar] [CrossRef]
- Scrocco, E.; Tomasi, J. Electronic Molecular Structure, Reactivity and Intermolecular Forces: An Euristic Interpretation by Means of Electrostatic Molecular Potentials. In Advances in Quantum Chemistry; Löwdin, P.-O., Ed.; Academic Press: Cambridge, MA, USA, 1978; Volume 11, pp. 115–193. [Google Scholar]
- Demir, P.; Akman, F. Molecular structure, spectroscopic characterization, HOMO and LUMO analysis of PU and PCL grafted onto PEMA-co-PHEMA with DFT quantum chemical calculations. J. Mol. Struct. 2017, 1134, 404–415. [Google Scholar] [CrossRef]
- Akman, F. A comparative study based on molecular structure, spectroscopic, electronic, thermodynamic and NBO analysis of some nitrogen-containing monomers. Polym. Bull. 2021, 78, 663–693. [Google Scholar] [CrossRef]
- Muthu, S.; Renuga, S. Vibrational spectra and normal coordinate analysis of 2-hydroxy-3-(2-methoxyphenoxy) propyl carbamate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 313–325. [Google Scholar] [CrossRef]
- Janani, S.; Rajagopal, H.; Muthu, S.; Aayisha, S.; Raja, M. Molecular structure, spectroscopic (FT-IR, FT-Raman, NMR), HOMO-LUMO, chemical reactivity, AIM, ELF, LOL and Molecular docking studies on 1-Benzyl-4-(N-Boc-amino)piperidine. J. Mol. Struct. 2021, 1230, 129657. [Google Scholar] [CrossRef]
- Tahenti, M.; Gatfaoui, S.; Issaoui, N.; Roisnel, T.; Marouani, H. A tetrachlorocobaltate(II) salt with 2-amino-5-picolinium: Synthesis, theoretical and experimental characterization. J. Mol. Struct. 2020, 1207, 127781. [Google Scholar] [CrossRef]
- Padmanabhan, J.; Parthasarathi, R.; Subramanian, V.; Chattaraj, P.K. Electrophilicity-Based Charge Transfer Descriptor. J. Phys. Chem. A 2007, 111, 1358–1361. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the Reactivity of Captodative Ethylenes in Polar Cycloaddition Reactions. A Theoretical Study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J. Frontier Orbitals and Organic Chemical Reactions. J. Prakt. Chem. 1978, 320, 879–880. [Google Scholar] [CrossRef]
- Mondal, S.; Mandal, S.M.; Mondal, T.K.; Sinha, C. Structural characterization of new Schiff bases of sulfamethoxazole and sulfathiazole, their antibacterial activity and docking computation with DHPS protein structure. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 150, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Demircioğlu, Z.; Kaştaş, Ç.A.; Büyükgüngör, O. Theoretical analysis (NBO, NPA, Mulliken Population Method) and molecular orbital studies (hardness, chemical potential, electrophilicity and Fukui function analysis) of (E)-2-((4-hydroxy-2-methylphenylimino)methyl)-3-methoxyphenol. J. Mol. Struct. 2015, 1091, 183–195. [Google Scholar] [CrossRef]
- Rose, M.R.; Lau, S.S.; Prasse, C.; Sivey, J.D. Exotic Electrophiles in Chlorinated and Chloraminated Water: When Conventional Kinetic Models and Reaction Pathways Fall Short. Environ. Sci. Technol. Lett. 2020, 7, 360–370. [Google Scholar] [CrossRef]
- Stavber, S.; Jereb, M.; Zupan, M. Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides. Synthesis 2008, 2008, 1487–1513. [Google Scholar] [CrossRef]
- Robert, J.; Ouellette, J.D.R. Chapter 13. Electrophilic Aromatic Substitution. Organic Chemistry; Academic Press: London, UK; San Diego, CA, USA, 2018; pp. 375–407. [Google Scholar] [CrossRef]
- Profant, V.; Johannessen, C.; Blanch, E.W.; Bouř, P.; Baumruk, V. Effects of sulfation and the environment on the structure of chondroitin sulfate studied via Raman optical activity. Phys. Chem. Chem. Phys. 2019, 21, 7367–7377. [Google Scholar] [CrossRef]
- Sagaama, A.; Issaoui, N.; Al-Dossary, O.; Kazachenko, A.S.; Wojcik, M.J. Non covalent interactions and molecular docking studies on morphine compound. J. King Saud Univ. Sci. 2021, 33, 101606. [Google Scholar] [CrossRef]
- Kazachenko, A.; Akman, F.; Abir, S.; Issaoui, N.; Malyar, Y.; Vasilieva, N.; Borovkova, V. Theoretical and experimental study of guar gum sulfation. J. Mol. Model. 2021, 27, 5. [Google Scholar] [CrossRef] [PubMed]
- Lutoshkin, M.A.; Kazachenko, A.S. Assessment of various density functionals and solvation models to describe acid-base, spectral and complexing properties of thiobarbituric and barbituric acids in aqueous solution. J. Comput. Methods Sci. Eng. 2017, 17, 851–863. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tomilin, F.N.; Pozdnyakova, A.A.; Vasilyeva, N.Y.; Malyar, Y.N.; Kuznetsova, S.A.; Avramov, P.V. Theoretical DFT interpretation of infrared spectra of biologically active arabinogalactan sulphated derivatives. Chem. Pap. 2020, 74, 4103–4113. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Malyar, Y.N.; Ghatfaoui, S.; Issaoui, N.; Al-Dossary, O.; Wojcik, M.J.; Kazachenko, A.S.; Miroshnikova, A.V.; Berezhnaya, Y.D. A density functional theory calculations of infrared spectra of galactomannan butyl ether. J. Mol. Struct. 2022, 1251, 131998. [Google Scholar] [CrossRef]
- Mainreck, N.; Brézillon, S.; Sockalingum, G.D.; Maquart, F.-X.; Manfait, M.; Wegrowski, Y. Rapid characterization of glycosaminoglycans using a combined approach by infrared and Raman microspectroscopies. J. Pharm. Sci. 2011, 100, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Akman, F. Spectroscopic investigation, HOMO–LUMO energies, natural bond orbital (NBO) analysis and thermodynamic properties of two–armed macroinitiator containing coumarin with DFT quantum chemical calculations. Can. J. Phys. 2016, 94, 583–593. [Google Scholar] [CrossRef]
- Shklyaev, O.E.; Kubicki, J.D.; Watts, H.D.; Crespi, V.H. Constraints on Iβ cellulose twist from DFT calculations of 13C NMR chemical shifts. Cellulose 2014, 21, 3979–3991. [Google Scholar] [CrossRef]
- Gerbst, A.; Nikolaev, A.; Yashunsky, D.; Shashkov, A.; Dmitrenok, A.; Nifantiev, N. Theoretical and NMR-based Conformational Analysis of Phosphodiester-linked Disaccharides. Sci. Rep. 2017, 7, 8934. [Google Scholar] [CrossRef]
- Issaoui, N.; Rekik, N.; Oujia, B.; Wójcik, M. Anharmonic effects on theoretical IR line shapes of medium strong H(D) bonds. Int. J. Quantum Chem. 2009, 109, 483–499. [Google Scholar] [CrossRef]
- Rekik, N.; Issaoui, N.; Oujia, B.; Wójcik, M. Theoretical IR spectral density of H-bond in liquid phase: Combined effects of anharmonicities, Fermi resonances, direct and indirect relaxations. J. Mol. Liq. 2008, 141, 104–109. [Google Scholar] [CrossRef]
- Jomaa, I.; Noureddine, O.; Gatfaoui, S.; Issaoui, N.; Roisnel, T.; Marouani, H. Experimental, computational, and in silico analysis of (C8H14N2)2[CdCl6] compound. J. Mol. Struct. 2020, 1213, 128186. [Google Scholar] [CrossRef]
- Gatfaoui, S.; Sagaama, A.; Issaoui, N.; Roisnel, T.; Marouani, H. Synthesis, experimental, theoretical study and molecular docking of 1-ethylpiperazine-1,4-diium bis(nitrate). Solid State Sci. 2020, 106, 106326. [Google Scholar] [CrossRef]
- Levdansky, V.A.; Levdansky, A.V.; Kuznetsov, B.N. Sulfation of betulin with chlorosulfonic acid in dimethylformamide and dioxane. Russ. J. Bioorg. Chem. 2014, 40, 748–751. [Google Scholar] [CrossRef]
- Natalia, Y.V.; Alexander, V.L.; Alexander, S.K.; Galina, P.S.; Boris, N.K. Modification of Sulfated Arabinogalactan with Amino Acids by Ion Exchange Method. J. Sib. Fed. Univ. Chem. 2016, 1, 20–28. [Google Scholar] [CrossRef]
- Zagorodni, A. Ion Exchange Materials: Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2006; p. 456. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.K.T.; Millam, J. Gauss View, version 5; Semichem Inc.: Shawnee, KS, USA, 2010. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Tawada, Y.; Tsuneda, T.; Yanagisawa, S.; Yanai, T.; Hirao, K. A Long-Range Corrected Time-Dependent Density Functional Theory. J. Chem. Phys. 2004, 120, 8425–8433. [Google Scholar] [CrossRef]

| No. | Number of Catalyst Cycles | Time, h | Sulfur Content, wt % |
|---|---|---|---|
| 1 | 1 | 0.5 | 5,1 |
| 2 | 1 | 1.0 | 7,2 |
| 3 | 1 | 1.5 | 9.7 |
| 4 | 1 | 2.0 | 10.1 |
| 5 | 1 | 2.5 | 10.1 |
| 6 | 2 | 2.0 | 10.0 |
| 7 | 3 | 2.0 | 9.9 |
| 8 | 4 | 2.0 | 9.9 |
| Parameter (eV) | BE | BES | ||
|---|---|---|---|---|
| CAM-B3LYP | B3LYP | CAM-B3LYP | B3LYP | |
| EHOMO | −8.0636 | −6.4970 | −7.6140 | −6.0592 |
| ELUMO | 2.0841 | 0.6027 | −1.0534 | −1.7704 |
| Energy band gap 1 (∆E1 = ELUMO − EHOMO) | 10.1477 | 7.0997 | 6.5607 | 4.2888 |
| EHOMO-1 | −8.3901 | −6.7860 | −8.0834 | −6.5468 |
| ELUMO+1 | 2.8428 | 1.5094 | −0.9562 | −1.6803 |
| Energy band gap 2 [∆E2 = (ELUMO+1) − (EHOMO-1)] | 11.2329 | 8.2954 | 7.1272 | 4.8665 |
| Chemical potential (μ) | −2.9897 | −2.9471 | −4.3337 | −3.9148 |
| Softness (ς) | 0.1971 | 0.2817 | 0.3048 | 0.4663 |
| Ionization energy (I) | 8.0636 | 6.4970 | 7.6140 | 6.0592 |
| Electron affinity (A) | −2.0841 | −0.6027 | 1.0534 | 1.7704 |
| Electronegativity (χ) | 2.9897 | 2.9471 | 4.3337 | 3.9148 |
| Chemical hardness (η) | 5.0738 | 3.5499 | 3.2803 | 2.1444 |
| Electrophilicity index (ω) | 0.8808 | 1.2234 | 2.8626 | 3.5734 |
| Maximum charge transfer index (ΔNmax) | 0.5892 | 0.8302 | 1.3211 | 1.8256 |
| Nucleophilic index (N) | 1.1353 | 0.8174 | 0.3493 | 0.2798 |
| Optical softness (σ˳) | 0.0985 | 0.1409 | 0.1524 | 0.2332 |
| BE | BES | ||||||
|---|---|---|---|---|---|---|---|
| Label | Symbol | CAM-B3LYP | B3LYP | Label | Symbol | CAM-B3LYP | B3LYP |
| 1 | C | −0.20309 | −0.2021 | 1 | C | −0.22973 | −0.22872 |
| 2 | C | 0.18481 | 0.18906 | 2 | C | 0.18324 | 0.18934 |
| 3 | C | 0.01401 | 0.01816 | 3 | C | 0.00964 | 0.01468 |
| 4 | C | −0.05888 | −0.0508 | 4 | C | −0.07512 | −0.06891 |
| 5 | C | 0.05783 | 0.05865 | 5 | C | 0.05565 | 0.05635 |
| 6 | C | −0.20435 | −0.19927 | 6 | C | −0.20148 | −0.19788 |
| 7 | C | −0.19031 | −0.19141 | 7 | C | −0.19045 | −0.1918 |
| 8 | C | −0.1909 | −0.19068 | 8 | C | −0.19249 | −0.19222 |
| 9 | C | 0.04579 | 0.04836 | 9 | C | 0.04544 | 0.04774 |
| 10 | C | −0.06271 | −0.0556 | 10 | C | −0.07765 | −0.06991 |
| 11 | C | 0.07032 | 0.07734 | 11 | C | 0.06934 | 0.07627 |
| 12 | C | −0.06643 | −0.06291 | 12 | C | −0.0649 | −0.06222 |
| 13 | C | −0.18767 | −0.18994 | 13 | C | −0.18787 | −0.19012 |
| 14 | C | −0.19296 | −0.19412 | 14 | C | −0.19225 | −0.19329 |
| 15 | C | −0.21532 | −0.2128 | 15 | C | −0.21202 | −0.20973 |
| 16 | C | −0.17216 | −0.17377 | 16 | C | −0.18315 | −0.18554 |
| 17 | C | 0.01376 | 0.02268 | 17 | C | −6.45E-4 | 0.00905 |
| 18 | C | −0.07535 | −0.07321 | 18 | C | −0.07845 | −0.07622 |
| 19 | C | −0.19513 | −0.19369 | 19 | C | −0.1947 | −0.1942 |
| 20 | C | −0.18731 | −0.18628 | 20 | C | −0.19282 | −0.1934 |
| 21 | C | −0.11263 | −0.11347 | 21 | C | −0.11335 | −0.11266 |
| 22 | O | −0.56017 | −0.55848 | 22 | O | −0.57467 | −0.58 |
| 23 | C | −0.29325 | −0.29311 | 23 | C | −0.30288 | −0.30325 |
| 24 | C | −0.3145 | −0.31645 | 24 | C | −0.31418 | −0.31685 |
| 25 | C | −0.32162 | −0.32217 | 25 | C | −0.31836 | −0.31921 |
| 26 | C | 0.05309 | 0.05169 | 26 | C | 0.03722 | 0.03911 |
| 27 | C | −0.31074 | −0.31217 | 27 | C | −0.31306 | −0.31459 |
| 28 | C | −0.33367 | −0.33536 | 28 | C | −0.33572 | −0.33768 |
| 29 | C | 0.15887 | 0.16828 | 29 | C | 0.16241 | 0.16996 |
| 30 | C | −0.36417 | −0.36923 | 30 | C | −0.36498 | −0.36949 |
| 31 | C | −0.2996 | −0.29698 | 31 | C | −0.29878 | −0.29611 |
| 32 | O | −0.54748 | −0.54509 | 32 | O | −0.54583 | −0.55022 |
| 33 | H | 0.1048 | 0.10177 | 33 | H | 0.12979 | 0.12677 |
| 34 | H | 0.09295 | 0.09169 | 34 | H | 0.09161 | 0.09019 |
| 35 | H | 0.08495 | 0.08254 | 35 | H | 0.09965 | 0.09586 |
| 36 | H | 0.05337 | 0.05213 | 36 | H | 0.13211 | 0.13113 |
| 37 | H | 0.10024 | 0.09727 | 37 | H | 0.08427 | 0.0821 |
| 38 | H | 0.0719 | 0.07075 | 38 | H | 0.13803 | 0.13532 |
| 39 | H | 0.0885 | 0.0889 | 39 | H | 0.07823 | 0.07888 |
| 40 | H | 0.09337 | 0.09139 | 40 | H | 0.08748 | 0.08604 |
| 41 | H | 0.08774 | 0.08523 | 41 | H | 0.07866 | 0.07653 |
| 42 | H | 0.08704 | 0.08641 | 42 | H | 0.09884 | 0.09788 |
| 43 | H | 0.06821 | 0.06817 | 43 | H | 0.10117 | 0.09971 |
| 44 | H | 0.08341 | 0.08274 | 44 | H | 0.07921 | 0.0789 |
| 45 | H | 0.09383 | 0.0913 | 45 | H | 0.0897 | 0.08761 |
| 46 | H | 0.08689 | 0.08867 | 46 | H | 0.09033 | 0.09176 |
| 47 | H | 0.08982 | 0.08987 | 47 | H | 0.08277 | 0.08326 |
| 48 | H | 0.08967 | 0.08751 | 48 | H | 0.09089 | 0.08839 |
| 49 | H | 0.09365 | 0.09198 | 49 | H | 0.08868 | 0.08699 |
| 50 | H | 0.08919 | 0.08727 | 50 | H | 0.09186 | 0.0901 |
| 51 | H | 0.09946 | 0.09543 | 51 | H | 0.1076 | 0.10509 |
| 52 | H | 0.08699 | 0.08781 | 52 | H | 0.08374 | 0.08357 |
| 53 | H | 0.08231 | 0.0818 | 53 | H | 0.08349 | 0.08281 |
| 54 | H | 0.06581 | 0.06409 | 54 | H | 0.12012 | 0.11787 |
| 55 | H | 0.09886 | 0.09787 | 55 | H | 0.09005 | 0.08945 |
| 56 | H | 0.1011 | 0.10052 | 56 | H | 0.11287 | 0.11204 |
| 57 | H | 0.09352 | 0.09118 | 57 | H | 0.08921 | 0.08717 |
| 58 | H | 0.08797 | 0.08677 | 58 | H | 0.08784 | 0.08778 |
| 59 | H | 0.30411 | 0.30184 | 59 | H | 0.1285 | 0.12782 |
| 60 | H | 0.10399 | 0.10187 | 60 | H | 0.09061 | 0.08926 |
| 61 | H | 0.09188 | 0.09124 | 61 | H | 0.09559 | 0.09434 |
| 62 | H | 0.09807 | 0.09677 | 62 | H | 0.0856 | 0.08599 |
| 63 | H | 0.0944 | 0.09438 | 63 | H | 0.09401 | 0.09461 |
| 64 | H | 0.09969 | 0.09982 | 64 | H | 0.09462 | 0.09426 |
| 65 | H | 0.09838 | 0.09805 | 65 | H | 0.0911 | 0.09123 |
| 66 | H | 0.09449 | 0.0942 | 66 | H | 0.09448 | 0.09386 |
| 67 | H | 0.10248 | 0.10202 | 67 | H | 0.09695 | 0.09611 |
| 68 | H | 0.09944 | 0.09872 | 68 | H | 0.1333 | 0.13009 |
| 69 | H | 0.0819 | 0.08019 | 69 | H | 0.11729 | 0.11281 |
| 70 | H | 0.1016 | 0.10022 | 70 | H | 0.10554 | 0.10448 |
| 71 | H | 0.10214 | 0.10139 | 71 | H | 0.09637 | 0.09664 |
| 72 | H | 0.09582 | 0.0964 | 72 | H | 0.09323 | 0.09188 |
| 73 | H | 0.09797 | 0.09658 | 73 | H | 0.10952 | 0.1082 |
| 74 | H | 0.09717 | 0.09619 | 74 | H | 0.09666 | 0.09747 |
| 75 | H | 0.09912 | 0.09985 | 75 | H | 0.10339 | 0.10164 |
| 76 | H | 0.10177 | 0.1 | 76 | H | 0.11443 | 0.11385 |
| 77 | H | 0.11392 | 0.11365 | 77 | H | 0.10407 | 0.1031 |
| 78 | H | 0.10872 | 0.10765 | 78 | H | 0.11323 | 0.1131 |
| 79 | H | 0.11357 | 0.11327 | 79 | H | 0.09275 | 0.09116 |
| 80 | H | 0.09079 | 0.08897 | 80 | H | 0.08375 | 0.0818 |
| 81 | H | 0.08773 | 0.08596 | 81 | S | 1.40967 | 1.41359 |
| 82 | H | 0.30721 | 0.30457 | 82 | O | −0.62093 | −0.6217 |
| - | - | - | - | 83 | O | −0.52194 | −0.52206 |
| - | - | - | - | 84 | O | −0.64718 | −0.64532 |
| - | - | - | - | 85 | Na | 0.59859 | 0.60197 |
| - | - | - | - | 86 | S | 1.44977 | 1.45217 |
| - | - | - | - | 87 | O | −0.53987 | −0.53873 |
| - | - | - | - | 88 | O | −0.62287 | −0.62426 |
| - | - | - | - | 89 | O | −0.65891 | −0.65632 |
| - | - | - | - | 90 | Na | 0.60311 | 0.60553 |
| BE | BES | ||
|---|---|---|---|
| O-H | 3671 and 3670 | C-H (CH2=) | 3107 and 3032 |
| C-H (CH2=) | 3107 and 3032 | C-H | 3033–2901 |
| C-H | 3038–2835 | C-H (=C-CH3) | 2998 and 2905 |
| C-H (=C-CH3) | 3002 and 2906 | C=C | 1658 |
| C-H (CH2-OH) | 2954 and 2870 | O-S | 1227, 1209, 1074, 1068, 941, 931 |
| CH (CH-OH) | 2940 and 2925 | CH2 (O-) | 2984, 1474, 1210 |
| C=C | 1657 | Na-O | 307 and 295 |
| BE | BES | ||||||
|---|---|---|---|---|---|---|---|
| Hydrogen Atom | Chemical Shift (ppm) | Carbon Atom | Chemical Shift (ppm) | Hydrogen Atom | Chemical Shift (ppm) | Carbon Atom | Chemical Shift (ppm) |
| 80-H | 5.1189 | 29-C | 140.0163 | 79-H | 5.156 | 29-C | 140.1273 |
| 81-H | 5.0522 | 31-C | 93.0736 | 80-H | 5.0961 | 31-C | 93.0158 |
| 69-H | 3.8844 | 2-C | 67.3998 | 68-H | 4.2101 | 2-C | 80.5108 |
| 35-H | 3.4006 | 26-C | 61.5732 | 35-H | 4.1063 | 26-C | 63.9693 |
| 70-H | 3.2142 | 10-C | 44.2637 | 69-H | 3.644 | 10-C | 42.9424 |
| 58-H | 3.0378 | 4-C | 42.8496 | 58-H | 3.1113 | 18-C | 41.8903 |
| 44-H | 2.2612 | 18-C | 41.2406 | 36-H | 2.7828 | 21-C | 40.439 |
| 34-H | 2.2327 | 17-C | 40.3549 | 33-H | 2.6308 | 4-C | 40.0096 |
| 57-H | 2.2152 | 21-C | 40.2103 | 57-H | 2.311 | 17-C | 38.9887 |
| 79-H | 2.0347 | 9-C | 38.2528 | 44-H | 2.2933 | 9-C | 38.1393 |
| 55-H | 2.0335 | 11-C | 36.727 | 34-H | 2.124 | 11-C | 36.4112 |
| 77-H | 1.9339 | 3-C | 32.7851 | 38-H | 2.1138 | 3-C | 32.9181 |
| 49-H | 1.9317 | 5-C | 32.6781 | 78-H | 2.0739 | 5-C | 32.3275 |
| 51-H | 1.8729 | 12-C | 29.4602 | 55-H | 2.0641 | 12-C | 29.5149 |
| 39-H | 1.8035 | 20-C | 26.4429 | 76-H | 1.9848 | 20-C | 26.7763 |
| 53-H | 1.796 | 6-C | 25.4542 | 54-H | 1.9815 | 6-C | 25.9807 |
| 78-H | 1.7151 | 8-C | 24.085 | 49-H | 1.9654 | 8-C | 23.9728 |
| 47-H | 1.6804 | 16-C | 22.1573 | 53-H | 1.9604 | 19-C | 22.9769 |
| 43-H | 1.6769 | 19-C | 22.0667 | 43-H | 1.944 | 16-C | 21.3784 |
| 42-H | 1.6543 | 15-C | 20.998 | 51-H | 1.844 | 15-C | 20.8974 |
| 48-H | 1.6326 | 23-C | 20.0705 | 39-H | 1.7884 | 23-C | 18.9603 |
| 37-H | 1.6259 | 13-C | 18.8465 | 77-H | 1.7473 | 13-C | 18.6958 |
| 46-H | 1.6253 | 1-C | 17.938 | 42-H | 1.7136 | 30-C | 16.8118 |
| 40-H | 1.582 | 30-C | 16.8562 | 47-H | 1.6841 | 1-C | 14.6996 |
| 33-H | 1.563 | 14-C | 14.6112 | 46-H | 1.6692 | 27-C | 14.5464 |
| 41-H | 1.5611 | 27-C | 13.6592 | 48-H | 1.654 | 14-C | 14.3916 |
| 74-H | 1.5597 | 28-C | 13.0177 | 40-H | 1.6504 | 28-C | 12.8152 |
| 54-H | 1.5306 | 7-C | 12.1492 | 73-H | 1.6266 | 7-C | 12.3059 |
| 45-H | 1.5145 | 25-C | 7.8876 | 45-H | 1.5462 | 24-C | 7.7267 |
| 68-H | 1.494 | 24-C | 7.6367 | 41-H | 1.5179 | 25-C | 7.5684 |
| 52-H | 1.4371 | - | - | 52-H | 1.5156 | - | - |
| 56-H | 1.3537 | - | - | 37-H | 1.4956 | - | - |
| 59-H | 1.3506 | - | - | 56-H | 1.4865 | - | - |
| 50-H | 1.2945 | - | - | 67-H | 1.4568 | - | - |
| 76-H | 1.2901 | - | - | 75-H | 1.3469 | - | - |
| 72-H | 1.2891 | - | - | 59-H | 1.3319 | - | - |
| 36-H | 1.2623 | - | - | 50-H | 1.3168 | - | - |
| 60-H | 1.2384 | - | - | 60-H | 1.2996 | - | - |
| 61-H | 1.196 | - | - | 71-H | 1.2321 | - | - |
| 38-H | 1.1732 | - | - | 70-H | 1.1811 | - | - |
| 71-H | 1.1562 | - | - | 63-H | 1.1485 | - | - |
| 64-H | 1.1243 | - | - | 65-H | 1.1308 | - | - |
| 75-H | 1.1019 | - | - | 74-H | 1.1244 | - | - |
| 66-H | 1.0916 | - | - | 64-H | 0.965 | - | - |
| 63-H | 1.0154 | - | - | 62-H | 0.9579 | - | - |
| 65-H | 0.9691 | - | - | 61-H | 0.9354 | - | - |
| 67-H | 0.8833 | - | - | 66-H | 0.8736 | - | - |
| 82-H | 0.8301 | - | - | 72-H | 0.5335 | - | - |
| 62-H | 0.7253 | - | - | - | - | - | - |
| 73-H | 0.6054 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazachenko, A.S.; Akman, F.; Vasilieva, N.Y.; Issaoui, N.; Malyar, Y.N.; Kondrasenko, A.A.; Borovkova, V.S.; Miroshnikova, A.V.; Kazachenko, A.S.; Al-Dossary, O.; et al. Catalytic Sulfation of Betulin with Sulfamic Acid: Experiment and DFT Calculation. Int. J. Mol. Sci. 2022, 23, 1602. https://doi.org/10.3390/ijms23031602
Kazachenko AS, Akman F, Vasilieva NY, Issaoui N, Malyar YN, Kondrasenko AA, Borovkova VS, Miroshnikova AV, Kazachenko AS, Al-Dossary O, et al. Catalytic Sulfation of Betulin with Sulfamic Acid: Experiment and DFT Calculation. International Journal of Molecular Sciences. 2022; 23(3):1602. https://doi.org/10.3390/ijms23031602
Chicago/Turabian StyleKazachenko, Aleksandr S., Feride Akman, Natalya Yu. Vasilieva, Noureddine Issaoui, Yuriy N. Malyar, Aleksandr A. Kondrasenko, Valentina S. Borovkova, Angelina V. Miroshnikova, Anna S. Kazachenko, Omar Al-Dossary, and et al. 2022. "Catalytic Sulfation of Betulin with Sulfamic Acid: Experiment and DFT Calculation" International Journal of Molecular Sciences 23, no. 3: 1602. https://doi.org/10.3390/ijms23031602
APA StyleKazachenko, A. S., Akman, F., Vasilieva, N. Y., Issaoui, N., Malyar, Y. N., Kondrasenko, A. A., Borovkova, V. S., Miroshnikova, A. V., Kazachenko, A. S., Al-Dossary, O., Wojcik, M. J., Berezhnaya, Y. D., & Elsuf’ev, E. V. (2022). Catalytic Sulfation of Betulin with Sulfamic Acid: Experiment and DFT Calculation. International Journal of Molecular Sciences, 23(3), 1602. https://doi.org/10.3390/ijms23031602










