Sorption and Magnetic Properties of Oxalato-Based Trimetallic Open Framework Stabilized by Charge-Assisted Hydrogen Bonds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
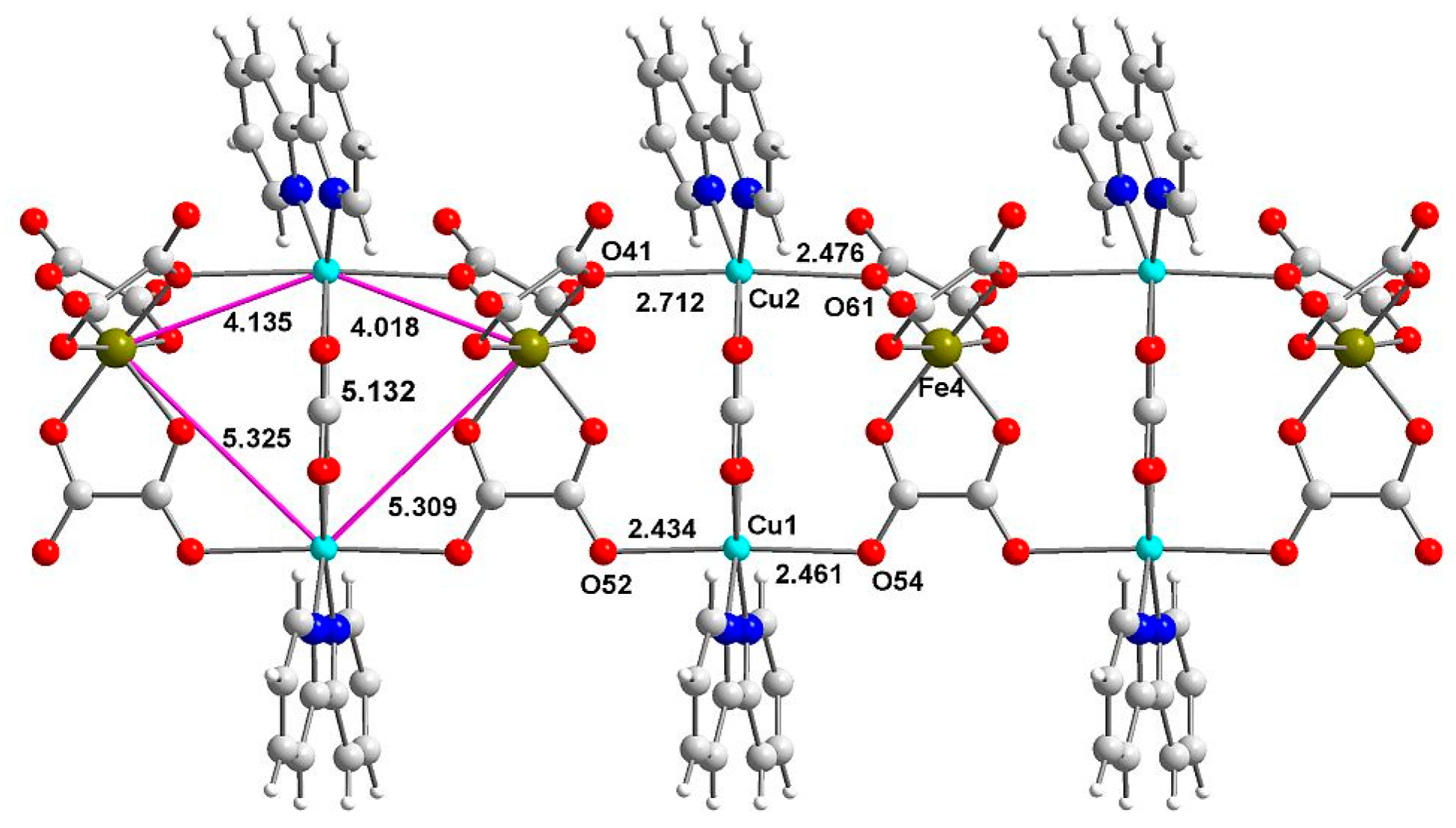
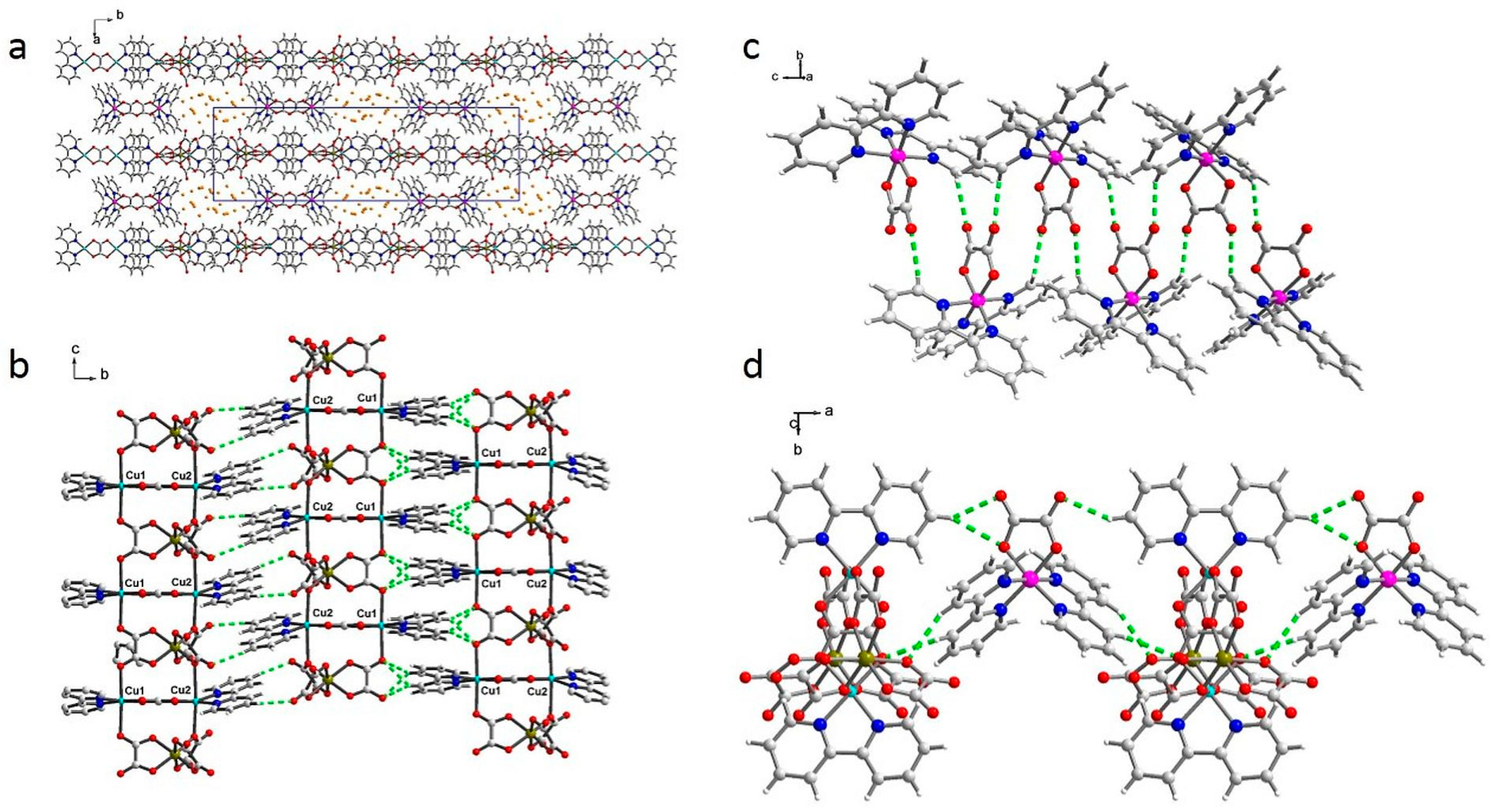
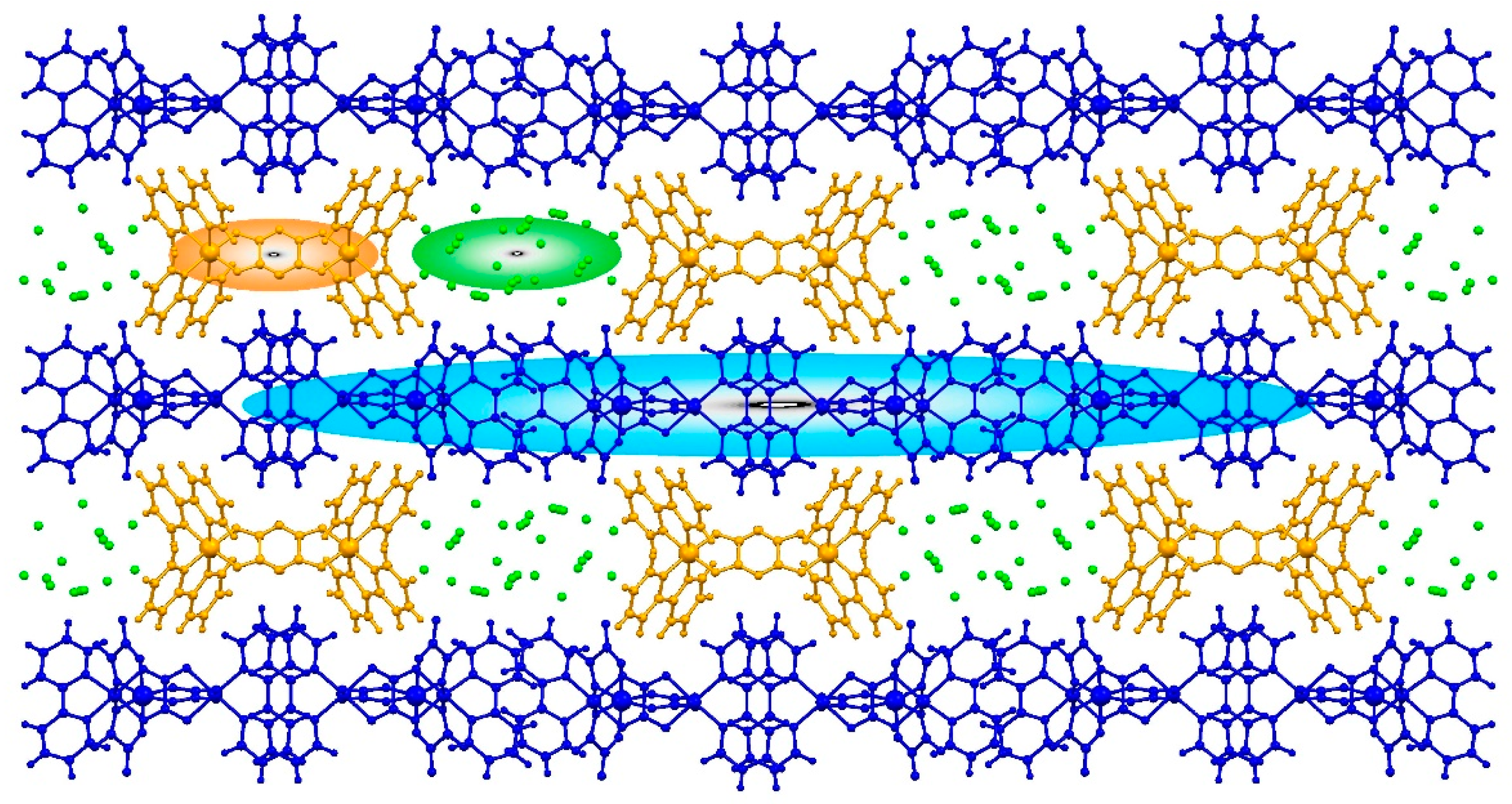
2.2. Structure Description
2.3. Thermal Analysis
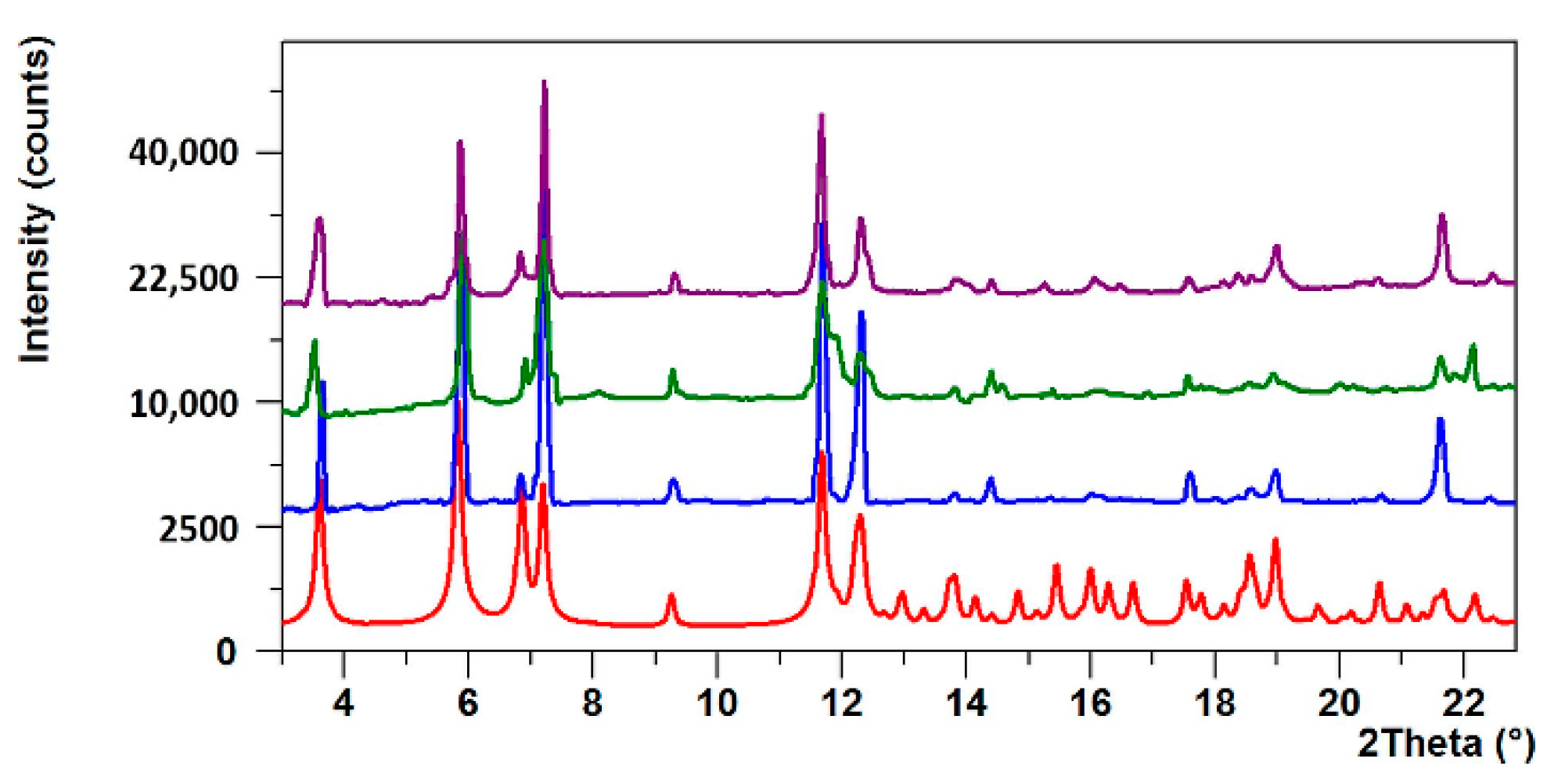
2.4. Powder Experiments
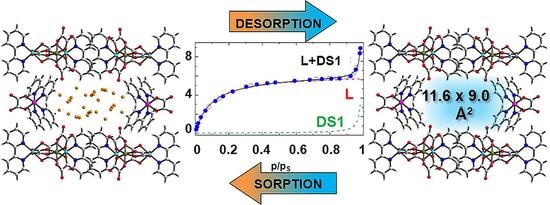
2.5. Sorption Properties
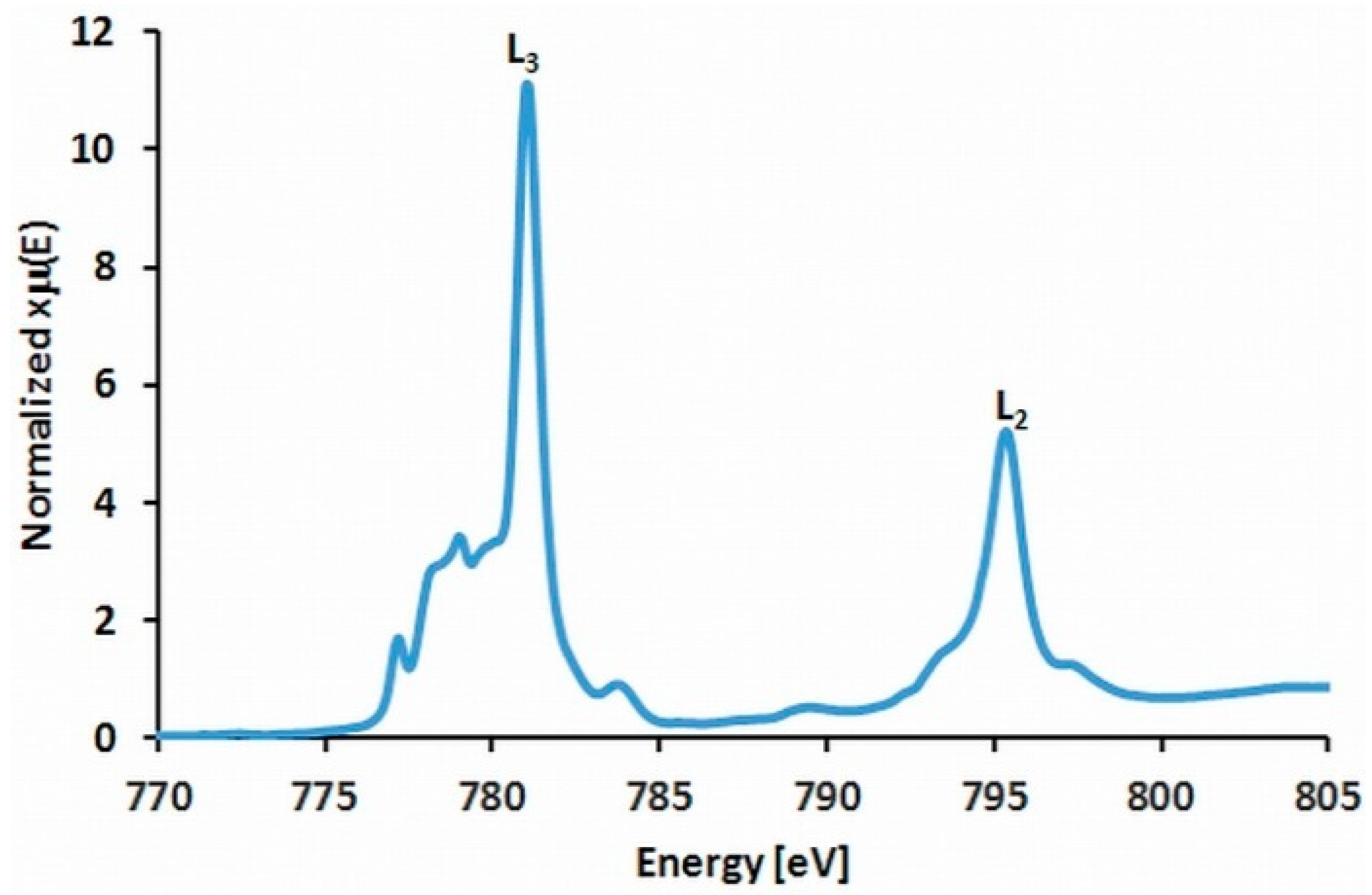
2.6. XAS Experiments
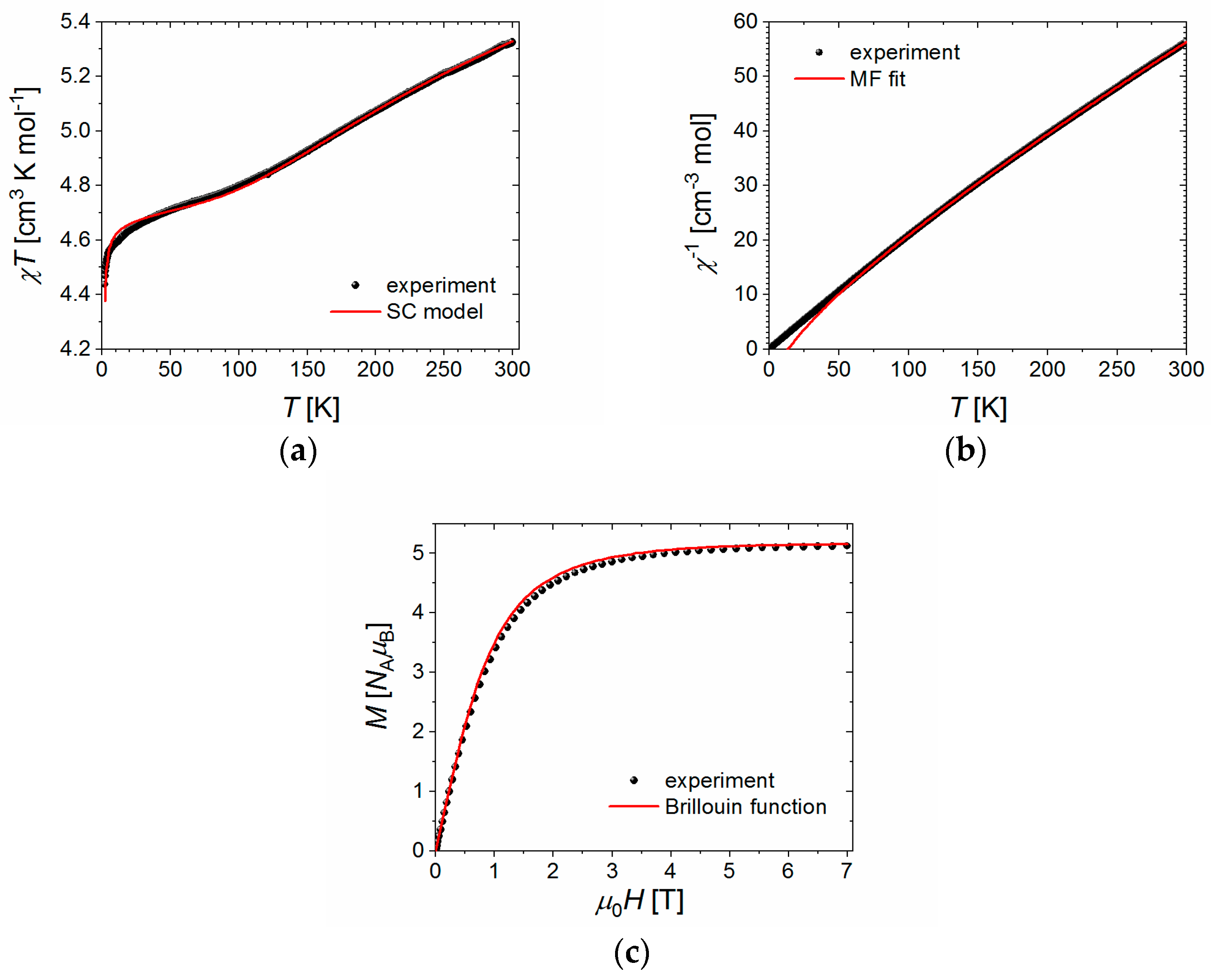
2.7. Magnetic Properties
2.7.1. Simple Dimer Model
2.7.2. Molecular Field (MF) Prediction
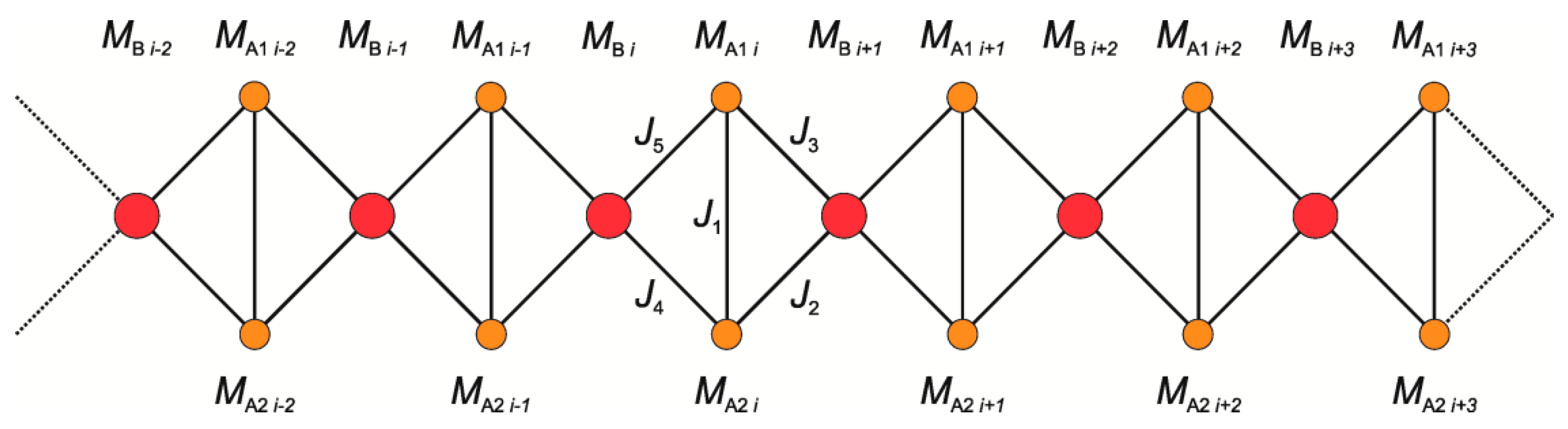
2.7.3. Semiclassical (SC) Model
2.7.4. MS and SC Results
2.7.5. Magnetostructural Comparison
3. Materials and Methods
3.1. Materials and General Procedure
3.2. Synthesis of {[Co(bpy)2(ox)][{Cu2(bpy)2ox}Fe(ox)3]}n·8.5nH2O 1
3.3. Single Crystal X-ray Diffraction Measurements
3.4. Water Adsorption-Desorption Measurements
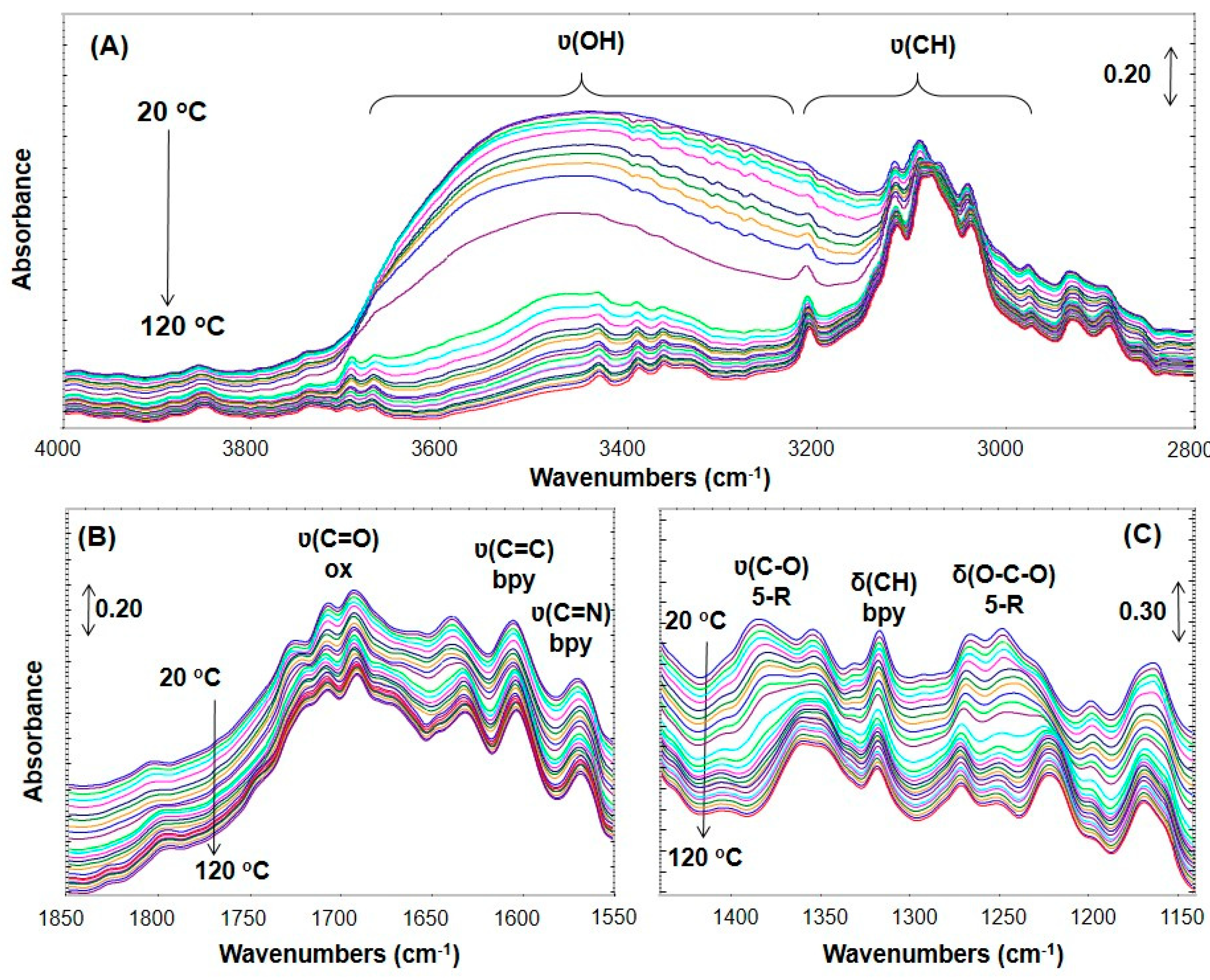
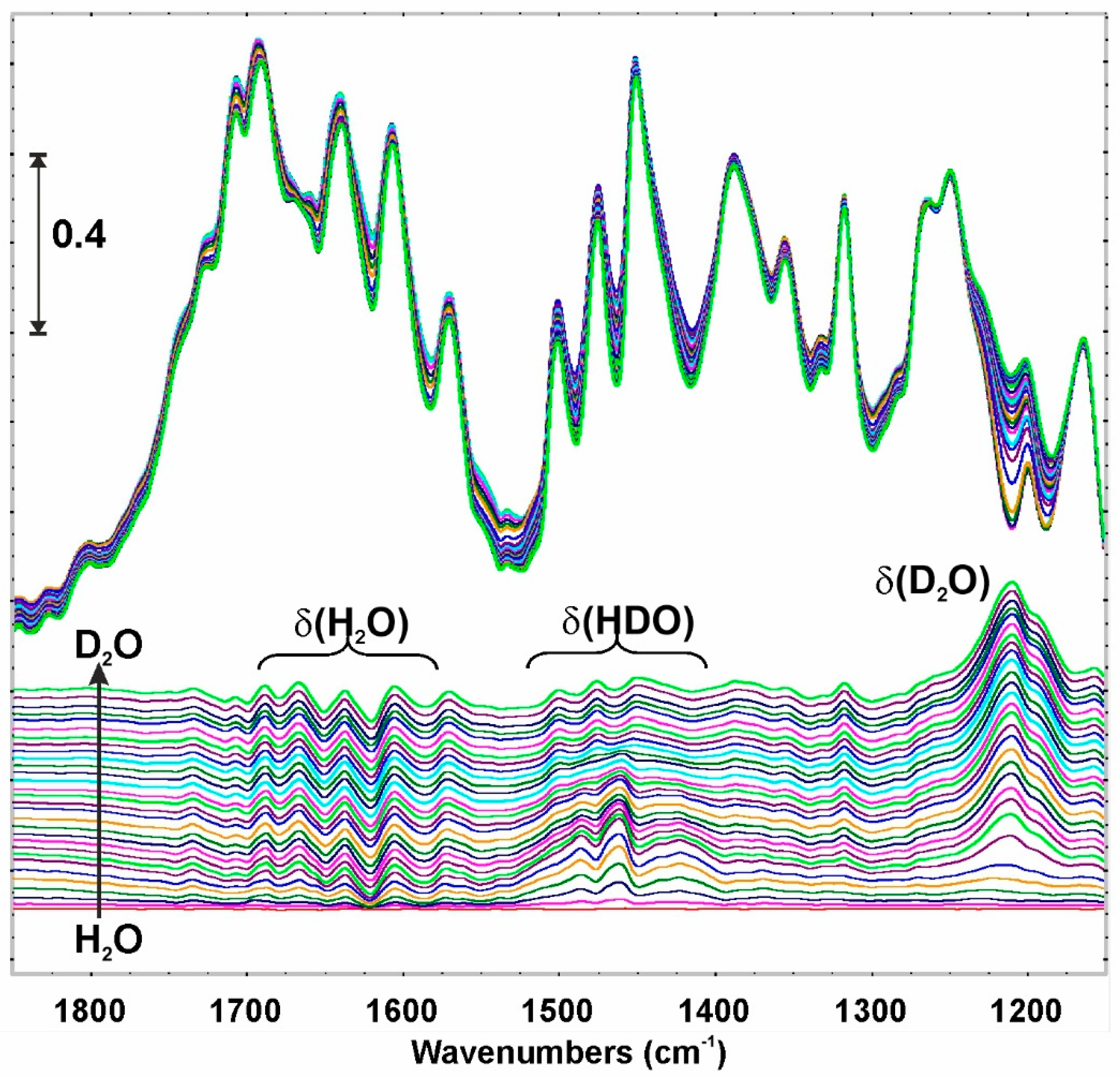
3.5. In-Situ DRIFT Investigations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keene, T.D.; Zimmermann, I.; Neels, A.; Sereda, O.; Hauser, J.; Bonin, M.; Hursthouse, M.B.; Price, D.J.; Decurtins, S. Heterocyclic amine directed synthesis of metal(II)-oxalates: Investigating the magnetic properties of two complete series of chains with S = 5/2 to S = 1/2. Dalt. Trans. 2010, 39, 4937–4950. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, G.; Andruh, M.; Lloret, F.; Julve, M. Bis(Oxalato)Chromium(III) complexes: Versatile tectons in designing heterometallic coordination compounds. Coord. Chem. Rev. 2011, 255, 161–185. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Martí-Gastaldo, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Kanižaj, L.; Molčanov, K.; Torić, F.; Pajić, D.; Lončarić, I.; Šantić, A.; Jurić, M. Ladder-LIKE [CrCu] coordination polymers containing unique bridging modes of [Cr(C2O4)3]3− and Cr2O72−. Dalt. Trans. 2019, 48, 7891–7898. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Tang, T.H.; Li, Y.; Xu, S. Versatile oxalato-bridging modes: A novel three-dimensional framework structure of manganese(ii) oxalate complex [MnC2O4]·0.5H2O and the relationship with other manganese(ii) oxalates. Dalt. Trans. 2021, 50, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.; Zhang, J.; Yan, X.; Zhu, D. Step by step crystal-to-crystal transformation from 1D K2Cu(C2O4)2(H2O)4 (1) to 1D K2Cu(C2O4)2(H2O)2 (2) and then 1D K2Cu(C2O4)2 (3) by dehydration. CrystEngComm 2016, 18, 5062–5065. [Google Scholar] [CrossRef]
- Fernandes, T.S.; Melo, W.D.C.; Kalinke, L.H.G.; Rabelo, R.; Valdo, A.K.; Da Silva, C.C.; Martins, F.T.; Amorós, P.; Lloret, F.; Julve, M.; et al. 2D and 3D mixed MII/CuII metal-organic frameworks (M = Ca and Sr) with: N,N′-2,6-pyridinebis(oxamate) and oxalate: Preparation and magneto-structural study. Dalt. Trans. 2018, 47, 11539–11553. [Google Scholar] [CrossRef]
- Alexandru, M.G.; Visinescu, D.; Shova, S.; Andruh, M.; Lloret, F.; Cano, J.; Julve, M. Three different types of bridging ligands in a 3d-3d′-3d″ heterotrimetallic chain. Dalt. Trans. 2018, 47, 1010–1013. [Google Scholar] [CrossRef]
- Li, A.M.; Rentschler, E. 1,2,4-triazole schiff base directed synthesis of polynuclear iron complexes: Investigating the magnetic properties going from a dimer to a 1D chain to a 3D framework. Polyhedron 2018, 154, 364–372. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Martí-Gastaldo, C. A “cation-less” oxalate-based ferromagnet formed by neutral bimetallic layers: {[Co(H2O)2]3[Cr(ox)3]2(18-crown-6)2}∞ (ox = oxalate dianion; 18-Crown-6 = C12H24O6). Inorg. Chem. 2007, 46, 8108–8110. [Google Scholar] [CrossRef]
- Sadakiyo, M.; Ōkawa, H.; Shigematsu, A.; Ohba, M.; Yamada, T.; Kitagawa, H. Promotion of low-humidity proton conduction by controlling hydrophilicity in layered metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- García-Couceiro, U.; Castillo, O.; Luque, A.; García-Terán, J.P.; Beobide, G.; Román, P. Rational design of 2D magnetic metal-organic coordination polymers assembled from oxalato and dipyridyl spacers. Cryst. Growth Des. 2006, 6, 1839–1847. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, C.; Desplanches, C.; Clemente-Juan, J.M.; Clemente-León, M.; Coronado, E. Photomagnetic properties of an Fe(II) spin-crossover complex of 6-(3,5-diamino-2,4,6-triazinyl)-2,2′-bipyridine and its insertion into 2D and 3D bimetallic oxalate-based networks. Dalt. Trans. 2017, 46, 2680–2689. [Google Scholar] [CrossRef] [PubMed]
- Dazem, C.L.F.; Ndosiri, B.N.; Nfor, E.N.; Köferstein, R.; Shankhari, P.; Fokwa, B.P.T.; Nenwa, J. Synthesis, structures, thermal and magnetic properties of two Bis(Oxalato)Cuprate(II) hybrid salts containing pyridinium derivative cations. J. Mol. Struct. 2020, 1203, 127399. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Martí-Gastaldo, C. Single chain magnets based on the oxalate ligand. J. Am. Chem. Soc. 2008, 130, 14987–14989. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Ferrero, E.; Almeida, M.; Waerenborgh, J.C. Oxalate-based 3D chiral magnets: The series [ZII(bpy)3][ClO4][MIIFeIII(ox)3] (ZII = Fe, Ru; MII = Mn, Fe; bpy = 2,2′-bipyridine; ox = oxalate dianion). Eur. J. Inorg. Chem. 2005, 11, 2064–2070. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Waerenborgh, J.C. Multifunctional magnetic materials obtained by insertion of spin-crossover feIII complexes into chiral 3D bimetallic oxalate-based ferromagnets. Inorg. Chem. 2011, 50, 9122–9130. [Google Scholar] [CrossRef]
- López-Jordà, M.; Giménez-Marqués, M.; Desplanches, C.; Mínguez Espallargas, G.; Clemente-León, M.; Coronado, E. Insertion of a [FeII(pyimH)3]2+ [pyimH = 2-(1H-Imidazol-2-yl)pyridine] spin-crossover complex inside a ferromagnetic lattice based on a chiral 3D bimetallic oxalate network. Eur. J. Inorg. Chem. 2016, 13–14, 2187–2192. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Behera, J.N. Organic-Inorganic hybrid three-dimensional metal sulfite-oxalates with honeycomb-like structures. Dalt. Trans. 2017, 46, 5911–5917. [Google Scholar] [CrossRef]
- Ma, L.-N.; Li, X.-Y.; Shi, W.-J.; Li, Y.-Z.; Liu, G.; Hou, L.; Wang, Y.-Y. Luminescent and magnetic properties of coordination polymers induced by coordinating modes of a Bis(Oxamate) ligand. Chempluschem 2019, 84, 62–68. [Google Scholar] [CrossRef]
- Xu, N.; Xing, Y.; Liu, X.; Song, D.; Ma, L.; Sun, X. Synthesis, structure and photoluminescent properties of [C 6N2H14][Nd2(C2O4)2(SO4)2(H2O)4]·H2O, a new organically templated Neodymlum(III) oxalate-sulfate. Z. Anorg. Allg. Chem. 2009, 635, 558–562. [Google Scholar] [CrossRef]
- Evans, J.S.O.; Bénard, S.; Yu, P.; Clément, R. Ferroelectric alignment of NLO chromophores in layered inorganic lattices: Structure of a stilbazolium metal-oxalate from powder diffraction data. Chem. Mater. 2001, 13, 3813–3816. [Google Scholar] [CrossRef]
- Lü, Y.; Zhang, X.; Cui, X.B.; Xu, J.Q. A Series of compounds based on [P2W18O62]6− and transition metal mixed organic ligand complexes with high catalytic properties for styrene epoxidation. Inorg. Chem. 2018, 57, 11123–11134. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.N.; Zhao, C.X.; Shi, X.M.; Zhang, H.; Wu, W.; Cui, X.B. Three new compounds based on similar molybdenum-vanadium clusters and several types of copper complexes. CrystEngComm 2018, 20, 969–977. [Google Scholar] [CrossRef]
- Tominaka, S.; Coudert, F.X.; Dao, T.D.; Nagao, T.; Cheetham, A.K. Insulator-to-Proton-conductor transition in a dense metal-organic framework. J. Am. Chem. Soc. 2015, 137, 6428–6431. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Wang, H.-N.; Wang, L.-S.; Zou, Y.-H.; Zhou, Z.-Y. Enhanced proton conductivity of a MOF-808 framework through anchoring organic acids to the zirconium clusters by post-synthetic modification. CrystEngComm 2019, 21, 3146–3150. [Google Scholar] [CrossRef]
- Thétiot, F.; Duhayon, C.; Venkatakrishnan, T.S.; Sutter, J.-P. Supramolecular hybrid architectures Including an open framework with reversible sorption properties. Cryst. Growth Des. 2008, 8, 1870–1877. [Google Scholar] [CrossRef]
- Mouchaham, G.; Roques, N.; Brandès, S.; Duhayon, C.; Sutter, J.P. Self-assembly of Zr(C2O4)44− metallotectons and bisimidazolium cations: Influence of the dication on H-bonded framework dimensionality and material potential porosity. Cryst. Growth Des. 2011, 11, 5424–5433. [Google Scholar] [CrossRef]
- Vegas, V.G.; Maldonado, N.; Castillo, O.; Gómez-García, C.J.; Amo-Ochoa, P. Multifunctional coordination polymers based on copper with modified nucleobases, easily modulated in size and conductivity. J. Inorg. Biochem. 2019, 200, 110805. [Google Scholar] [CrossRef]
- Coronado, E.; Galán Mascarós, J.R.; Martí-Gastaldo, C. Controlling the dimensionality of oxalate-based bimetallic complexes: The ferromagnetic chain {[K(18-Crown-6)][Mn(Bpy)Cr(Ox)3]}∞(18-Crown-6 = C12H24O6, Ox = C2 O42−, Bpy = C10H8N2). Polyhedron 2007, 26, 2101–2104. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Marino, N.; De Munno, G.; Lloret, F.; Julve, M.; Pardo, E.; Armentano, D. Selective guest inclusion in oxalate-based Iron(III) magnetic coordination polymers. Inorg. Chem. 2016, 55, 11160–11169. [Google Scholar] [CrossRef]
- Kanižaj, L.; Dubraja, L.A.; Torić, F.; Pajić, D.; Molčanov, K.; Wenger, E.; Jurić, M. Dimensionality controlled by light exposure: 1D: Versus 3D oxalate-bridged [CuFe] coordination polymers based on an [Fe(C2O4)3]3− metallotecton. Inorg. Chem. Front. 2019, 6, 3327–3335. [Google Scholar] [CrossRef]
- Oshio, H.; Nagashima, U. Design of a homonuclear ferromagnetic chain: Structures and Magnetic properties of oxalato-bridged Copper(II) complexes with one-dimensional structures. Inorg. Chem. 1992, 31, 3295–3301. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Shi, Y.; Toms, B.B.; Dixit, N.; Kumari, N.; Mishra, L.; Goodisman, J.; Dabrowiak, J.C. Cytotoxicity of Cu(II) and Zn(II) 2,2′-bipyridyl complexes: Dependence of IC50 on recovery time. Chem. Res. Toxicol. 2010, 23, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Castillo, O.; Muga, I.; Luque, A.; Gutiérrez-Zorrilla, J.M.; Sertucha, J.; Vitoria, P.; Román, P. Synthesis, chemical characterization, x-ray crystal structure and magnetic properties of oxalato-bridged Copper(II) binuclear complexes with 2,2′-bipyridine and diethylenetriamine as peripheral ligands. Polyhedron 1999, 18, 1235–1245. [Google Scholar] [CrossRef]
- Llunel, S.M.; Casanova, D.; Cirera, J.; Bofill, J.M.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. University of Barcelona, S. SHAPE v. 2.1. Program for the Calculations of Continous Shape Measures of Polygonal and Polyhedral Molecular Fragments. 2013. Available online: http://www.ee.ub.edu/index.php/downloads/viewcategory/4-registerd-download (accessed on 26 January 2022).
- Muzioł, T.M.; Tereba, N.; Podgajny, R.; Kȩdziera, D.; Wrzeszcz, G. Solvent-assisted structural conversion involving bimetallic complexes based on the Tris(Oxalato)Ferrate(III) unit with the green → blue → red crystal color sequence. Dalt. Trans. 2019, 48, 11536–11546. [Google Scholar] [CrossRef]
- Muzioł, T.M.; Wrzeszcz, G. Spontaneous resolution of heterometallic complex: Cis-[Co(NH3)4(H2O)2][Fe(Ox)3] 2H2O—A rare example for labile Iron(III) complex. Polyhedron 2016, 109, 138–146. [Google Scholar] [CrossRef]
- Muzioł, T.M.; Wrzeszcz, G.; Chrząszcz, Ł. Hydrogen bond and π Stacking assisted formation of Tris(Oxalato) Ferrate(III) based crystals of heteronuclear compounds. Polyhedron 2011, 30, 169–177. [Google Scholar] [CrossRef]
- Sreekantan Nair Lalithambika, S.; Golnak, R.; Winter, B.; Atak, K. Electronic structure of aqueous [Co(Bpy)3]2+/3+ electron mediators. Inorg. Chem. 2019, 58, 4731–4740. [Google Scholar] [CrossRef] [Green Version]
- Yaghoobi Nia, N.; Farahani, P.; Sabzyan, H.; Zendehdel, M.; Oftadeh, M. A Combined computational and experimental study of the [Co(Bpy)3]2+/3+ complexes as one-electron outer-sphere redox couples in dye-sensitized solar cell electrolyte media. Phys. Chem. Chem. Phys. 2014, 16, 11481–11491. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Zhao, X.-J.; Cai, H. Crystal structure of Tris(2,2′-Bipyridine)Cobalt(in) triperchlorate dihydrate, [Co(C10H8N2)3](ClO4)3·2H2O. Z. Krist.-New Cryst. Struct. 2004, 219, 463–465. [Google Scholar] [CrossRef]
- Brown, I.D. Recent developments in the methods and applications of the bond valence model. Chem. Rev. 2009, 109, 6858–6919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitajgorodskij, A.I. Molecular Crystals and Molecules; Academic Press: New York, NY, USA, 1973; pp. 18–21. [Google Scholar]
- Lin, R.B.; He, Y.; Li, P.; Wang, H.; Zhou, W.; Chen, B. Multifunctional porous hydrogen-bonded organic framework materials. Chem. Soc. Rev. 2019, 48, 1362–1389. [Google Scholar] [CrossRef] [PubMed]
- Baptistella, G.B.; Manica, G.C.M.; de Souza, S.W.; Santana, F.S.; Fachini, L.G.; Hughes, D.L.; de Sá, E.L.; Picheth, G.; Soares, J.F.; Rego, F.G.M.; et al. An oxalate-bridged oxidovanadium(IV) binuclear complex that improves the in vitro cell uptake of a fluorescent glucose analog. Polyhedron 2021, 198, 115071. [Google Scholar] [CrossRef]
- Kamga, I.N.; Ndosiri, B.N.; Nana, A.N.; Roman, T.; Pouamo, L.S.; Nenwa, J. Synthesis, crystal structure, thermal analysis and magnetic properties of two bis(oxalato)cuprate(II) hybrid salts crystallizing with lattice oxalic acid molecules. Polyhedron 2021, 205, 115291. [Google Scholar] [CrossRef]
- Dridi, R.; Essghaier, B.; Cherni, S.N.; Zid, M.F. Synthesis, crystal structure, Hirshfeld surface analysis and determination of antioxidant activity for a new mononuclear tris-oxalato-chromium(III) complex. J. Mol. Struct. 2021, 1240, 130560. [Google Scholar] [CrossRef]
- Jesus, A.J.L.; Redinha, J.S. Charge-assisted intramolecular hydrogen bonds in disubstituted cyclohexane derivatives. J. Phys. Chem. A 2011, 115, 14069–14077. [Google Scholar] [CrossRef]
- Yamagishi, H.; Sato, H.; Hori, A.; Sato, Y.; Matsuda, R.; Kato, K.; Aida, T. Self-assembly of lattices with high structural complexity from a geometrically simple molecule. Science 2018, 361, 1242–1246. [Google Scholar] [CrossRef] [Green Version]
- Pavlishchuk, A.V.; Kolotilov, S.V.; Zeller, M.; Thompson, L.K.; Fritsky, I.O.; Addison, A.W.; Hunter, A.D. A triple-decker heptadecanuclear (CuII)15(CrIII)2 complex assembled from pentanuclear metallacrowns. Eur. J. Inorg. Chem. 2010, 15, 4851–4858. [Google Scholar] [CrossRef]
- D’Arcy, R.L.; Watt, I.C. Analysis of sorption isotherms of non-homogeneous sorbents. Trans. Faraday Soc. 1970, 66, 1236–1245. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Furmaniak, S.; Wiśniewski, M.; Werengowska, K.; Gauden, P.A.; Kowalczyk, P. New findings on the influence of carbon surface curvature on energetics of benzene adsorption from gaseous phase. Chem. Phys. Lett. 2016, 645, 157–163. [Google Scholar] [CrossRef]
- Furmaniak, S.; Wiśniewski, M.; Werengowska-Ciećwierz, K.; Terzyk, A.P.; Hata, K.; Gauden, P.A.; Kowalczyk, P.; Szybowicz, M. Water at curved carbon surface: Mechanisms of adsorption revealed by first calorimetric study. J. Phys. Chem. C 2015, 119, 2703–2715. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2004; pp. 168–171. [Google Scholar]
- Castellucci, E.; Angeloni, L.; Neto, N.; Sbrana, G. IR and raman spectra of A 2,2′-bipyridine single crystal: Internal modes. Chem. Phys. 1979, 43, 365–373. [Google Scholar] [CrossRef]
- Strukl, J.S.; Walter, J.L. Infrared and raman spectra of heterocyclic compounds-III. the infrared studies and normal vibrations of 2,2′-bipyridine. Spectrochim. Acta Part A Mol. Spectrosc. 1971, 27, 209–221. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Walker, W.R. Infrared spectra of hydroxy-bridged copper(I1) compounds. Inorg. Chem. 1965, 105, 1382–1386. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley: Hoboken, NJ, USA, 2009; Volume A, pp. 180–184, Volume B, pp. 79–82. [Google Scholar]
- Persson, P.; Axe, K. Adsorption of oxalate and malonate at the water-goethite interface: Molecular surface speciation from IR spectroscopy. Geochim. Cosmochim. Acta 2005, 69, 541–552. [Google Scholar] [CrossRef]
- Clausén, M.; Öhman, L.O.; Axe, K.; Persson, P. Spectroscopic studies of aluminum and gallium complexes with oxalate and malonate in aqueous solution. J. Mol. Struct. 2003, 648, 225–235. [Google Scholar] [CrossRef]
- Fujita, J.; Martell, A.E.; Nakamoto, K. Infrared spectra of metal chelate compounds. VII. normal coordinate treatments on 1:2 and 1:3 oxalato complexes. J. Chem. Phys. 1962, 36, 331–338. [Google Scholar] [CrossRef]
- Hocking, R.K.; George, S.D.; Raymond, K.N.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. Fe L-edge X-ray absorption spectroscopy determination of differential orbital covalency of siderophore model compounds: Electronic structure contributions to high stability constants. J. Am. Chem. Soc. 2010, 132, 4006–4015. [Google Scholar] [CrossRef] [Green Version]
- Baker, M.L.; Mara, M.W.; Yan, J.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. K- and L-edge X-ray absorption spectroscopy (XAS) and resonant inelastic x-ray scattering (RIXS) determination of differential orbital covalency (DOC) of transition metal sites. Coord. Chem. Rev. 2017, 345, 182–208. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Curély, J.; Georges, R. Theoretical study of isotropic ferrimagnetic chains composed of classical spins alternating with quantum systems. Phys. Rev. B 1992, 46, 3520–3526. [Google Scholar] [CrossRef]
- Pascanut, C.; Dragoe, N.; Berthet, P. Magnetic and transport properties of cobalt-doped perovskites SrTi1−XCoxO3 (x ≤ 0.5). J. Magn. Magn. Mater. 2006, 305, 6–11. [Google Scholar] [CrossRef]
- Setifi, F.; Setifi, Z.; Konieczny, P.; Glidewell, C.; Benmansour, S.; Gómez-García, C.J.; Grandjean, F.; Long, G.J.; Pelka, R.; Reedijk, J. Synthesis, structure and magnetic properties of an unusual oligonuclear Iron(III)-Cobalt(III) compound with oxido-, sulfato- and cyanido-bridging ligands. Polyhedron 2019, 157, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Julve, M.; Gleizes, A.; Chamoreau, L.M.; Ruiz, E.; Verdaguer, M. Antiferromagnetic interactions in Copper(II) µ-oxalato dinuclear complexes: The role of the counterion. Eur. J. Inorg. Chem. 2018, 2018, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Gleizes, A.; Julve, M.; Verdaguer, M.; Real, J.A.; Faus, J.; Solans, X. Two different (oxalato)(bipyridine)copper(II) complexes in one single crystal. Crystal structures and magnetic properties of [Cu2(bipy)2(H2O)2(C2O4)]X2·[Cu(bipy)(C2O4)](X = NO3−, BF4− or ClO4−). J. Chem. Soc. Dalt. Trans. 1992, 3209–3216. [Google Scholar] [CrossRef]
- Julve, M.; Verdaguer, M.; Gleizes, A.; Philoche-Levisalles, M.; Kahn, O. Design of μ-oxalato copper(II) binuclear complexes exhibiting expected magnetic properties. Inorg. Chem. 1984, 23, 3808–3818. [Google Scholar] [CrossRef]
- Johnson, R.C. Convenient procedure for the preparation of potassium trioxalatoferrate(III). J. Chem. Educ. 1972, 47, 702. [Google Scholar] [CrossRef]
- Diaz, C.; Ribas, J.; Sanz, N.; Solans, X.; Font-Bardía, M. Synthesis, crystal structure and magnetic properties of catena-poly[(2,2′-Bipyridylcopper)-di-μ-thiocyanato] and bis(2,2′-bipyridyl)thiocyanatocopper(II) perchlorate. Inorg. Chim. Acta 1999, 286, 169–174. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for x-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [Green Version]
- Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Krug, M.; Weiss, M.S.; Heinemann, U.; Mueller, U. XDSAPP: A graphical user interface for the convenient processing of diffraction data using XDS. J. Appl. Crystallogr. 2012, 45, 568–572. [Google Scholar] [CrossRef]
- CrysAlis Red and CrysAlis CCD; Oxford Diffraction Ltd.: Oxfordshire, UK, 2000.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, Release 2.1e; Crystal Impact GbR: Bonn, Germany, 2001. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Wiśniewski, M.; Bieniek, A.; Bolibok, P.; Koter, S.; Bryk, P.; Kowalczyk, P.; Terzyk, A.P. Mechanistic aspects of water adsorption-desorption in porphyrin containing MOFs. Microporous Mesoporous Mater. 2019, 290, 109649. [Google Scholar] [CrossRef]
- Szymański, G.S.; Wiśniewski, M.; Olejnik, P.; Koter, S.; Castro, E.; Echegoyen, L.; Terzyk, A.P.; Plonska-Brzezinska, M.E. Correlation between the Catalytic and electrocatalytic properties of nitrogen-doped carbon nanoonions and the polarity of the carbon surface: Experimental and theoretical investigations. Carbon 2019, 151, 120–129. [Google Scholar] [CrossRef]
- Pina-Salazar, E.Z.; Urita, K.; Hayashi, T.; Futamura, R.; Vallejos-Burgos, F.; Włoch, J.; Kowalczyk, P.; Wiśniewski, M.; Sakai, T.; Moriguchi, I.; et al. Water adsorption property of hierarchically nanoporous detonation nanodiamonds. Langmuir 2017, 33, 11180–11188. [Google Scholar] [CrossRef]
- Andruh, M. Heterotrimetallic complexes in molecular magnetism. Chem. Commun. 2018, 54, 3559–3577. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Kuzniak, E.; Liberka, M.; Chorazy, S.; Sieklucka, B.; Podgajny, R. Modular approach towards functional multimetallic coordination clusters. Coord. Chem. Rev. 2020, 419, 213394. [Google Scholar] [CrossRef]
- Mueller, U.; Förster, R.; Hellmig, M.; Huschmann, F.U.; Kastner, A.; Malecki, P.; Pühringer, S.; Röwer, M.; Sparta, K.; Steffien, M.; et al. The macromolecular crystallography beamlines at BESSY II of the helmholtz-zentrum Berlin: Current status and perspectives. Eur. Phys. J. Plus 2015, 130, 141–150. [Google Scholar] [CrossRef]
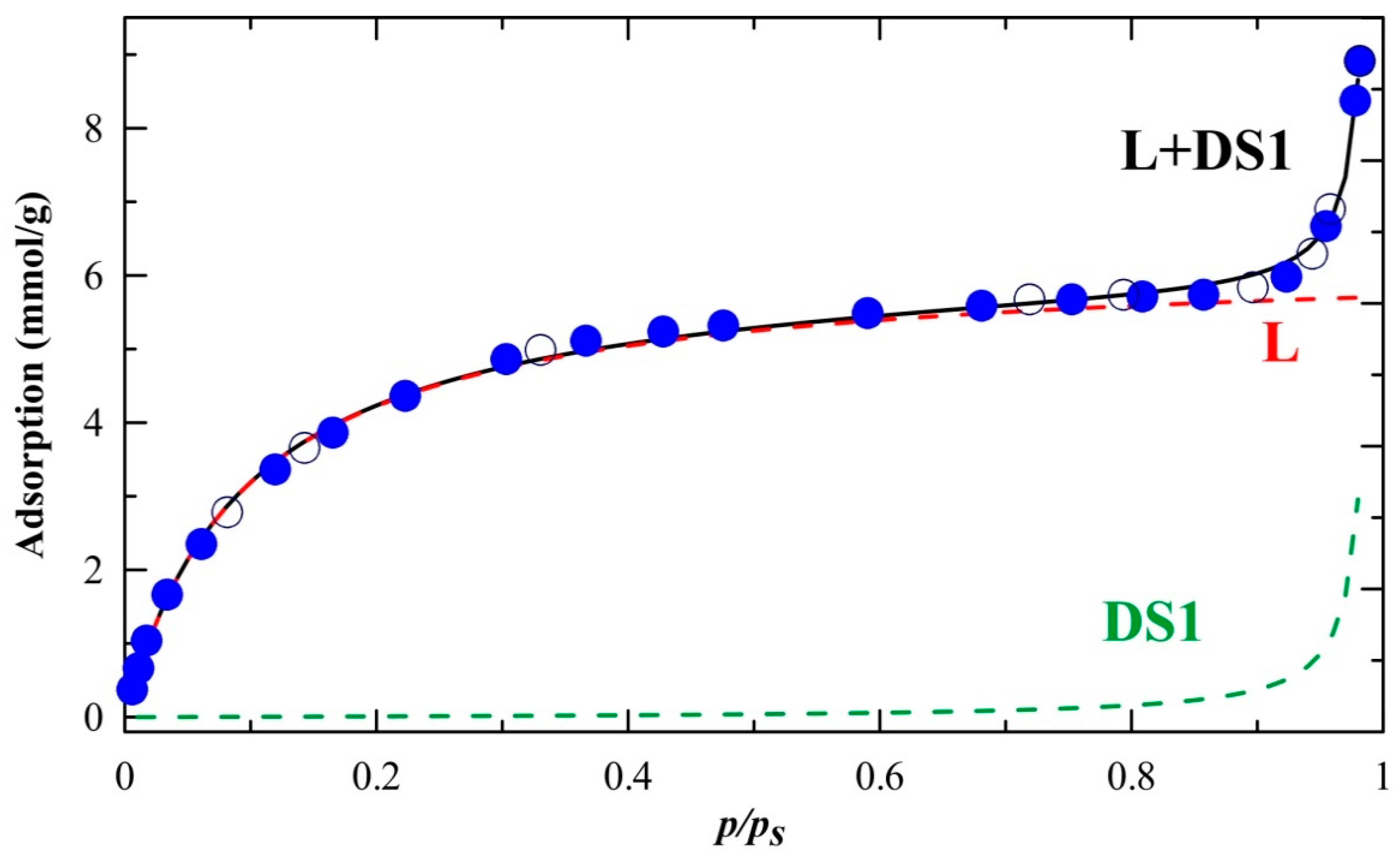
| Parameter | Estimate | Std. Error | p-Value |
|---|---|---|---|
| amL [mmol/g] | 6.259 | 0.074 | 7.7 × 10−25 |
| KL | 3.27 | 0.15 | 2.5 × 10−14 |
| C | 1.0073 | 0.0016 | 1.8 × 10−40 |
| a0 [mmol/g] | 0.0386 | 0.0052 | 7.6 × 10−7 |
| R2 | 0.9997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muzioł, T.M.; Tereba, N.; Podgajny, R.; Pełka, R.; Czernia, D.; Wiśniewski, M.; Koter, S.; Wrzeszcz, G. Sorption and Magnetic Properties of Oxalato-Based Trimetallic Open Framework Stabilized by Charge-Assisted Hydrogen Bonds. Int. J. Mol. Sci. 2022, 23, 1556. https://doi.org/10.3390/ijms23031556
Muzioł TM, Tereba N, Podgajny R, Pełka R, Czernia D, Wiśniewski M, Koter S, Wrzeszcz G. Sorption and Magnetic Properties of Oxalato-Based Trimetallic Open Framework Stabilized by Charge-Assisted Hydrogen Bonds. International Journal of Molecular Sciences. 2022; 23(3):1556. https://doi.org/10.3390/ijms23031556
Chicago/Turabian StyleMuzioł, Tadeusz Mikołaj, Natalia Tereba, Robert Podgajny, Robert Pełka, Dominik Czernia, Marek Wiśniewski, Stanisław Koter, and Grzegorz Wrzeszcz. 2022. "Sorption and Magnetic Properties of Oxalato-Based Trimetallic Open Framework Stabilized by Charge-Assisted Hydrogen Bonds" International Journal of Molecular Sciences 23, no. 3: 1556. https://doi.org/10.3390/ijms23031556
APA StyleMuzioł, T. M., Tereba, N., Podgajny, R., Pełka, R., Czernia, D., Wiśniewski, M., Koter, S., & Wrzeszcz, G. (2022). Sorption and Magnetic Properties of Oxalato-Based Trimetallic Open Framework Stabilized by Charge-Assisted Hydrogen Bonds. International Journal of Molecular Sciences, 23(3), 1556. https://doi.org/10.3390/ijms23031556