Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action
Abstract
:1. Introduction
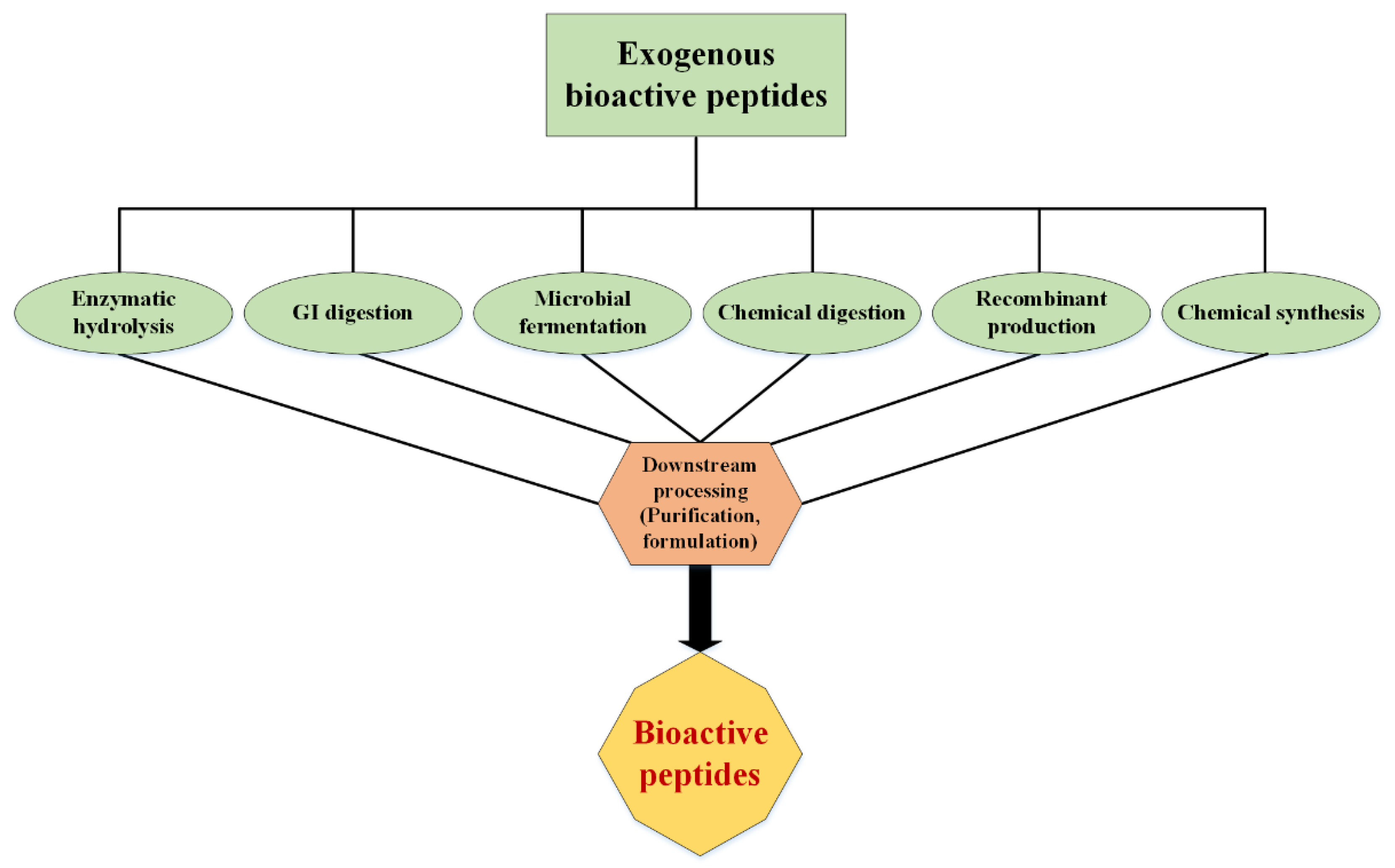
2. Production of Bioactive Peptides
2.1. Enzymatic Hydrolysis
Microbial Fermentation
2.2. Chemical Synthesis of Peptides
2.3. Recombinant Productions
3. The Sources of Bioactive Peptides
3.1. Animal Sources
3.1.1. Marine Sources
3.1.2. Milk Products
3.1.3. Egg Products
3.1.4. Meat Products
3.1.5. Venom Peptidomics: A Cure for the Deathtrap
3.1.6. Other Animals
3.2. Plant Sources
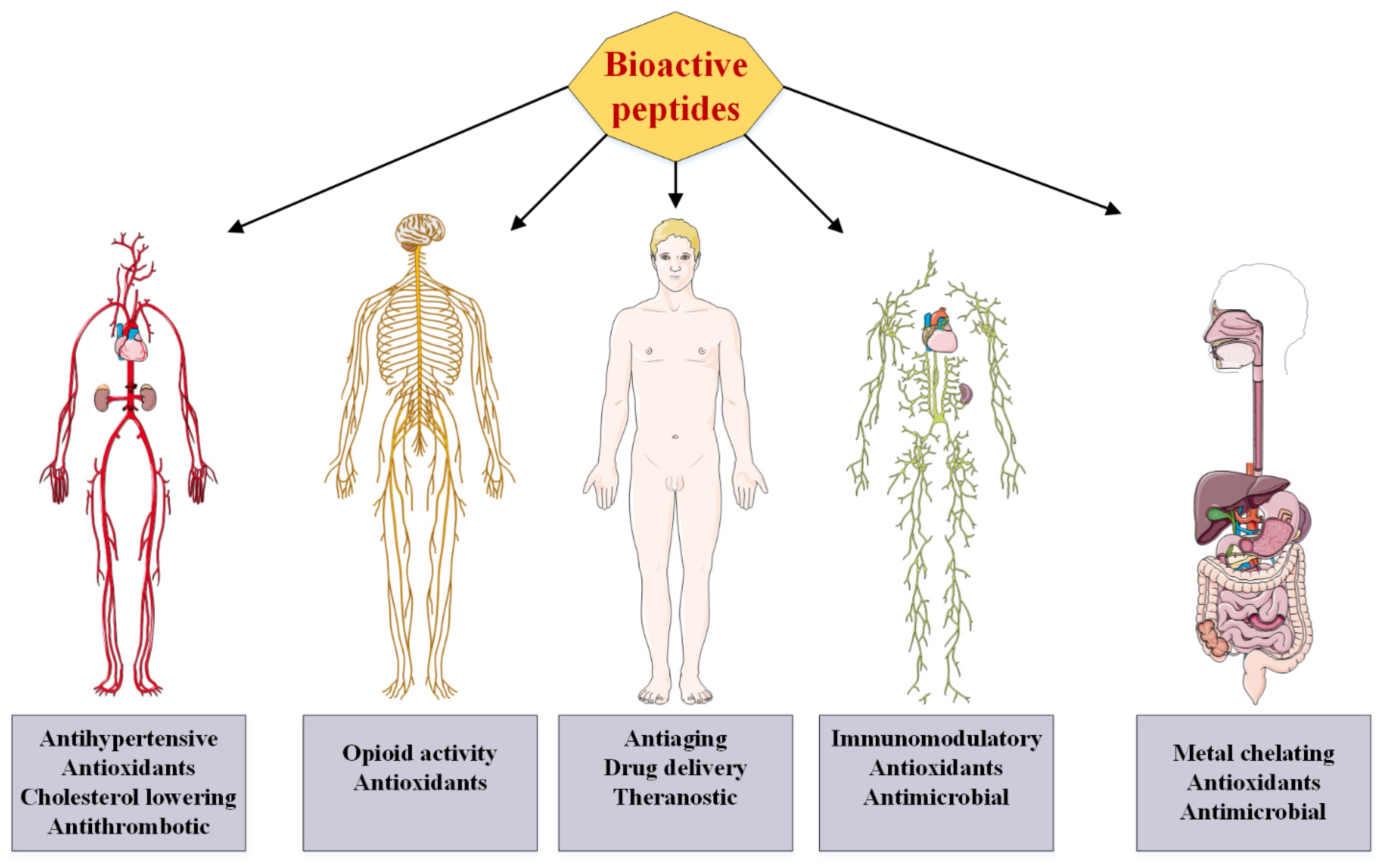
4. Medicinal Applications and Proposed Mechanism of Actions of BPs
4.1. Antioxidant Activity of BP and Its Mechanism of Action
4.1.1. Effect of Amino Acid Contents on Antioxidant Activity of Peptides
4.1.2. Effect of Peptide Size on Antioxidant Activity
4.1.3. The Role of Hydrophobicity of Peptides in Their Antioxidant Activity
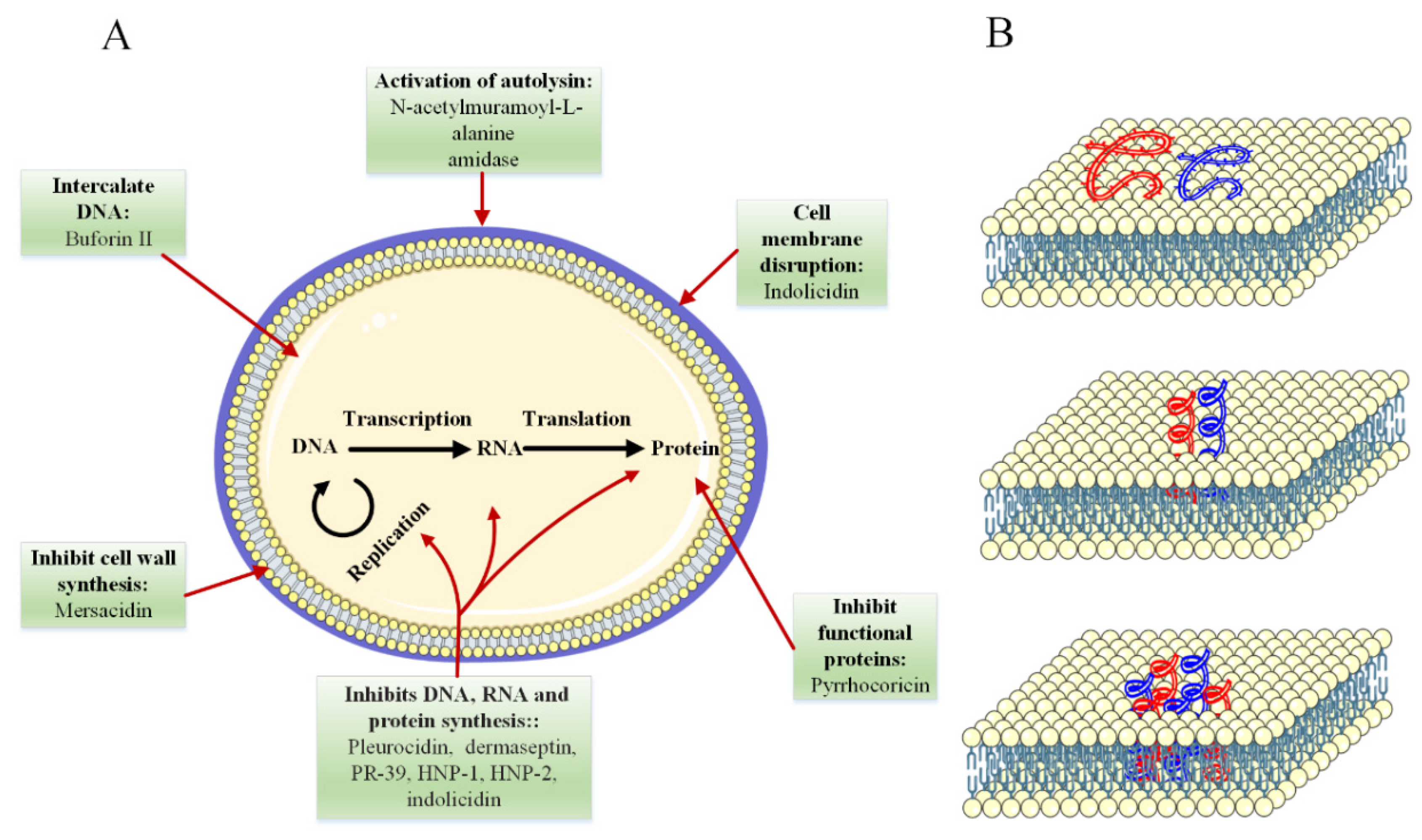
4.2. Mechanism of Antimicrobial Activity
4.3. Antihypertensive Peptide and Its Mechanism of Action
4.4. Mechanisms of Opioid Activity
- (a)
- Muscle relaxants such as probantin and belladonna group such as atropine;
- (b)
- Vascular dilators such as papaverine hydrochloride or nitroglycerin;
- (c)
- Anti-inflammatory drugs such as indomethacin, ibuprofen, and phenylbutazone;
- (d)
- Non-narcotic analgesics such as aspirin and acetaminophen;
- (e)
- Narcotic analgesics such as Demerol and methadone hydrochloride.
4.5. Mineral-Binding Peptides
4.6. Blood-Lipid-Lowering Effect
4.7. Antiobesity Effect
4.8. Antidiabetic Activity
4.9. Antiaging Peptides
5. Cyclic Peptides: One Step Ahead of Linear Peptides
6. Use of Computer-Based Techniques in Peptide Research
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A review on bioactive peptides: Physiological functions, bioavailability and safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef] [PubMed]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.M.; Munekata, P.E.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Hamley, I.W. Small bioactive peptides for biomaterials design and therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, M.; Ménard, O.; Cahu, A.; Nogret, I.; Briard-Bion, V.; Cudennec, B.; Cuinet, I.; Le Ruyet, P.; Baudry, C.; Dupont, D. Addition of dairy lipids and probiotic L. fermentum in infant formulas modulates proteolysis and lipolysis with moderate consequences on gut physiology and metabolism in Yucatan piglets. Front. Nutr. 2021, 8, 20. [Google Scholar] [CrossRef]
- Akbarian, M. Insulin therapy; a valuable legacy and its future perspective. Int. J. Biol. Macromol. 2021, 181, 1224–1230. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Lee, B.H.; Oh, D.H. Current trends and perspectives of bioactive peptides. Crit. Rev. Food Sci. Nutr. 2018, 58, 2273–2284. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Timmer, M.; Polanowski, A.; Lubec, G.; Trziszka, T. Manufacturing of peptides exhibiting biological activity. Amino Acids 2013, 44, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Räder, A.F.; Weinmüller, M.; Reichart, F.; Schumacher-Klinger, A.; Merzbach, S.; Gilon, C.; Hoffman, A.; Kessler, H. Orally active peptides: Is there a magic bullet? Angew. Chem. Int. Ed. 2018, 57, 14414–14438. [Google Scholar] [CrossRef]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meisel, H.; Walsh, D.; Murray, B.; FitzGerald, R. ACE inhibitory peptides. In Nutraceutical Proteins and Peptides in Health and Disease; CRC Press LLC: Boca Raton, FL, USA, 2006; pp. 269–315. [Google Scholar]
- Franěk, F.; Hohenwarter, O.; Katinger, H. Plant protein hydrolysates: Preparation of defined peptide fractions promoting growth and production in animal cells cultures. Biotechnol. Prog. 2000, 16, 688–692. [Google Scholar] [CrossRef]
- Barati, M.; Javanmardi, F.; Mousavi Jazayeri, S.M.H.; Jabbari, M.; Rahmani, J.; Barati, F.; Nickho, H.; Davoodi, S.H.; Roshanravan, N.; Mousavi Khaneghah, A. Techniques, perspectives, and challenges of bioactive peptide generation: A comprehensive systematic review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1488–1520. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Danquah, M.K. Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol. Adv. 2011, 29, 272–277. [Google Scholar] [CrossRef]
- Dionysius, D.; Milne, J. Antibacterial peptides of bovine lactoferrin: Purification and characterization. J. Dairy Sci. 1997, 80, 667–674. [Google Scholar] [CrossRef]
- Yeoh, Y.Q.; Yu, J.; Polyak, S.W.; Horsley, J.R.; Abell, A.D. Photopharmacological control of cyclic antimicrobial peptides. ChemBioChem 2018, 19, 2591–2597. [Google Scholar] [CrossRef]
- Marciniak, A.; Suwal, S.; Naderi, N.; Pouliot, Y.; Doyen, A. Enhancing enzymatic hydrolysis of food proteins and production of bioactive peptides using high hydrostatic pressure technology. Trends Food Sci. Technol. 2018, 80, 187–198. [Google Scholar] [CrossRef]
- Naderi, N.; House, J.D.; Pouliot, Y.; Doyen, A. Effects of high hydrostatic pressure processing on hen egg compounds and egg products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 707–720. [Google Scholar] [CrossRef] [Green Version]
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef]
- Song, A.A.-L.; In, L.L.; Lim, S.H.E.; Rahim, R.A. A review on Lactococcus lactis: From food to factory. Microb. Cell Factories 2017, 16, 1–15. [Google Scholar]
- Griffiths, M.W.; Tellez, A.M. Lactobacillus helveticus: The proteolytic system. Front. Microbiol. 2013, 4, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Solid-state fermentation for L-lactic acid production from agro wastes using Lactobacillus delbrueckii. Process Biochem. 2006, 41, 759–763. [Google Scholar] [CrossRef]
- Sasaki, M.; Bosman, B.W.; Tan, P.S. Comparison of proteolytic activities in various lactobacilli. J. Dairy Res. 1995, 62, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-W.; Tsai, J.-S.; Pan, B.S. Purification of angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int. Dairy J. 2007, 17, 641–647. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Meisel, H. Influence of trypsin action in yoghurt milk on the release of caseinophosphopeptide-rich fractions and physical properties of the fermented products. Int. J. Dairy Technol. 2005, 58, 119–124. [Google Scholar] [CrossRef]
- Soleymanzadeh, N.; Mirdamadi, S.; Kianirad, M. Antioxidant activity of camel and bovine milk fermented by lactic acid bacteria isolated from traditional fermented camel milk (Chal). Dairy Sci. Technol. 2016, 96, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, S.; Matsuura, K.; Gotou, T.; Nishimura, S.; Kajimoto, O.; Yabune, M.; Kajimoto, Y.; Yamamoto, N. Antihypertensive effect of casein hydrolysate in a placebo-controlled study in subjects with high-normal blood pressure and mild hypertension. Br. J. Nutr. 2005, 94, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Kim, S.-H. Recent biotechnological trends in lactic acid bacterial fermentation for food processing industries. Syst. Microbiol. Biomanuf. 2021, 2, 14–40. [Google Scholar] [CrossRef]
- Conibear, A.C.; Watson, E.E.; Payne, R.J.; Becker, C.F. Native chemical ligation in protein synthesis and semi-synthesis. Chem. Soc. Rev. 2018, 47, 9046–9068. [Google Scholar] [CrossRef]
- Katsoyannis, P. Synthesis of insulin. Science 1966, 154, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Vigneaud, V.D.; Ressler, C.; Swan, C.J.M.; Roberts, C.W.; Katsoyannis, P.G.; Gordon, S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. J. Am. Chem. Soc. 1953, 75, 4879–4880. [Google Scholar] [CrossRef]
- Kent, S.B. Novel protein science enabled by total chemical synthesis. Protein Sci. 2019, 28, 313–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, B.L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov. 2003, 2, 587–593. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Albericio, F.; El-Faham, A. Choosing the right coupling reagent for peptides: A twenty-five-year journey. Org. Process Res. Dev. 2018, 22, 760–772. [Google Scholar] [CrossRef]
- Lawrenson, S.B.; Arav, R.; North, M. The greening of peptide synthesis. Green Chem. 2017, 19, 1685–1691. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N. Solid-phase synthesis of sulfur containing heterocycles. J. Sulfur Chem. 2018, 39, 544–577. [Google Scholar] [CrossRef]
- Banga, A.K. Therapeutic Peptides and Proteins: Formulation, Processing, and Delivery Systems; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Yu, H.M.; Chen, S.T.; Wang, K.T. Enhanced coupling efficiency in solid-phase peptide synthesis by microwave irradiation. J. Org. Chem. 1992, 57, 4781–4784. [Google Scholar] [CrossRef]
- Goodwin, D.; Simerska, P.; Toth, I. Peptides as therapeutics with enhanced bioactivity. Curr. Med. Chem. 2012, 19, 4451–4461. [Google Scholar] [CrossRef]
- Li, W.; O’Brien-Simpson, N.M.; Hossain, M.A.; Wade, J.D. The 9-fluorenylmethoxycarbonyl (Fmoc) group in chemical peptide synthesis—Its past, present, and future. Aust. J. Chem. 2019, 73, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Ingham, A.B.; Moore, R.J. Recombinant production of antimicrobial peptides in heterologous microbial systems. Biotechnol. Appl. Biochem. 2007, 47, 1–9. [Google Scholar] [PubMed]
- Antosova, Z.; Mackova, M.; Kral, V.; Macek, T. Therapeutic application of peptides and proteins: Parenteral forever? Trends Biotechnol. 2009, 27, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, A.; Cai, Y.; Lindberg, I. Production of bioactive peptides in an in vitro system. Anal. Biochem. 2007, 366, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, L.; Blomberg, L.; Flegel, M.; Lepsa, L.; Nilsson, B.; Verlander, M. Large-scale synthesis of peptides. Pept. Sci. 2000, 55, 227–250. [Google Scholar] [CrossRef]
- Drucker, D.J.; Dritselis, A.; Kirkpatrick, P. Liraglutide. Nat. Rev. Drug Discov. 2010, 9, 267–268. [Google Scholar] [CrossRef]
- Akbarian, M.; Yousefi, R.; Moosavi-Movahedi, A.A.; Ahmad, A.; Uversky, V.N. Modulating insulin fibrillation using engineered B-chains with mutated C-termini. Biophys. J. 2019, 117, 1626–1641. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive peptides from marine processing waste and shellfish: A Review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Mohebbi, G.H.; Nabipour, I.; Vazirizadeh, A. The Sea, the future pharmacy. ISMJ 2014, 17, 748–788. [Google Scholar]
- King, G. Venoms to drugs: Translating venom peptides into therapeutics. Aust. Biochem. 2013, 44, 13–15. [Google Scholar]
- Jo, C.; Khan, F.F.; Khan, M.I.; Iqbal, J. Marine bioactive peptides: Types, structures, and physiological functions. Food Rev. Int. 2017, 33, 44–61. [Google Scholar] [CrossRef]
- Voultsiadou, E. Demosponge distribution in the eastern Mediterranean: A NW–SE gradient. Helgol. Mar. Res. 2005, 59, 237–251. [Google Scholar] [CrossRef]
- Wesson, K.J.; Hamann, M.T. Keenamide A, a bioactive cyclic peptide from the marine mollusk Pleurobranchus forskalii. J. Nat. Prod. 1996, 59, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N.; Maurizi, L.K.; Pannell, L.K.; Williams, D.E.; Dilip de Silva, E.; Lassota, P.; Allen, T.M. Papuamides A–D, HIV-inhibitory and cytotoxic depsipeptides from the Sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Zampella, A.; Sepe, V.; Luciano, P.; Bellotta, F.; Monti, M.C.; D’Auria, M.V.; Jepsen, T.; Petek, S.; Adeline, M.-T.; Laprévôte, O.; et al. Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 2008, 73, 5319–5327. [Google Scholar] [CrossRef]
- Barkia, I.; Al-Haj, L.; Abdul Hamid, A.; Zakaria, M.; Saari, N.; Zadjali, F. Technology, Indigenous marine diatoms as novel sources of bioactive peptides with antihypertensive and antioxidant properties. Int. J. Food Sci. Technol. 2019, 54, 1514–1522. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Konosu, S. Bioactive marine metabolites VII. Structures of discodermins B, C, and D, antimicrobial peptides from the marine sponge discodermia kiiensis. Tetrahedron Lett. 1985, 26, 855–856. [Google Scholar] [CrossRef]
- Wu, Q.-X.; Crews, M.S.; Draskovic, M.; Sohn, J.; Johnson, T.A.; Tenney, K.; Valeriote, F.A.; Yao, X.-J.; Bjeldanes, L.F.; Crews, P. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Org. Lett. 2010, 12, 4458–4461. [Google Scholar] [CrossRef] [Green Version]
- Nogle, L.M.; Marquez, B.L.; Gerwick, W.H. Wewakazole, a novel cyclic dodecapeptide from a Papua New Guinea Lyngbya m ajuscula. Org. Lett. 2003, 5, 3–6. [Google Scholar] [CrossRef]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R.; Baker, H.L.; Bewley, C.A. Celebesides A–C and theopapuamides B–D, depsipeptides from an Indonesian sponge that inhibit HIV-1 entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erba, E.; Bassano, L.; Di Liberti, G.; Muradore, I.; Chiorino, G.; Ubezio, P.; Vignati, S.; Codegoni, A.; Desiderio, M.; Faircloth, G. Cell cycle phase perturbations and apoptosis in tumour cells induced by aplidine. Br. J. Cancer 2002, 86, 1510–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andavan, G.S.B.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suenaga, K.; Mutou, T.; Shibata, T.; Itoh, T.; Kigoshi, H.; Yamada, K. Isolation and stereostructure of aurilide, a novel cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. Tetrahedron Lett. 1996, 37, 6771–6774. [Google Scholar] [CrossRef]
- Xing, H.; Tong, M.; Jiang, N.; Zhang, X.; Hu, H.; Pan, H.; Li, D. Antitumour bioactive peptides isolated from marine organisms. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1077–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Wang, Y.; Hua, H.; Li, D.; Tang, C. Marine antitumor peptide dolastatin 10: Biological activity, structural modification and synthetic chemistry. Mar. Drugs 2021, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Freitas, V.M.; Rangel, M.; Bisson, L.F.; Jaeger, R.G.; Machado-Santelli, G.M. The geodiamolide H, derived from brazilian sponge Geodia corticostylifera, regulates actin cytoskeleton, migration and invasion of breast cancer cells cultured in three-dimensional environment. J. Cell. Physiol. 2008, 216, 583–594. [Google Scholar] [CrossRef]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef] [Green Version]
- Donia, M.S.; Wang, B.; Dunbar, D.C.; Desai, P.V.; Patny, A.; Avery, M.; Hamann, M.T. Mollamides B and C, cyclic hexapeptides from the Indonesian tunicate Didemnum molle. J. Nat. Prod. 2008, 71, 941–945. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-L.; Yi, Y.-H.; Wu, H.-M.; Xu, Q.-Z.; Tang, H.-F.; Zhou, D.-Z.; Lin, H.-W.; Wang, Z.-H. Isolation and structure of the cytotoxic cycloheptapeptide phakellistatin 13. J. Nat. Prod. 2003, 66, 146–148. [Google Scholar] [CrossRef]
- Wang, Y.-K.; He, H.-L.; Wang, G.-F.; Wu, H.; Zhou, B.-C.; Chen, X.-L.; Zhang, Y.-Z. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 2010, 8, 255–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liao, H.; Liu, L.-Y.; Sun, F.; Chen, H.-F.; Jiao, W.-H.; Zhu, H.-R.; Yang, F.; Huang, G.; Zeng, D.-Q. Phakefustatins A–C: Kynurenine-bearing cycloheptapeptides as RXRα modulators from the marine sponge Phakellia fusca. Org. Lett. 2020, 22, 6703–6708. [Google Scholar] [CrossRef] [PubMed]
- Kerr, R.G.; Kerr, S.S. Marine natural products as therapeutic agents. Expert Opin. Ther. Pat. 1999, 9, 1207–1222. [Google Scholar] [CrossRef]
- Ferranti, P.; Traisci, M.V.; Picariello, G.; Nasi, A.; Boschi, V.; Siervo, M.; Falconi, C.; Chianese, L.; Addeo, F. Casein proteolysis in human milk: Tracing the pattern of casein breakdown and the formation of potential bioactive peptides. J. Dairy Res. 2004, 71, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Jäkälä, P.; Vapaatalo, H. Antihypertensive peptides from milk proteins. Pharmaceuticals 2010, 3, 251–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiozzi, R.Z.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Samperi, R.; Laganà, A. Purification and identification of endogenous antioxidant and ACE-inhibitory peptides from donkey milk by multidimensional liquid chromatography and nanoHPLC-high resolution mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5657–5666. [Google Scholar] [CrossRef] [PubMed]
- El-Salam, M.A.; El-Shibiny, S. Bioactive peptides of buffalo, camel, goat, sheep, mare, and yak milks and milk products. Food Rev. Int. 2013, 29, 1–23. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy protein hydrolysates: Peptides for health benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef] [Green Version]
- Mudgil, P.; Kamal, H.; Yuen, G.C.; Maqsood, S. Characterization and identification of novel antidiabetic and anti-obesity peptides from camel milk protein hydrolysates. Food Chem. 2018, 259, 46–54. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Dipeptidyl peptidase IV (DPP-IV) inhibitory properties of camel milk protein hydrolysates generated with trypsin. J. Funct. Foods 2017, 34, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Nongonierma, A.B.; Paolella, S.; Mudgil, P.; Maqsood, S.; FitzGerald, R.J. Identification of novel dipeptidyl peptidase IV (DPP-IV) inhibitory peptides in camel milk protein hydrolysates. Food Chem. 2018, 244, 340–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudgil, P.; Baby, B.; Ngoh, Y.-Y.; Vijayan, R.; Gan, C.-Y.; Maqsood, S. Identification and molecular docking study of novel cholesterol esterase inhibitory peptides from camel milk proteins. J. Dairy Sci. 2019, 102, 10748–10759. [Google Scholar] [CrossRef] [PubMed]
- Wali, A.; Yanhua, G.; Ishimov, U.; Yili, A.; Aisa, H.A.; Salikhov, S. Therapeutics, Isolation and identification of three novel antioxidant peptides from the Bactrian camel milk Hydrolysates. Int. J. Pept. Res. Ther. 2020, 26, 641–650. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Q.; Ji, Z.; Shu, G.; Chen, H.J.L. Production and fermentation characteristics of angiotensin-I-converting enzyme inhibitory peptides of goat milk fermented by a novel wild Lactobacillus plantarum 69. Lebensm. Wiss. Technol. 2018, 91, 532–540. [Google Scholar] [CrossRef]
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; do Prado, D.Z.; Buzalaf, M.A.R.; de Magalhães Padilha, P.; de Oliveira, D.E.; Fleuri, L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef]
- Davalos, A.; Miguel, M.; Bartolome, B.; Lopez-Fandino, R. Antioxidant activity of peptides derived from egg white proteins by enzymatic hydrolysis. J. Food Prot. 2004, 67, 1939–1944. [Google Scholar] [CrossRef]
- Walzem, R.; Dillard, C.; German, J.B. Whey components: Millennia of evolution create functionalities for mammalian nutrition: What we know and what we may be overlooking. Crit. Rev. Food Sci. Nutr. 2002, 42, 353–375. [Google Scholar] [CrossRef]
- Yu, Z.; Yin, Y.; Zhao, W.; Wang, F.; Yu, Y.; Liu, B.; Liu, J.; Chen, F. Characterization of ACE-inhibitory peptide associated with antioxidant and anticoagulation properties. J. Food Sci. 2011, 76, C1149–C1155. [Google Scholar] [CrossRef]
- Sun, X.; Chakrabarti, S.; Fang, J.; Yin, Y.; Wu, J. Low-molecular-weight fractions of Alcalase hydrolyzed egg ovomucin extract exert anti-inflammatory activity in human dermal fibroblasts through the inhibition of tumor necrosis factor–mediated nuclear factor κB pathway. Nutr. Res. 2016, 36, 648–657. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Oey, I.; Bremer, P.; Carne, A.; Silcock, P. Bioactive peptides derived from egg proteins: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2508–2530. [Google Scholar] [CrossRef]
- You, S.-J.; Udenigwe, C.C.; Aluko, R.E.; Wu, J. Multifunctional peptides from egg white lysozyme. Food Res. Int. 2010, 43, 848–855. [Google Scholar] [CrossRef]
- Mine, Y.; Kovacs-Nolan, J. New insights in biologically active proteins and peptides derived from hen egg. World’s Poult. Sci. J. 2006, 62, 87–96. [Google Scholar] [CrossRef]
- Abdou, A.M.; Higashiguchi, S.; Aboueleinin, A.; Kim, M.; Ibrahim, H.R. Antimicrobial peptides derived from hen egg lysozyme with inhibitory effect against Bacillus species. Food Control 2007, 18, 173–178. [Google Scholar] [CrossRef]
- Lafarga, T.; Hayes, M. Bioactive peptides from meat muscle and by-products: Generation, functionality and application as functional ingredients. Meat Sci. 2014, 98, 227–239. [Google Scholar] [CrossRef]
- Möller, N.P.; Scholz-Ahrens, K.E.; Roos, N.; Schrezenmeir, J. Bioactive peptides and proteins from foods: Indication for health effects. Eur. J. Nutr. 2008, 47, 171–182. [Google Scholar] [CrossRef]
- Arihara, K.; Nakashima, Y.; Mukai, T.; Ishikawa, S.; Itoh, M. Peptide inhibitors for angiotensin I-converting enzyme from enzymatic hydrolysates of porcine skeletal muscle proteins. Meat Sci. 2001, 57, 319–324. [Google Scholar] [CrossRef]
- Jang, A.; Lee, M. Purification and identification of angiotensin converting enzyme inhibitory peptides from beef hydrolysates. Meat Sci. 2005, 69, 653–661. [Google Scholar] [CrossRef]
- Stadnik, J.; Kęska, P. Meat and fermented meat products as a source of bioactive peptides. Acta Sci. Pol. Technol. Aliment. 2015, 14, 181–190. [Google Scholar] [CrossRef]
- Saiga, A.; Tanabe, S.; Nishimura, T. Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment. J. Agric. Food Chem. 2003, 51, 3661–3667. [Google Scholar] [CrossRef]
- Di Bernardini, R.; Mullen, A.M.; Bolton, D.; Kerry, J.; O’Neill, E.; Hayes, M. Assessment of the angiotensin-I-converting enzyme (ACE-I) inhibitory and antioxidant activities of hydrolysates of bovine brisket sarcoplasmic proteins produced by papain and characterisation of associated bioactive peptidic fractions. Meat Sci. 2012, 90, 226–235. [Google Scholar] [CrossRef]
- Culler, R.; Smith, G.; Cross, H. Relationship of myofibril fragmentation index to certain chemical, physical and sensory characteristics of bovine longissimus muscle. J. Food Sci. 1978, 43, 1177–1180. [Google Scholar] [CrossRef]
- Haard, N.F.; Simpson, B.K.; Pan, B.S. Sarcoplasmic proteins and other nitrogenous compounds. In Seafood Proteins; Springer: Berlin/Heidelberg, Germany, 1994; pp. 13–39. [Google Scholar]
- Robinson, S.D.; Safavi-Hemami, H. Venom peptides as pharmacological tools and therapeutics for diabetes. Neuropharmacology 2017, 127, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Li, Q.; Bandyopadhyay, P.K.; Gajewiak, J.; Yandell, M.; Papenfuss, A.T.; Purcell, A.W.; Norton, R.S.; Safavi-Hemami, H. Hormone-like peptides in the venoms of marine cone snails. Gen. Comp. Endocrinol. 2017, 244, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.D.; Undheim, E.A.; Ueberheide, B.; King, G.F. Venom peptides as therapeutics: Advances, challenges and the future of venom-peptide discovery. Expert Rev. Proteom. 2017, 14, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Ianzer, D.; Konno, K.; Marques-Porto, R.; Portaro, F.C.V.; Stöcklin, R.; de Camargo, A.C.M.; Pimenta, D.C. Identification of five new bradykinin potentiating peptides (BPPs) from Bothrops jararaca crude venom by using electrospray ionization tandem mass spectrometry after a two-step liquid chromatography. Peptides 2004, 25, 1085–1092. [Google Scholar] [CrossRef]
- Lima, M.E.D.; Martin-Eauclaire, M.-F. The toxins purified from Tityus serrulatus (Lutz & Mello) venom. J. Toxicol. Toxin Rev. 1995, 14, 457–481. [Google Scholar]
- Dai, L.; Corzo, G.; Naoki, H.; Andriantsiferana, M.; Nakajima, T. Purification, structure–function analysis, and molecular characterization of novel linear peptides from scorpion Opisthacanthus madagascariensis. Biochem. Biophys. Res. Commun. 2002, 293, 1514–1522. [Google Scholar] [CrossRef]
- Xiong, X.; Menting, J.G.; Disotuar, M.M.; Smith, N.A.; Delaine, C.A.; Ghabash, G.; Agrawal, R.; Wang, X.; He, X.; Fisher, S.J. A structurally minimized yet fully active insulin based on cone-snail venom insulin principles. Nat. Struct. Mol. Biol. 2020, 27, 615–624. [Google Scholar] [CrossRef]
- Miljanich, G. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef]
- Seo, M.-D.; Won, H.-S.; Kim, J.-H.; Mishig-Ochir, T.; Lee, B.-J. Antimicrobial peptides for therapeutic applications: A review. Molecules 2012, 17, 12276–12286. [Google Scholar] [CrossRef] [Green Version]
- Pometto, A.; Shetty, K.; Paliyath, G.; Levin, R.E. Food Biotechnology; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Cheikhyoussef, A.; Pogori, N.; Chen, W.; Zhang, H. Antimicrobial proteinaceous compounds obtained from bifidobacteria: From production to their application. Int. J. Food Microbiol. 2008, 125, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect antimicrobial peptides, a mini review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Kamala, K.; Sivaperumal, P.; Kamath, S.M.; Thilagaraj, W.R.; Rajaram, R. Marine actinobacteria as a source for emerging biopharmaceuticals. Encycl. Mar. Biotechnol. 2020, 4, 2095–2105. [Google Scholar]
- Mohanrasu, K.; Rao, R.G.R.; Sudhakar, M.; Raja, R.; Jeyakanthan, J.; Arun, A. Marine microbial pharmacognosy: Prospects and perspectives. In Marine Niche: Applications in Pharmaceutical Sciences; Springer: Berlin/Heidelberg, Germany, 2020; pp. 89–110. [Google Scholar]
- D’Orazio, N.; Gammone, M.A.; Gemello, E.; De Girolamo, M.; Cusenza, S.; Riccioni, G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar. Drugs 2012, 10, 812–833. [Google Scholar] [CrossRef] [Green Version]
- Rollins-Smith, L.A.; Reinert, L.K.; O’Leary, C.J.; Houston, L.E.; Woodhams, D.C. Antimicrobial peptide defenses in amphibian skin. Integr. Comp. Biol. 2005, 45, 137–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou Aleinein, R.; Hamoud, R.; Schäfer, H.; Wink, M. Molecular cloning and expression of ranalexin, a bioactive antimicrobial peptide from Rana catesbeiana in Escherichia coli and assessments of its biological activities. Appl. Microbiol. Biotechnol. 2013, 97, 3535–3543. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.E.; Badillo-Corona, J.A.; Ramírez-Sotelo, G.; Oliver-Salvador, C. Biologically active and antimicrobial peptides from plants. BioMed Res. Int. 2015, 2015, 102129. [Google Scholar] [CrossRef] [Green Version]
- Jenssen, H.; Hamill, P.; Hancock, R.E. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006, 19, 491–511. [Google Scholar] [CrossRef] [Green Version]
- Boman, H.G. Innate immunity and the normal microflora. Immunol. Rev. 2000, 173, 5–16. [Google Scholar] [CrossRef]
- Powers, J.-P.S.; Rozek, A.; Hancock, R.E. Structure–activity relationships for the β-hairpin cationic antimicrobial peptide polyphemusin I. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2004, 1698, 239–250. [Google Scholar] [CrossRef]
- Stotz, H.U.; Thomson, J.; Wang, Y. Plant defensins: Defense, development and application. Plant Signal. Behav. 2009, 4, 1010–1012. [Google Scholar] [CrossRef] [Green Version]
- Nawrot, R.; Barylski, J.; Nowicki, G.; Broniarczyk, J.; Buchwald, W.; Goździcka-Józefiak, A. Plant antimicrobial peptides. Folia Microbiol. 2014, 59, 181–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stec, B. Plant thionins—The structural perspective. Cell. Mol. Life Sci. CMLS 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, J.; Xu, C.; Ren, F.; Peng, C.; Wu, G.; Zhao, J. Purification, characterization, and molecular cloning of the gene of a seed-specific antimicrobial protein from pokeweed. Plant Physiol. 2000, 122, 1015–1024. [Google Scholar] [CrossRef] [Green Version]
- Terras, F.R.; Eggermont, K.; Kovaleva, V.; Raikhel, N.V.; Osborn, R.W.; Kester, A.; Rees, S.B.; Torrekens, S.; Van Leuven, F.; Vanderleyden, J. Small cysteine-rich antifungal proteins from radish: Their role in host defense. Plant Cell 1995, 7, 573–588. [Google Scholar]
- Ye, X.; Ng, T.; Rao, P. Cicerin and arietin, novel chickpea peptides with different antifungal potencies. Peptides 2002, 23, 817–822. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G. APD: The antimicrobial peptide database. Nucleic Acids Res. 2004, 32 (Suppl. S1), D590–D592. [Google Scholar] [CrossRef] [Green Version]
- del Mar Yust, M.; Pedroche, J.; Megías, C.; Girón-Calle, J.; Alaiz, M.; Millán, F.; Vioque, J. Rapeseed protein hydrolysates: A source of HIV protease peptide inhibitors. Food Chem. 2004, 87, 387–392. [Google Scholar] [CrossRef]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef]
- Craik, D.J. Discovery and applications of the plant cyclotides. Toxicon 2010, 56, 1092–1102. [Google Scholar] [CrossRef]
- Shao, F.; Hu, Z.; Xiong, Y.-M.; Huang, Q.-Z.; Wang, C.-G.; Zhu, R.-H.; Wang, D.-C. A new antifungal peptide from the seeds of Phytolacca americana: Characterization, amino acid sequence and cDNA cloning. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1999, 1430, 262–268. [Google Scholar] [CrossRef]
- De Caleya, R.F.; Gonzalez-Pascual, B.; García-Olmedo, F.; Carbonero, P. Susceptibility of phytopathogenic bacteria to wheat purothionins in vitro. Appl. Microbiol. 1972, 23, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Hördegen, P.; Cabaret, J.; Hertzberg, H.; Langhans, W.; Maurer, V. In vitro screening of six anthelmintic plant products against larval Haemonchus contortus with a modified methyl-thiazolyl-tetrazolium reduction assay. J. Ethnopharmacol. 2006, 108, 85–89. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, H.N.; Sharma, N.K. Cationic bioactive peptide from the seeds of Benincasa hispida. Int. J. Pept. 2014, 2014, 156060. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, U. Stress, subjective and objective health. Int. J. Soc. Welf. 2006, 15, S41–S48. [Google Scholar] [CrossRef]
- Pitkänen, A.; Kharatishvili, I.; Karhunen, H.; Lukasiuk, K.; Immonen, R.; Nairismägi, J.; Gröhn, O.; Nissinen, J. Epileptogenesis in experimental models. Epilepsia 2007, 48, 13–20. [Google Scholar] [CrossRef]
- Almroth, B.C.; Albertsson, E.; Sturve, J.; Förlin, L. Oxidative stress, evident in antioxidant defences and damage products, in rainbow trout caged outside a sewage treatment plant. Ecotoxicol. Environ. Saf. 2008, 70, 370–378. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Seo, H.-S.; Kwak, S.-Y.; Lee, Y.-S. Antioxidative activities of histidine containing caffeic acid-dipeptides. Bioorganic Med. Chem. Lett. 2010, 20, 4266–4272. [Google Scholar] [CrossRef]
- Aluko, R. Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2015; pp. 105–140. [Google Scholar]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Ohshima, T. Bioactive marine peptides: Nutraceutical value and novel approaches. Adv. Food Nutr. Res. 2012, 65, 73–105. [Google Scholar] [PubMed]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Je, J.-Y.; Kim, S.-K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Li, X.X.; Han, L.J.; Chen, L.J. In vitro antioxidant activity of protein hydrolysates prepared from corn gluten meal. J. Sci. Food Agric. 2008, 88, 1660–1666. [Google Scholar] [CrossRef]
- Ranathunga, S.; Rajapakse, N.; Kim, S.-K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2006, 222, 310–315. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Sato, H.; Feix, J.B.; Frank, D.W. Identification of superoxide dismutase as a cofactor for the pseudomonas type III toxin, ExoU. Biochemistry 2006, 45, 10368–10375. [Google Scholar] [CrossRef]
- Tincu, J.A.; Taylor, S.W. Antimicrobial peptides from marine invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. [Google Scholar] [CrossRef] [Green Version]
- Rydlo, T.; Miltz, J.; Mor, A. Eukaryotic antimicrobial peptides: Promises and premises in food safety. J. Food Sci. 2006, 71, R125–R135. [Google Scholar] [CrossRef]
- Cutrona, K.J.; Kaufman, B.A.; Figueroa, D.M.; Elmore, D.E. Role of arginine and lysine in the antimicrobial mechanism of histone-derived antimicrobial peptides. FEBS Lett. 2015, 589, 3915–3920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, L.; Bai, X.; Luan, N.; Yao, H.; Zhang, Z.; Liu, W.; Chen, Y.; Yan, X.; Rong, M.; Lai, R. A designed tryptophan-and lysine/arginine-rich antimicrobial peptide with therapeutic potential for clinical antibiotic-resistant Candida albicans vaginitis. J. Med. Chem. 2016, 59, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-T.; Kuo, T.-Y.; Chiang, J.-C.; Pei, M.-J.; Yang, W.-T.; Yu, H.-C.; Lin, S.-B.; Chen, W.-J. Design and synthesis of cationic antimicrobial peptides with improved activity and selectivity against Vibrio spp. Int. J. Antimicrob. Agents 2008, 32, 130–138. [Google Scholar] [CrossRef]
- Jin, Y.; Hammer, J.; Pate, M.; Zhang, Y.; Zhu, F.; Zmuda, E.; Blazyk, J. Antimicrobial activities and structures of two linear cationic peptide families with various amphipathic β-sheet and α-helical potentials. Antimicrob. Agents Chemother. 2005, 49, 4957–4964. [Google Scholar] [CrossRef] [Green Version]
- Layek, B.; Lipp, L.; Singh, J. Cell penetrating peptide conjugated chitosan for enhanced delivery of nucleic acid. Int. J. Mol. Sci. 2015, 16, 28912–28930. [Google Scholar] [CrossRef] [Green Version]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Wittkopf, N.; Neurath, M.F.; Becker, C. Immune-epithelial crosstalk at the intestinal surface. J. Gastroenterol. 2014, 49, 375–387. [Google Scholar] [CrossRef]
- Kayser, H.; Meisel, H. Stimulation of human peripheral blood lymphocytes by bioactive peptides derived from bovine milk proteins. FEBS Lett. 1996, 383, 18–20. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Nan, Y.H.; Park, Y.; Kim, J.I.; Park, I.-S.; Hahm, K.-S.; Shin, S.Y. Cell specificity, anti-inflammatory activity, and plausible bactericidal mechanism of designed Trp-rich model antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2009, 1788, 1193–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahwa, R.; Goyal, A.; Bansal, P.; Jialal, I. Chronic Inflammation; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Van Linthout, S.; Tschöpe, C. Inflammation—Cause or consequence of heart failure or both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dürr, M.; Peschel, A. Chemokines meet defensins: The merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect. Immun. 2002, 70, 6515–6517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izadpanah, A.; Gallo, R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390. [Google Scholar] [CrossRef]
- Oppenheim, J.; Biragyn, A.; Kwak, L.; Yang, D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann. Rheum. Dis. 2003, 62 (Suppl. S2), ii17–ii21. [Google Scholar] [CrossRef] [Green Version]
- Auvynet, C.; Rosenstein, Y. Multifunctional host defense peptides: Antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef]
- Agier, J.; Efenberger, M.; Brzezińska-Błaszczyk, E. Cathelicidin impact on inflammatory cells. Cent.-Eur. J. Immunol. 2015, 40, 225. [Google Scholar] [CrossRef]
- Singh, M.; Mukhopadhyay, K. Alpha-melanocyte stimulating hormone: An emerging anti-inflammatory antimicrobial peptide. BioMed Res. Int. 2014, 2014, 874610. [Google Scholar] [CrossRef]
- Pradeepa, R.; Mohan, V. Hypertension & pre-hypertension in developing countries. Indian J. Med. Res. 2008, 128, 688. [Google Scholar]
- Messerli, F.H.; Bangalore, S.; Bavishi, C.; Rimoldi, S.F. Angiotensin-converting enzyme inhibitors in hypertension: To use or not to use? J. Am. Coll. Cardiol. 2018, 71, 1474–1482. [Google Scholar] [CrossRef]
- Messerli, F.H. Combinations in the treatment of hypertension: ACE inhibitors and calcium antagonists. Am. J. Hypertens. 1999, 12 (Suppl. S5), 86S–90S. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.; Recio, I.; Ramos, M.; Delgado, M.; Aleixandre, M. Antihypertensive effect of peptides obtained from Enterococcus faecalis-fermented milk in rats. J. Dairy Sci. 2006, 89, 3352–3359. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M.; Aleixandre, M.; Ramos, M.; Lopez-Fandino, R. Effect of simulated gastrointestinal digestion on the antihypertensive properties of ACE-inhibitory peptides derived from ovalbumin. J. Agric. Food Chem. 2006, 54, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Su, R.; He, Z. Transformation of antimicrobial into bradykinin-potentiating peptides during peptic hydrolysis of bovine haemoglobin: Identification, release kinetics and reaction network of peptides. J. Sci. Food Agric. 2007, 87, 461–469. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kim, Y.-T.; Byun, H.-G.; Nam, K.-S.; Joo, D.-S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef]
- Fujita, H.; Yoshikawa, M. LKPNM: A prodrug-type ACE-inhibitory peptide derived from fish protein. Immunopharmacology 1999, 44, 123–127. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef]
- Aondona, M.M.; Ikya, J.K.; Ukeyima, M.T.; Gborigo, T.J.A.; Aluko, R.E.; Girgih, A.T. In vitro antioxidant and antihypertensive properties of sesame seed enzymatic protein hydrolysate and ultrafiltration peptide fractions. J. Food Biochem. 2021, 45, e13587. [Google Scholar] [CrossRef]
- Dang, Y.; Zhou, T.; Hao, L.; Cao, J.; Sun, Y.; Pan, D. In vitro and in vivo studies on the angiotensin-converting enzyme inhibitory activity peptides isolated from broccoli protein hydrolysate. J. Agric. Food Chem. 2019, 67, 6757–6764. [Google Scholar] [CrossRef]
- Koyama, M.; Naramoto, K.; Nakajima, T.; Aoyama, T.; Watanabe, M.; Nakamura, K. Purification and identification of antihypertensive peptides from fermented buckwheat sprouts. J. Agric. Food Chem. 2013, 61, 3013–3021. [Google Scholar] [CrossRef]
- Wakasa, Y.; Zhao, H.; Hirose, S.; Yamauchi, D.; Yamada, Y.; Yang, L.; Ohinata, K.; Yoshikawa, M.; Takaiwa, F. Antihypertensive activity of transgenic rice seed containing an 18-repeat novokinin peptide localized in the nucleolus of endosperm cells. Plant Biotechnol. J. 2011, 9, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Pruimboom, L.; Van Dam, A. Chronic pain: A non-use disease. Med. Hypotheses 2007, 68, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Torrance, N.; Elliott, A.M.; Lee, A.J.; Smith, B.H. Severe chronic pain is associated with increased 10 year mortality. A cohort record linkage study. Eur. J. Pain 2010, 14, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Saiki, C.B. Cancer pain management. Mayo Clin. Proc. 2015, 90, 1428–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wootton, M. Morphine is not the only analgesic in palliative care: Literature review. J. Adv. Nurs. 2004, 45, 527–532. [Google Scholar] [CrossRef]
- Haegerstam, G.A. Pathophysiology of bone pain: A review. Acta Orthop. Scand. 2001, 72, 308–317. [Google Scholar] [CrossRef] [Green Version]
- Orr, P.M.; Shank, B.C.; Black, A.C. The role of pain classification systems in pain management. Crit. Care Nurs. Clin. N. Am. 2017, 29, 407–418. [Google Scholar] [CrossRef]
- Rakowski, J.A.; Holloway, R.W.; Ahmad, S.; Jeppson, C.N.; James, J.A.; Ghurani, G.B.; Bigsby, G.E.; Kendrick, J.E. A prospective randomized trial of intravenous ketorolac vs. acetaminophen administered with opioid patient-controlled analgesia in gynecologic surgery. Gynecol. Oncol. 2019, 155, 468–472. [Google Scholar] [CrossRef]
- Memar, R.; Farokhpur, M.; Mesripur, A. Medical Pharmacology (Nursing-Midwifery-Anesthesiology); Andishe Rafie Pub: Tehran, Iran, 2020. [Google Scholar]
- Bousso, R.S.; Poles, K.; de Almeida Lopes Monteiro da Cruz, D. Nursing concepts and theories. Rev. Esc. Enferm. USP 2014, 48, 141–145. [Google Scholar] [CrossRef] [Green Version]
- Dickason, R.M.; Chauhan, V.; Mor, A.; Ibler, E.; Kuehnle, S.; Mahoney, D.; Armbrecht, E.; Dalawari, P. Racial differences in opiate administration for pain relief at an academic emergency department. West. J. Emerg. Med. 2015, 16, 372. [Google Scholar] [CrossRef]
- De Vega, M.J.P.; Ferrer-Montiel, A.; González-Muñiz, R. Recent progress in non-opioid analgesic peptides. Arch. Biochem. Biophys. 2018, 660, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Papini, A.M. From morphine to endogenous opioid peptides, eg, endorphins: The endless quest for the perfect painkiller. Substantia 2018, 2, 81–91. [Google Scholar]
- Takahashi, A. Enkephalin. In Handbook of Hormones; Elsevier: Amsterdam, The Netherlands, 2021; pp. 91–94. [Google Scholar]
- Cullen, J.M.; Cascella, M. Physiology, Enkephalin; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Teschemacher, H.; Koch, G.; Brantl, V. Milk protein-derived opioid receptor ligands. Pept. Sci. 1997, 43, 99–117. [Google Scholar] [CrossRef]
- Zioudrou, C.; Streaty, R.A.; Klee, W.A. Opioid peptides derived from food proteins. The exorphins. J. Biol. Chem. 1979, 254, 2446–2449. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Takahashi, M.; Yang, S. Delta opioid peptides derived from plant proteins. Curr. Pharm. Des. 2003, 9, 1325–1330. [Google Scholar] [CrossRef]
- Walther, B.; Sieber, R. Bioactive proteins and peptides in foods. Int. J. Vitam. Nutr. Res. 2011, 81, 181. [Google Scholar] [CrossRef]
- Miquel, E.; Gómez, J.Á.; Alegría, A.; Barberá, R.; Farré, R.; Recio, I. Identification of casein phosphopeptides released after simulated digestion of milk-based infant formulas. J. Agric. Food Chem. 2005, 53, 3426–3433. [Google Scholar] [CrossRef]
- Gagnaire, V.; Pierre, A.; Molle, D.; Leonil, J. Phosphopeptides interacting with colloidal calcium phosphate isolated by tryptic hydrolysis of bovine casein micelles. J. Dairy Res. 1996, 63, 405–422. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Ros, E. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis 2000, 151, 357–379. [Google Scholar] [CrossRef]
- Athmani, N.; Dehiba, F.; Allaoui, A.; Barkia, A.; Bougatef, A.; Lamri-Senhadji, M.Y.; Nasri, M.; Boualga, A. Sardina pilchardus and Sardinella aurita protein hydrolysates reduce cholesterolemia and oxidative stress in rat fed high cholesterol diet. J. Exp. Integr. Med. 2015, 5, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Affane, F.; Louala, S.; el Imane Harrat, N.; Bensalah, F.; Chekkal, H.; Allaoui, A.; Lamri-Senhadji, M. Hypolipidemic, antioxidant and antiatherogenic property of sardine by-products proteins in high-fat diet induced obese rats. Life Sci. 2018, 199, 16–22. [Google Scholar] [CrossRef]
- Ktari, N.; Mnafgui, K.; Nasri, R.; Hamden, K.; Bkhairia, I.; Hadj, A.B.; Boudaouara, T.; Elfeki, A.; Nasri, M. Hypoglycemic and hypolipidemic effects of protein hydrolysates from zebra blenny (Salaria basilisca) in alloxan-induced diabetic rats. Food Funct. 2013, 4, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Futamura, Y.; Miwa, K.; Awano, T.; Yamauchi, K.; Kanamaru, Y.; Tadashi, K.; Kuwata, T. Identification of novel hypocholesterolemic peptides derived from bovine milk β-lactoglobulin. Biochem. Biophys. Res. Commun. 2001, 281, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pupovac, J.; Anderson, G.H. Dietary peptides induce satiety via cholecystokinin—A and peripheral opioid receptors in rats. J. Nutr. 2002, 132, 2775–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, S.P.; Goswami, A.; Kalia, K.; Kate, A.S. Plant-derived bioactive peptides: A treatment to cure diabetes. Int. J. Pept. Res. Ther. 2020, 26, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Sivaraman, K.; Shanthi, C. Matrikines for therapeutic and biomedical applications. Life Sci. 2018, 214, 22–33. [Google Scholar] [CrossRef]
- Leroux, R.; Ringenbach, C.; Marchand, T.; Peschard, O.; Mondon, P.; Criton, P. A new matrikine-derived peptide up-regulates longevity genes for improving extracellular matrix architecture and connections of dermal cell with its matrix. Int. J. Cosmet. Sci. 2020, 42, 53–59. [Google Scholar] [CrossRef]
- Fuller-Iglesias, H.; Smith, J.; Antonucci, T.C. Theories of aging from a life-course and life-span perspective. In Annual Review of Gerontology and Geriatrics. Life-Course Perspectives on Late Life Health Inequalities; Springer: New York, NY, USA, 2009; Volume 29, Chapter 1. [Google Scholar]
- Tosato, M.; Zamboni, V.; Ferrini, A.; Cesari, M. The aging process and potential interventions to extend life expectancy. Clin. Interv. Aging 2007, 2, 401. [Google Scholar]
- Podolskiy, D.I.; Gladyshev, V.N. Intrinsic versus extrinsic cancer risk factors and aging. Trends Mol. Med. 2016, 22, 833–834. [Google Scholar] [CrossRef] [Green Version]
- Fields, K.; Falla, T.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Wieland, T.; Bodanszky, M. The World of Peptides: A Brief History of Peptide Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Rahnamaeian, M.; Vilcinskas, A. Short antimicrobial peptides as cosmetic ingredients to deter dermatological pathogens. Appl. Microbiol. Biotechnol. 2015, 99, 8847–8855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, K.; Seyer, J.M.; Raghow, R.; Kang, A.H. Regulation of extracellular matrix production by chemically synthesized subfragments of type I collagen carboxy propeptide. Biochemistry 1991, 30, 7097–7104. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Almeida, I.F. Trending anti-aging peptides. Cosmetics 2020, 7, 91. [Google Scholar] [CrossRef]
- Maeda, I.; Kai, S.; Taniguchi, S.; Inoue, A.; Li, H.; Kesamaru, H.; Nose, T. Angiotensin I converting enzyme-inhibiting peptides purified from elastase-degraded elastin prepared from pig aorta. Curr. Enzym. Inhib. 2018, 14, 67–74. [Google Scholar] [CrossRef]
- Kluczyk, A.; Ludwiczak, J.; Modzel, M.; Kuczer, M.; Cebrat, M.; Biernat, M.; Bąchor, R. Argireline: Needle-free botox as analytical challenge. Chem. Biodivers. 2021, 18, e2000992. [Google Scholar] [CrossRef]
- Field, M.; Splevins, A.; Picaut, P.; Van der Schans, M.; Langenberg, J.; Noort, D.; Foster, K. AbobotulinumtoxinA (Dysport®), onabotulinumtoxinA (Botox®), and incobotulinumtoxinA (Xeomin®) neurotoxin content and potential implications for duration of response in patients. Toxins 2018, 10, 535. [Google Scholar] [CrossRef] [Green Version]
- Hussack, G.; Hirama, T.; Ding, W.; MacKenzie, R.; Tanha, J. Engineered single-domain antibodies with high protease resistance and thermal stability. PLoS ONE 2011, 6, e28218. [Google Scholar] [CrossRef] [Green Version]
- Fukuzumi, T.; Ju, L.; Bode, J.W. Chemoselective cyclization of unprotected linear peptides by α-ketoacid–hydroxylamine amide-ligation. Org. Biomol. Chem. 2012, 10, 5837–5844. [Google Scholar] [CrossRef]
- Cini, E.; Bifulco, G.; Menchi, G.; Rodriquez, M.; Taddei, M. Synthesis of enantiopure 7-substituted azepane-2-carboxylic acids as templates for conformationally constrained peptidomimetics. Eur. J. Org. Chem. 2012, 2012, 2133–2141. [Google Scholar] [CrossRef]
- Tapeinou, A.; Matsoukas, M.T.; Simal, C.; Tselios, T. Review cyclic peptides on a merry-go-round; towards drug design. Pept. Sci. 2015, 104, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kondejewski, L.H.; Farmer, S.W.; Wishart, D.S.; Kay, C.M.; Hancock, R.W.; Hodges, R.S. Modulation of structure and antibacterial and hemolytic activity by ring size in cyclic gramicidin S analogs. J. Biol. Chem. 1996, 271, 25261–25268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gause, G.F.; Brazhnikova, M.G. Gramicidin S and its use in the treatment of infected wounds. Nature 1944, 154, 703. [Google Scholar] [CrossRef]
- Qin, C.; Bu, X.; Wu, X.; Guo, Z. A chemical approach to generate molecular diversity based on the scaffold of cyclic decapeptide antibiotic tyrocidine A. J. Comb. Chem. 2003, 5, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Álvarez, S.; Pardal, E.; Sánchez-Nieto, D.; Navarro, M.; Caballero, M.D.; Mateos, M.V.; Martín, A. Plitidepsin: Design, development, and potential place in therapy. Drug Des. Dev. Ther. 2017, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, S.L.; Crabtree, G.R. The mechanism of action of cyclosporin A and FK506. Immunol. Today 1992, 13, 136–142. [Google Scholar] [CrossRef]
- Gallay, P.A.; Lin, K. Profile of alisporivir and its potential in the treatment of hepatitis C. Drug Des. Dev. Ther. 2013, 7, 105. [Google Scholar] [CrossRef] [Green Version]
- Dubinin, M.V.; Starinets, V.S.; Talanov, E.Y.; Mikheeva, I.B.; Belosludtseva, N.V.; Belosludtsev, K.N. Alisporivir improves mitochondrial function in skeletal muscle of mdx mice but suppresses mitochondrial dynamics and biogenesis. Int. J. Mol. Sci. 2021, 22, 9780. [Google Scholar] [CrossRef]
- Campas-Moya, C. Romidepsin for the treatment of cutaneous T-cell lymphoma. Drugs Today 2009, 45, 787–795. [Google Scholar] [CrossRef]
- Bertino, E.M.; Otterson, G.A. Romidepsin: A novel histone deacetylase inhibitor for cancer. Expert Opin. Investig. Drugs 2011, 20, 1151–1158. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577. [Google Scholar] [CrossRef]
- De Hoog, M.; Mouton, J.W.; van den Anker, J.N. Vancomycin: Pharmacokinetics and administration regimens in neonates. Clin. Pharmacokinet. 2004, 43, 417–440. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.; Orsburn, B. In silico approach to accelerate the development of mass spectrometry-based proteomics methods for detection of viral proteins: Application to COVID-19. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Usmani, S.S.; Kumar, R.; Bhalla, S.; Kumar, V.; Raghava, G.P. In silico tools and databases for designing peptide-based vaccine and drugs. Adv. Protein Chem. Struct. Biol. 2018, 112, 221–263. [Google Scholar] [PubMed]
| Peptide | Organism | Function | Ref. |
|---|---|---|---|
| Peptide extracts | Bacillariophyceae | Antihypertensive/antioxidant | [59] |
| Peptide extracts | Discodermiu kiiensis | Antimicrobial | [60] |
| Azonazine | Aspergillus insulicola | Anti-inflammatory | [61] |
| Wewakazole | L. majuscula | Anticancer | [62] |
| Mirabamide A-C-D | Sponges | anti-HIV | [63] |
| Aplidine | Aplidine | Anticancer | [64] |
| Arenastatin A | Dysidea arenaria | Anticancer | [65] |
| Aurilide | Dolabella auricularia | Anticancer | [66] |
| Didemnin | Trididemnum sp. | Anticancer | [67] |
| Dolastatin | Dolabella auricularia | Anticancer | [68] |
| Geodiamolide H | Geodia sp. | Anticancer | [69] |
| Homophymines | Homophymia sp. | Anticancer | [58] |
| Jaspamide | Jaspis sp., Hemiastrella sp. | Anticancer | [70] |
| Kahalalide F | Elysia rufescens, Spisula polynyma | Anticancer | [65] |
| Keenamide A | Pleurobranchus forskalii | Anticancer | [56] |
| Mollamide | Didemnum molle | Anticancer | [71] |
| Phakellistatins | Phakellia carteri | Anticancer | [72] |
| Tamandarins A and B | Didemnum sp. | Anticancer | [73] |
| Precursor Protein | Peptide Sequence | Bioactivity | Ref. |
|---|---|---|---|
| Hydrolysates of camel milk protein | KDLWDDFKGL and MPSKPPLL | Antidiabetic | [81] |
| Hydrolysates of camel milk protein | LPVPG | Antidiabetic | [82] |
| Hydrolysates of camel milk protein | FLQY, FQLGASPY, ILDKEGIDY, ILELA, LLQLEAIR, LPVP, LQALHQGQIV, MPVQA, and SPVVPF | Antidiabetic | [83] |
| Hydrolysates of camel milk protein | KFQWGY, SQDWSFY, and YWYPPQ | Inhibition of cholesterol esterase | [84] |
| Bactrian camel milk hydrolysate | RLDG QGRPRVWLGR, TPDNIDIW LGGIAEPQVKR, and VAYSDDGENWTEYRDQGAVEGK | Antioxidant | [85] |
| Fermented camel milk (Leuconostoc lactis) | MVPYPQR | ACE inhibitor | [28] |
| Fermented goat milk (Lactobacillus plantarum 69) | ND | ACE inhibitor | [86] |
| Hydrolyzed goat milk | ND | Antimicrobial activity | [87] |
| Classification | Example | Host | Applications | Ref. |
|---|---|---|---|---|
| Bradykinin potentiating peptides | TsTX-Ka and TsTX-KO | Bothrops jararaca | Hypotensive effects, ACE inhibitor | [108] |
| BPPs | Tityus serrulatus Bothrops jararaca | ACE inhibitor | [109] | |
| Antimicrobial peptides | IsCTs | Opisthacanthus madagascariensis | Antimicrobial Cytolytic activity | [110] |
| Hormonelike peptides | Mini-Ins | Conus geographus | Insulin-like activity | [111] |
| Therapeutic peptides | Ziconotide | Conus magus | Pain killer | [112] |
| Plant | Peptide | Peptide Size | Biological Activity | Ref. |
|---|---|---|---|---|
| Hevea brasiliensis | Heveins | 43 residues, 4.7 kDa | Antibacterial and antifungal | [127] |
| Phaseolus vulgaris | ND | 2.2 and 6 kDa | Antibacterial and antifungal | [131,132] |
| Brassica napus | Peptides | ND | Antiviral | [133] |
| Capsella bursa-pastoris | Shepherins | 28 residues | Antibacterial and antifungal | [134] |
| Higher plants | Thionins | 45–47 residues | Antibacterial | [126,127,128] |
| Oldenlandia affinis | Cyclotides | 28–37 residues | Antibacterial, Antifungal, Insecticide, Nematicide | [126,135] |
| Phytolacca americana | PAFP-S | 36–37 residues | Antibacterial | [136] |
| Triticum aestivum | Alpha-1-purothionin | 45 residues | Antibacterial | [137] |
| Triticum aestivum | Defensins | 5 kDa | Antibacterial and antifungal | [138] |
| Benincasa hispida | Hispidulin | 5.7 kDa | Antibacterial and antifungal | [139] |
| Name | Source | Application | Ref. |
|---|---|---|---|
| Gramicidin S | Bacillus brevis | Antibiotic activity towards Gram-negative and Gram-positive and even several pathogenic fungi. | [234,235] |
| Tyrocidine | Bacillus brevis | By antibiotic action, it can disrupt the cell membrane function. | [236] |
| Plitidepsin | Aplidium albicans | Antitumor, antiviral, and immunosuppressive activities. | [237] |
| Cyclosporin A | Tolypocladium inflatum | As a calcineurin inhibitor, it can decrease the function of lymphocytes. | [238] |
| Alisporivir | Chemically synthesized from ciclosporin | Inhibits cyclophilin A, and it is believed that it may have a potential effect on Alzheimer’s disease and hepatitis C. | [239,240] |
| Romidepsin | Chromobacterium violaceum | By apoptotic activity, it has an anticancer activity on many types of malignant cell lines. | [241,242] |
| Ziconotide | Conus magus | Acts as an analgesic agent; strong pain killer. | [243] |
| Vancomycin | Amycolatopsis orientalis | A board range antibacterial compound that is used in many bacterial infections. | [244] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. https://doi.org/10.3390/ijms23031445
Akbarian M, Khani A, Eghbalpour S, Uversky VN. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. International Journal of Molecular Sciences. 2022; 23(3):1445. https://doi.org/10.3390/ijms23031445
Chicago/Turabian StyleAkbarian, Mohsen, Ali Khani, Sara Eghbalpour, and Vladimir N. Uversky. 2022. "Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action" International Journal of Molecular Sciences 23, no. 3: 1445. https://doi.org/10.3390/ijms23031445
APA StyleAkbarian, M., Khani, A., Eghbalpour, S., & Uversky, V. N. (2022). Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. International Journal of Molecular Sciences, 23(3), 1445. https://doi.org/10.3390/ijms23031445







