Combined Modification of Fiber Materials by Enzymes and Metal Nanoparticles for Chemical and Biological Protection
Abstract
:1. Introduction
2. Results
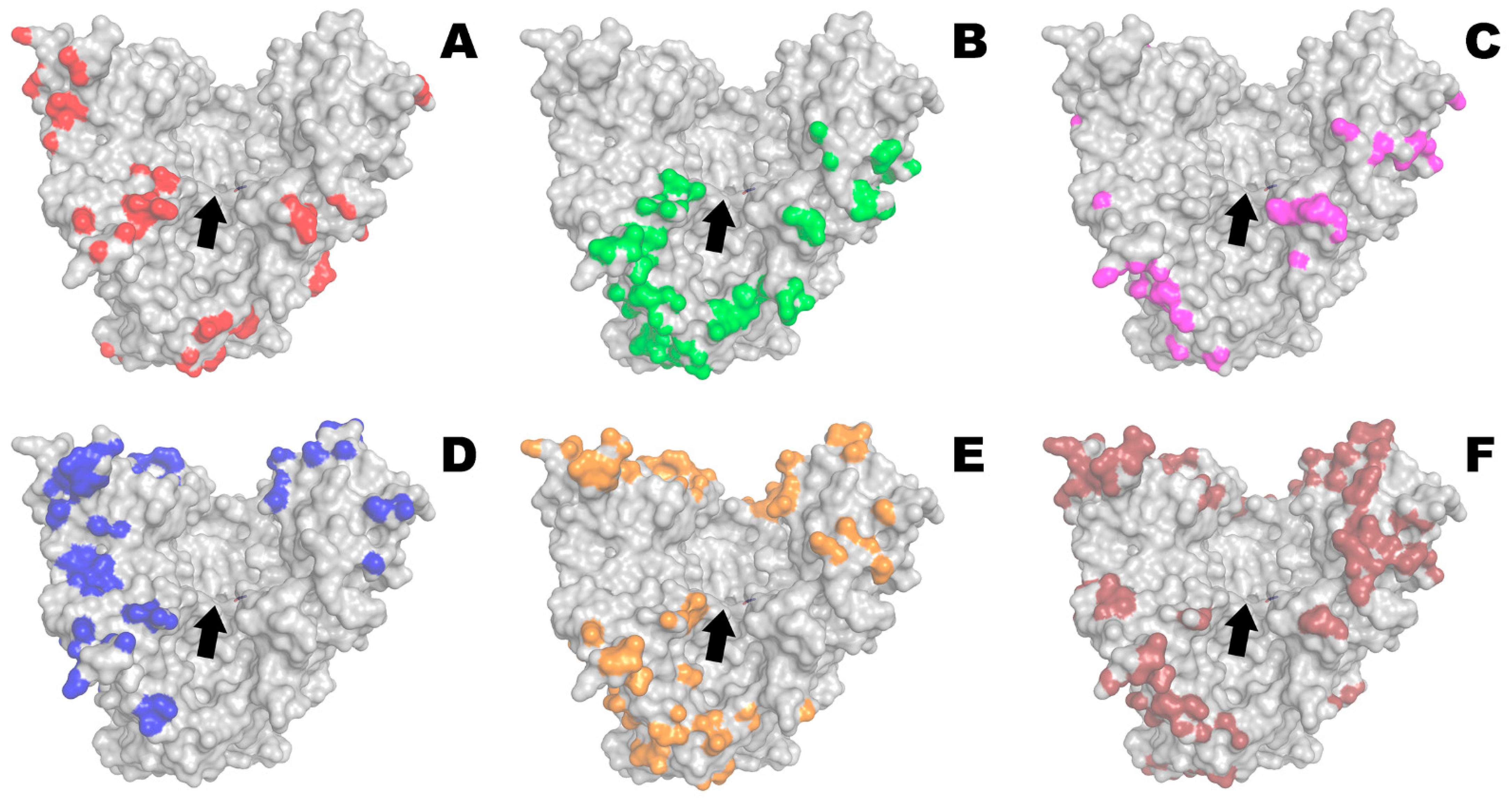
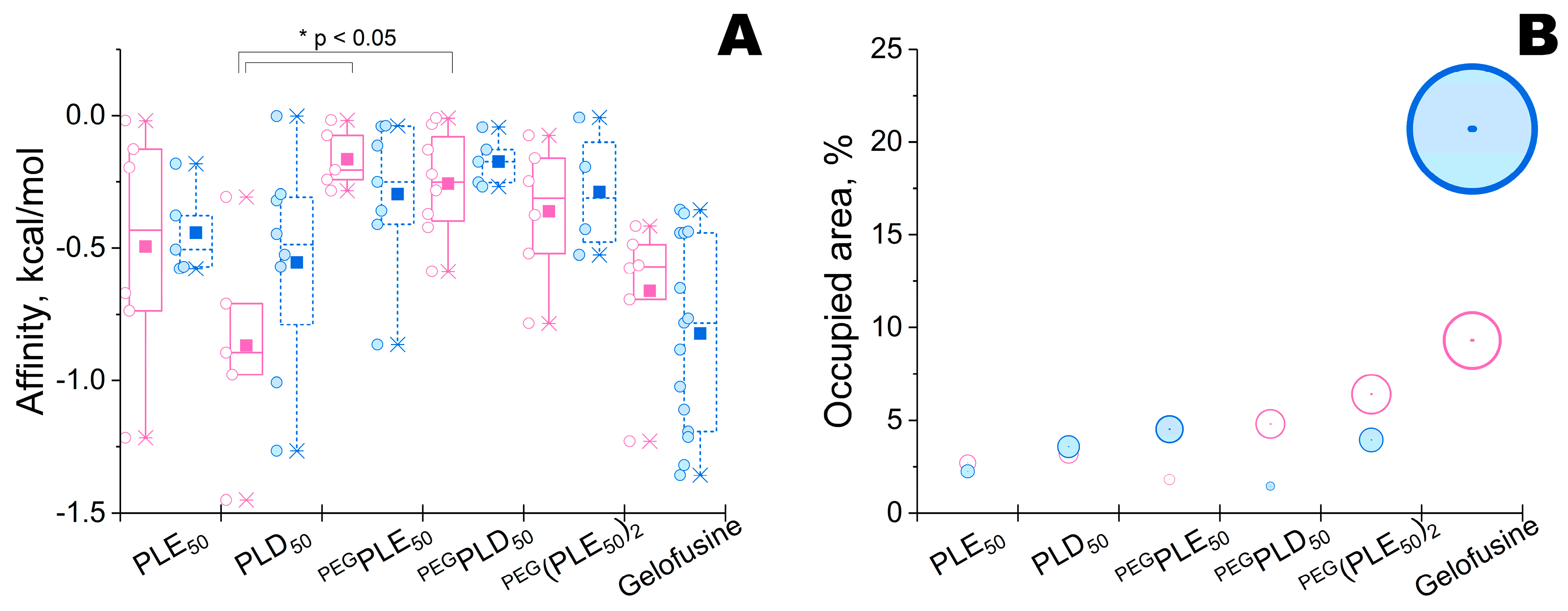
2.1. Modeling of Enzyme–Polyelectrolyte Complexes and Their Activity within Bacterial Cellulose
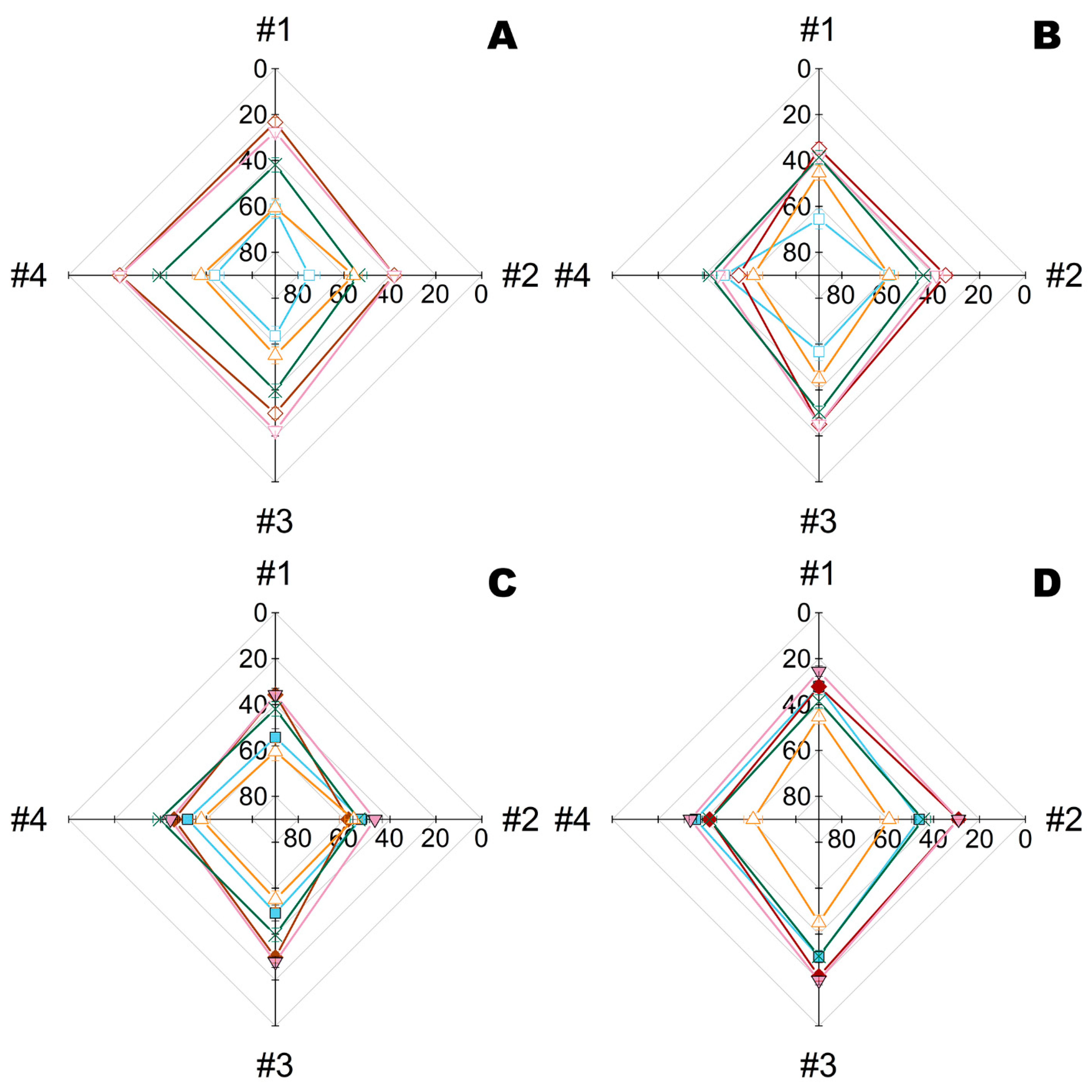
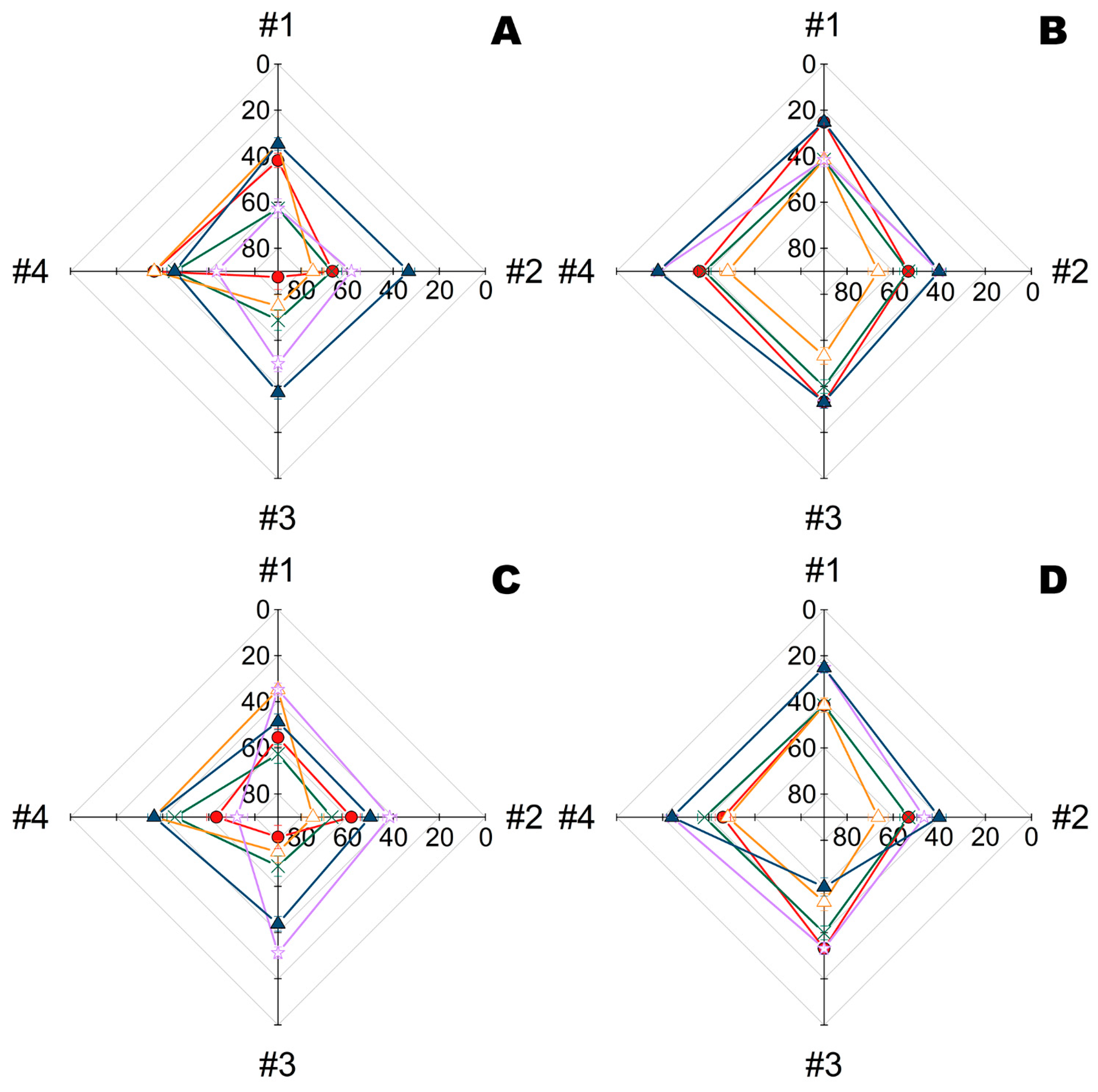
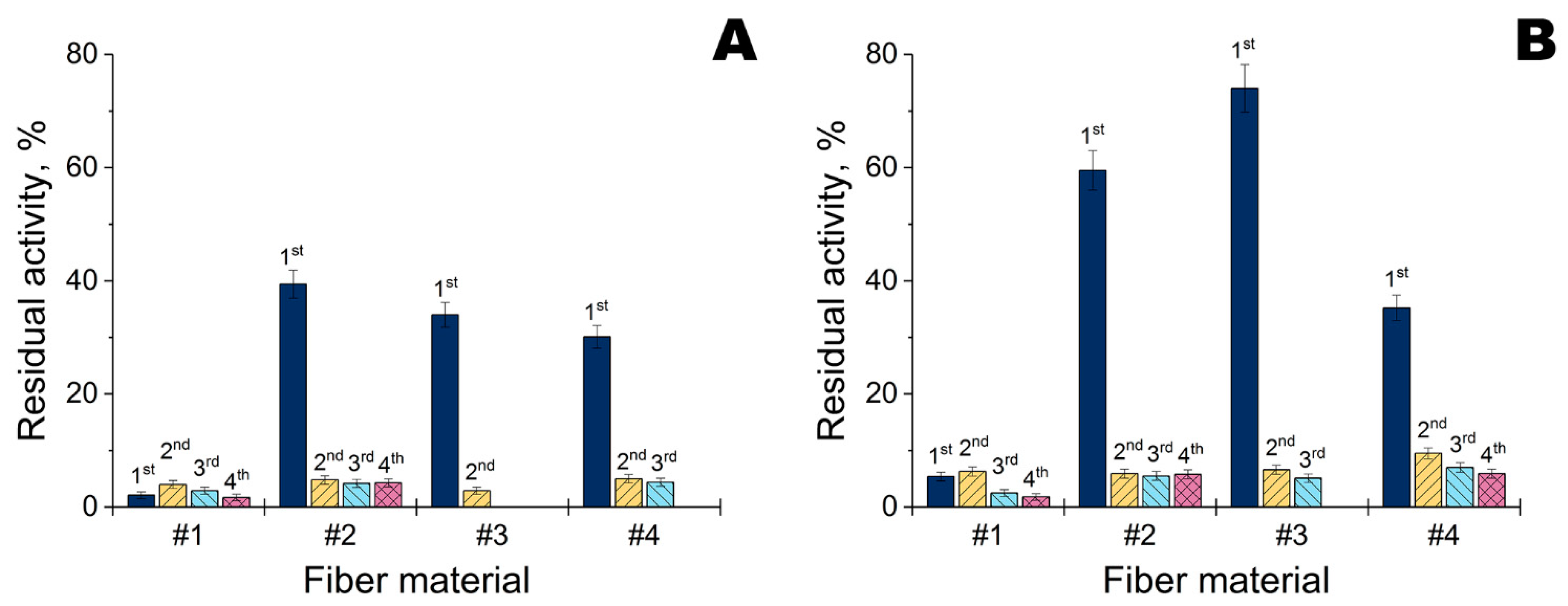
2.2. Combination of Metal Nanoparticles with Enzymes within Fiber Materials
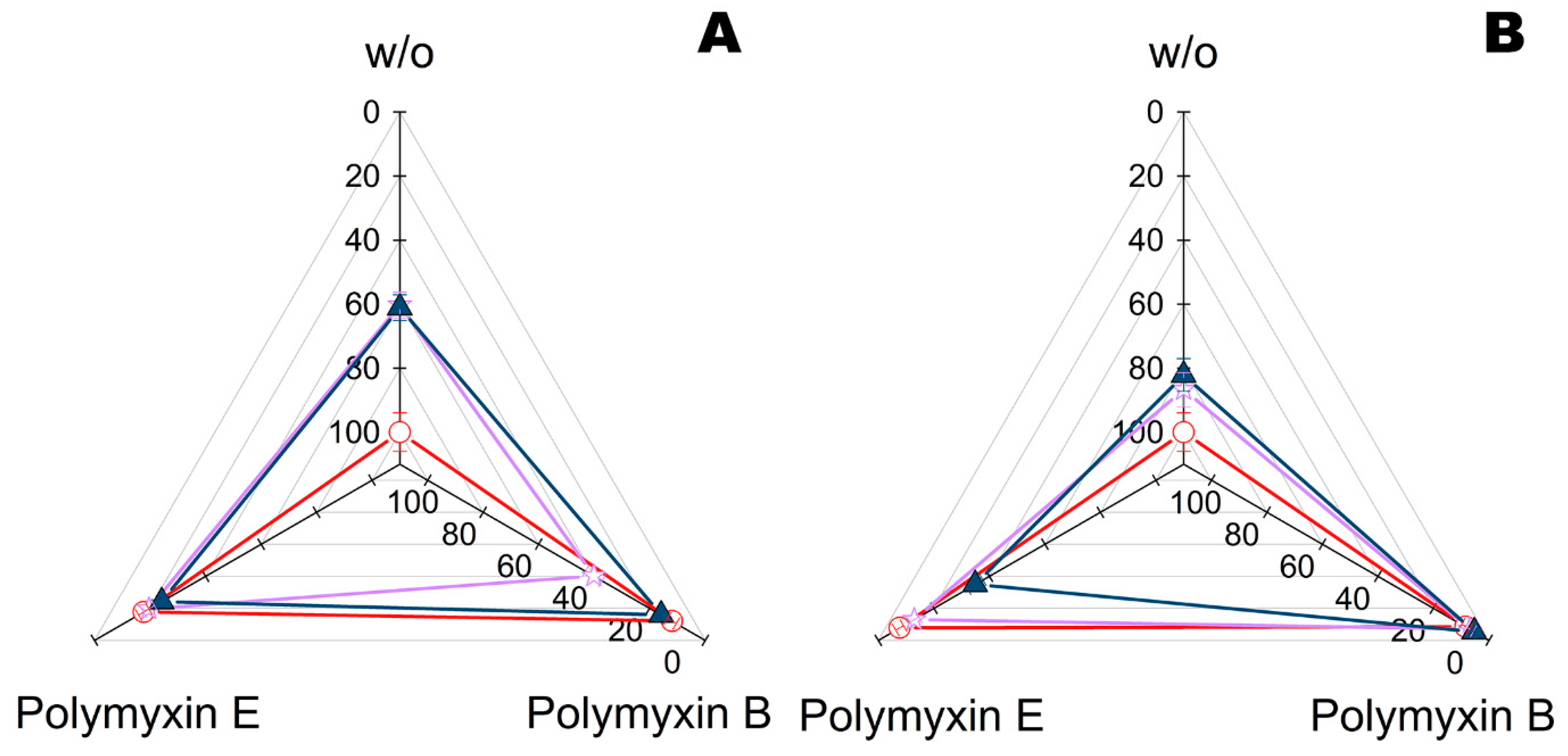
2.3. Combination of Polymyxins with Enzymes within Fiber Materials
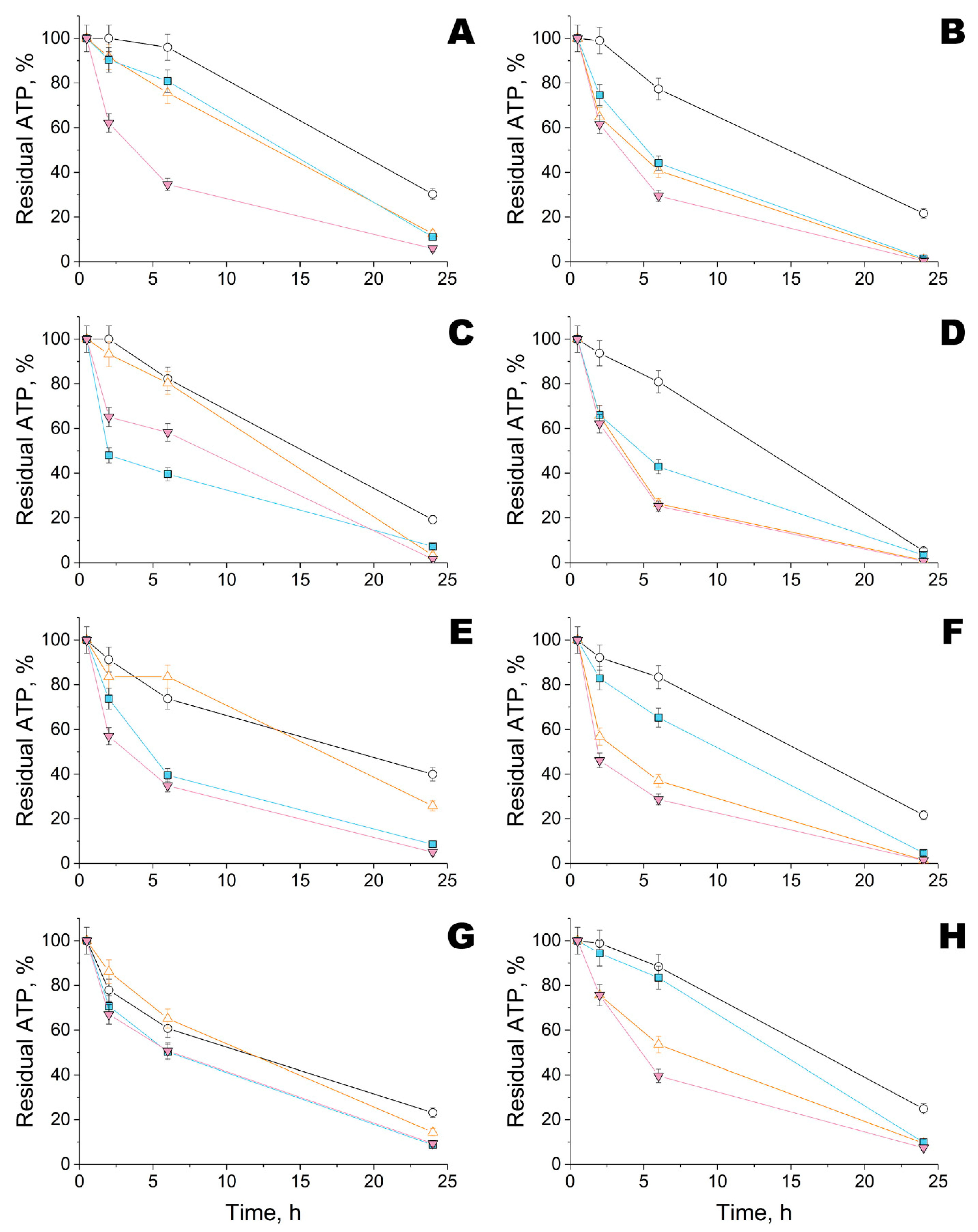
2.4. Activity of His6-OPH within Modified Fiber Materials
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of Metal Nanoparticles
4.3. Preparation and Antibacterial Activity of Modified Bacterial Cellulose (BC)
4.4. Preparation and Antibacterial Activity of Modified Fibrous Materials
4.5. Hydrolytic Activity of Modified Fibrous Materials
4.6. Computational Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapbamrer, R.; Ajchamon Thammachai, A. Factors affecting use of personal protective equipment and pesticide safety practices: A systematic review. Environ. Res. 2020, 185, e109444. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.G.S.; Soares, T.K.M.; Linhares, M.A.; Ghisi, N.C. Occupational exposure to pesticides: Genetic danger to farmworkers and manufacturing workers-A meta-analytical review. Sci. Total Environ. 2020, 748, e141382. [Google Scholar] [CrossRef] [PubMed]
- Tuñón-Molina, A.; Martí, M.; Muramoto, Y.; Noda, T.; Takayama, K.; Serrano-Aroca, Á. Antimicrobial Face Shield: Next Generation of Facial Protective Equipment against SARS-CoV-2 and Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2021, 22, 9518. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2019, 49, 97–138. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Recent Progress in Antimicrobial Nanomaterials. Nanomaterials 2020, 10, 2315. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Zhou, Y.; Zhou, Y. Multifunctional Antibacterial Materials for the Control of Hazardous Microbes and Chemicals: A Review. ACS ES&T Water 2021, 1, 479–497. [Google Scholar] [CrossRef]
- Vitchuli, N.; Shi, Q.; Nowak, J.; Kay, K.; Caldwell, J.M.; Breidt, F.; Bourham, M.; McCord, M.; Zhang, X. Multifunctional ZnO/Nylon 6 nanofiber mats by an electrospinning-electrospraying hybrid process for use in protective applications. Sci. Technol. Adv. Mater. 2011, 12, e055004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denet, E.; Espina-Benitez, M.B.; Pitault, I.; Pollet, T.; Blaha, D.; Bolzinger, M.-A.; Rodriguez-Nava, V.; Briançon, S. Metal oxide nanoparticles for the decontamination of toxic chemical and biological compounds. Int. J. Pharm. 2020, 583, e119373. [Google Scholar] [CrossRef]
- Vanangamudi, A.; Hamzah, S.; Singh, G. Synthesis of hybrid hydrophobic composite air filtration membranes for antibacterial activity and chemical detoxification with high particulate filtration efficiency (PFE). Chem. Eng. J. 2015, 260, 801–808. [Google Scholar] [CrossRef]
- Sharma, N.; Chaudhary, M.; Butola, B.S.; Jeyabalaji, J.K.; Pathak, D.P.; Sharma, R.K. Preparation, characterization and evaluation of the zinc titanate and silver nitrate incorporated wipes for topical chemical and biological decontamination. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 183–196. [Google Scholar] [CrossRef]
- Amitai, G.; Murata, H.; Andersen, J.D.; Koepsel, R.R.; Russell, A.J. Decontamination of chemical and biological warfare agents with a single multi-functional material. Biomaterials 2010, 31, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-Y.; Park, M.-K.; Kwak, S.-Y. Silver-titania/polyurethane composite nanofibre mat for chemical and biological warfare protection. Int. J. Nanotechnol. 2013, 10, 771–788. [Google Scholar] [CrossRef]
- Gopinath, A.; Krishna, K. Dual role of chemically functionalized activated carbon fibres: Investigation of parameters influencing the degradation of organophosphorus compounds and antibacterial behaviour. J. Chem. Technol. Biotechnol. 2019, 94, 611–617. [Google Scholar] [CrossRef]
- Frolov, G.; Lyagin, I.; Senko, O.; Stepanov, N.; Pogorelsky, I.; Efremenko, E. Metal nanoparticles for improving bactericide functionality of usual fibers. Nanomaterials 2020, 10, 1724. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Charges’ interaction in polyelectrolyte (nano)complexing of His6-OPH with peptides: Unpredictable results due to imperfect or useless concept? Int. J. Biol. Macromol. 2019, 140, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Stepanov, N.; Presnov, D.; Efremenko, E. Bacterial Cellulose Containing Combinations of Antimicrobial Peptides with Various QQ Enzymes as a Prototype of an “Enhanced Antibacterial” Dressing: In Silico and In Vitro Data. Pharmaceutics 2020, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, S.D.; Efremenko, E.N. (Eds.) Organophosphorus Neurotoxins, 1st ed.; Publishing Center RIOR: Moscow, Russia, 2020; p. 380. [Google Scholar] [CrossRef]
- Lyagin, I.; Efremenko, E. Enzymes, Reacting with Organophosphorus Compounds as Detoxifiers: Diversity and Functions. Int. J. Mol. Sci. 2021, 22, 1761. [Google Scholar] [CrossRef] [PubMed]
- Aslanli, A.; Lyagin, I.; Efremenko, E. Novel approach to Quorum Quenching: Rational design of antibacterials in combination with hexahistidine-tagged organophosphorus hydrolase. Biol. Chem. 2018, 399, 869–879. [Google Scholar] [CrossRef]
- Lyagin, I.V.; Efremenko, E.N. Biomolecular engineering of biocatalysts hydrolyzing neurotoxic organophosphates. Biochimie 2018, 144, 115–121. [Google Scholar] [CrossRef]
- Efremenko, E.N.; Lyagin, I.V.; Klyachko, N.L.; Bronich, T.; Zavyalova, N.V.; Jiang, Y.; Kabanov, A.V. A simple and highly effective catalytic nanozyme scavenger for organophosphorus neurotoxins. J. Control. Release 2017, 247, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Vogel, J.; Quax, W.J. Enzymatic Quorum Quenching in Biofilms. In Quorum Sensing: Molecular Mechanism and Biotechnological Application; Tommonaro, G., Ed.; Academic Press: London, UK, 2019; pp. 173–193. [Google Scholar] [CrossRef]
- Efremenko, E.; Votchitseva, Y.; Plieva, F.; Galaev, I.; Mattiasson, B. Purification of His6-organophosphate hydrolase using monolithic supermacroporous polyacrylamide cryogels developed for immobilized metal affinity chromatography. Appl. Microbiol. Biotechnol. 2006, 70, 558–563. [Google Scholar] [CrossRef]
- Votchitseva, Y.A.; Efremenko, E.N.; Aliev, T.K.; Varfolomeyev, S.D. Properties of hexahistidine-tagged organophosphate hydrolase. Biochemistry 2006, 71, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, N.A.; Efremenko, E.N. “Deceived” concentrated immobilized cells as biocatalyst for intensive bacterial cellulose production from various sources. Catalysts 2018, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Aslanli, A.; Stepanov, N.; Razheva, T.; Podorozhko, E.A.; Lyagin, I.; Lozinsky, V.I.; Efremenko, E.N. Enzymatically functionalized composite materials based on nanocellulose and poly(vinyl alcohol) cryogel and possessing antimicrobial activity. Materials 2019, 12, 3619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leont’ev, V.K.; Pogorel’skii, I.P.; Frolov, G.A.; Karasenkov, Y.N.; Gusev, A.A.; Latuta, N.V.; Borozdkin, L.L.; Stefantsova, D.S. Antibacterial Properties of Aqueous Colloid Solutions of Metal and Metal Oxide Nanoparticles against Dental Plaque Bacteria. Nanotechnol. Russ. 2018, 13, 195–198. [Google Scholar] [CrossRef]
- Ismayilov, I.T.; Stepanov, N.A.; Efremenko, E.N.; Abbasov, V.M. Evaluation of biocidal properties of vegetable oil-based corrosion inhibitors using bioluminescent enzymatic method. Mosc. Univ. Chem. Bull. 2015, 70, 197–201. [Google Scholar] [CrossRef]
- Stepanov, N.; Senko, O.; Perminova, I.; Efremenko, E. A new approach to assess the effect of various humic compounds on the metabolic activity of cells participating in methanogenesis. Sustainability 2019, 11, 3158. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [Green Version]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucleic Acids Res. 2007, 35 (Suppl. 2), W522–W525. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and Auto-DockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large Scale, Deep Monitoring and Fine Analytics for the User Community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyagin, I.; Stepanov, N.; Frolov, G.; Efremenko, E. Combined Modification of Fiber Materials by Enzymes and Metal Nanoparticles for Chemical and Biological Protection. Int. J. Mol. Sci. 2022, 23, 1359. https://doi.org/10.3390/ijms23031359
Lyagin I, Stepanov N, Frolov G, Efremenko E. Combined Modification of Fiber Materials by Enzymes and Metal Nanoparticles for Chemical and Biological Protection. International Journal of Molecular Sciences. 2022; 23(3):1359. https://doi.org/10.3390/ijms23031359
Chicago/Turabian StyleLyagin, Ilya, Nikolay Stepanov, George Frolov, and Elena Efremenko. 2022. "Combined Modification of Fiber Materials by Enzymes and Metal Nanoparticles for Chemical and Biological Protection" International Journal of Molecular Sciences 23, no. 3: 1359. https://doi.org/10.3390/ijms23031359
APA StyleLyagin, I., Stepanov, N., Frolov, G., & Efremenko, E. (2022). Combined Modification of Fiber Materials by Enzymes and Metal Nanoparticles for Chemical and Biological Protection. International Journal of Molecular Sciences, 23(3), 1359. https://doi.org/10.3390/ijms23031359





