The Role of Podoplanin in Skin Diseases
Abstract
:1. Introduction
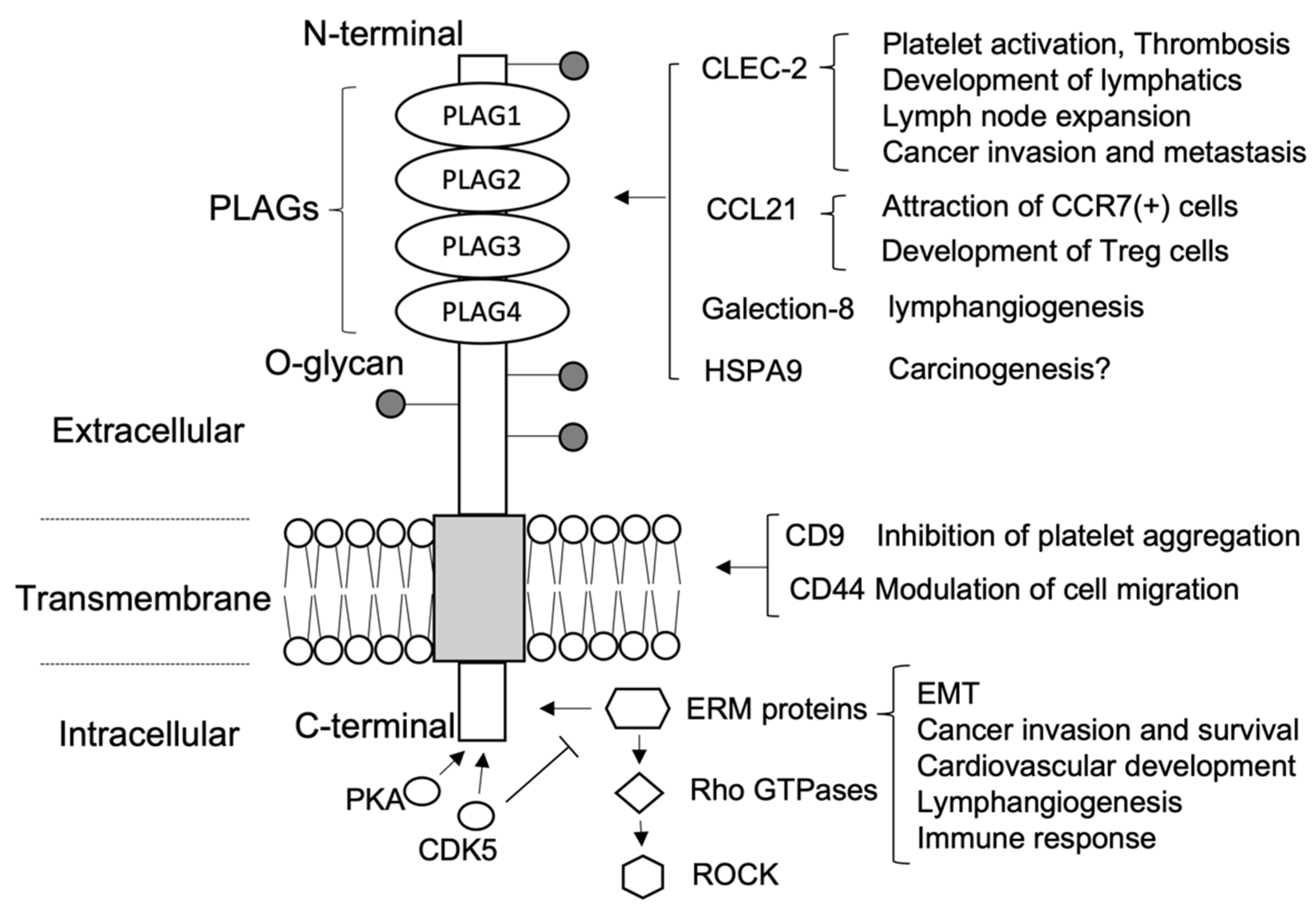
2. Podoplanin Structure and Targeting Agents
2.1. Extracellular Domain
2.2. Transmembrane Domain
2.3. Intracellular Domain
3. Podoplanin in Normal Skin
4. Podoplanin in Inflammatory Skin Diseases
4.1. Psoriasis
4.2. Allergic Contact Dermatitis
5. Podoplanin in Wound Healing
6. Podoplanin in Skin Malignancies
6.1. Melanoma
6.2. Cutaneous SCC
6.3. Extramammary Paget’s Disease (EMPD)
6.4. Mycosis Fungoides and Sezary Syndrome
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Breiteneder-Geleff, S.; Matsui, K.; Soleiman, A.; Meraner, P.; Poczewski, H.; Kalt, R.; Schaffner, G.; Kerjaschki, D. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 1997, 151, 1141–1152. [Google Scholar] [PubMed]
- Quintanilla, M.; Montero-Montero, L.; Renart, J.; Martín-Villar, E. Podoplanin in inflammation and cancer. Int. J. Mol. Sci. 2019, 20, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, M.K.; Kato, Y.; Kitano, T.; Osawa, M. Conservation of a platelet activating domain of Aggrus/podoplanin as a platelet aggregation-inducing factor. Gene 2006, 378, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Martín-Villar, E.; Scholl, F.G.; Gamallo, C.; Yurrita, M.M.; Muñoz-Guerra, M.; Cruces, J.; Quintanilla, M. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int. J. Cancer 2005, 113, 899–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanderbilt, J.N.; Dobbs, L.G. Characterization of the gene and promoter for RTI40, a differentiation marker of type I alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 1998, 19, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Honma, M.; Minami-Hori, M.; Takahashi, H.; Iizuka, H. Podoplanin expression in wound and hyperproliferative psoriatic epidermis: Regulation by TGF-beta and STAT-3 activating cytokines, IFN-γ, IL-6, and IL-22. J. Dermatol. Sci. 2012, 65, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schacht, V.; Ramirez, M.I.; Hong, Y.K.; Hirakawa, S.; Feng, D.; Harvey, N.; Williams, M.; Dvorak, A.M.; Dvorak, H.F.; Oliver, G.; et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003, 22, 3546–3556. [Google Scholar] [CrossRef]
- Mahtab, E.A.; Wijffels, M.C.; Van Den Akker, N.M.; Hahurij, N.D.; Lie-Venema, H.; Wisse, L.J.; Deruiter, M.C.; Uhrin, P.; Zaujec, J.; Binder, B.R.; et al. Cardiac malformations and myocardial abnormalities in podoplanin knockout mouse embryos: Correlation with abnormal epicardial development. Dev. Dyn. 2008, 237, 847–857. [Google Scholar] [CrossRef]
- de Winde, C.M.; Makris, S.; Millward, L.J.; Cantoral-Rebordinos, J.A.; Benjamin, A.C.; Martínez, V.G.; Acton, S.E. Fibroblastic reticular cell response to dendritic cells requires coordinated activity of podoplanin, CD44 and CD9. J. Cell Sci. 2021, 134, jcs258610. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Dieterich, L.C.; Tacconi, C.; Sesartic, M.; He, Y.; Brunner, L.; Kwon, O.; Detmar, M. An important role of podoplanin in hair follicle growth. PLoS ONE 2019, 14, e0219938. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, N.; Inoue, O.; Morimoto, M.; Tatsumi, N.; Nagatomo, H.; Ueta, K.; Shirai, T.; Sasaki, T.; Otake, S.; Tamura, S.; et al. Platelets play an essential role in murine lung development through Clec-2/podoplanin interaction. Blood 2018, 132, 1167–1179. [Google Scholar] [CrossRef] [PubMed]
- Tsukiji, N.; Osada, M.; Sasaki, T.; Shirai, T.; Satoh, K.; Inoue, O.; Umetani, N.; Mochizuki, C.; Saito, T.; Kojima, S.; et al. Cobalt hematoporphyrin inhibits CLEC-2-podoplanin interaction, tumor metastasis, and arterial/venous thrombosis in mice. Blood Adv. 2018, 2, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Shirai, T.; Tsukiji, N.; Otake, S.; Tamura, S.; Ichikawa, J.; Osada, M.; Satoh, K.; Ozaki, Y.; Suzuki-Inoue, K. Functional characterization of recombinant snake venom rhodocytin: Rhodocytin mutant blocks CLEC-2/podoplanin-dependent platelet aggregation and lung metastasis. J. Thromb. Haemost. 2018, 16, 960–972. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Fujita, N.; Kunita, A.; Sato, S.; Kaneko, M.; Osawa, M.; Tsuruo, T. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J. Biol. Chem. 2003, 278, 51599–51605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Núñez, L.; Langan, S.A.; Nash, G.B.; Watson, S.P. The physiological and pathophysiological roles of platelet CLEC-2. Thromb. Haemost. 2013, 109, 991–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki-Inoue, K.; Osada, M.; Ozaki, Y. Physiologic and pathophysiologic roles of interaction between C-type lectin-like receptor 2 and podoplanin: Partners from in utero to adulthood. J. Thromb. Haemost. 2017, 15, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Bertozzi, C.C.; Schmaier, A.A.; Mericko, P.; Hess, P.R.; Zou, Z.; Chen, M.; Chen, C.Y.; Xu, B.; Lu, M.M.; Zhou, D.; et al. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 2010, 116, 661–670. [Google Scholar] [CrossRef] [Green Version]
- Takagi, S.; Sato, S.; Oh-hara, T.; Takami, M.; Koike, S.; Mishima, Y.; Hatake, K.; Fujita, N. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS ONE 2013, 8, e73609. [Google Scholar] [CrossRef]
- Tal, O.; Lim, H.Y.; Gurevich, I.; Milo, I.; Shipony, Z.; Ng, L.G.; Angeli, V.; Shakhar, G. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J. Exp. Med. 2011, 208, 2141–2153. [Google Scholar] [CrossRef] [Green Version]
- Acton, S.E.; Astarita, J.L.; Malhotra, D.; Lukacs-Kornek, V.; Franz, B.; Hess, P.R.; Jakus, Z.; Kuligowski, M.; Fletcher, A.L.; Elpek, K.G.; et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity 2012, 37, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Acton, S.E.; Farrugia, A.J.; Astarita, J.L.; Mourão-Sã, D.; Jenkins, R.P.; Nye, E.; Hooper, S.; van Blijswijk, J.; Rogers, N.C.; Snelgrove, K.J.; et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature 2014, 514, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Tsuneki, M.; Maruyama, S.; Yamazaki, M.; Xu, B.; Essa, A.; Abé, T.; Babkair, H.; Cheng, J.; Yamamoto, T.; Saku, T. Extracellular heat shock protein A9 is a novel interaction partner of podoplanin in oral squamous cell carcinoma cells. BioChem. Biophys. Res. Commun. 2013, 434, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Cueni, L.N.; Detmar, M. Galectin-8 interacts with podoplanin and modulates lymphatic endothelial cell functions. Exp. Cell Res. 2009, 315, 1715–1723. [Google Scholar] [CrossRef] [Green Version]
- Fuertbauer, E.; Zaujec, J.; Uhrin, P.; Raab, I.; Weber, M.; Schachner, H.; Bauer, M.; Schütz, G.J.; Binder, B.R.; Sixt, M.; et al. Thymic medullar conduits-associated podoplanin promotes natural regulatory T cells. Immunol. Lett. 2013, 154, 31–41. [Google Scholar] [CrossRef]
- Tejchman, A.; Lamerant-Fayel, N.; Jacquinet, J.C.; Bielawska-Pohl, A.; Mleczko-Sanecka, K.; Grillon, C.; Chouaib, S.; Ugorski, M.; Kieda, C. Tumor hypoxia modulates podoplanin/CCL21 interactions in CCR7+ NK cell recruitment and CCR7+ tumor cell mobilization. Oncotarget 2017, 8, 31876–31887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerman, K.; Tardiveau, C.; Martins, F.; Coudert, J.; Girard, J.P. Single-cell analysis reveals heterogeneity of high endothelial venules and different regulation of genes controlling lymphocyte entry to lymph nodes. Cell Rep. 2019, 26, 3116–3131.e5. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, Y.; Sato, S.; Naito, M.; Kato, Y.; Mishima, K.; Arai, H.; Tsuruo, T.; Fujita, N. Tetraspanin family member CD9 inhibits Aggrus/podoplanin-induced platelet aggregation and suppresses pulmonary metastasis. Blood 2008, 112, 1730–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Villar, E.; Fernández-Munñz, B.; Parsons, M.; Yurrita, M.M.; Megías, D.; Pérez-Gómez, E.; Jones, G.E.; Quintanilla, M. Podoplanin associates with CD44 to promote directional cell migration. Mol. Biol. Cell 2010, 21, 4387–4399. [Google Scholar] [CrossRef] [Green Version]
- Marhaba, R.; Zöller, M. CD44 in cancer progression: Adhesion, migration and growth regulation. J. Mol. Histol. 2004, 35, 211–231. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef] [Green Version]
- Yin, T.; Wang, G.; He, S.; Liu, Q.; Sun, J.; Wang, Y. Human cancer cells with stem cell-like phenotype exhibit enhanced sensitivity to the cytotoxicity of IL-2 and IL-15 activated natural killer cells. Cell Immunol. 2016, 300, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Montero-Montero, L.; Renart, J.; Ramírez, A.; Ramos, C.; Shamhood, M.; Jarcovsky, R.; Quintanilla, M.; Martin-Villar, E. Interplay between podoplanin, CD44s and CD44v in squamous carcinoma cells. Cells 2020, 9, 2200. [Google Scholar] [CrossRef] [PubMed]
- Renart, J.; Carrasco-Ramírez, P.; Fernández-Muñoz, B.; Martín-Villar, E.; Montero, L.; Yurrita, M.M.; Quintanilla, M. New insights into the role of podoplanin in epithelial-mesenchymal transition. Int. Rev. Cell Mol. Biol. 2015, 317, 185–239. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.; Rayes, J.; Miyashita, T.; Ishii, G.; Retzbach, E.P.; Sheehan, S.A.; Takemoto, A.; Chang, Y.W.; Yoneda, K.; Asai, J.; et al. Podoplanin: An emerging cancer biomarker and therapeutic target. Cancer Sci. 2018, 109, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.; Retzbach, E.P.; Ramirez, M.I.; Liu, T.; Li, H.; Miller, W.T.; Goldberg, G.S. PKA and CDK5 can phosphorylate specific serines on the intracellular domain of podoplanin (PDPN) to inhibit cell motility. Exp. Cell Res. 2015, 335, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoh, Y.; Hoffman, R.M. Hair follicle-associated-pluripotent (HAP) stem cells. Cell Cycle 2017, 16, 2169–2175. [Google Scholar] [CrossRef] [Green Version]
- Williams, R.; Westgate, G.E.; Pawlus, A.D.; Sikkink, S.K.; Thornton, M.J. Age-related changes in female scalp dermal sheath and dermal fibroblasts: How the hair follicle environment impacts hair aging. J. Investig. Dermatol. 2021, 141, 1041–1051. [Google Scholar] [CrossRef]
- Bianchi, R.; Russo, E.; Bachmann, S.B.; Proulx, S.T.; Sesartic, M.; Smaadahl, N.; Watson, S.P.; Buckley, C.D.; Halin, C.; Detmar, M. Postnatal deletion of podoplanin in lymphatic endothelium results in blood filling of the lymphatic system and impairs dendritic cell migration to lymph nodes. Arter. Thromb. Vasc. Biol. 2017, 37, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Boehncke, W.H.; Schön, M.P. Psoriasis. Lancet 2015, 386, 983–994. [Google Scholar] [CrossRef]
- Amoruso, G.F.; Nisticò, S.P.; Iannone, L.; Russo, E.; Rago, G.; Patruno, C.; Bennardo, L. Ixekizumab may improve renal function in Psoriasis. Healthcare 2021, 9, 543. [Google Scholar] [CrossRef]
- Passante, M.; Dastoli, S.; Nisticò, S.P.; Bennardo, L.; Patruno, C. Effectiveness of brodalumab in acrodermatitis continua of Hallopeau: A case report. Dermatol. Ther. 2020, 33, e13170. [Google Scholar] [CrossRef] [PubMed]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noack, M.; Ndongo-Thiam, N.; Miossec, P. Role of podoplanin in the high interleukin-17A secretion resulting from interactions between activated lymphocytes and psoriatic skin-derived mesenchymal cells. Clin. Exp. Immunol. 2016, 186, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honma, M.; Fujii, M.; Iinuma, S.; Minami-Hori, M.; Takahashi, H.; Ishida-Yamamoto, A.; Iizuka, H. Podoplanin expression is inversely correlated with granular layer/filaggrin formation in psoriatic epidermis. J. Dermatol. 2013, 40, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Shibuya, T.; Hayashi, K.; Iinuma, S.; Fujii, M.; Ishida-Yamamoto, A. Suppression of podoplanin expression during differentiation of epidermal keratinocytes. J. Dermatol. 2019, 46, 922–924. [Google Scholar] [CrossRef]
- Shibuya, T.; Honma, M.; Fujii, M.; Iinuma, S.; Ishida-Yamamoto, A. Podoplanin suppresses the cell adhesion of epidermal keratinocytes via functional regulation of beta1-integrin. Arch. Dermatol. Res. 2019, 311, 45–53. [Google Scholar] [CrossRef]
- Nylander, A.N.; Ponath, G.D.; Axisa, P.P.; Mubarak, M.; Tomayko, M.; Kuchroo, V.K.; Pitt, D.; Hafler, D.A. Podoplanin is a negative regulator of Th17 inflammation. JCI Insight 2017, 2, e92321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, G.; Anumanthan, A.; Brown, J.A.; Greenfield, E.A.; Zhu, B.; Freeman, G.J. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat. Immunol. 2008, 9, 176–185. [Google Scholar] [CrossRef]
- Honda, T.; Egawa, G.; Grabbe, S.; Kabashima, K. Update of immune events in the murine contact hypersensitivity model: Toward the understanding of allergic contact dermatitis. J. Investig. Dermatol. 2013, 133, 303–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astarita, J.L.; Cremasco, V.; Fu, J.; Darnell, M.C.; Peck, J.R.; Nieves-Bonilla, J.M.; Song, K.; Kondo, Y.; Woodruff, M.C.; Gogineni, A.; et al. The CLEC-2-podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat. Immunol. 2015, 16, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Bajenoff, M.; Egen, J.G.; Koo, L.Y.; Laugier, J.P.; Brau, F.; Glaichenhaus, N.; Germain, R.N. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 2006, 25, 989–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Link, A.; Vogt, T.K.; Favre, S.; Britschgi, M.R.; Acha-Orbea, H.; Hinz, B.; Cyster, J.G.; Luther, S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, D.; Fletcher, A.L.; Astarita, J.; Lukacs-Kornek, V.; Tayalia, P.; Gonzalez, S.F.; Elpek, K.G.; Chang, S.K.; Knoblich, K.; Hemler, M.E.; et al. Immunological Genome Project Consortium. Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat. Immunol. 2012, 13, 499–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sixt, M.; Kanazawa, N.; Selg, M.; Samson, T.; Roos, G.; Reinhardt, D.P.; Pabst, R.; Lutz, M.B.; Sorokin, L. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 2005, 22, 19–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asai, J.; Hirakawa, S.; Sakabe, J.; Kishida, T.; Wada, M.; Nakamura, N.; Takenaka, H.; Mazda, O.; Urano, T.; Suzuki-Inoue, K.; et al. Platelets regulate the migration of keratinocytes via podoplanin/CLEC-2 signaling during cutaneous wound healing in mice. Am. J. Pathol. 2016, 186, 101–108. [Google Scholar] [CrossRef]
- Asai, J.; Takenaka, H.; Hirakawa, S.; Sakabe, J.; Hagura, A.; Kishimoto, S.; Maruyama, K.; Kajiya, K.; Kinoshita, S.; Tokura, Y.; et al. Topical simvastatin accelerates wound healing in diabetes by enhancing angiogenesis and lymphangiogenesis. Am. J. Pathol. 2012, 181, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Asai, J.; Ii, M.; Thorne, T.; Losordo, D.W.; D’Amore, P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 2007, 170, 1178–1191. [Google Scholar] [CrossRef] [Green Version]
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Maruyama, Y.; Maruyama, K.; Kato, Y.; Kajiya, K.; Moritoh, S.; Yamamoto, K.; Matsumoto, Y.; Sawane, M.; Kerjaschki, D.; Nakazawa, T.; et al. The effect of podoplanin inhibition on lymphangiogenesis under pathological conditions. Investig. OphthalMol. Vis. Sci. 2014, 55, 4813–4822. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Nicolls, M.R.; Tian, W.; Rockson, S.G. Lymphatic dysfunction, leukotrienes, and lymphedema. Annu. Rev. Physiol. 2018, 80, 49–70. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, T.; He, Z. ADSCs enhance VEGFR3-mediated lymphangiogenesis via METTL3-mediated VEGF-C m(6)A modification to improve wound healing of diabetic foot ulcers. Mol. Med. 2021, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Plaza, J.A.; Ortega, P.F.; Bengana, C.; Stockman, D.L.; Suster, S. Immunolabeling pattern of podoplanin (d2-40) may distinguish basal cell carcinomas from trichoepitheliomas: A clinicopathologic and immunohistochemical study of 49 cases. Am. J. DermatoPathol. 2010, 32, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Neinaa, Y.M.E.; El-Ashmawy, A.A.; Alshenawy, H.A.; Dorgham, W.L. The prognostic value of podoplanin expression in nonmelanoma skin cancers: Correlation with lymphatic vessel density. Am. J. DermatoPathol. 2020, 42, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.; Konishi, E.; Arita, T.; Ikemoto, C.; Takenaka, H.; Yanagisawa, A.; Katoh, N.; Asai, J. Podoplanin expression in cancer-associated fibroblasts predicts aggressive behavior in melanoma. J. Cutan. Pathol. 2014, 41, 561–567. [Google Scholar] [CrossRef]
- Cho, Z.; Konishi, E.; Kanemaru, M.; Isohisa, T.; Arita, T.; Kawai, M.; Tsutsumi, M.; Mizutani, H.; Takenaka, H.; Ozawa, T.; et al. Podoplanin expression in peritumoral keratinocytes predicts aggressive behavior in extramammary Paget’s disease. J. Dermatol. Sci. 2017, 87, 29–35. [Google Scholar] [CrossRef]
- Gülseren, D.; Gököz, Ö.; Karahan, S.; Karaduman, A. Podoplanin expression in cutaneous squamous cell carcinomas and its relationship to histopathological prognostic factors. J. Histotechnol. 2020, 43, 147–152. [Google Scholar] [CrossRef]
- Lobl, M.; Grinnell, M.; Phillips, A.; Abels, J.; Wysong, A. The correlation between immunohistochemistry findings and metastasis in squamous cell carcinoma: A review. Dermatol. Surg. 2021, 47, 313–318. [Google Scholar] [CrossRef]
- Sasaki, K.; Sugai, T.; Ishida, K.; Osakabe, M.; Amano, H.; Kimura, H.; Sakuraba, M.; Kashiwa, K.; Kobayashi, S. Analysis of cancer-associated fibroblasts and the epithelial-mesenchymal transition in cutaneous basal cell carcinoma, squamous cell carcinoma, and malignant melanoma. Hum. Pathol. 2018, 79, 1–8. [Google Scholar] [CrossRef]
- El-Ashmawy, A.A.; Shamloula, M.M.; Elfar, N.N. Podoplanin as a predictive marker for identification of high-risk mycosis fungoides patients: An immunohistochemical study. Indian J. Derm. 2020, 65, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Temam, S.; El-Naggar, A.; Zhou, X.; Liu, D.D.; Lee, J.J.; Mao, L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer 2006, 107, 563–569. [Google Scholar] [CrossRef]
- Dumoff, K.L.; Chu, C.; Xu, X.; Pasha, T.; Zhang, P.J.; Acs, G. Low D2-40 immunoreactivity correlates with lymphatic invasion and nodal metastasis in early-stage squamous cell carcinoma of the uterine cervix. Mod. Pathol. 2005, 18, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Ishii, G.; Nagai, K.; Nagano, T.; Kojika, M.; Murata, Y.; Atsumi, N.; Nishiwaki, Y.; Miyazaki, E.; Kumamoto, T.; et al. Low podoplanin expression of tumor cells predicts poor prognosis in pathological stage IB squamous cell carcinoma of the lung, tissue microarray analysis of 136 patients using 24 antibodies. Lung Cancer 2009, 63, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Ishii, G.; Nagai, K.; Atsumi, N.; Fujii, S.; Yamada, A.; Yamane, Y.; Hishida, T.; Nishimura, M.; Yoshida, J.; et al. Expression of podoplanin, CD44, and p63 in squamous cell carcinoma of the lung. Cancer Sci. 2009, 100, 2054–2059. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Onimaru, M.; Koga, T.; Takeshita, M.; Yano, T.; Maehara, Y.; Nakamura, S.; Sueishi, K. High podoplanin expression in cancer cells predicts lower incidence of nodal metastasis in patients with lung squamous cell carcinoma. Pathol. Res. Pract. 2011, 207, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Ishii, G.; Ito, T.; Aoyagi, K.; Ohtaki, Y.; Nagai, K.; Sasaki, H.; Ochiai, A. Podoplanin-positive fibroblasts enhance lung adenocarcinoma tumor formation: Podoplanin in fibroblast functions for tumor progression. Cancer Res. 2011, 71, 4769–4779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Ishii, G.; Hoshino, A.; Hashimoto, H.; Neri, S.; Kuwata, T.; Higashi, M.; Nagai, K.; Ochiai, A. Tumor promoting effect of podoplanin-positive fibroblasts is mediated by enhanced RhoA activity. BioChem. Biophys. Res. Commun. 2012, 422, 194–199. [Google Scholar] [CrossRef]
- Luanpitpong, S.; Wang, L.; Castranova, V.; Dinu, C.Z.; Issaragrisil, S.; Chen, Y.C.; Rojanasakul, Y. Induction of cancer-associated fibroblast-like cells by carbon nanotubes dictates its tumorigenicity. Sci. Rep. 2016, 6, 39558. [Google Scholar] [CrossRef]
- Miyata, K.; Takemoto, A.; Okumura, S.; Nishio, M.; Fujita, N. Podoplanin enhances lung cancer cell growth in vivo by inducing platelet aggregation. Sci. Rep. 2017, 7, 4059. [Google Scholar] [CrossRef] [Green Version]
- Scholl, F.G.; Gamallo, C.; Vilaró, S.; Quintanilla, M. Identification of PA2.26 antigen as a novel cell-surface mucin-type glycoprotein that induces plasma membrane extensions and increased motility in keratinocytes. J. Cell Sci. 1999, 112, 4601–4613. [Google Scholar] [CrossRef]
- Martin-Villar, E.; Borda-D’Agua, B.; Carrasco-Ramirez, P.; Renart, J.; Parsons, M.; Quintanilla, M.; Jones, G.E. Podoplanin mediates ECM degradation by squamous carcinoma cells through control of invadopodia stability. Oncogene 2015, 34, 4531–4544. [Google Scholar] [CrossRef] [Green Version]
- Suchanski, J.; Tejchman, A.; Zacharski, M.; Piotrowska, A.; Grzegrzolka, J.; Chodaczek, G.; Nowinska, K.; Rys, J.; Dziegiel, P.; Kieda, C.; et al. Podoplanin increases the migration of human fibroblasts and affects the endothelial cell network formation: A possible role for cancer-associated fibroblasts in breast cancer progression. PLoS ONE 2017, 12, e0184970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takemoto, A.; Okitaka, M.; Takagi, S.; Takami, M.; Sato, S.; Nishio, M.; Okumura, S.; Fujita, N. A critical role of platelet TGF-beta release in podoplanin-mediated tumour invasion and metastasis. Sci. Rep. 2017, 7, 42186. [Google Scholar] [CrossRef] [PubMed]
- Mir Seyed Nazari, P.; Riedl, J.; Pabinger, I.; Ay, C. The role of podoplanin in cancer-associated thrombosis. Thromb. Res. 2018, 164 (Suppl. 1), S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Inoue, K.; Tsukiji, N.; Shirai, T.; Osada, M.; Inoue, O.; Ozaki, Y. Platelet CLEC-2: Roles beyond hemostasis. Semin. Thromb. Hemost. 2018, 44, 126–134. [Google Scholar] [CrossRef]
- Takemoto, A.; Miyata, K.; Fujita, N. Platelet-activating factor podoplanin: From discovery to drug development. Cancer Metastasis Rev. 2017, 36, 225–234. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Takemoto, A.; Takagi, S.; Takatori, K.; Sato, S.; Takami, M.; Fujita, N. Targeting a novel domain in podoplanin for inhibiting platelet-mediated tumor metastasis. Oncotarget 2016, 7, 3934–3946. [Google Scholar] [CrossRef] [Green Version]
- Suzuki-Inoue, K. Platelets and cancer-associated thrombosis: Focusing on the platelet activation receptor CLEC-2 and podoplanin. Blood 2019, 134, 1912–1918. [Google Scholar] [CrossRef]
- Yoshida, T.; Ishii, G.; Goto, K.; Neri, S.; Hashimoto, H.; Yoh, K.; Niho, S.; Umemura, S.; Matsumoto, S.; Ohmatsu, H.; et al. Podoplanin-positive cancer-associated fibroblasts in the tumor microenvironment induce primary resistance to EGFR-TKIs in lung adenocarcinoma with EGFR mutation. Clin. Cancer Res. 2015, 21, 642–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nisticò, S. Primary mucosal melanoma presenting with a unilateral nasal obstruction of the left inferior turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Yoshikawa, S.; Minagawa, A.; Takenouchi, T.; Yokota, K.; Uchi, H.; Noma, N.; Nakamura, Y.; Asai, J.; Kato, J.; et al. Clinical and histopathological characteristics and survival analysis of 4594 Japanese patients with melanoma. Cancer Med. 2019, 8, 2146–2156. [Google Scholar] [CrossRef]
- Ochoa-Alvarez, J.A.; Krishnan, H.; Shen, Y.; Acharya, N.K.; Han, M.; McNulty, D.E.; Hasegawa, H.; Hyodo, T.; Senga, T.; Geng, J.G.; et al. Plant lectin can target receptors containing sialic acid, exemplified by podoplanin, to inhibit transformed cell growth and migration. PLoS ONE 2012, 7, e41845. [Google Scholar] [CrossRef] [PubMed]
- Shinada, M.; Kato, D.; Kamoto, S.; Yoshimoto, S.; Tsuboi, M.; Yoshitake, R.; Eto, S.; Ikeda, N.; Saeki, K.; Hashimoto, Y.; et al. PDPN is expressed in various types of canine tumors and its silencing induces apoptosis and cell cycle arrest in canine malignant melanoma. Cells 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, M.K.; Nakamura, T.; Kunita, A.; Fukayama, M.; Abe, S.; Nishioka, Y.; Yamada, S.; Yanaka, M.; Saidoh, N.; Yoshida, K.; et al. ChLpMab-23: Cancer-specific human-mouse chimeric anti-podoplanin antibody exhibits antitumor activity via antibody-dependent cellular cytotoxicity. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Kunita, A.; Fukayama, M.; Abe, S.; Nishioka, Y.; Uchida, H.; Tahara, H.; Yamada, S.; Yanaka, M.; Nakamura, T.; et al. Antiglycopeptide mouse monoclonal antibody LpMab-21 exerts antitumor activity against human podoplanin through antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 20–24. [Google Scholar] [CrossRef]
- Kaneko, M.K.; Yamada, S.; Nakamura, T.; Abe, S.; Nishioka, Y.; Kunita, A.; Fukayama, M.; Fujii, Y.; Ogasawara, S.; Kato, Y. Antitumor activity of chLpMab-2, a human-mouse chimeric cancer-specific antihuman podoplanin antibody, via antibody-dependent cellular cytotoxicity. Cancer Med. 2017, 6, 768–777. [Google Scholar] [CrossRef]
- Yamada, S.; Ogasawara, S.; Kaneko, M.K.; Kato, Y. LpMab-23: A cancer-specific monoclonal antibody against human podoplanin. Monoclon. Antib. Immunodiagn. Immunother. 2017, 36, 72–76. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Pan, Y.; Zhao, X.; Yan, B.; Ruan, C.; Xia, L.; Zhao, Y. Blocking podoplanin suppresses growth and pulmonary metastasis of human malignant melanoma. BMC Cancer 2019, 19, 599. [Google Scholar] [CrossRef]
- Kamoto, S.; Shinada, M.; Kato, D.; Yoshimoto, S.; Ikeda, N.; Tsuboi, M.; Yoshitake, R.; Eto, S.; Hashimoto, Y.; Takahashi, Y.; et al. Phase I/II clinical trial of the anti-podoplanin monoclonal antibody therapy in dogs with malignant melanoma. Cells 2020, 9, 2529. [Google Scholar] [CrossRef]
- Schacht, V.; Dadras, S.S.; Johnson, L.A.; Jackson, D.G.; Hong, Y.K.; Detmar, M. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am. J. Pathol. 2005, 166, 913–921. [Google Scholar] [CrossRef] [Green Version]
- De Vinicius, L.V.; Scapulatempo, C.; Perpetuo, N.M.; Mohamed, F.; de Carvalho, T.S.; de Oliveira, A.T.; Segalla, J.G.; Carvalho, A.L. Prognostic and risk factors in patients with locally advanced cutaneous squamous cell carcinoma of the trunk and extremities. J. Ski. Cancer 2011, 2011, 420796. [Google Scholar] [CrossRef]
- Cañueto, J.; Cardeñoso-Álvarez, E.; Cosano-Quero, A.; Santos-Briz, Á.; Fernández-López, E.; Pérez-Losada, J.; Román-Curto, C. The expression of podoplanin is associated with poor outcome in cutaneous squamous cell carcinoma. J. Cutan. Pathol. 2017, 44, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska-Zdrojowy, M.; Szepietowski, J.C.; Matusiak, Ł.; Dzięgiel, P.; Puła, B. Expression of podoplanin in non-melanoma skin cancers and actinic keratosis. Anticancer Res. 2016, 36, 1591–1597. [Google Scholar] [PubMed]
- Martín-Villar, E.; Megías, D.; Castel, S.; Yurrita, M.M.; Vilaró, S.; Quintanilla, M. Podoplanin binds ERM proteins to activate RhoA and promote epithelial-mesenchymal transition. J. Cell Sci. 2006, 119, 4541–4553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scholl, F.G.; Gamallo, C.; Quintanilla, M. Ectopic expression of PA2.26 antigen in epidermal keratinocytes leads to destabilization of adherens junctions and malignant progression. Lab. Investig. 2000, 80, 1749–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sesartić, M.; Ikenberg, K.; Yoon, S.Y.; Detmar, M. Keratinocyte-expressed podoplanin is dispensable for multi-step skin carcinogenesis. Cells 2020, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Eddy, R.J.; Weidmann, M.D.; Sharma, V.P.; Condeelis, J.S. Tumor cell invadopodia: Invasive protrusions that orchestrate metastasis. Trends Cell Biol. 2017, 27, 595–607. [Google Scholar] [CrossRef]
- Arita, T.; Kondo, J.; Kaneko, Y.; Tsutsumi, M.; Kanemaru, M.; Matsui, M.; Arakawa, Y.; Katoh, N.; Inoue, M.; Asai, J. Novel ex vivo disease model for extramammary Paget’s disease using the cancer tissue-originated spheroid method. J. Dermatol. Sci. 2020, 99, 185–192. [Google Scholar] [CrossRef]
- Fujii, K.; Hamada, T.; Shimauchi, T.; Asai, J.; Fujisawa, Y.; Ihn, H.; Katoh, N. Cutaneous lymphoma in Japan, 2012-2017: A nationwide study. J. Derm. Sci. 2020, 97, 187–193. [Google Scholar] [CrossRef]
- Jankowska-Konsur, A.; Kobierzycki, C.; Grzegrzółka, J.; Piotrowska, A.; Gomulkiewicz, A.; Glatzel-Plucinska, N.; Reich, A.; Podhorska-Okołów, M.; Dzięgiel, P.; Szepietowski, J.C. Podoplanin expression correlates with disease progression in mycosis fungoides. Acta Derm. Venereol. 2017, 97, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Karpova, M.B.; Fujii, K.; Jenni, D.; Dummer, R.; Urosevic-Maiwald, M. Evaluation of lymphangiogenic markers in Sezary syndrome. Leuk. Lymphoma 2011, 52, 491–501. [Google Scholar] [CrossRef]
| Disease | Cell Type | Role |
|---|---|---|
| Psoriasis | Keratinocytes | Epidermal elongation |
| Th17 cells | IL-17 ↓ | |
| Monocytes | IL-17 ↑ | |
| Allergic contact dermatitis | LECs | Mediate migration of DCs and LCs |
| FRCs | Lymph node expansion | |
| Wound healing | LECs | Lymphangiogenesis |
| Keratinocytes | Re-epithelialization | |
| Melanoma | CAFs | Biomarker |
| Tumor cells | amoeboid invasion and dedifferentiation | |
| cSCC | Tumor cells | EMT, invadopodia |
| EMPD | Keratinocytes | Invadopodia |
| Mycosis fungoides | Basal cell layer Malignant lymphocytes LECs | lymphangiogenesis |
| Sézary syndrome | LECs | lymphangiogenesis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asai, J. The Role of Podoplanin in Skin Diseases. Int. J. Mol. Sci. 2022, 23, 1310. https://doi.org/10.3390/ijms23031310
Asai J. The Role of Podoplanin in Skin Diseases. International Journal of Molecular Sciences. 2022; 23(3):1310. https://doi.org/10.3390/ijms23031310
Chicago/Turabian StyleAsai, Jun. 2022. "The Role of Podoplanin in Skin Diseases" International Journal of Molecular Sciences 23, no. 3: 1310. https://doi.org/10.3390/ijms23031310
APA StyleAsai, J. (2022). The Role of Podoplanin in Skin Diseases. International Journal of Molecular Sciences, 23(3), 1310. https://doi.org/10.3390/ijms23031310






