Vesicle Fusion as a Target Process for the Action of Sphingosine and Its Derived Drugs
Abstract
:1. Lipids and Exocytosis
2. The Direct Interaction between Sphingolipids and SNAREs
3. Sphingolipids Alter the Single Fusion Properties of Neurotransmitter Release
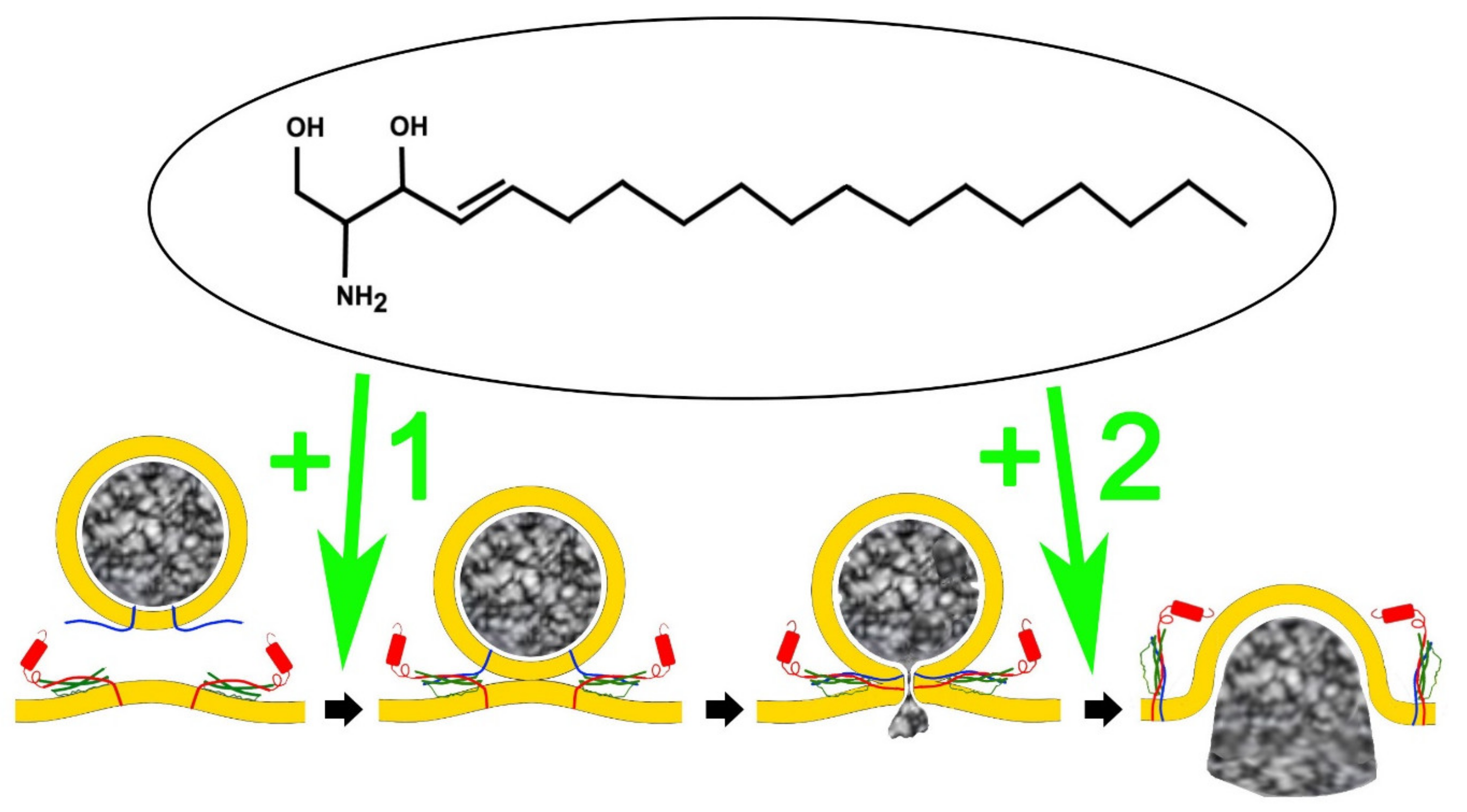
4. An Analog of Sphingosine, FTY-720 (Fingolimod) Mimics Signalling Lipid Potentiation of Exocytosis
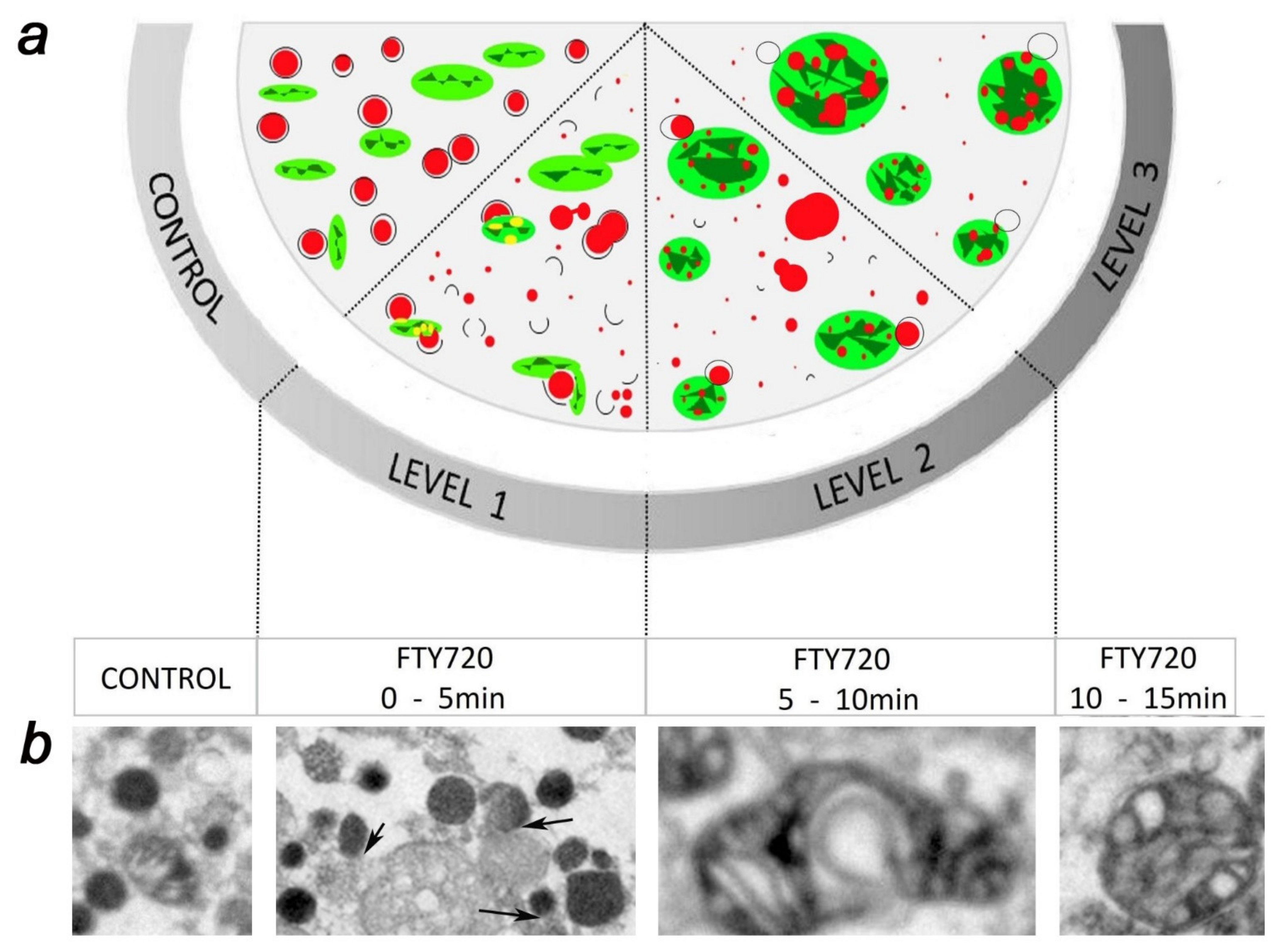
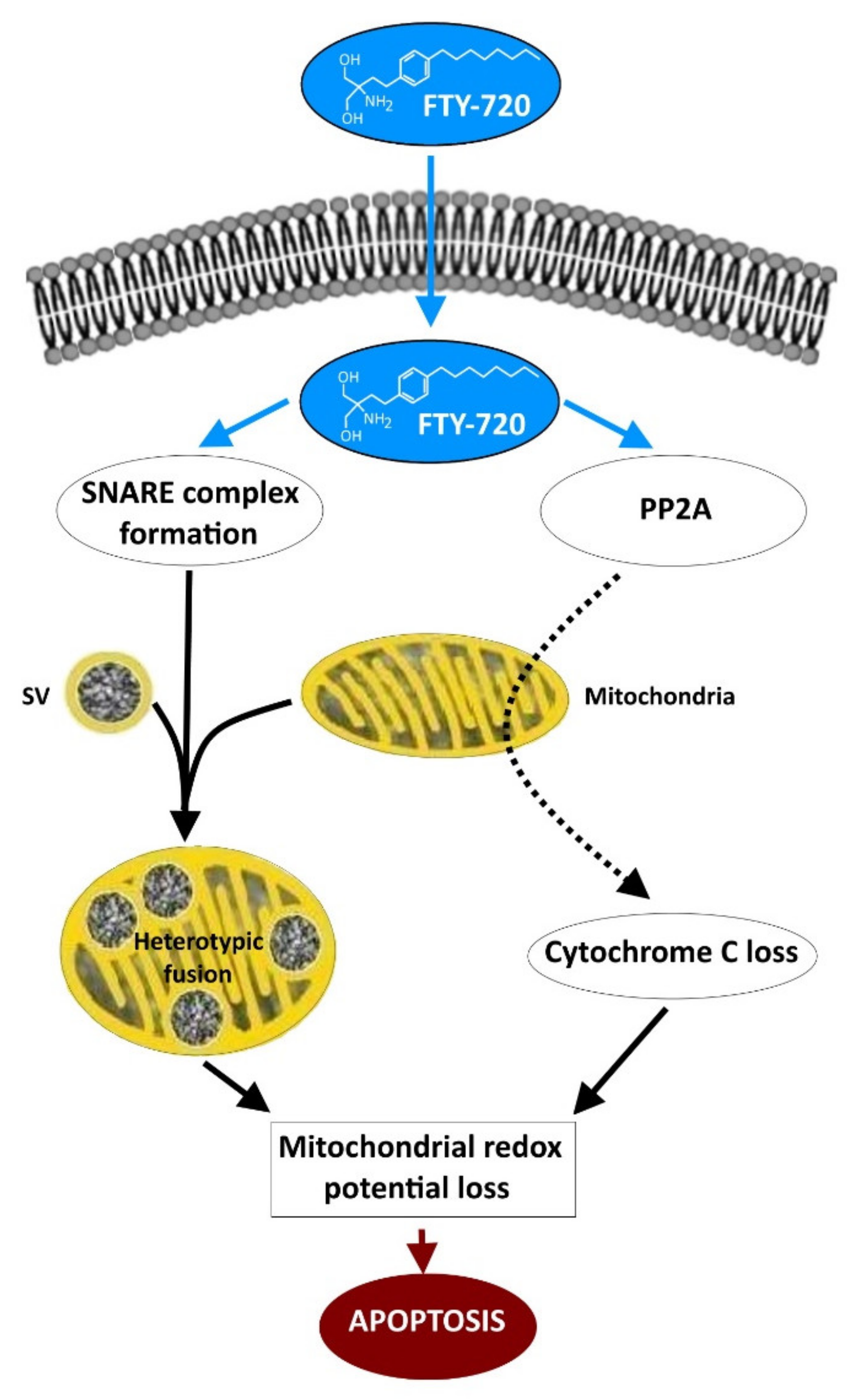
5. FTY-720 Induces the Heterotypic Fusion of Organelles
6. The Potential Use of FTY-720 in the Treatment of Neuron-Related Syndromes and Cancers
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Almers, W. Exocytosis. Annu. Rev. Physiol. 1990, 52, 607–624. [Google Scholar] [CrossRef]
- Betz, W.J.; Angleson, J.K. The synaptic vesicle cycle. Annu. Rev. Physiol. 1998, 60, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Jahn, R.; Lang, T.; Sudhof, T.C. Membrane fusion. Cell 2003, 112, 519–533. [Google Scholar] [CrossRef] [Green Version]
- Chanaday, N.L.; Cousin, M.A.; Milosevic, I.; Watanabe, S.; Morgan, J.R. The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms. J. Neurosci. 2019, 39, 8209–8216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trifaro, J.M.; Gasman, S.; Gutierrez, L.M. Cytoskeletal control of vesicle transport and exocytosis in chromaffin cells. Acta Physiol. 2008, 192, 165–172. [Google Scholar] [CrossRef]
- Gutierrez, L.M. New insights into the role of the cortical cytoskeleton in exocytosis from neuroendocrine cells. Int. Rev. Cell. Mol. Biol. 2012, 295, 109–137. [Google Scholar] [PubMed]
- James, D.J.; Martin, T.F. CAPS and Munc13: CATCHRs that SNARE Vesicles. Front. Endocrinol. 2013, 4, 187. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Tran, V.; Marty, A. Calcium-dependent docking of synaptic vesicles. Trends Neurosci. 2021, 44, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Gundersen, C.B. Fast, synchronous neurotransmitter release: Past, present and future. Neuroscience 2020, 439, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Anantharam, A.; Kreutzberger, A.J.B. Unraveling the mechanisms of calcium-dependent secretion. J. Gen. Physiol. 2019, 151, 417–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, M.K.; Scheller, R.H. Molecular correlates of synaptic vesicle docking and fusion. Curr. Opin. Neurobiol. 1994, 4, 324–329. [Google Scholar] [CrossRef]
- Burgoyne, R.D.; Morgan, A. Analysis of regulated exocytosis in adrenal chromaffin cells: Insights into NSF/SNAP/SNARE function. BioEssays 1998, 20, 328–335. [Google Scholar] [CrossRef]
- Zhang, Y.; Hughson, F.M. Chaperoning SNARE Folding and Assembly. Annu. Rev. Biochem. 2021, 90, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Xiao, H. Mechanism of membrane fusion: Protein-protein interaction and beyond. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 250–257. [Google Scholar]
- Pan, C.Y.; Wu, A.Z.; Chen, Y.T. Lysophospholipids regulate excitability and exocytosis in cultured bovine chromaffin cells. J Neurochem. 2007, 102, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Arbault, S.; Bouret, Y.; Guille, M.; Lemaitre, F.; Verchier, Y. Regulation of exocytosis in chromaffin cells by trans-insertion of lysophosphatidylcholine and arachidonic acid into the outer leaflet of the cell membrane. ChemBioChem 2006, 7, 1998–2003. [Google Scholar] [CrossRef]
- Eberhard, D.A.; Cooper, C.L.; Low, M.G.; Holz, R.W. Evidence that the inositol phospholipids are necessary for exocytosis. Loss of inositol phospholipids and inhibition of secretion in permeabilized cells caused by a bacterial phospholipase C and removal of ATP. Biochem. J. 1990, 268, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyagi, K.; Sugaya, T.; Umeda, M.; Yamamoto, S.; Terakawa, S.; Takahashi, M. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J. Biol. Chem. 2005, 280, 17346–17352. [Google Scholar] [CrossRef] [Green Version]
- Eberhard, D.A.; Holz, R.W. Calcium promotes the accumulation of polyphosphoinositides in intact and permeabilized bovine adrenal chromaffin cells. Cell. Mol. Neurobiol. 1991, 11, 357–370. [Google Scholar] [CrossRef] [Green Version]
- Wen, P.J.; Osborne, S.L.; Zanin, M.; Low, P.C.; Wang, H.T.; Schoenwaelder, S.M.; Jackson, S.P.; Wedlich-Soldner, R.; Vanhaesebroeck, B.; Keating, D.J.; et al. Phosphatidylinositol(4,5)bisphosphate coordinates actin-mediated mobilization and translocation of secretory vesicles to the plasma membrane of chromaffin cells. Nat. Commun. 2011, 2, 491. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Bruns, D.; Wenzel, D.; Riedel, D.; Holroyd, P.; Thiele, C.; Jahn, R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001, 20, 2202–2213. [Google Scholar] [CrossRef] [Green Version]
- Hess, D.T.; Slater, T.M.; Wilson, M.C.; Skene, J.H. The 25 kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J. Neurosci. 1992, 12, 4634–4641. [Google Scholar] [CrossRef] [Green Version]
- Veit, M.; Sollner, T.H.; Rothman, J.E. Multiple palmitoylation of synaptotagmin and the t-SNARE SNAP-25. FEBS Lett. 1996, 385, 119–123. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo, S.; Linder, M.E. SNAP-25 palmitoylation and plasma membrane targeting require a functional secretory pathway. Mol. Biol. Cell. 1998, 9, 585–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Washbourne, P.; Cansino, V.; Mathews, J.R.; Graham, M.; Burgoyne, R.D.; Wilson, M.C. Cysteine residues of SNAP-25 are required for SNARE disassembly and exocytosis, but not for membrane targeting. Biochem. J. 2001, 357, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Baekkeskov, S.; Kanaani, J. Palmitoylation cycles and regulation of protein function (Review). Mol. Membr. Biol. 2009, 26, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Veit, M.; Becher, A.; Ahnert-Hilger, G. Synaptobrevin 2 is palmitoylated in synaptic vesicles prepared from adult, but not from embryonic brain. Mol. Cell. Neurosci. 2000, 15, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Chen, X.; Arac, D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006, 16, 339–350. [Google Scholar] [CrossRef]
- Chamberlain, L.H.; Burgoyne, R.D. Cysteine-string protein: The chaperone at the synapse. J. Neurochem. 2000, 74, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Evans, G.J.; Morgan, A.; Burgoyne, R.D. Tying everything together: The multiple roles of cysteine string protein (CSP) in regulated exocytosis. Traffic 2003, 4, 653–659. [Google Scholar] [CrossRef]
- Graham, M.E.; Burgoyne, R.D. Comparison of cysteine string protein (Csp) and mutant alpha-SNAP overexpression reveals a role for csp in late steps of membrane fusion in dense-core granule exocytosis in adrenal chromaffin cells. J. Neurosci. 2000, 20, 1281–1289. [Google Scholar] [CrossRef] [Green Version]
- Balsinde, J.; Winstead, M.V.; Dennis, E.A. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett. 2002, 531, 2–6. [Google Scholar] [CrossRef]
- Winstead, M.V.; Balsinde, J.; Dennis, E.A. Calcium-independent phospholipase A(2): Structure and function. Biochim. Biophys. Acta 2000, 1488, 28–39. [Google Scholar] [CrossRef]
- Darios, F.; Wasser, C.; Shakirzyanova, A.; Giniatullin, A.; Goodman, K.; Munoz-Bravo, J.L.; Raingo, J.; Jorgacevski, J.; Kreft, M.; Zorec, R.; et al. Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron 2009, 62, 683–694. [Google Scholar] [CrossRef] [Green Version]
- Rickman, C.; Davletov, B. Arachidonic acid allows SNARE complex formation in the presence of Munc18. Chem. Biol. 2005, 12, 545–553. [Google Scholar] [CrossRef] [Green Version]
- Connell, E.; Darios, F.; Broersen, K.; Gatsby, N.; Peak-Chew, S.Y.; Rickman, C.; Davletov, B. Mechanism of arachidonic acid action on syntaxin-Munc18. EMBO Rep. 2007, 8, 414–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darios, F.; Ruiperez, V.; Lopez, I.; Villanueva, J.; Gutierrez, L.M.; Davletov, B. Alpha- synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis. EMBO Rep. 2010, 11, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Martinez, V.; Villanueva, J.; Torregrosa-Hetland, C.J.; Bittman, R.; Higdon, A.; Darley- Usmar, V.M.; Davletov, B.; Gutierrez, L.M. Lipid metabolites enhance secretion acting on SNARE microdomains and altering the extent and kinetics of single release events in bovine adrenal chromaffin cells. PLoS ONE 2013, 8, e75845. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Martinez, V.; Montes, M.A.; Villanueva, J.; Gimenez-Molina, Y.; de Toledo, G.A.; Gutierrez, L.M. Sphingomyelin derivatives increase the frequency of microvesicle and granule fusion in chromaffin cells. Neuroscience 2015, 295, 117–125. [Google Scholar] [CrossRef]
- Camoletto, P.G.; Vara, H.; Morando, L.; Connell, E.; Marletto, F.P.; Giustetto, M.; Sassoe-Pognetto, M.; Van Veldhoven, P.P.; Ledesma, M.D. Synaptic vesicle docking: Sphingosine regulates syntaxin1 interaction with Munc18. PLoS ONE 2009, 4, e5310. [Google Scholar] [CrossRef]
- Pan, C.Y.; Lee, H.; Chen, C.L. Lysophospholipids elevate [Ca2+]i and trigger exocytosis in bovine chromaffin cells. Neuropharmacology 2006, 51, 18–26. [Google Scholar] [CrossRef]
- Wu, A.Z.; Ohn, T.L.; Shei, R.J.; Wu, H.F.; Chen, Y.C.; Lee, H.C.; Dai, D.F.; Wu, S.N. Permissive Modulation of Sphingosine-1-Phosphate-Enhanced Intracellular Calcium on BKCa Channel of Chromaffin Cells. Int. J. Mol. Sci. 2021, 22, 2175. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, J.; Torregrosa-Hetland, C.J.; García-Martínez, V.; Francés, M.M.; Viniegra, S.; Gutiérrez, L.M. The F-actin cortex in chromaffin granule dynamics and fusion: A minireview. J. Mol. Neurosci. 2012, 48, 323–327. [Google Scholar] [CrossRef]
- Gutiérrez, L.M.; Villanueva, J. The role of F-actin in the transport and secretion of chromaffin granules: An historic perspective. Pflug. Arch. 2018, 470, 181–186. [Google Scholar] [CrossRef]
- Becherer, U.; Rettig, J. Vesicle pools, docking, priming, and release. Cell Tissue Res. 2006, 326, 393–407. [Google Scholar] [CrossRef]
- Zimmerberg, J.; Curran, M.; Cohen, F.S. A lipid/protein complex hypothesis for exocytotic fusion pore formation. Ann. New York Acad. Sci. 1991, 635, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lindau, M.; de Toledo, G.A. The fusion pore. Biochim. Biophys. Acta Mol. Cell Res. 2003, 1641, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Karatekin, E. Toward a unified picture of the exocytotic fusion pore. FEBS Lett. 2003, 592, 3563–3585. [Google Scholar] [CrossRef] [Green Version]
- Lindau, M.; Neher, E. Patch-Clamp Techniques for Time-Resolved Capacitance Measurements in Single Cells. Pflug. Arch. Eur. J. Physiol. 1988, 411, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Houy, S.; Martins, J.S.; Mohrmann, R.; Sørensen, J.B. Measurements of Exocytosis by Capacitance Recordings and Calcium Uncaging in Mouse Adrenal Chromaffin Cells. Methods Mol. Biol. 2021, 2233, 233–251. [Google Scholar]
- Wightman, R.M.; Jankowski, J.A.; Kennedy, R.T.; Kawagoe, K.T.; Schroeder, T.J.; Leszczyszyn, D.J.; Near, J.A.; Diliberto, E.J., Jr.; Viveros, O.H. Temporally resolved catecholamine spikes correspond to single vesicle release from individual chromaffin cells. Proc. Natl. Acad. Sci. USA 1991, 88, 10754–10758. [Google Scholar] [CrossRef] [Green Version]
- Álvarez de Toledo, G.; Montes, M.A.; Montenegro, P.; Borges, R. Phases of the exocytotic fusion pore. FEBS Lett. 2018, 592, 3532–3541. [Google Scholar] [CrossRef] [Green Version]
- Flasker, A.; Jorgacevski, J.; Calejo, A.I.; Kreft, M.; Zorec, R. Vesicle size determines unitary exocytic properties and their sensitivity to sphingosine. Mol. Cell. Endocrinol. 2013, 376, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.J.; Delaney, T.L.; Zanin, M.P.; Haberberger, R.V.; Pitson, S.M.; Huang, J.; Alford, S.; Cologna, S.M.; Keating, D.J.; Gong, L.W. Extracellular and intracellular sphingosine-1-phosphate distinctly regulates exocytosis in chromaffin cells. J. Neurochem. 2019, 149, 729–746. [Google Scholar] [CrossRef]
- Abbineni, P.S.; Coorssen, J.R. Sphingolipids modulate docking, Ca2+ sensitivity and membrane fusion of native cortical vesicles. Int. J. Biochem. Cell Biol. 2018, 104, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Riganti, L.; Antonucci, F.; Gabrielli, M.; Prada, I.; Giussani, P.; Viani, P.; Valtorta, F.; Menna, E.; Matteoli, M.; Verderio, C. Sphingosine-1-Phosphate (S1P) Impacts Presynaptic Functions by Regulating Synapsin I Localization in the Presynaptic Compartment. J. Neurosci. 2016, 36, 4624–4634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, J.M.; Gandía, L.; García, A.G. Permissive role of sphingosine on calcium-dependent endocytosis in chromaffin cells. Pflug. Arch. 2010, 460, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Kahan, B.D. FTY720: A new immunosuppressive agent with novel mechanism(s) of action. Transplant. Proc. 1988, 30, 2210–2213. [Google Scholar] [CrossRef]
- Chiba, K.; Yanagawa, Y.; Masubuchi, Y.; Kataoka, H.; Kawaguchi, T.; Ohtsuki, M.; Hoshino, Y. FTY720, a novel immunosuppressant, induces sequestration of circulating mature lymphocytes by acceleration of lymphocyte homing in rats. I. FTY720 selectively decreases the number of circulating mature lymphocytes by acceleration of lymphocyte homing. J. Immunol. 1998, 160, 5037–5044. [Google Scholar]
- Brinkmann, V. FTY720 (fingolimod) in Multiple Sclerosis: Therapeutic effects in the immune and the central nervous system. Br. J. Pharmacol. 2009, 158, 1173–1182. [Google Scholar] [CrossRef] [Green Version]
- Strader, C.R.; Pearce, C.J.; Oberlies, N.H. Fingolimod (FTY720): A recently approved multiple sclerosis drug based on a fungal secondary metabolite. J. Nat. Prod. 2011, 74, 900–907. [Google Scholar] [CrossRef]
- Koch-Henriksen, N.; Magyari, M. Apparent changes in the epidemiology and severity of multiple sclerosis. Nat. Rev. Neurol. 2021, 17, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.D.; Jorgacevski, J.; Flasker, A.; Zorec, R.; Garcia-Martinez, V.; Villanueva, J.; Gutierrez, L.M.; Leese, C.; Bal, M.; Nosyreva, E.; et al. Sphingomimetic multiple sclerosis drug FTY720 activates vesicular synaptobrevin and augments neuroendocrine secretion. Sci. Rep. 2017, 7, 5958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez-Molina, Y.; García-Martínez, V.; Villanueva, J.; Davletov, B.; Gutiérrez, L.M. Multiple sclerosis drug FTY-720 toxicity is mediated by the heterotypic fusion of organelles in neuroendocrine cells. Sci. Rep. 2019, 9, 18471. [Google Scholar] [CrossRef] [Green Version]
- Trkov, S.; Stenovec, M.; Kreft, M.; Potokar, M.; Parpura, V.; Davletov, B.; Zorec, R. Fingolimod—A sphingosine-like molecule inhibits vesicle mobility and secretion in astrocytes. Glia 2012, 60, 1406–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signaling: Lessons from sphingolipids. Nat. Rev. Mol. Cell. Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Anastasiadou, S.; Knoll, B. The multiple sclerosis drug fingolimod (FTY720) stimulates neuronal gene expression, axonal growth and regeneration. Exp. Neurol. 2016, 279, 243–260. [Google Scholar] [CrossRef] [Green Version]
- Brunkhorst, R.; Vutukuri, R.; Pfeilschifter, W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front. Cell Neurosci. 2014, 8, 283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipriani, R.; Chara, J.C.; Rodriguez-Antiguedad, A.; Matute, C. FTY720 attenuates excitotoxicity and neuroinflammation. J. Neuroinflammation 2015, 12, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, Y.; Suzuki, H.; Sozen, T.; Rolland, W.; Zhang, J.H. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke 2010, 41, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Nazari, M.; Keshavarz, S.; Rafati, A.; Namavar, M.R.; Haghani, M. Fingolimod (FTY720) improves hippocampal synaptic plasticity and memory deficit in rats following focal cerebral ischemia. Brain Res. Bull. 2016, 124, 95–102. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; Gao, J.; Liang, S.; Hao, Y.; Sun, C.; Xia, W.; Cao, Y.; Wu, L. Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuroinflammation in the rat model of autism. Life Sci. 2017, 173, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Miguez, A.; Garcia-Diaz Barriga, G.; Brito, V.; Straccia, M.; Giralt, A.; Gines, S.; Canals, J.M.; Alberch, J. Fingolimod (FTY720) enhances hippocampal synaptic plasticity and memory in Huntington’s disease by preventing p75NTR up-regulation and astrocyte-mediated inflammation. Hum. Mol. Genet. 2015, 24, 4958–4970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asle-Rousta, M.; Kolahdooz, Z.; Oryan, S.; Ahmadiani, A.; Dargahi, L. FTY720 (fingolimod) attenuates beta-amyloid peptide (Abeta42)-induced impairment of spatial learning and memory in rats. J. Mol. Neurosci. 2013, 50, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Han, M.; Wei, X.; Guo, Y.; Shi, H.; Zhang, X.; Perez, R.; Lou, H. FTY720 Attenuates 6-OHDA-Associated Dopaminergic Degeneration in Cellular and Mouse Parkinsonian Models. Neurochem. Res. 2017, 42, 686–696. [Google Scholar] [CrossRef]
- Vidal-Martinez, G.; Segura-Ulate, I.; Yang, B.; Diaz-Pacheco, V.; Barragan, J.A.; De-Leon Esquivel, J.; Chaparro, S.A.; Vargas-Medrano, J.; Perez, R.G. FTY720-Mitoxy reduces synucleinopathy and neuroinflammation, restores behavior and mitochondria function, and increases GDNF expression in Multiple System Atrophy mouse models. Exp. Neurol. 2020, 325, 113120. [Google Scholar] [CrossRef] [PubMed]
- Lange, I.; Espinoza-Fuenzalida, I.; Ali, M.W.; Serrano, L.E.; Koomoa, D.T. FTY-720 induces apoptosis in neuroblastoma via multiple signaling pathways. Oncotarget 2017, 8, 109985–109999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolodziej, M.A.; Al Barim, B.; Nagl, J.; Weigand, M.A.; Uhl, E.; Uhle, F.; Di Fazio, P.; Schwarm, F.P.; Stein, M. Sphingosine-1-phosphate analogue FTY720 exhibits a potent anti-proliferative effect on glioblastoma cells. Int. J. Oncol. 2020, 57, 1039–1046. [Google Scholar]
- Perla, A.S.; Fratini, L.; Cardoso, P.S.; de Farias, C.B.; da Cunha Jaeger, M.; Roesler, R. Fingolimod (FTY720) reduces viability and survival and increases histone H3 acetylation in medulloblastoma cells. Pediatr. Hematol. Oncol. 2020, 37, 170–175. [Google Scholar] [CrossRef] [PubMed]
- McCracken, A.N.; McMonigle, R.J.; Tessier, J.; Fransson, R.; Perryman, M.S.; Chen, B.; Keebaugh, A.; Selwan, E.; Barr, S.A.; Kim, S.M.; et al. Phosphorylation of a constrained azacyclic FTY720 analog enhances anti-leukemic activity without inducing S1P receptor activation. Leukemia 2017, 31, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.K.; Spiegel, S. Sphingosine-1-phosphate signaling: A novel target for simultaneous adjuvant treatment of triple negative breast cancer and chemotherapy-induced neuropathic pain. Adv. Biol. Regul. 2020, 75, 100670. [Google Scholar] [CrossRef]
- Carbone, M.L.; Lacal, P.M.; Messinese, S.; De Giglio, L.; Pozzilli, C.; Persechino, S.; Mazzanti, C.; Failla, C.M.; Pagnanelli, G. Multiple Sclerosis Treatment and Melanoma Development. Int. J. Mol. Sci. 2020, 21, 2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Tian, F.; Ma, H.; Wang, H.; Yang, W.; Liu, Z.; Liao, A. FTY720 induces ferroptosis and autophagy via PP2A/AMPK pathway in multiple myeloma cells. Life Sci. 2020, 260, 118077. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Li, X.K.; Enosawa, S.; Shinomiya, T. A new immunosuppressant, FTY720, induces bcl-2-associated apoptotic cell death in human lymphocytes. Immunology 1996, 89, 518–523. [Google Scholar] [CrossRef]
- Wolf, B.B.; Schuler, M.; Li, W.; Eggers-Sedlet, B.; Lee, W.; Tailor, P.; Fitzgerald, P.; Mills, G.B.; Green, D.R. Defective cytochrome c-dependent caspase activation in ovarian cancer cell lines due to diminished or absent apoptotic protease activating factor-1 activity. J. Biol. Chem. 2001, 276, 34244–34251. [Google Scholar] [CrossRef] [Green Version]
- Nagahara, Y.; Matsuoka, Y.; Saito, K.; Ikekita, M.; Higuchi, S.; Shinomiya, T. Coordinate involvement of cell cycle arrest and apoptosis strengthen the effect of FTY720. Jpn. J. Cancer Res. 2001, 92, 680–687. [Google Scholar] [CrossRef]
- Davletov, B.; Connell, E.; Darios, F. Regulation of SNARE fusion machinery by fatty acids. Cell Mol. Life Sci. 2007, 64, 1597–1608. [Google Scholar] [CrossRef]
- Lang, T.; Halemani, N.D.; Rammner, B. Interplay between lipids and the proteinaceous membrane fusion machinery. Prog. Lipid Res. 2008, 47, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martinez, V.; Gimenez-Molina, Y.; Villanueva, J.; Darios, F.D.; Davletov, B.; Gutiérrez, L.M. Emerging evidence for the modulation of exocytosis by signalling lipids. FEBS Lett. 2018, 592, 3493–3503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H. FTY720 in CNS injuries: Molecular mechanisms and therapeutic potential. Brain Res. Bull. 2020, 164, 75–82. [Google Scholar] [CrossRef]
- Lee, T.; Moon, H.S.; Kim, S.W.; Shrestha, J.; Shin, S.M.; Lee, J.Y.; Kim, S.; Park, E.Y.; Baek, D.J. Synthesis and Biological Evaluation of FTY720 (Fingolimod) Derivatives with Aromatic Head Group as Anticancer Agents. Chem. Pharm. Bull. 2018, 66, 1015–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villanueva, J.; Gimenez-Molina, Y.; Davletov, B.; Gutiérrez, L.M. Vesicle Fusion as a Target Process for the Action of Sphingosine and Its Derived Drugs. Int. J. Mol. Sci. 2022, 23, 1086. https://doi.org/10.3390/ijms23031086
Villanueva J, Gimenez-Molina Y, Davletov B, Gutiérrez LM. Vesicle Fusion as a Target Process for the Action of Sphingosine and Its Derived Drugs. International Journal of Molecular Sciences. 2022; 23(3):1086. https://doi.org/10.3390/ijms23031086
Chicago/Turabian StyleVillanueva, José, Yolanda Gimenez-Molina, Bazbek Davletov, and Luis M. Gutiérrez. 2022. "Vesicle Fusion as a Target Process for the Action of Sphingosine and Its Derived Drugs" International Journal of Molecular Sciences 23, no. 3: 1086. https://doi.org/10.3390/ijms23031086
APA StyleVillanueva, J., Gimenez-Molina, Y., Davletov, B., & Gutiérrez, L. M. (2022). Vesicle Fusion as a Target Process for the Action of Sphingosine and Its Derived Drugs. International Journal of Molecular Sciences, 23(3), 1086. https://doi.org/10.3390/ijms23031086





