The Effect of TGF-β1 Reduced Functionality on the Expression of Selected Synaptic Proteins and Electrophysiological Parameters: Implications of Changes Observed in Acute Hepatic Encephalopathy
Abstract
1. Introduction
2. Results
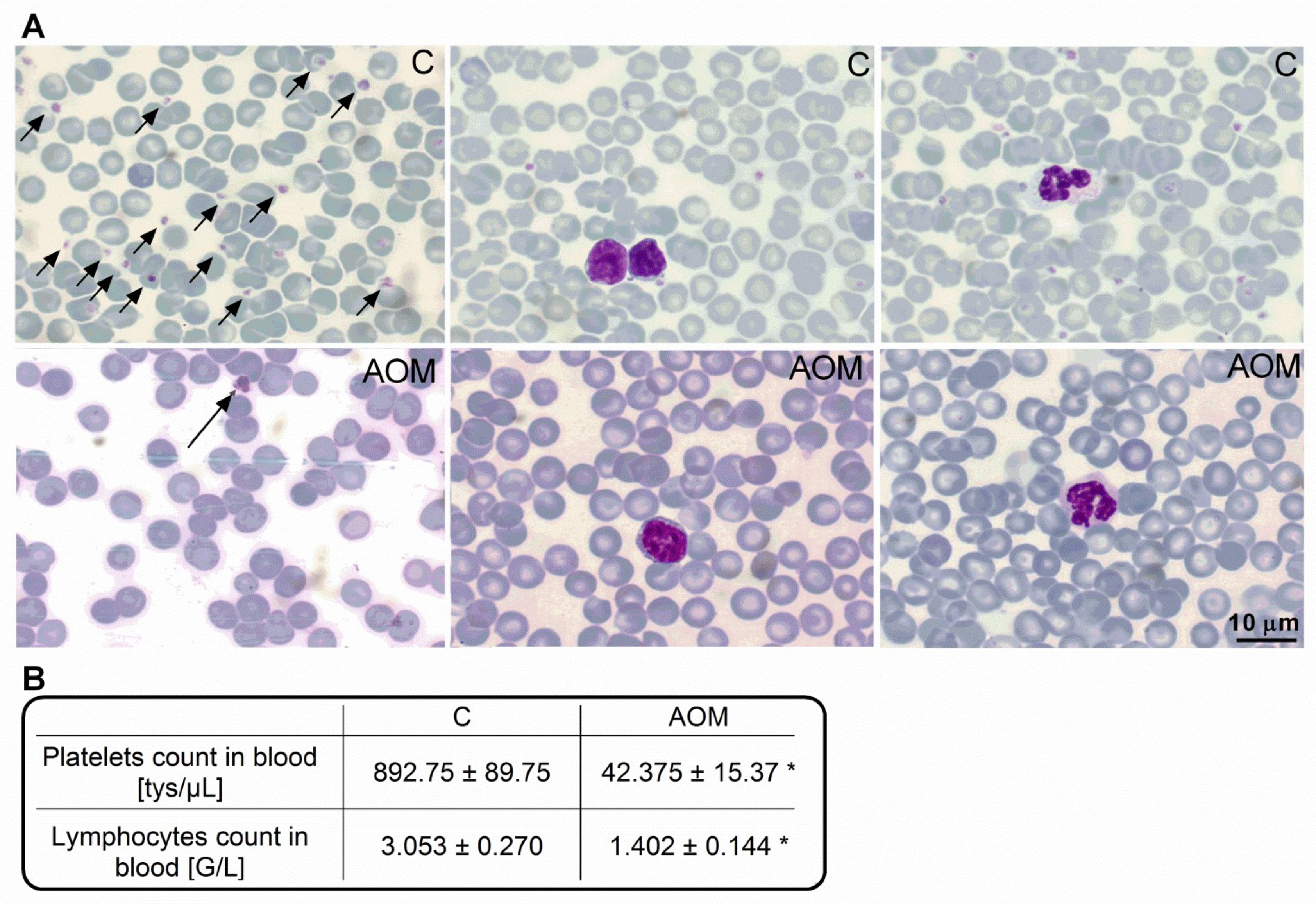
2.1. Blood Morphology and Biochemical Characteristics of the AOM Model
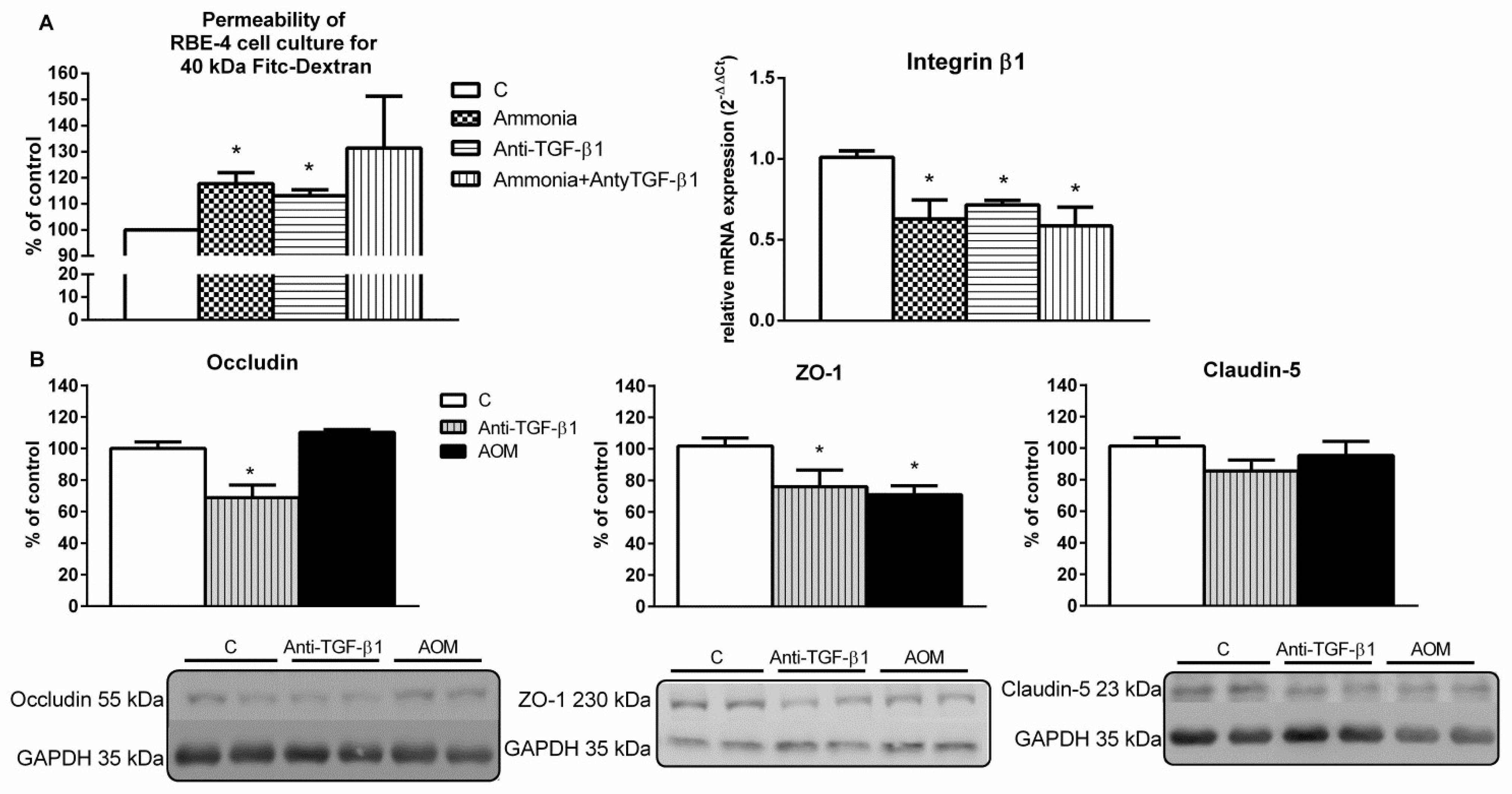
2.2. Endothelial Cells Monolayer Permeability Measurement and the Expression of Blood–Brain Barrier Proteins in the Frontal Cortex Homogenates from AOM and Anti-TGF-β1 Mice
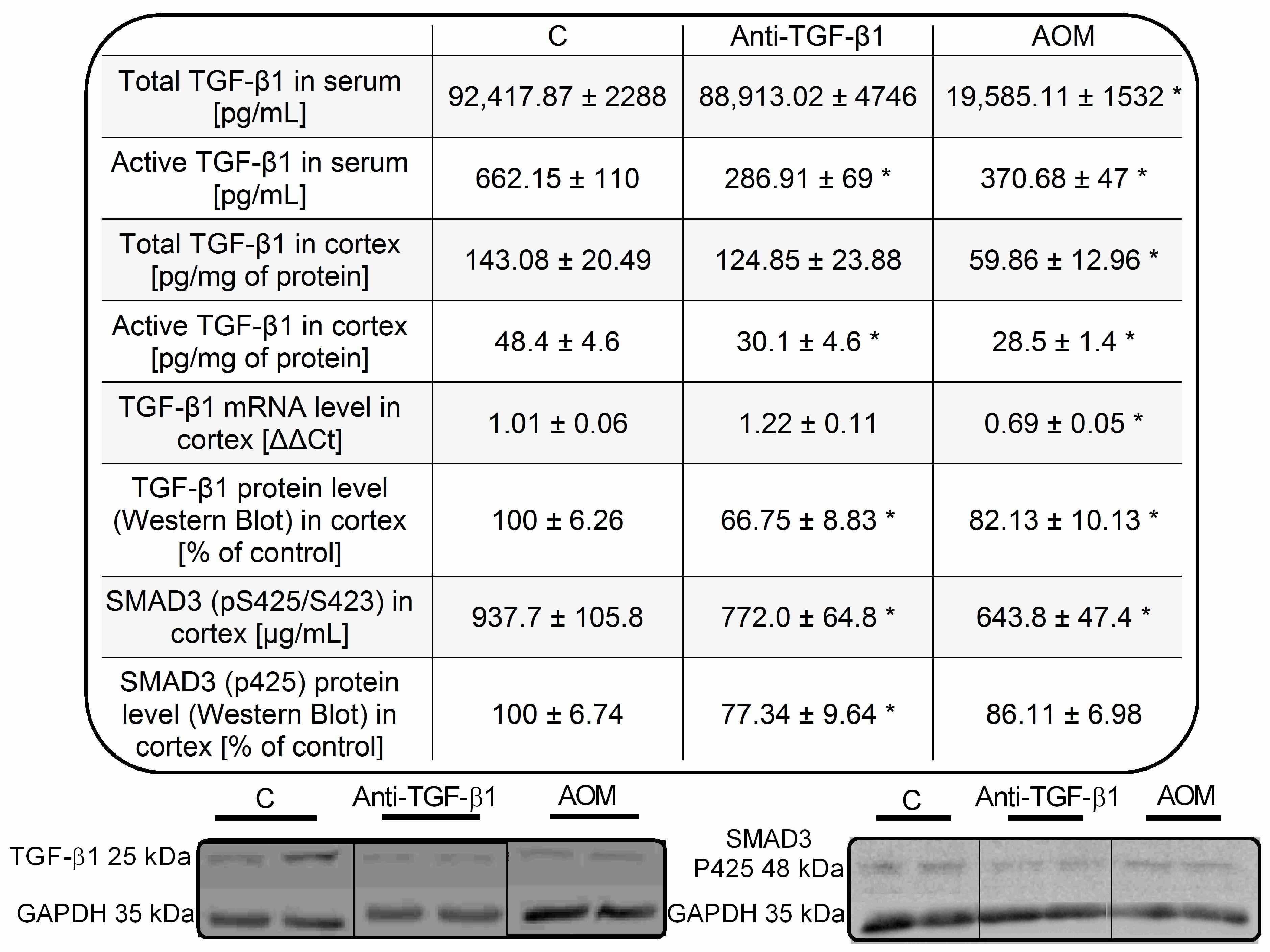
2.3. Parameters of TGF-β1 Signaling in Serum and Frontal Cortex Homogenates from AOM and Anti-TGF-β1 Mice
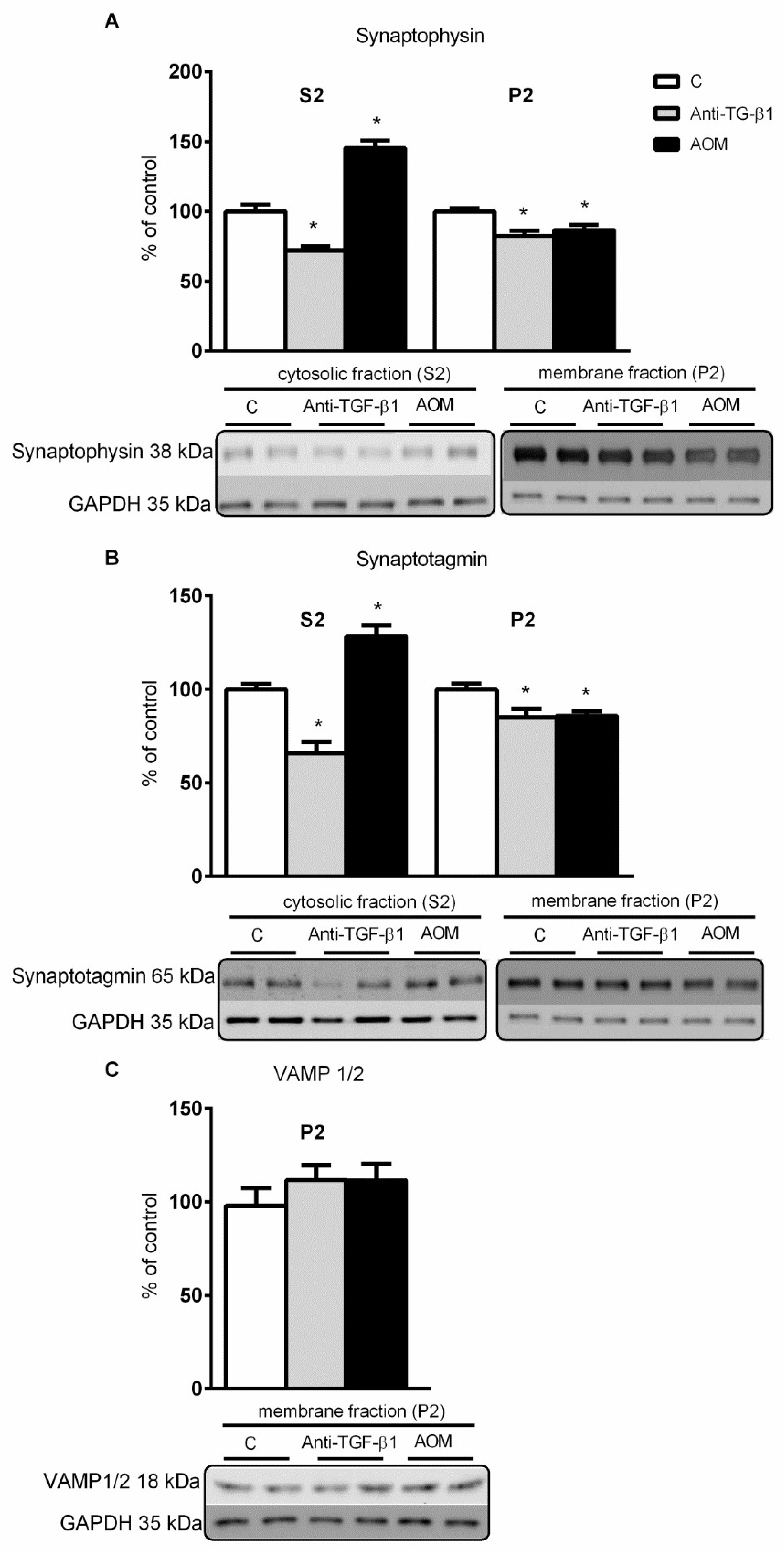
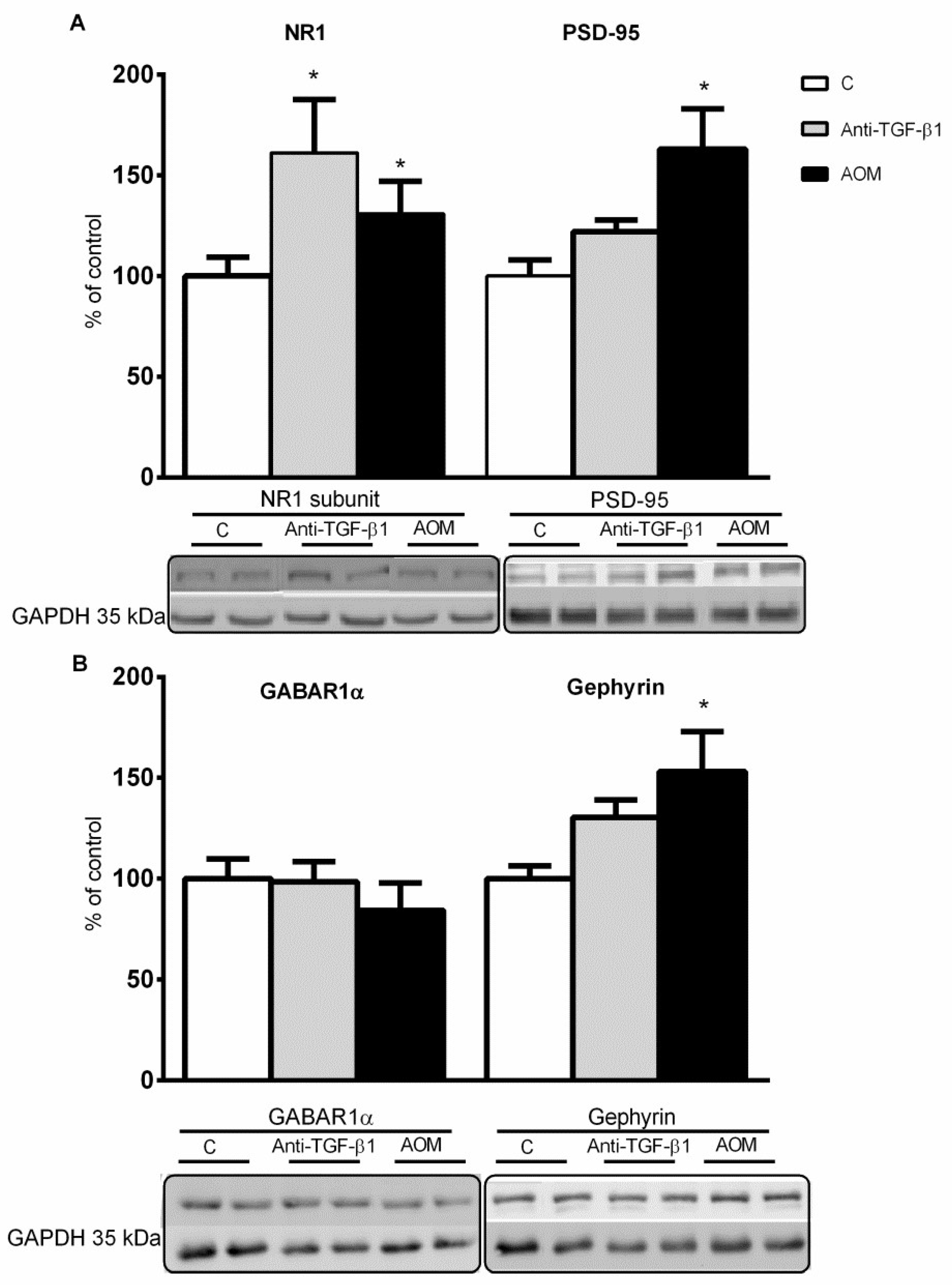
2.4. Expression and Distribution of Synaptic Proteins in Frontal Cortex Homogenates from AOM, and Anti- TGF-β1 Mice
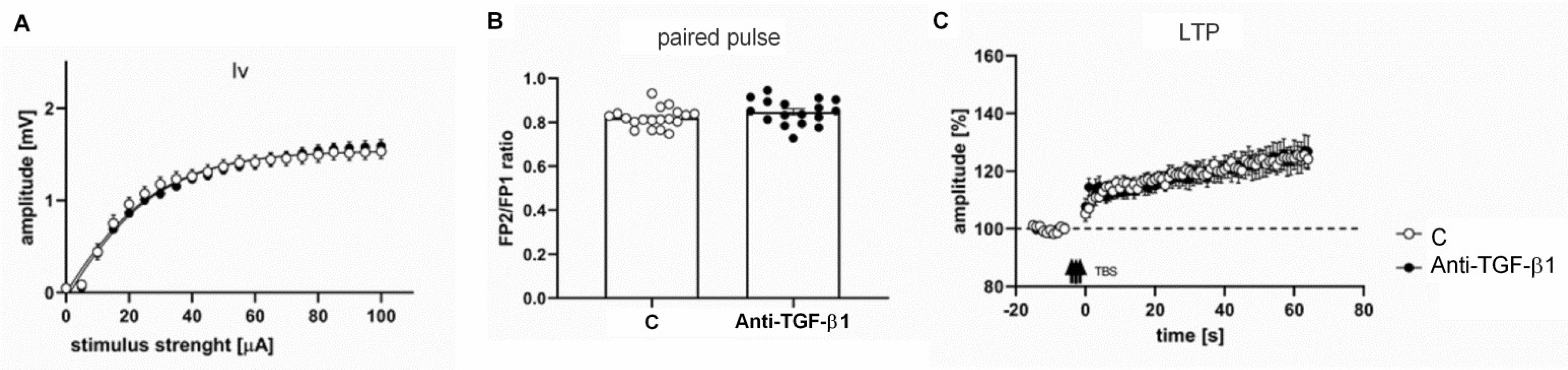
2.5. Electrophysiological Responses of Frontal Cortical Slices from Anti-TGF-β1 Mice
3. Discussion
4. Materials and Methods
4.1. Animal Models
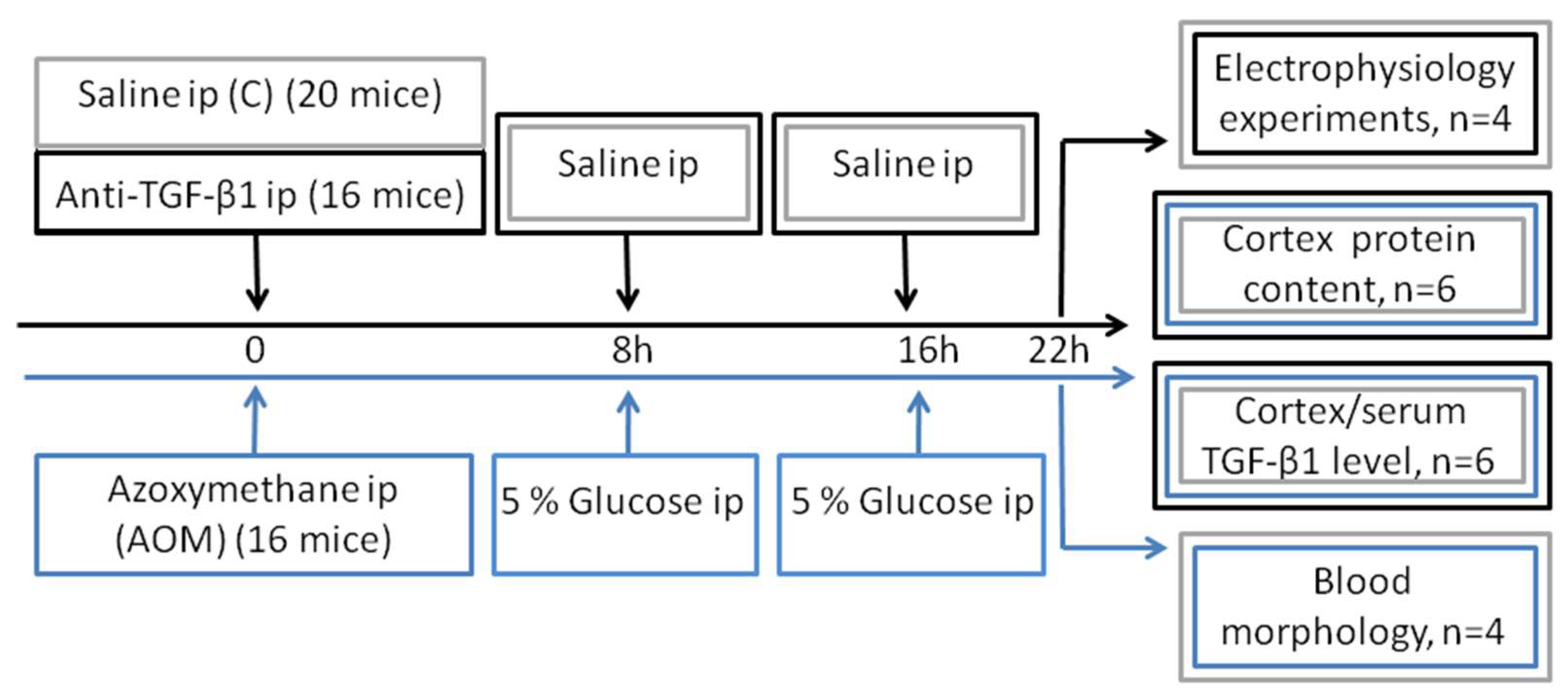
4.2. Study Design and Animal Groups
4.3. Analysis of Biochemical Parameters
4.4. TGF-β1 Level Determination
4.5. Endothelial Cell Line Culture
4.6. Permeability Assay
4.7. RNA Isolation and Real-Time PCR Analysis
4.8. Immunoblotting Analyses
4.9. Electrophysiological Analysis
4.9.1. Treatment of Animals and Brain Slice Preparation
4.9.2. Field Potential Recording and LTP Induction
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALF | Acute liver failure |
| AOM | Azoxymethane |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| FITC | Fluorescein-5-isothiocyanate |
| FPs | Whole-cell field potentials |
| GABA | γ-aminobutyric acid |
| GABAA | γ-aminobutyric acid type A receptor |
| GABAR1α | subunit of GABAA receptor 1α |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HE | Hepatic encephalopathy |
| LAP | Latency-associated peptide |
| LLC | Large latent complex |
| LTBP | Latent TGF-β-binding protein |
| LTP | Long-term potentiation |
| MBEC4 | Mouse brain capillary endothelial cell line |
| NMDA | N-methyl-D-aspartate |
| PBS | Phosphate-saline buffer |
| PSD-95 | Postsynaptic density protein 95 |
| RBE-4 | Rat brain endothelial cell line |
| RT | Room temperature |
| SMAD3 | Mothers against decapentaplegic homolog 3 protein |
| TBS | Theta-burst stimulation |
| TGF-β1 | Transforming growth factor beta 1 |
| TSP-1 | Thrombospondin 1 |
| VAMP 1/2 | Vesicle-associated membrane proteins |
| WT | Wild-type |
| ZO-1 | Tight junction protein 1 or Zonula occludens-1 |
References
- Prakash, R.; Mullen, K.D. Mechanisms, Diagnosis and Management of Hepatic Encephalopathy. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Jones, E.A. Hepatic Encephalopathy: Molecular Mechanisms Underlying the Clinical Syndrome. J. Neurol. Sci. 1999, 170, 138–146. [Google Scholar] [CrossRef]
- Assoian, R.K.; Komoriya, A.; Meyers, C.A.; Miller, D.M.; Sporn, M.B. Transforming Growth Factor-Beta in Human Platelets. Identification of a Major Storage Site, Purification, and Characterization. J. Biol. Chem. 1983, 258, 7155–7160. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming Growth Factor-β in Stem Cells and Tissue Homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Böttner, M.; Krieglstein, K.; Unsicker, K. The Transforming Growth Factor-Betas: Structure, Signaling, and Roles in Nervous System Development and Functions. J. Neurochem. 2000, 75, 2227–2240. [Google Scholar] [CrossRef] [PubMed]
- Brionne, T.C.; Tesseur, I.; Masliah, E.; Wyss-Coray, T. Loss of TGF-Beta 1 Leads to Increased Neuronal Cell Death and Microgliosis in Mouse Brain. Neuron 2003, 40, 1133–1145. [Google Scholar] [CrossRef]
- Gomes, F.C.A.; Sousa, V.; de Oliveira Sousa, L. Emerging Roles for TGF-Beta1 in Nervous System Development. Int. J. Dev. Neurosci. 2005, 23, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, J.A.; Miller, M.W. Transforming Growth Factor Beta1 Modulates Cell Migration in Rat Cortex: Effects of Ethanol. Cereb. Cortex 2004, 14, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Flanders, K.C.; Ren, R.F.; Lippa, C.F. Transforming Growth Factor-Betas in Neurodegenerative Disease. Prog. Neurobiol. 1998, 54, 71–85. [Google Scholar] [CrossRef]
- Villapol, S.; Logan, T.T.; Symes, A.J. Role of TGF-β Signaling in Neurogenic Regions After Brain Injury. In Trends in Cell Signaling Pathways in Neuronal Fate Decision; Wislet-Gendebien, S., Ed.; InTech: Rijeka, Croatia, 2013; ISBN 978-953-51-1059-0. [Google Scholar]
- Vivien, D.; Ali, C. Transforming Growth Factor-Beta Signalling in Brain Disorders. Cytokine Growth Factor Rev. 2006, 17, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Farmer, P.M.; Mulakkan, T. The Pathogenesis of Hepatic Encephalopathy. Ann. Clin. Lab. Sci. 1990, 20, 91–97. [Google Scholar] [PubMed]
- Felipo, V. Hepatic Encephalopathy: Effects of Liver Failure on Brain Function. Nat. Rev. Neurosci. 2013, 14, 851–858. [Google Scholar] [CrossRef]
- Häussinger, D.; Butz, M.; Schnitzler, A.; Görg, B. Pathomechanisms in Hepatic Encephalopathy. Biol. Chem. 2021, 402, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Häussinger, D.; Kircheis, G.; Fischer, R.; Schliess, F.; vom Dahl, S. Hepatic Encephalopathy in Chronic Liver Disease: A Clinical Manifestation of Astrocyte Swelling and Low-Grade Cerebral Edema? J. Hepatol. 2000, 32, 1035–1038. [Google Scholar] [CrossRef]
- Monfort, P.; Muñoz, M.-D.; ElAyadi, A.; Kosenko, E.; Felipo, V. Effects of Hyperammonemia and Liver Failure on Glutamatergic Neurotransmission. Metab. Brain Dis. 2002, 17, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.; Ferenci, P.; Pifl, C.; Yurdaydin, C.; Ebner, J.; Lassmann, H.; Roth, E.; Hörtnagl, H. Hepatic Encephalopathy in Thioacetamide-Induced Acute Liver Failure in Rats: Characterization of an Improved Model and Study of Amino Acid-Ergic Neurotransmission. Hepatology 1989, 9, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Popek, M.; Bobula, B.; Sowa, J.; Hess, G.; Polowy, R.; Filipkowski, R.K.; Frontczak-Baniewicz, M.; Zabłocka, B.; Albrecht, J.; Zielińska, M. Cortical Synaptic Transmission and Plasticity in Acute Liver Failure Are Decreased by Presynaptic Events. Mol. Neurobiol. 2018, 55, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.R.; Tong, X.Y.; Curtis, K.M.; Ruiz-Cordero, R.; Shamaladevi, N.; Abuzamel, M.; Johnstone, J.; Gaidosh, G.; Rama Rao, K.V.; Norenberg, M.D. Decreased Astrocytic Thrombospondin-1 Secretion after Chronic Ammonia Treatment Reduces the Level of Synaptic Proteins: In Vitro and in Vivo Studies. J. Neurochem. 2014, 131, 333–347. [Google Scholar] [CrossRef]
- Crawford, S.E.; Stellmach, V.; Murphy-Ullrich, J.E.; Ribeiro, S.M.F.; Lawler, J.; Hynes, R.O.; Boivin, G.P.; Bouck, N. Thrombospondin-1 Is a Major Activator of TGF-Β1 In Vivo. Cell 1998, 93, 1159–1170. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Poczatek, M.; Schultz-Cherry, S.; Villain, M.; Murphy-Ullrich, J.E. The Activation Sequence of Thrombospondin-1 Interacts with the Latency-Associated Peptide to Regulate Activation of Latent Transforming Growth Factor-Beta. J. Biol. Chem. 1999, 274, 13586–13593. [Google Scholar] [CrossRef]
- Jayakumar, A.R.; Norenberg, M.D. Hyperammonemia in Hepatic Encephalopathy. J. Clin. Exp. Hepatol. 2018, 8, 272–280. [Google Scholar] [CrossRef]
- Jefferson, B.; Ali, M.; Grant, S.; Frampton, G.; Ploof, M.; Andry, S.; DeMorrow, S.; McMillin, M. Thrombospondin-1 Exacerbates Acute Liver Failure and Hepatic Encephalopathy Pathology in Mice by Activating Transforming Growth Factor Β1. Am. J. Pathol. 2020, 190, 347–357. [Google Scholar] [CrossRef]
- McMillin, M.; Galindo, C.; Pae, H.Y.; Frampton, G.; Di Patre, P.L.; Quinn, M.; Whittington, E.; DeMorrow, S. Gli1 Activation and Protection against Hepatic Encephalopathy Is Suppressed by Circulating Transforming Growth Factor Β1 in Mice. J. Hepatol. 2014, 61, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.A.; Frampton, G.A.; Seiwell, A.P.; Patel, N.S.; Jacobs, A.N.; DeMorrow, S. TGFβ1 Exacerbates Blood-Brain Barrier Permeability in a Mouse Model of Hepatic Encephalopathy via Upregulation of MMP9 and Downregulation of Claudin-5. Lab. Investig. 2015, 95, 903–913. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; Grant, S.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jefferson, B.; Thomas, A.; Brahmaroutu, A.; DeMorrow, S. Elevated Circulating TGFβ1 during Acute Liver Failure Activates TGFβR2 on Cortical Neurons and Exacerbates Neuroinflammation and Hepatic Encephalopathy in Mice. J. Neuroinflamm. 2019, 16, 69. [Google Scholar] [CrossRef]
- Araujo, A.P.B.; Diniz, L.P.; Eller, C.M.; de Matos, B.G.; Martinez, R.; Gomes, F.C.A. Effects of Transforming Growth Factor Beta 1 in Cerebellar Development: Role in Synapse Formation. Front. Cell. Neurosci. 2016, 10, 104. [Google Scholar] [CrossRef]
- Diniz, L.P.; Tortelli, V.; Matias, I.; Morgado, J.; Bérgamo Araujo, A.P.; Melo, H.M.; Seixas da Silva, G.S.; Alves-Leon, S.V.; de Souza, J.M.; Ferreira, S.T.; et al. Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Aβ Oligomers in Alzheimer’s Disease Model. J. Neurosci. 2017, 37, 6797–6809. [Google Scholar] [CrossRef]
- Diniz, L.P.; Almeida, J.C.; Tortelli, V.; Vargas Lopes, C.; Setti-Perdigão, P.; Stipursky, J.; Kahn, S.A.; Romão, L.F.; de Miranda, J.; Alves-Leon, S.V.; et al. Astrocyte-Induced Synaptogenesis Is Mediated by Transforming Growth Factor β Signaling through Modulation of D-Serine Levels in Cerebral Cortex Neurons. J. Biol. Chem. 2012, 287, 41432–41445. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.J.; Xiang, Y.-Y.; Martinez-Canabal, A.; Frankland, P.W.; Yang, B.B.; Lu, W.-Y. Increased Transforming Growth Factor-Β1 Modulates Glutamate Receptor Expression in the Hippocampus. Int. J. Physiol. Pathophysiol. Pharmacol. 2011, 3, 9–20. [Google Scholar]
- Bélanger, M.; Côté, J.; Butterworth, R.F. Neurobiological Characterization of an Azoxymethane Mouse Model of Acute Liver Failure. Neurochem. Int. 2006, 48, 434–440. [Google Scholar] [CrossRef]
- Matkowskyj, K.A.; Marrero, J.A.; Carroll, R.E.; Danilkovich, A.V.; Green, R.M.; Benya, R.V. Azoxymethane-Induced Fulminant Hepatic Failure in C57BL/6J Mice: Characterization of a New Animal Model. Am. J. Physiol. 1999, 277, G455–G462. [Google Scholar] [CrossRef] [PubMed]
- Walshe, T.E.; Saint-Geniez, M.; Maharaj, A.S.R.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. TGF-β Is Required for Vascular Barrier Function, Endothelial Survival and Homeostasis of the Adult Microvasculature. PLoS ONE 2009, 4, e5149. [Google Scholar] [CrossRef]
- Schiødt, F.V.; Balko, J.; Schilsky, M.; Harrison, M.E.; Thornton, A.; Lee, W.M. Acute Liver Failure Study Group Thrombopoietin in Acute Liver Failure. Hepatology 2003, 37, 558–561. [Google Scholar] [CrossRef]
- Giannini, E.G. Review Article: Thrombocytopenia in Chronic Liver Disease and Pharmacologic Treatment Options. Aliment. Pharmacol. Ther. 2006, 23, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.A.; Grace, N.D. Abnormal Hematological Indices in Cirrhosis. Can. J. Gastroenterol. 2009, 23, 441–445. [Google Scholar] [CrossRef]
- Gangireddy, V.G.R.; Kanneganti, P.C.; Sridhar, S.; Talla, S.; Coleman, T. Management of Thrombocytopenia in Advanced Liver Disease. Can. J. Gastroenterol. Hepatol. 2014, 28, 558–564. [Google Scholar] [CrossRef]
- Sakai, K.; Iwao, T.; Oho, K.; Toyonaga, A.; Sata, M. Propranolol Ameliorates Thrombocytopenia in Patients with Cirrhosis. J. Gastroenterol. 2002, 37, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Hancox, S.H.; Smith, B.C. Liver Disease as a Cause of Thrombocytopenia. QJM 2013, 106, 425–431. [Google Scholar] [CrossRef]
- Olariu, M.; Olariu, C.; Olteanu, D. Thrombocytopenia in Chronic Hepatitis C. J. Gastrointestin Liver Dis. 2010, 19, 381–385. [Google Scholar]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-Associated Immune Dysfunction: Distinctive Features and Clinical Relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Tuchendler, E.; Tuchendler, P.K.; Madej, G. Immunodeficiency Caused by Cirrhosis. Ceh 2018, 4, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Q.; Mao, W.; Fan, J.; Ye, B. Neutrophil-to-Lymphocyte Ratio Predicts Early Mortality in Patients with HBV-Related Decompensated Cirrhosis. Gastroenterol. Res. Pract. 2016, 2016, 4394650. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Xia, J.; Wang, X.; Huang, Y.; Li, B.; Meng, Z.; Gao, Y.; Qian, Z.; Liu, F.; et al. Baseline Neutrophil-to-Lymphocyte Ratio Is Independently Associated with 90-Day Transplant-Free Mortality in Patients with Cirrhosis. Front. Med. 2021, 8, 726950. [Google Scholar] [CrossRef] [PubMed]
- Blakytny, R.; Ludlow, A.; Martin, G.E.M.; Ireland, G.; Lund, L.R.; Ferguson, M.W.J.; Brunner, G. Latent TGF-Beta1 Activation by Platelets. J. Cell. Physiol. 2004, 199, 67–76. [Google Scholar] [CrossRef]
- Meyer, A.; Wang, W.; Qu, J.; Croft, L.; Degen, J.L.; Coller, B.S.; Ahamed, J. Platelet TGF-Β1 Contributions to Plasma TGF-Β1, Cardiac Fibrosis, and Systolic Dysfunction in a Mouse Model of Pressure Overload. Blood 2012, 119, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Budi, E.H. Specificity, Versatility, and Control of TGF-β Family Signaling. Sci. Signal. 2019, 12, eaav5183. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.B.; Rifkin, D.B. Regulation of the Bioavailability of TGF-β and TGF-β-Related Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021907. [Google Scholar] [CrossRef]
- Chaudhry, S.S.; Cain, S.A.; Morgan, A.; Dallas, S.L.; Shuttleworth, C.A.; Kielty, C.M. Fibrillin-1 Regulates the Bioavailability of TGFβ1. J. Cell Biol. 2007, 176, 355–367. [Google Scholar] [CrossRef]
- Horiguchi, M.; Ota, M.; Rifkin, D.B. Matrix Control of Transforming Growth Factor—Function. J. Biochem. 2012, 152, 321–329. [Google Scholar] [CrossRef]
- Goerge, T.; Ho-Tin-Noe, B.; Carbo, C.; Benarafa, C.; Remold-O’Donnell, E.; Zhao, B.-Q.; Cifuni, S.M.; Wagner, D.D. Inflammation Induces Hemorrhage in Thrombocytopenia. Blood 2008, 111, 4958–4964. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Konradt, C.; Corken, A.; Ware, J.; Nieswandt, B.; Di Paola, J.; Yu, M.; Wang, D.; Nieman, M.T.; Whiteheart, S.W.; et al. Hemostasis vs. Homeostasis: Platelets Are Essential for Preserving Vascular Barrier Function in the Absence of Injury or Inflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 24316–24325. [Google Scholar] [CrossRef]
- McCarty, J.H. Integrin-Mediated Regulation of Neurovascular Development, Physiology and Disease. Cell Adhes. Migr. 2009, 3, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.L. Integrin-Mediated Transforming Growth Factor-β Activation, a Potential Therapeutic Target in Fibrogenic Disorders. Am. J. Pathol. 2009, 175, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Margadant, C.; Sonnenberg, A. Integrin–TGF-β Crosstalk in Fibrosis, Cancer and Wound Healing. EMBO Rep. 2010, 11, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Izawa, Y.; Gu, Y.-H.; Osada, T.; Kanazawa, M.; Hawkins, B.T.; Koziol, J.A.; Papayannopoulou, T.; Spatz, M.; del Zoppo, G.J. Β1-Integrin–Matrix Interactions Modulate Cerebral Microvessel Endothelial Cell Tight Junction Expression and Permeability. J. Cereb. Blood Flow Metab. 2018, 38, 641–658. [Google Scholar] [CrossRef] [PubMed]
- Dohgu, S.; Takata, F.; Yamauchi, A.; Nakagawa, S.; Egawa, T.; Naito, M.; Tsuruo, T.; Sawada, Y.; Niwa, M.; Kataoka, Y. Brain Pericytes Contribute to the Induction and Up-Regulation of Blood–Brain Barrier Functions through Transforming Growth Factor-β Production. Brain Res. 2005, 1038, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Maki, T.; Maeda, M.; Miyamoto, N.; Liang, A.C.; Hayakawa, K.; Pham, L.-D.D.; Suwa, F.; Taguchi, A.; Matsuyama, T.; et al. Oligodendrocyte Precursor Cells Support Blood-Brain Barrier Integrity via TGF-β Signaling. PLoS ONE 2014, 9, e103174. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; McMillin, M.; Frampton, G.; Petrescu, A.D.; Williams, E.; Jaeger, V.; Kain, J.; DeMorrow, S. Direct Comparison of the Thioacetamide and Azoxymethane Models of Type A Hepatic Encephalopathy in Mice. Gene Expr. 2018, 18, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Obara-Michlewska, M.; Ding, F.; Popek, M.; Verkhratsky, A.; Nedergaard, M.; Zielinska, M.; Albrecht, J. Interstitial Ion Homeostasis and Acid-Base Balance Are Maintained in Oedematous Brain of Mice with Acute Toxic Liver Failure. Neurochem. Int. 2018, 118, 286–291. [Google Scholar] [CrossRef]
- Hamdani, E.H.; Popek, M.; Frontczak-Baniewicz, M.; Utheim, T.P.; Albrecht, J.; Zielińska, M.; Chaudhry, F.A. Perturbation of Astroglial Slc38 Glutamine Transporters by NH4+ Contributes to Neurophysiologic Manifestations in Acute Liver Failure. FASEB J. 2021, 35, e21588. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, K.; Zheng, F.; Unsicker, K.; Alzheimer, C. More than Being Protective: Functional Roles for TGF-β/Activin Signaling Pathways at Central Synapses. Trends Neurosci. 2011, 34, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Giráldez-Pérez, R.; Antolín-Vallespín, M.; Muñoz, M.; Sánchez-Capelo, A. Models of α-Synuclein Aggregation in Parkinson’s Disease. Acta Neuropathol. Commun. 2014, 2, 176. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.D.; de la Fuente, N.; Sánchez-Capelo, A. TGF-β/Smad3 Signalling Modulates GABA Neurotransmission: Implications in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 590. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.-L.; Wang, C.-Y.; Zhou, M.-H.; Duan, K.-Z.; Mei, Y.-A. TGF-Β1 Enhances Kv2.1 Potassium Channel Protein Expression and Promotes Maturation of Cerebellar Granule Neurons. J. Cell. Physiol. 2012, 227, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.D.; Antolín-Vallespín, M.; Tapia-González, S.; Sánchez-Capelo, A. Smad3 Deficiency Inhibits Dentate Gyrus LTP by Enhancing GABAA Neurotransmission. J. Neurochem. 2016, 137, 190–199. [Google Scholar] [CrossRef]
- Choii, G.; Ko, J. Gephyrin: A Central GABAergic Synapse Organizer. Exp. Mol. Med. 2015, 47, e158. [Google Scholar] [CrossRef] [PubMed]
- Zacchi, P.; Antonelli, R.; Cherubini, E. Gephyrin Phosphorylation in the Functional Organization and Plasticity of GABAergic Synapses. Front. Cell. Neurosci. 2014, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Gili, E.; Calafiore, M.; Failla, M.; Larosa, C.; Crimi, N.; Sortino, M.; Nicoletti, F.; Copani, A.; Vancheri, C. TGF-Β1 Targets the GSK-3β/β-Catenin Pathway via ERK Activation in the Transition of Human Lung Fibroblasts into Myofibroblasts. Pharmacol. Res. 2008, 57, 274–282. [Google Scholar] [CrossRef]
- Basile, A.; Gammal, S.; Mullen, K.; Jones, E.; Skolnick, P. Differential Responsiveness of Cerebellar Purkinje Neurons to GABA and Benzodiazepine Receptor Ligands in an Animal Model of Hepatic Encephalopathy. J. Neurosci. 1988, 8, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Bassett, M.L.; Mullen, K.D.; Skolnick, P.; Jones, E.A. Amelioration of Hepatic Encephalopathy by Pharmacologic Antagonism of the GABAA-Benzodiazepine Receptor Complex in a Rabbit Model of Fulminant Hepatic Failure. Gastroenterology 1987, 93, 1069–1077. [Google Scholar] [CrossRef]
- Gammal, S.H.; Basile, A.S.; Geller, D.; Skolnick, P.; Jones, E.A. Reversal of the Behavioral and Electrophysiological Abnormalities of an Animal Model of Hepatic Encephalopathy by Benzodiazepine Receptor Ligands. Hepatology 1990, 11, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.R.; Gammal, S.H.; Jones, E.A. Resistance to 3-Mercaptopropionic Acid-Induced Seizures in Hepatic Encephalopathy. Hepatogastroenterology 1997, 44, 766–769. [Google Scholar]
- Takahashi, K.; Kameda, H.; Kataoka, M.; Sanjou, K.; Harata, N.; Akaike, N. Ammonia Potentiates GABAA Response in Dissociated Rat Cortical Neurons. Neurosci. Lett. 1993, 151, 51–54. [Google Scholar] [CrossRef]
- Ahboucha, S.; Butterworth, R.F. Pathophysiology of Hepatic Encephalopathy: A New Look at GABA from the Molecular Standpoint. Metab. Brain Dis. 2004, 19, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Ahboucha, S.; Gamrani, H.; Baker, G. GABAergic Neurosteroids: The “Endogenous Benzodiazepines” of Acute Liver Failure. Neurochem. Int. 2012, 60, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Koeglsperger, T.; Li, S.; Brenneis, C.; Saulnier, J.L.; Mayo, L.; Carrier, Y.; Selkoe, D.J.; Weiner, H.L. Impaired Glutamate Recycling and GluN2B-Mediated Neuronal Calcium Overload in Mice Lacking TGF-Β1 in the CNS. Glia 2013, 61, 985–1002. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.A. A Brief History of Long-Term Potentiation. Neuron 2017, 93, 281–290. [Google Scholar] [CrossRef]
- Murphy, G. Starting from the Ground State Up: Factors Influencing Synaptic Plasticity. Biophys. J. 2015, 108, 997–998. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kashima, R.; Hata, A. The Role of TGF-β Superfamily Signaling in Neurological Disorders. Acta Biochim. Biophys. Sin. 2018, 50, 106–120. [Google Scholar] [CrossRef]
- Tichauer, J.E.; von Bernhardi, R. Transforming Growth Factor-β Stimulates β Amyloid Uptake by Microglia through Smad3-Dependent Mechanisms. J. Neurosci. Res. 2012, 90, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Tesseur, I.; Zou, K.; Esposito, L.; Bard, F.; Berber, E.; Can, J.V.; Lin, A.H.; Crews, L.; Tremblay, P.; Mathews, P.; et al. Deficiency in Neuronal TGF-β Signaling Promotes Neurodegeneration and Alzheimer’s Pathology. J. Clin. Investig. 2006, 116, 3060–3069. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Battaglia, G.; Bruno, V.; Bosco, P.; Carbonaro, V.; Giuffrida, M.L.; Drago, F.; Sortino, M.A.; Nicoletti, F.; Copani, A. TGF-Β1 Pathway as a New Target for Neuroprotection in Alzheimer’s Disease: Neuroprotective Role of TGF-Β1 in AD. CNS Neurosci. Ther. 2011, 17, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, M.; Milewski, K.; Skowrońska, M.; Gajos, A.; Ziemińska, E.; Beręsewicz, A.; Albrecht, J. Induction of Inducible Nitric Oxide Synthase Expression in Ammonia-Exposed Cultured Astrocytes Is Coupled to Increased Arginine Transport by Upregulated y(+)LAT2 Transporter. J. Neurochem. 2015, 135, 1272–1281. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popek, M.; Bobula, B.; Orzeł-Gajowik, K.; Zielińska, M. The Effect of TGF-β1 Reduced Functionality on the Expression of Selected Synaptic Proteins and Electrophysiological Parameters: Implications of Changes Observed in Acute Hepatic Encephalopathy. Int. J. Mol. Sci. 2022, 23, 1081. https://doi.org/10.3390/ijms23031081
Popek M, Bobula B, Orzeł-Gajowik K, Zielińska M. The Effect of TGF-β1 Reduced Functionality on the Expression of Selected Synaptic Proteins and Electrophysiological Parameters: Implications of Changes Observed in Acute Hepatic Encephalopathy. International Journal of Molecular Sciences. 2022; 23(3):1081. https://doi.org/10.3390/ijms23031081
Chicago/Turabian StylePopek, Mariusz, Bartosz Bobula, Karolina Orzeł-Gajowik, and Magdalena Zielińska. 2022. "The Effect of TGF-β1 Reduced Functionality on the Expression of Selected Synaptic Proteins and Electrophysiological Parameters: Implications of Changes Observed in Acute Hepatic Encephalopathy" International Journal of Molecular Sciences 23, no. 3: 1081. https://doi.org/10.3390/ijms23031081
APA StylePopek, M., Bobula, B., Orzeł-Gajowik, K., & Zielińska, M. (2022). The Effect of TGF-β1 Reduced Functionality on the Expression of Selected Synaptic Proteins and Electrophysiological Parameters: Implications of Changes Observed in Acute Hepatic Encephalopathy. International Journal of Molecular Sciences, 23(3), 1081. https://doi.org/10.3390/ijms23031081





