Polymeric Nanocomposites for Environmental and Industrial Applications
Abstract
1. Introduction
2. Preparation Methods
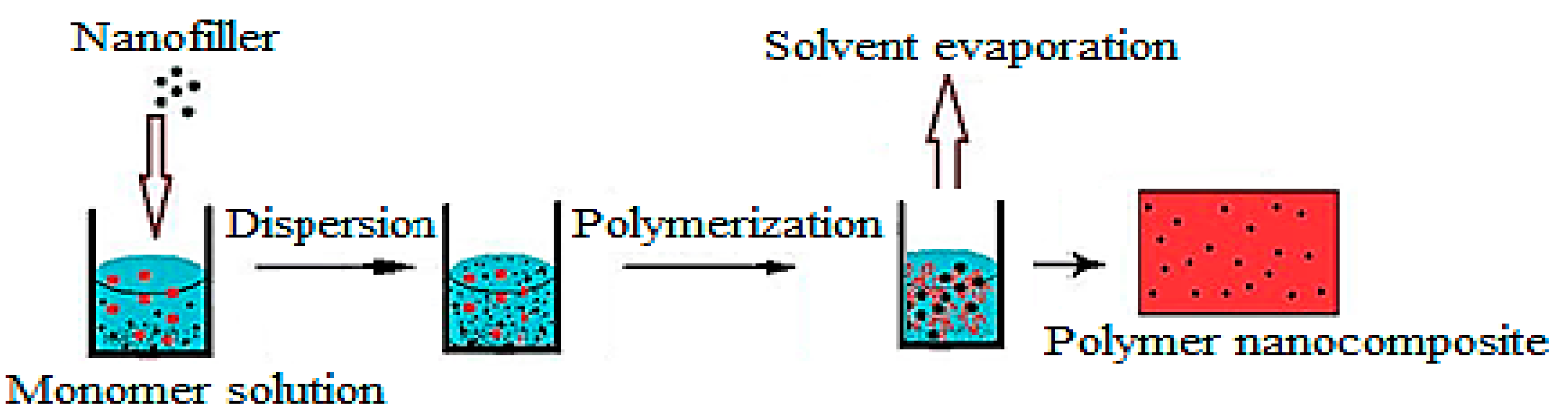
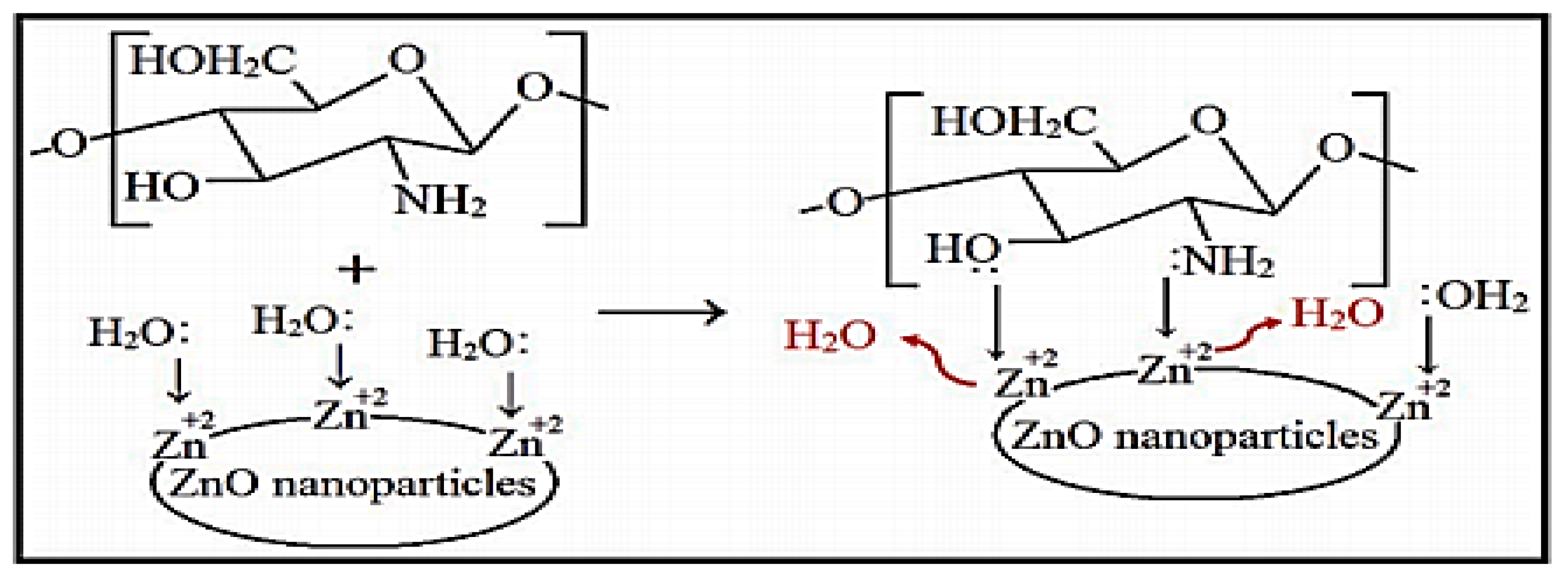
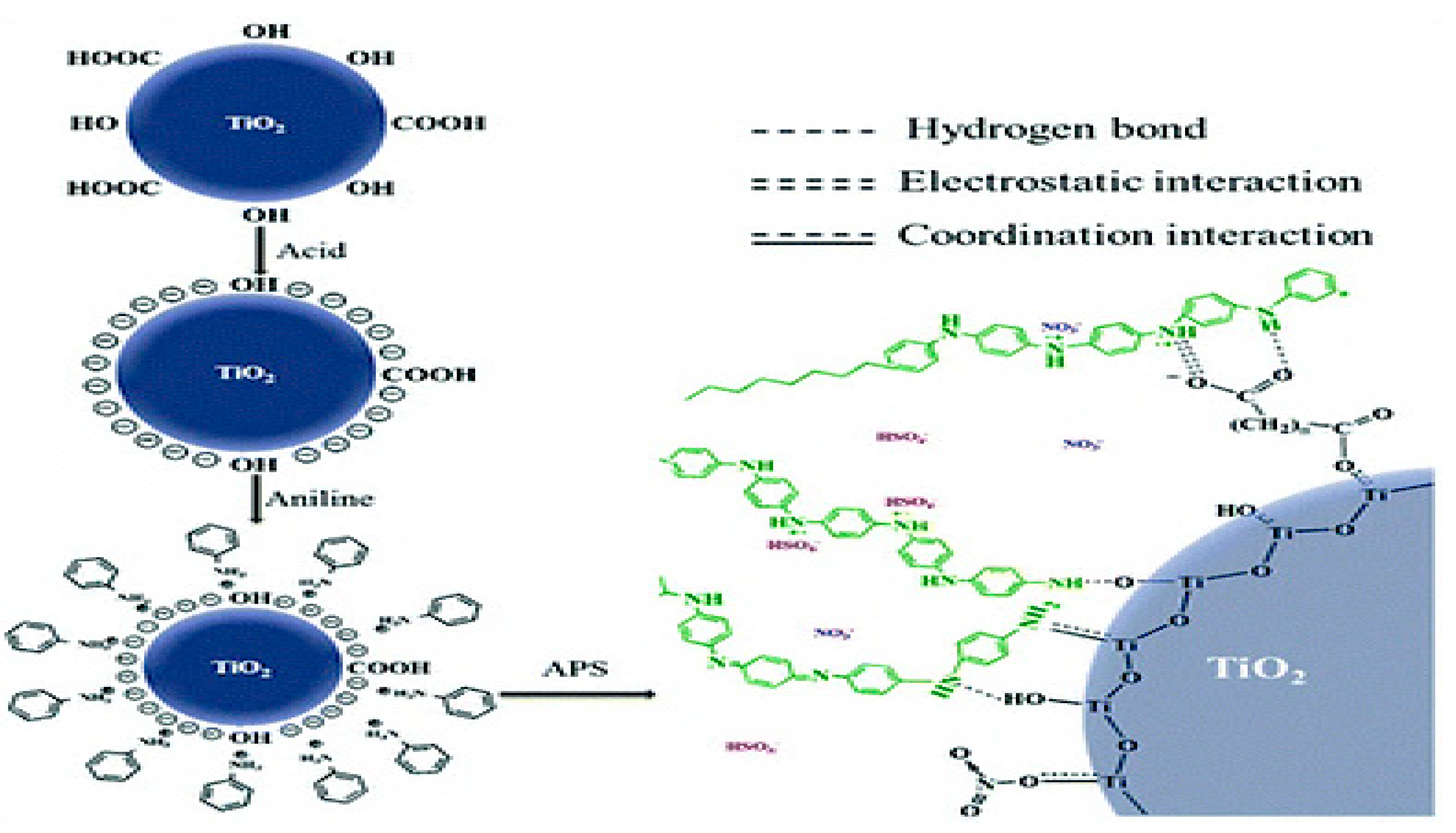
2.1. In Situ Synthesis
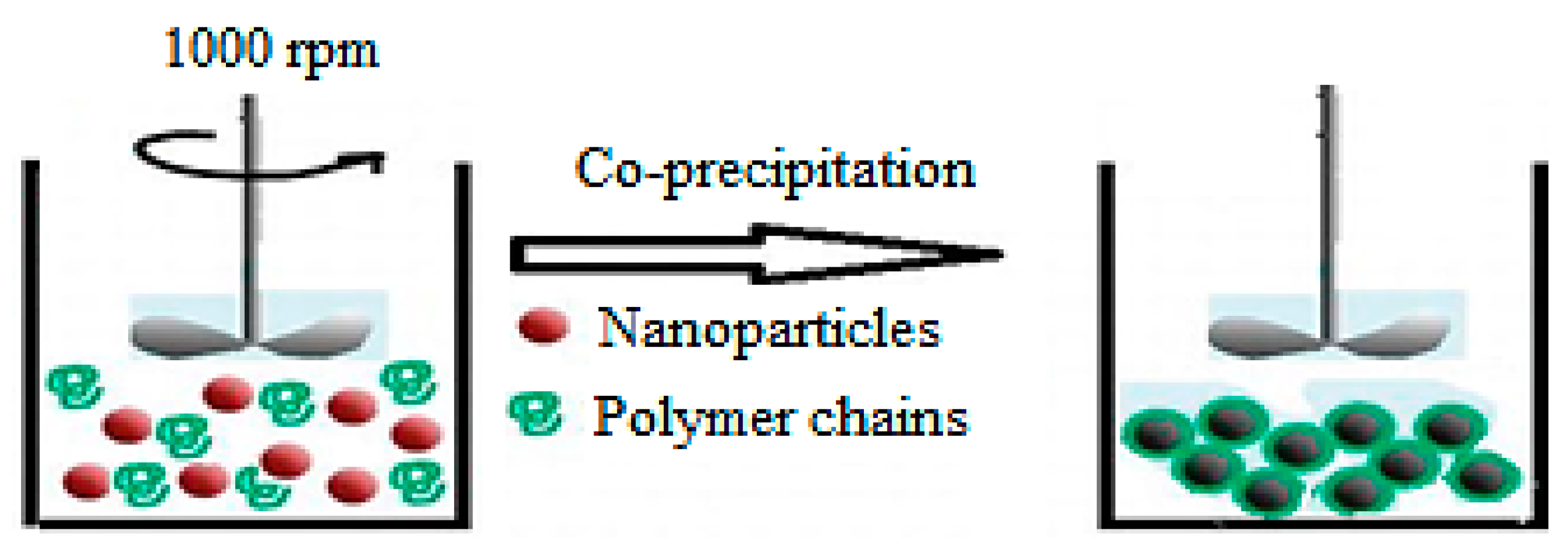
2.2. Solution Mixing
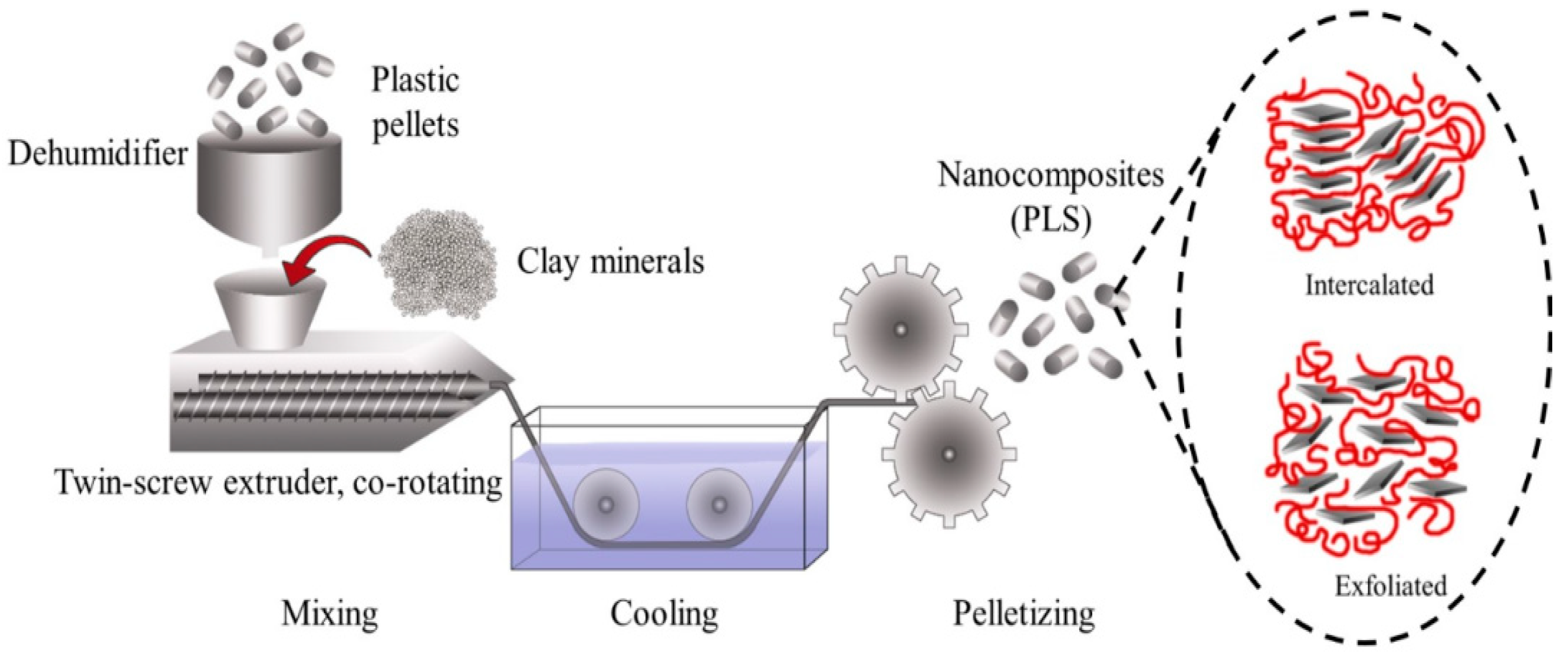
2.3. Melt Blending
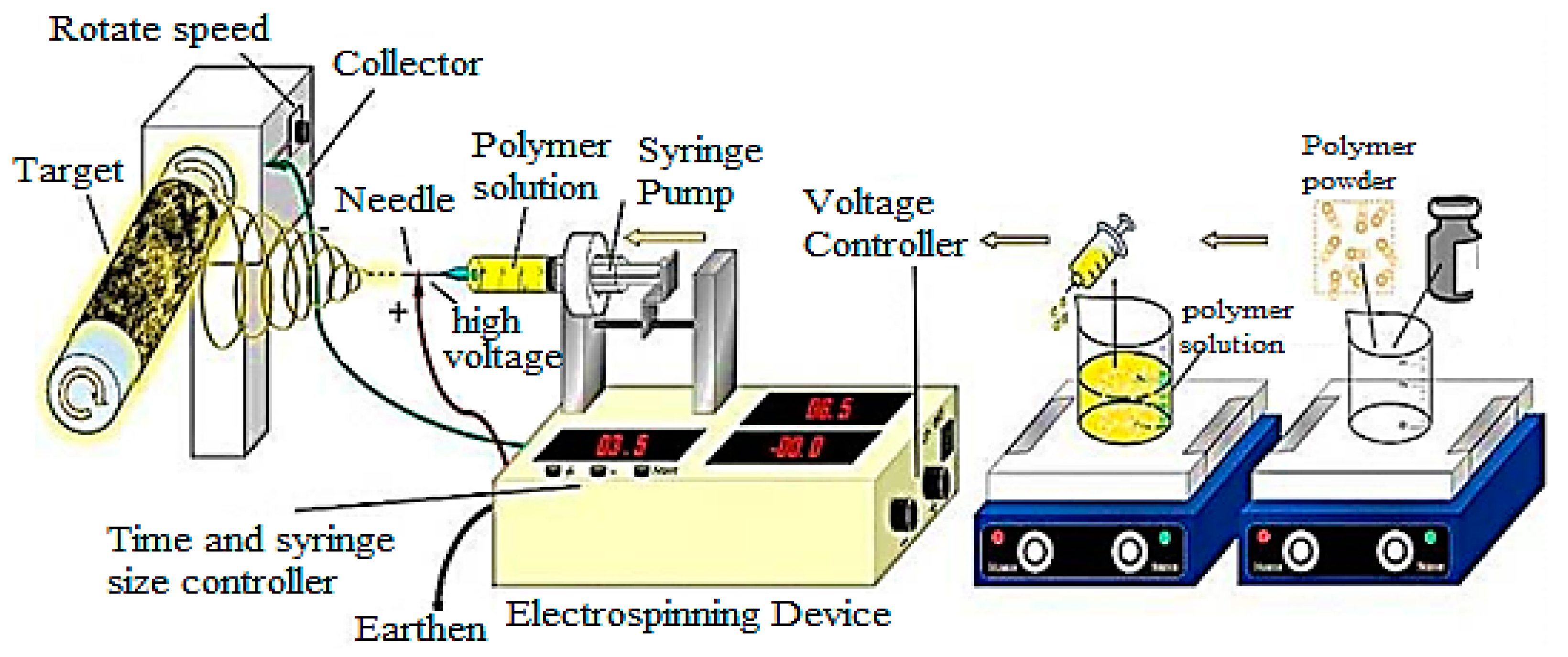
2.4. Electrospinning
2.5. Other Methods
3. Smart Polymer Nanocomposites
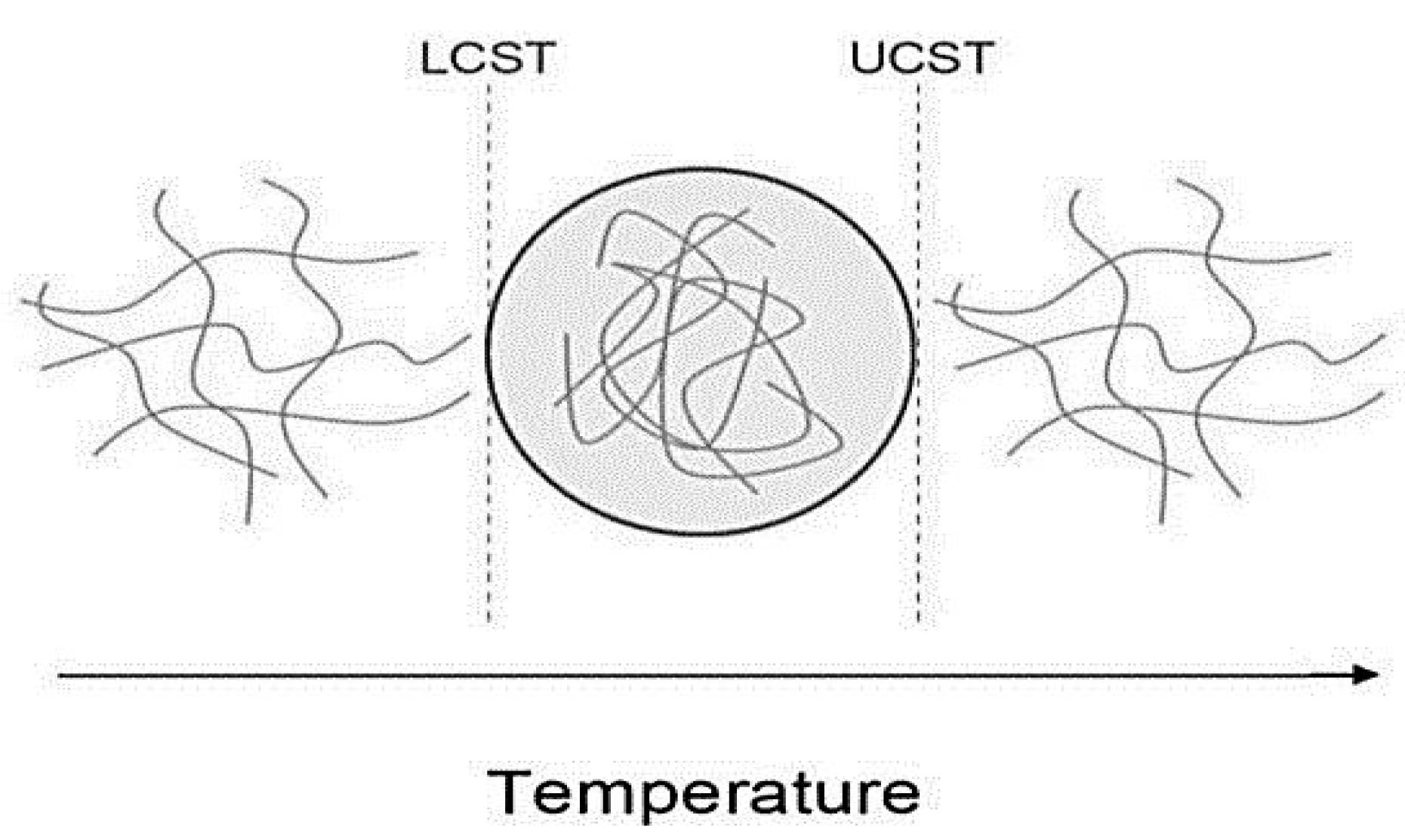
3.1. Thermo-Responsive Nanocomposites
3.2. Light-Responsive Nanocomposites
3.3. Responsive Nanocomposites Based on Electric Current
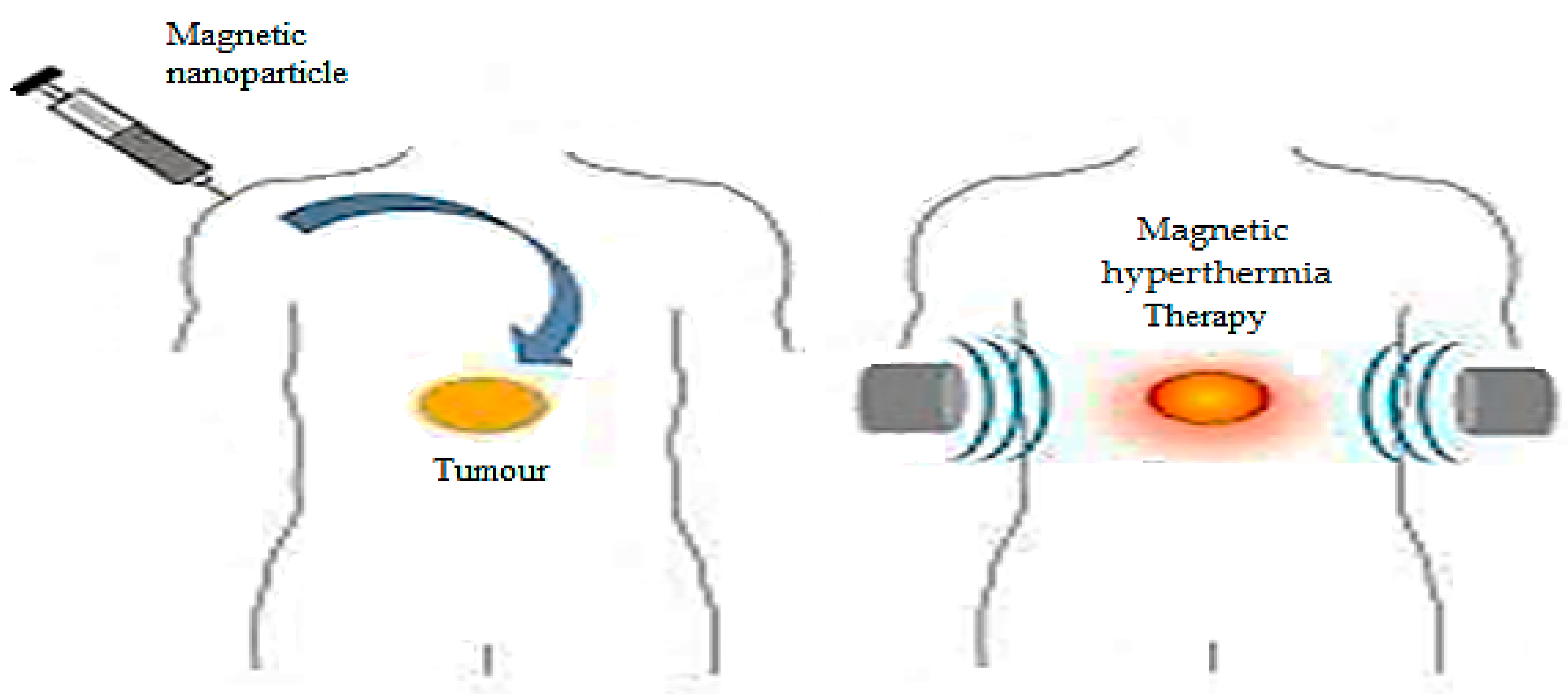
3.4. Magnetic Responsive Nanocomposites
4. Applications of Polymer Nanocomposites
4.1. Water Treatment
4.1.1. Dye Removal
| Polymer Nanocomposite | Dye | Results | Ref. |
|---|---|---|---|
| Chitosan/CuO nanocomposites beads | Congo red (CR) Eriochrome black T (EBT) | A total of 97% of dyes were removed within 2 h. Maximum adsorption capacity of CR and EBT were 119.70 and 235.70 mgg−1 | [98] |
| Molecularly imprinted Chitosan/TiO2 nanocomposite | Rose Bengal (RB) | The adsorption capacity for RB was 79.365 mg/g and enthalpy was 62.279 kJ mol−1 | [99] |
| Chitosan/ZnO nanocomposite | Methylene blue (MB) | 96.7% of MB dye was removed | [46] |
| ZnO/Cellulose nanocrystal nanocomposite | Methylene blue (MB) Malachite green (MG) | 93.55% and 99.02% of MB and MG were removed within 5 min. The absorption capacity was 46.77 and 49.51 mg/g for MB and MG | [97] |
| ZnO/Poly(methyl methacrylate) nanocomposite membrane | Methylene blue (MB) | About 100% of MB was removed within 80 min | [100] |
| Poly(methyl methacrylate)/Multiwall carbon nanotube nanocomposite | Methyl green (MG) | The Langmuir adsorption capacity for MG was 6.85 mmol/g at 25 °C | [101] |
| Polyacrylic acid/Fe3O4/Carboxylated cellulose nanocrystals nanocomposite | MB | The maximum adsorption capacity for MB was 332 mg g−1 | [102] |
| Fe3O4/Starch/Poly (acrylic acid) nanocomposite hydrogel | Methylene violet (MV) Congo red (CR) | A maximum of 93.83% and 99.32% CR and MV dyes with maximum adsorption of 96.7% and 97.5% | [103] |
| Polylactic acid/Graphene oxide/Chitosan nanocomposite | Crystal violet (CV) | 97.8 ± 0.5% of CV was removed | [104] |
| Polypyrrole/Zeolite nanocomposite | Reactive blue (RB) Reactive red (RR) | A total of 86.2% of RB and 88.3% of RR were adsorbed from synthetic solution | [105] |
4.1.2. Metal Ion Removal
| Polymer Nanocomposite | Metal Ion | Results | Ref. |
|---|---|---|---|
| Polyaniline/Reduced graphene oxide nanocomposite | Hg(II) | The adsorption capacity was 1000.00 mg/g | [117] |
| Fe3O4/starch/Poly(acrylic acid) nanocomposite hydrogel | Cu(II) Pb (II) | 95.4% of Cu(II) and 88.4% of Pb(II) were removed at pH of 6.0 and 5.5 | [103] |
| Graphene oxide/Chitosan/ Ferrite nanocomposite | Cr (VI) | The adsorption capacity for Cr(VI) was 270.27 mg g−1 at pH of 2.0. | [118] |
| Magnetic chitosan/Functionalized 3D graphene nanocomposite | Pb (II) | The efficiency of Pb(II) removal is 100% at pH of 8.5 within 18 min | [119] |
| Bacterial cellulose/Amorphous TiO2 nanocomposite | Pb(II) | A total of 90% of Pb(II) was removed in 120 min at pH 7 | [120] |
| Cellulose/TiO2 nanocomposite | Zn(II) Cd(II) Pb(II) | Maximum adsorption capacity for Zn(II), Cd(II) and Pb(II) was 102.04, 102.05 and 120.48 mg/g | [121] |
| Polyacrylamide/Sodium Montmorillonite nanocomposite | Ni (II) Co (II) | A total of 99.3% of Ni(II) and 98.7% of Co (II) was removed at pH 6. | [122] |
| Polyacrylamide/Bentonite hydrogel nanocomposite | Pb (II) Cd (II) | More than 95% of Pb (II) and Cd (II) were removed within first 20 min. Maximum adsorption capacity for Pb (II) and Cd (II) was 138.33 and 200.41 mg/g. | [123] |
| Modified mesoporous silica/Poly(methyl methacrylate) nanocomposites | Cu (II) | Maximum adsorption capacity for Cu (II) was 41.5 mg/g at pH 4 and 140 min | [124] |
| Xanthan gum grafted Polyaniline/ZnO nanocomposite | Cr(VI) | Maximum adsorption capacity was 346.18 mg g–1 for Cr(VI) | [125] |
4.1.3. Water Disinfection
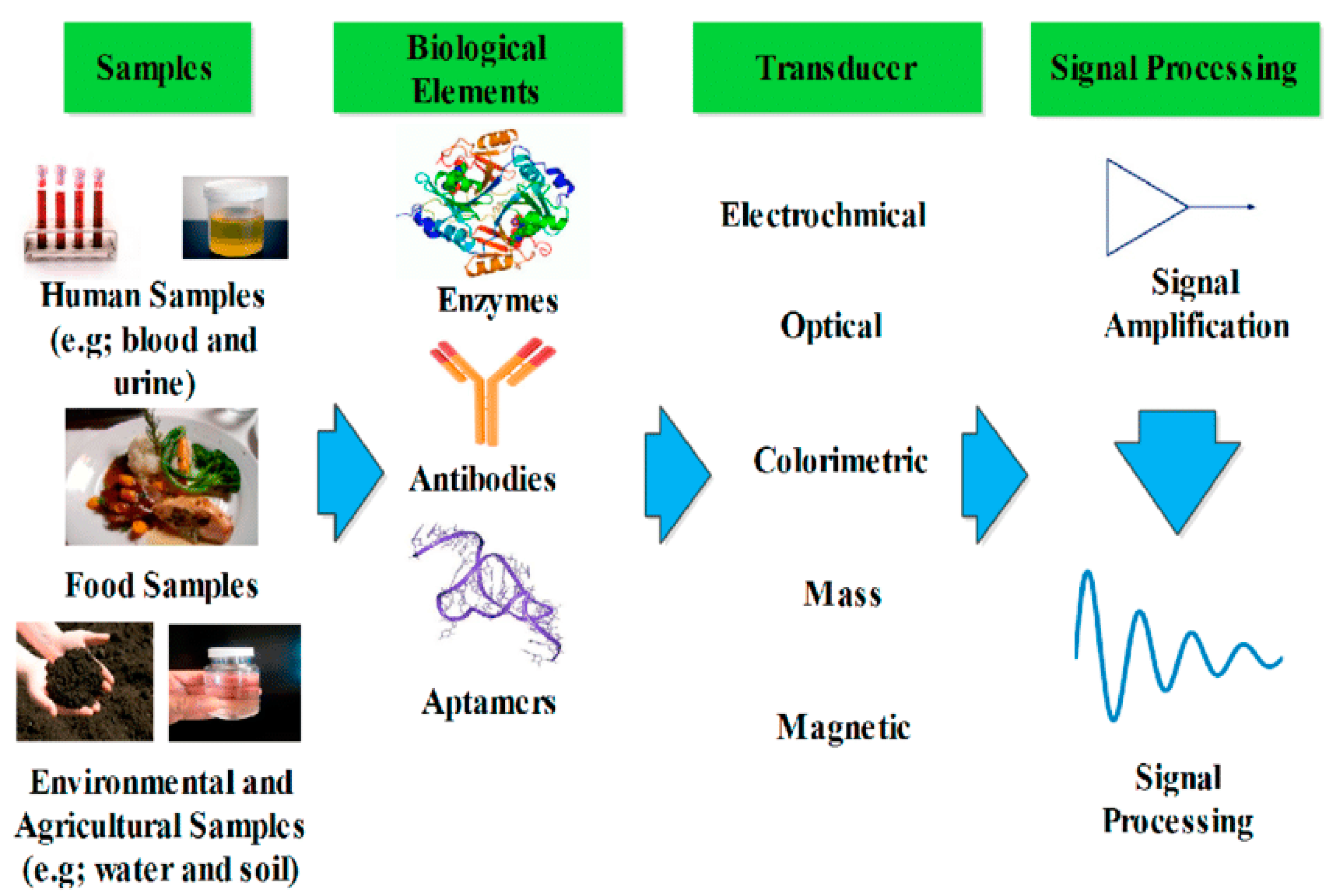
4.2. Sensor Devices
| Polymer Nanocomposite | Type of Sensor | Target | Results | Ref. |
|---|---|---|---|---|
| NiO– chitosan/ZnO/Zinc hexacyanoferrate nanocomposite film | Biosensor | Triolein | Optimum response: within 4 s linear concentration range: (50–700 mg/dL) Sensitivity: 0.05 A/mg/dL | [135] |
| GOx/MWCNTs-polyaniline nanocomposite. | Biosensor | Glucose | Electrical conductivity: 3.78 × 10−1 Scm−1 Response time: 5 s Linear concentration range: 0.5–22 mM | [149] |
| Polyaniline/MWCNTs/Au NPs nanocomposite modified glass carbon electrode | Biosensor | Glucose | Detection limit: 0.19 mM Sensitivity: 29.17 mA mM−1 cm−2 Concentration range: 0.0625–1.19 mM | [150] |
| Polypyrrole/MWCNTs/GOx nanocomposite modified glassy carbon electrode | Biosensor | Glucose | The linear range: up to 4 mM Sensitivity: 95 nAmM−1 Response time: 8 s | [151] |
| Polypyrrole/MWCNTs/Au NPs/ChOx | Biosensor | Cholesterol | Linear response: (2 × 10−3 to 8 × 10−3 M) Detection limit: 0.1 × 10−3 M Sensitivity: 10.12 mA mM−1 cm−2. | [152] |
| Polyaniline/ Functionalize MWCNT nanocomposite | Gas Sensor | Ammonia Vapor | High sensitivity (92% for100 ppm) Detection limit: (200 ppb) Response time: (9 s) Recovery time: (30 s) | [153] |
| Polypyrrole/Nitrogen-doped MWCNTs film fabricated on PI substrate | Gas Sensor | NO2 gas | The sensor possessed high response of 24.82% (Rg − Ra)/Ra × 100%) under 5 ppm of NO2. The sensor had outstanding selectivity, repeatability and stability | [154] |
| Ethylene diamine tetraacetic acid/Polyaniline/MWCNTs. with carbon electrode | Metal ion sensor | Pb+2 | Detection limit: 22 pM | [155] |
| Polypyrrole/MWCNTs deposited on electrode | Metal ion sensor | Pb+2 ions | Detection limit: 2.9 × 10−9 mol/L (S/N = 3) | [156] |
| Polyaniline/MWCNTs -3-aminopropyltriethoxysilane casted on glassy carbon electrode | Metal ion sensor | Cd+2 ions | Detection limit: 0.015 µM Linear concentration range:(0.05–50 µM) | [157] |
| Modified glassy carbon electrode with polythiophene/COOH -MWCNTs/reduced graphene oxide | Metal ion sensor | Hg+2 ions | Linear range: (0.1 to 25 µM) Limit of detection: (0.009 µM) Recovery: between 110.7 and 96.79% | [158] |
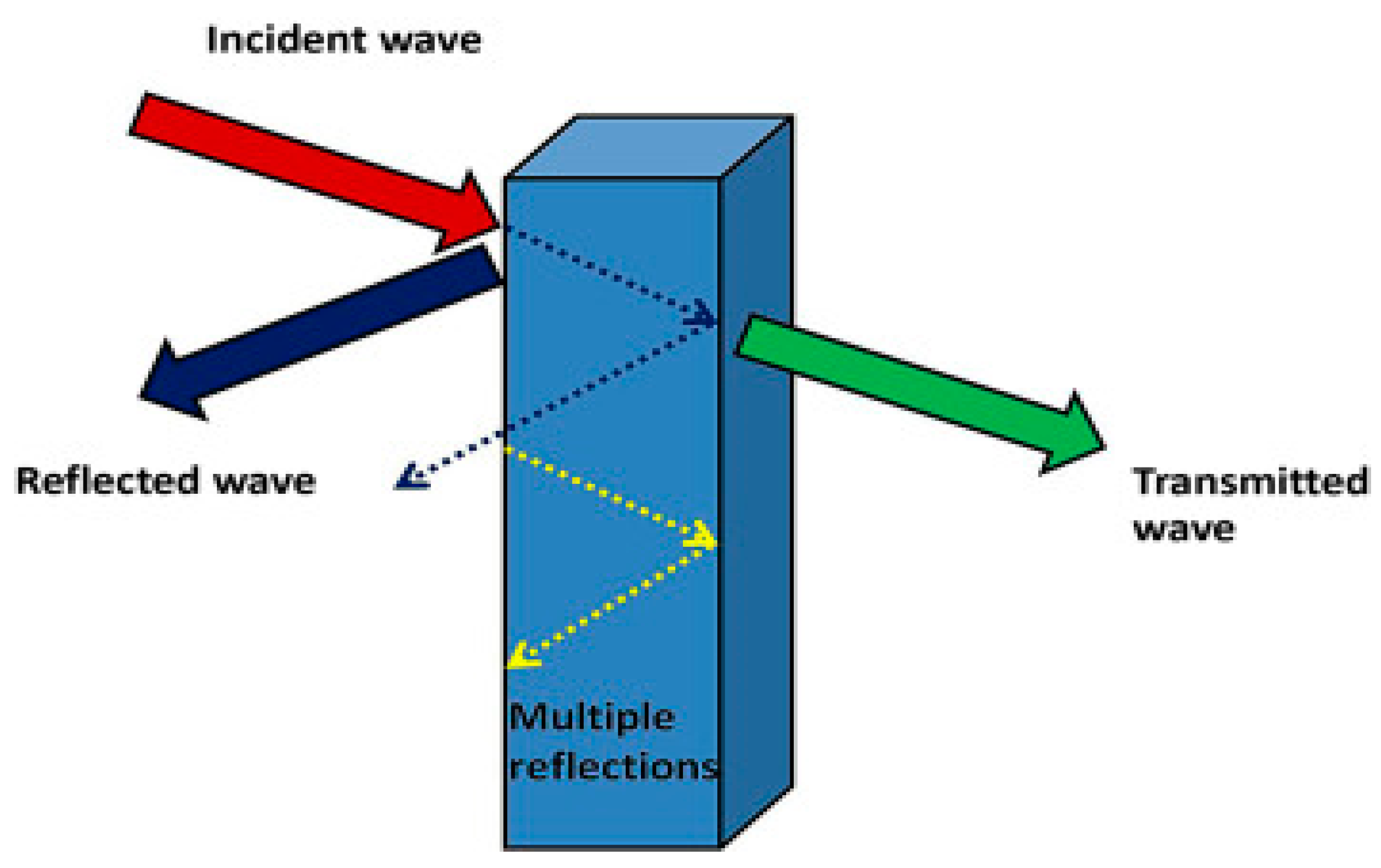
4.3. Electromagnetic Shielding in Aerospace Applications
| Polymer Nanocomposites | Thickness d(mm) | Shielding (dB) | References |
|---|---|---|---|
| Poly (methyl methacrylate)/Multi-walled carbon nanotubes | 0.06 | 27 | [174] |
| Nitrile butadiene rubber/Fe3O4 | 2 | 80–90 | [175] |
| Poly(vinyl alcohol)/Fe3O4 | 4.5 | 6 | [176] |
| Polyurethane/Multi-walled Carbon nanotubes | 0.1–0.2 | 20–29 | [177,178] |
| Polyacrylate/Multi-walled carbon nanotubes | 1.5 | 25 | [179] |
| Polypropylene/Carbon black | 2.8 | 40 | [180] |
| Polysulfone/Carbon nofiber | 1 | 45 | [181] |
| Polylactide/Graphene | 1.5 | 15 | [182] |
| Polyaniline/Grahene | 2.5 | 45.1 | [183] |
| Polyetherimide/Graphene | 2.3 | 44 | [184] |
| Poly (methyl methacrylate)/Single-walled carbon nanotubes | 4.5 | 40 | [185] |
4.4. Food Packaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, X.; Huang, C.; Wang, L.; Liang, L.; Cheng, Y.; Fei, W.; Li, Y. Recent Progress in Graphene/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2001105. [Google Scholar] [CrossRef]
- Chow, W.S.; Ishak, Z.A.M. Smart polymer nanocomposites: A review. Express Polym. Lett. 2020, 14, 416–435. [Google Scholar] [CrossRef]
- Alateyah, A.I.; Dhakal, H.; Zhang, Z. Processing, Properties, and Applications of Polymer Nanocomposites Based on Layer Silicates: A Review. Adv. Polym. Technol. 2013, 32. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, X.; Ellingford, C.; Zhang, Y.; Chen, S.; Zhou, K.; Zhang, D.; Bowen, C.R.; Wan, C. Interface design for high energy density polymer nanocomposites. Chem. Soc. Rev. 2019, 48, 4424–4465. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fahy, W.; Kim, S.; Kim, H.; Zhao, N.; Pilato, L.; Kafi, A.; Bateman, S.; Koo, J. Recent developments in polymers/polymer nanocomposites for additive manufacturing. Prog. Mater. Sci. 2020, 111, 100638. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Bhaduri, D.; Sarkar, B.; Rusmin, R.; Hou, D.; Khanam, R.; Sarkar, S.; Biswas, J.K.; Vithanage, M.; Bhatnagar, A.; et al. Clay–polymer nanocomposites: Progress and challenges for use in sustainable water treatment. J. Hazard. Mater. 2020, 383, 121125. [Google Scholar] [CrossRef]
- Yuan, M.; Hu, M.; Dai, F.; Fan, Y.; Deng, Z.; Deng, H.; Cheng, Y. Application of synthetic and natural polymers in surgical mesh for pelvic floor reconstruction. Mater. Des. 2021, 209, 109984. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef]
- Vera, M.; Mella, C.; Urbano, B.F. Smart Polymer Nanocomposites: Recent Advances and Perspectives. J. Chil. Chem. Soc. 2020, 65, 4973–4981. [Google Scholar] [CrossRef]
- Talaat, A.; Suraj, M.; Byerly, K.; Wang, A.; Wang, Y.; Lee, J.; Ohodnicki, P. Review on soft magnetic metal and inorganic oxide nanocomposites for power applications. J. Alloy Compd. 2021, 870, 159500. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539–2544. [Google Scholar] [CrossRef]
- Jawed, A.; Saxena, V.; Pandey, L. Engineered nanomaterials and their surface functionalization for the removal of heavy metals: A review. J. Water Process Eng. 2020, 33, 101009. [Google Scholar] [CrossRef]
- Wanna, Y.; Chindaduang, A.; Tumcharern, G.; Phromyothin, D.; Porntheerapat, S.; Nukeaw, J.; Hofmann, H.; Pratontep, S. Efficiency of SPIONs functionalized with polyethylene glycol bis(amine) for heavy metal removal. J. Magn. Magn. Mater. 2016, 414, 32–37. [Google Scholar] [CrossRef]
- Hashim, A.; Agool, I.R.; Kadhim, K.J. Modern Developments in Polymer Nanocomposites For Antibacterial and Antimicrobial Applications: A Review. J. Bionanosci. 2018, 12, 608–613. [Google Scholar] [CrossRef]
- Luan, J.; Wang, S.; Hu, Z.; Zhang, L. Synthesis Techniques, Properties and Applications of Polymer Nanocomposites. Curr. Org. Synth. 2012, 9, 114–136. [Google Scholar] [CrossRef]
- Saraswathi, M.S.S.A.; Nagendran, A.; Rana, D. Tailored polymer nanocomposite membranes based on carbon, metal oxide and silicon nanomaterials: A review. J. Mater. Chem. A 2019, 7, 8723–8745. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.E.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the Processing and Properties of Polymer Nanocomposites and Nanocoatings and Their Applications in the Packaging, Automotive and Solar Energy Fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef]
- Shameem, M.M.; Sasikanth, S.; Annamalai, R.; Raman, R.G. A brief review on polymer nanocomposites and its applications. In Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2021; Volume 45, pp. 2536–2539. [Google Scholar]
- Porel, S.; Venkatram, N.; Rao, D.N.; Radhakrishnan, T.P. In situ synthesis of metal nanoparticles in polymer matrix and their optical limiting applications. J. Nanosci. Nanotechnol. 2007, 7, 1887–1892. [Google Scholar] [CrossRef]
- Kashihara, K.; Uto, Y.; Nakajima, T. Rapid in situ synthesis of polymer-metal nanocomposite films in several seconds using a CO2 laser. Sci. Rep. 2018, 8, 14719. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.M.; Dalod, A.R.M.; Balci, M.H.; Glaum, J.; Einarsrud, M.-A. In Situ Synthesis of Hybrid Inorganic–Polymer Nanocomposites. Polymers 2018, 10, 1129. [Google Scholar] [CrossRef]
- Akpan, E.; Shen, X.; Wetzel, B.; Friedrich, K. Design and Synthesis of Polymer Nanocomposites. In Polymer Composites with Functionalized Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 47–83. [Google Scholar] [CrossRef]
- Lü, T.; Zhang, S.; Qi, D.; Zhang, D.; Zhao, H. Thermosensitive poly(N-isopropylacrylamide)-grafted magnetic nanoparticles for efficient treatment of emulsified oily wastewater. J. Alloy Compd. 2016, 688, 513–520. [Google Scholar] [CrossRef]
- Munnawar, I.; Iqbal, S.S.; Anwar, M.N.; Batool, M.; Tariq, S.; Faitma, N.; Khan, A.L.; Khan, A.U.; Nazar, U.; Jamil, T.; et al. Synergistic effect of Chitosan-Zinc Oxide Hybrid Nanoparticles on antibiofouling and water disinfection of mixed matrix polyethersulfone nanocomposite membranes. Carbohydr. Polym. 2017, 175, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xu, Z.; Chen, L.; Shan, M.; Tian, X.; Yang, C.; Lv, H.; Qian, X. A facile method to produce graphene oxide-g-poly(L-lactic acid) as an promising reinforcement for PLLA nanocomposites. Chem. Eng. J. 2014, 237, 291–299. [Google Scholar] [CrossRef]
- Singla, P.; Mehta, R.; Upadhyay, S. Microwave assisted in situ ring-opening polymerization of polylactide/clay nanocomposites: Effect of clay loading. Appl. Clay Sci. 2014, 95, 67–73. [Google Scholar] [CrossRef]
- de Oliveira, A.D.; Beatrice, C.A.G. Polymer nanocomposites with different types of nanofiller. In Nanocomposites-Recent Evolutions; IntechOpen Limited: Rijeka, Croatia, 2018; pp. 103–104. Available online: https://www.intechopen.com/chapters/64843 (accessed on 21 October 2021). [CrossRef]
- Cheng, S.; Grest, G.S. Dispersing Nanoparticles in a Polymer Film via Solvent Evaporation. ACS Macro Lett. 2016, 5, 694–698. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Diez-Vicente, A.L. ZnO-reinforced poly(3-hydroxybutyrate-co-3-hydroxyvalerate) bionanocomposites with antimicrobial function for food packaging. ACS Appl. Mater. Interfaces 2014, 6, 9822–9834. [Google Scholar] [CrossRef] [PubMed]
- Mehr, R.K.; Peighambardoust, S.J. Preparation and Characterization of Corn Starch/Clay Nanocomposite Films: Effect of Clay Content and Surface Modification. Starch-Stärke 2018, 70, 1700251. [Google Scholar] [CrossRef]
- Hussein, L.I.; Abdaleem, A.H.; Darwish, M.S.A.; ElSawy, M.A.; Mostafa, M.H.; Hassan, A. Chitosan/TiO2 nanocomposites: Effect of microwave heating and solution mixing techniques on physical properties. Egypt J. Chem. 2020, 63, 449–460. [Google Scholar] [CrossRef]
- Di, Y.; Iannace, S.; Di Maio, E.; Nicolais, L. Nanocomposites by melt intercalation based on polycaprolactone and organoclay. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 670–678. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef] [PubMed]
- Anžlovar, A.; Kržan, A.; Žagar, E. Degradation of PLA/ZnO and PHBV/ZnO composites prepared by melt processing. Arab. J. Chem. 2018, 11, 343–352. [Google Scholar] [CrossRef]
- Carli, L.N.; Crespo, J.D.S.; Mauler, R.S. PHBV nanocomposites based on organomodified montmorillonite and halloysite: The effect of clay type on the morphology and thermal and mechanical properties. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1601–1608. [Google Scholar] [CrossRef]
- Darwish, M.S.A.; Mostafa, M.H.; Hussein, L.I.; Abdaleem, A.H.; Elsawy, M.A. Preparation, characterization, mechanical and biodegradation behavior of polypropylene—chitosan/ZnO nanocomposite. Polym. Technol. Mater. 2021, 60, 1630–1640. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.A. Clay-Based Polymer Nanocomposites: Essential Work of Fracture. Polymers 2021, 13, 2399. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Wang, X.; Lin, T. Needleless Electrospinning: Developments and Performances. In Nanofibers—Production, Properties and Functional Applications; IntechOpen Limited: Rijeka, Croatia, 2011; pp. 17–36. Available online: https://www.intechopen.com/chapters/23290 (accessed on 1 October 2021). [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Yu, W.; Lan, C.-H.; Wang, S.-J.; Fang, P.-F.; Sun, Y.-M. Influence of zinc oxide nanoparticles on the crystallization behavior of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofibers. Polymers 2010, 51, 2403–2409. [Google Scholar] [CrossRef]
- Cai, J.; Lei, M.; Zhang, Q.; He, J.-R.; Chen, T.; Liu, S.; Fu, S.-H.; Li, T.-T.; Liu, G.; Fei, P. Electrospun composite nanofiber mats of Cellulose@Organically modified montmorillonite for heavy metal ion removal: Design, characterization, evaluation of absorption performance. Compos. Part A Appl. Sci. Manuf. 2017, 92, 10–16. [Google Scholar] [CrossRef]
- Mahdieh, Z.M.; Mottaghitalab, V.; Piri, N.; Haghi, A.K. Conductive chitosan/multi walled carbon nanotubes electrospun nanofiber feasibility. Korean J. Chem. Eng. 2011, 29, 111–119. [Google Scholar] [CrossRef]
- Chan KH, K.; Wong, S.Y.; Tiju, W.C.; Li, X.; Kotaki, M.; He, C.B. Morphologies and electrical properties of electrospun poly [(R)-3-hydroxybutyrate-co-(R)-3-hydroxyvalerate]/multiwalled carbon nanotubes fibers. J. Appl. Polym. Sci. 2020, 116, 1030–1035. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Multiwalled Carbon Nanotube (MWCNT) Reinforced Cellulose Fibers by Electrospinning. ACS Appl. Mater. Interfaces 2010, 2, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Talal, J.H.; Mohammed, D.B.; Jawad, K.H. Fabrication of Hydrophobic Nanocomposites Coating Using Electrospinning Technique for Various Substrate. J. Phys. Conf. Ser. 2018, 1032, 012033. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Elsawy, M.A.; Darwish, M.S.; Hussein, L.I.; Abdaleem, A.H. Microwave-Assisted preparation of Chitosan/ZnO nanocomposite and its application in dye removal. Mater. Chem. Phys. 2020, 248, 122914. [Google Scholar] [CrossRef]
- López-Cruz, A.; Barrera, C.; Calero-DdelC, V.L.; Rinaldi, C. Water dispersible iron oxide nanoparticles coated with covalently linked chitosan. J. Mater. Chem. 2009, 19, 6870–6876. [Google Scholar] [CrossRef]
- Yan, L.; Chang, P.R.; Zheng, P. Preparation and characterization of starch-grafted multiwall carbon nanotube composites. Carbohydr. Polym. 2011, 84, 1378–1383. [Google Scholar] [CrossRef]
- Hoogenboom, R. Temperature-Responsive Polymers: Properties, Synthesis, and Applications. In Smart Polymers and their Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–44. [Google Scholar] [CrossRef]
- Niskanen, J.; Tenhu, H. How to manipulate the upper critical solution temperature (UCST)? Polym. Chem. 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Yaghoubi, Z.; Basiri-Parsa, J. Modification of ultrafiltration membrane by thermo-responsive Bentonite-poly(N-isopropylacrylamide) nanocomposite to improve its antifouling properties. J. Water Process. Eng. 2020, 34, 101067. [Google Scholar] [CrossRef]
- Tatiana, N.M.; Cornelia, V.; Tatia, R.; Aurica, C. Hybrid collagen/pNIPAAM hydrogel nanocomposites for tissue engineering application. Colloid Polym. Sci. 2018, 296, 1555–1571. [Google Scholar] [CrossRef]
- Demir Oğuz, Ö.; Ege, D. Rheological and Mechanical Properties of Thermoresponsive Methylcellulose/Calcium Phosphate-Based Injectable Bone Substitutes. Materials 2018, 11, 604. [Google Scholar] [CrossRef]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.; Liu, Y.; Chang, S.; Chen, H.; Chen, J.-H. Surface Plasmonic Sensors: Sensing Mechanism and Recent Applications. Sensors 2021, 21, 5262. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Kinnear, C.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Plasmonic polymer nanocomposites. Nat. Rev. Mater. 2018, 3, 375–391. [Google Scholar] [CrossRef]
- Zhu, C.; Lu, Y.; Peng, J.; Chen, J.; Yu, S. Photothermally sensitive poly (N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels as remote light-controlled liquid microvalves. Adv. Funct. Mater. 2012, 22, 4017–4022. [Google Scholar] [CrossRef]
- Li, H.; Yao, Y.; Shi, H.; Lei, Y.; Huang, Y.; Wang, K.; He, X.; Liu, J. A near-infrared light-responsive nanocomposite for photothermal release of H2S and suppression of cell viability. J. Mater. Chem. B 2019, 7, 5992–5997. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Yang, N.; Zhu, M.; Xu, G.; Liu, N.; Yu, C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J. Mater. Chem. B 2020, 8, 3908–3917. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; He, L.; Tang, Y.; Yang, L.; Wu, B.; Ni, J. Facile design and development of photoluminescent graphene quantum dots grafted dextran/glycol-polymeric hydrogel for thermoresponsive triggered delivery of buprenorphine on pain management in tissue implantation. J. Photochem. Photobiol. B Biol. 2019, 197, 111530. [Google Scholar] [CrossRef] [PubMed]
- Ratna, D.; Karger-Kocsis, J. Recent advances in shape memory polymers and composites: A review. J. Mater. Sci. 2008, 43, 254–269. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Zheng, X.; Li, H.-F.; Lin, J.-M. Application of carbon-based nanomaterials in sample preparation: A review. Anal. Chim. Acta 2013, 784, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, Z.; Chen, C.; Shi, K.; Zhang, L.; Ju, X.-J.; Wang, W.; Xie, R.; Chu, L.-Y. Reduced Graphene Oxide-Containing Smart Hydrogels with Excellent Electro-Response and Mechanical Properties for Soft Actuators. ACS Appl. Mater. Interfaces 2017, 9, 15758–15767. [Google Scholar] [CrossRef] [PubMed]
- Soto, G.; Meiorin, C.; Actis, D.; Zélis, P.M.; Londoño, O.M.; Muraca, D.; Mosiewicki, M.; Marcovich, N. Magnetic nanocomposites based on shape memory polyurethanes. Eur. Polym. J. 2018, 109, 8–15. [Google Scholar] [CrossRef]
- Li, J.; Zheng, L.; Cai, H.; Sun, W.; Shen, M.; Zhang, G.; Shi, X. Polyethyleneimine-mediated synthesis of folic acid-targeted iron oxide nanoparticles for in vivo tumor MR imaging. Biomaterials 2013, 34, 8382–8392. [Google Scholar] [CrossRef] [PubMed]
- Nitin, N.; LaConte, L.; Zurkiya, O.; Hu, X.; Bao, G. Functionalization and peptide-based delivery of magnetic nanoparticles as an intracellular MRI contrast agent. JBIC J. Biol. Inorg. Chem. 2004, 9, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Maenosono, S.; Saita, S. Theoretical Assessment of FePt Nanoparticles as Heating Elements for Magnetic Hyperthermia. IEEE Trans. Magn. 2006, 42, 1638–1642. [Google Scholar] [CrossRef]
- Stauffer, P.; Cetas, T.C.; Jones, R.C. Magnetic Induction Heating of Ferromagnetic Implants for Inducing Localized Hyperthermia in Deep-Seated Tumors. IEEE Trans. Biomed. Eng. 1984, 31, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Latorre, M.; Rinaldi, C. Applications of magnetic nanoparticles in medicine: Magnetic fluid hyperthermia. Puerto Rico Health Sci. J. 2009, 28, 227–238. [Google Scholar]
- Niemirowicz, K.; Markiewicz, K.; Wilczewska, A.; Car, H. Magnetic nanoparticles as new diagnostic tools in medicine. Adv. Med. Sci. 2012, 57, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Ilg, P.; Kröger, M. Dynamics of interacting magnetic nanoparticles: Effective behavior from competition between Brownian and Néel relaxation. Phys. Chem. Chem. Phys. 2020, 22, 22244–22259. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.; Stibor, I. Preparation and characterization of magnetite–PDMS composites by magnetic induction heating. Mater. Chem. Phys. 2015, 164, 163–169. [Google Scholar] [CrossRef]
- Yang, J.; Fan, L.; Xu, Y.; Xia, J. Iron oxide nanoparticles with different polymer coatings for photothermal therapy. J. Nanopart. Res. 2017, 19, 333. [Google Scholar] [CrossRef]
- Bardajee, G.R.; Hooshyar, Z.; Rastgo, F. Kappa carrageenan-g-poly (acrylic acid)/SPION nanocomposite as a novel stimuli-sensitive drug delivery system. Colloid Polym. Sci. 2013, 291, 2791–2803. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Gupta, R. Magnetically mediated release of ciprofloxacin from polyvinyl alcohol based superparamagnetic nanocomposites. J. Mater. Sci. Mater. Electron. 2011, 22, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Lemine, O. Magnetic Hyperthermia Therapy Using Hybrid Magnetic Nanostructures. In Hybrid Nanostructures for Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 125–138. [Google Scholar] [CrossRef]
- Kawai, N.; Kobayashi, D.; Yasui, T.; Umemoto, Y.; Mizuno, K.; Okada, A.; Tozawa, K.; Kobayashi, T.; Kohri, K. Evaluation of side effects of radiofrequency capacitive hyperthermia with magnetite on the blood vessel walls of tumor metastatic lesion surrounding the abdominal large vessels: An agar phantom study. Vasc. Cell 2014, 6, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Nardo, S.J.; DeNardo, G.L.; Miers, L.A.; Natarajan, A.; Foreman, A.R.; Gruettner, C.; Adamson, G.N.; Ivkov, R. Development of Tumor Targeting Bioprobes (111In-Chimeric L6 Monoclonal Antibody Nanoparticles) for Alternating Magnetic Field Cancer Therapy. Clin. Cancer Res. 2005, 11, 7087s–7092s. [Google Scholar] [CrossRef] [PubMed]
- Ivkov, R.; DeNardo, S.J.; Daum, W.; Foreman, A.R.; Goldstein, R.C.; Nemkov, V.S.; DeNardo, G.L. Application of High Amplitude Alternating Magnetic Fields for Heat Induction of Nanoparticles Localized in Cancer. Clin. Cancer Res. 2005, 11, 7093s–7103s. [Google Scholar] [CrossRef]
- Darwish, M.S.; Nguyen, N.H.; Ševců, A.; Stibor, I.; Smoukov, S.K. Dual-modality self-heating and antibacterial polymer-coated nanoparticles for magnetic hyperthermia. Mater. Sci. Eng. C 2016, 63, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Akharame, M.O.; Fatoki, O.S.; Opeolu, B.O.; Olorunfemi, D.I.; Oputu, O.U. Polymeric Nanocomposites (PNCs) for Wastewater Remediation: An Overview. Polym. Technol. Eng. 2018, 57, 1801–1827. [Google Scholar] [CrossRef]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Tam, K.C. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Khan, M.A.; Govindasamy, R.; Ahmad, A.; Siddiqui, M.; Alshareef, S.; Hakami, A.; Rafatullah, M. Carbon Based Polymeric Nanocomposites for Dye Adsorption: Synthesis, Characterization, and Application. Polymers 2021, 13, 419. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Orasugh, J.T.; Banerjee, P.; Dutta, S.; Ali, M.S.; Das, D.; Bhattacharya, A.; Chattopadhyay, D. Facile one-pot in-situ synthesis of novel graphene oxide-cellulose nanocomposite for enhanced azo dye adsorption at optimized conditions. Carbohydr. Polym. 2020, 246, 116661. [Google Scholar] [CrossRef]
- Peng, N.; Hu, D.; Zeng, J.; Li, Y.; Liang, L.; Chang, C. Superabsorbent Cellulose–Clay Nanocomposite Hydrogels for Highly Efficient Removal of Dye in Water. ACS Sustain. Chem. Eng. 2016, 4, 7217–7224. [Google Scholar] [CrossRef]
- Wang, N.; Chen, J.; Wang, J.; Feng, J.; Yan, W. Removal of methylene blue by Polyaniline/TiO2 hydrate: Adsorption kinetic, isotherm and mechanism studies. Powder Technol. 2019, 347, 93–102. [Google Scholar] [CrossRef]
- Gelaw, T.B.; Sarojini, B.K.; Kodoth, A.K. Review of the Advancements on Polymer/Metal Oxide Hybrid Nanocomposite-Based Adsorption Assisted Photocatalytic Materials for Dye Removal. ChemistrySelect 2021, 6, 9300–9310. [Google Scholar] [CrossRef]
- Kumar, P.S.; Selvakumar, M.; Babu, S.G.; Jaganathan, S.K.; Karuthapandian, S.; Chattopadhyay, S. Novel CuO/chitosan nanocomposite thin film: Facile hand-picking recoverable, efficient and reusable heterogeneous photocatalyst. RSC Adv. 2015, 5, 57493–57501. [Google Scholar] [CrossRef]
- Shaikh, T.; Rathore, A.; Kaur, H. Poly (Lactic Acid) Grafting of TiO2Nanoparticles: A Shift in Dye Degradation Performance of TiO2 from UV to Solar Light. ChemistrySelect 2017, 2, 6901–6908. [Google Scholar] [CrossRef]
- Karagoz, S.; Kiremitler, N.B.; Sakir, M.; Salem, S.; Onses, M.S.; Sahmetlioglu, E.; Ceylan, A.; Yilmaz, E. Synthesis of Ag and TiO2 modified polycaprolactone electrospun nanofibers (PCL/TiO2-Ag NFs) as a multifunctional material for SERS, photocatalysis and antibacterial applications. Ecotoxicol. Environ. Saf. 2020, 188, 109856. [Google Scholar] [CrossRef]
- Farzana, M.H.; Meenakshi, S. Photo-decolorization and detoxification of toxic dyes using titanium dioxide impregnated chitosan beads. Int. J. Biol. Macromol. 2014, 70, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, F.; Sadighian, S.; Hosseini-Monfared, H.; Mahmoodi, N.M. Dye removal and kinetics of adsorption by magnetic chitosan nanoparticles. Desalin. Water Treat. 2016, 57, 24378–24386. [Google Scholar] [CrossRef]
- Esvandi, Z.; Foroutan, R.; Peighambardoust, S.J.; Akbari, A.; Ramavandi, B. Uptake of anionic and cationic dyes from water using natural clay and clay/starch/MnFe2O4 magnetic nanocomposite. Surfaces Interfaces 2020, 21, 100754. [Google Scholar] [CrossRef]
- Guan, Y.; Yu, H.-Y.; Abdalkarim, S.Y.H.; Wang, C.; Tang, F.; Marek, J.; Chen, W.-L.; Militky, J.; Yao, J.-M. Green one-step synthesis of ZnO/cellulose nanocrystal hybrids with modulated morphologies and superfast absorption of cationic dyes. Int. J. Biol. Macromol. 2019, 132, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Choubey, A.K. Investigation of adsorption of organic dyes present in wastewater using chitosan beads immobilized with bio fabricated CuO nanoparticles. J. Mol. Struct. 2021, 1242, 130749. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abdelbar, N.M.; Mohamed, A.A. Molecular imprinted chitosan-TiO2 nanocomposite for the selective removal of Rose Bengal from wastewater. Int. J. Biol. Macromol. 2018, 107, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.I.; Khafagy, R.M.; Hussien, M.S.A.; Sakr, G.B.; Ibrahim, M.A.; Yahia, I.S.; Zahran, H.Y. Enhancing the structural, optical, electrical, properties and photocatalytic applications of ZnO/PMMA nanocomposite membranes: Towards multifunctional membranes. J. Mater. Sci. Mater. Electron. 2021, 1–26. [Google Scholar] [CrossRef]
- Hussein, M.A.; Albeladi, H.K.; Elsherbiny, A.S.; El-Shishtawy, R.M.; Al-romaizan, A.N. Cross-linked poly (methyl methacrylate)/multiwall carbon nanotube nanocomposites for environmental treatment. Adv. Polym. Technol. 2018, 37, 3240–3251. [Google Scholar] [CrossRef]
- Samadder, R.; Akter, N.; Roy, A.C.; Uddin, M.; Hossen, J.; Azam, S. Magnetic nanocomposite based on polyacrylic acid and carboxylated cellulose nanocrystal for the removal of cationic dye. RSC Adv. 2020, 10, 11945–11956. [Google Scholar] [CrossRef]
- Saberi, A.; Alipour, E.; Sadeghi, M. Superabsorbent magnetic Fe3O4-based starch-poly (acrylic acid) nanocomposite hydrogel for efficient removal of dyes and heavy metal ions from water. J. Polym. Res. 2019, 26, 271. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, K.; Liu, H.; Wang, L.; Xiao, X.; Dou, D.; Fan, Y. Three-dimensional polylactic acid@graphene oxide/chitosan sponge bionic filter: Highly efficient adsorption of crystal violet dye. Int. J. Biol. Macromol. 2018, 113, 792–803. [Google Scholar] [CrossRef]
- Senguttuvan, S.; Janaki, V.; Senthilkumar, P.; Kamala-Kannan, S. Polypyrrole/zeolite composite–A nanoadsorbent for reactive dyes removal from synthetic solution. Chemosphere 2022, 287, 132164. [Google Scholar] [CrossRef]
- Agboola, O.; Fayomi, O.; Ayodeji, A.; Ayeni, A.; Alagbe, E.; Sanni, S.; Okoro, E.; Moropeng, L.; Sadiku, R.; Kupolati, K.; et al. A Review on Polymer Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation. Membranes 2021, 11, 139. [Google Scholar] [CrossRef] [PubMed]
- Momina; Ahmad, K. Study of different polymer nanocomposites and their pollutant removal efficiency: Review. Polymers 2021, 217, 123453. [Google Scholar] [CrossRef]
- Ebrahim, S.; Shokry, A.; Ibrahim, H.; Soliman, M. Polyaniline/akaganéite nanocomposite for detoxification of noxious Cr(VI) from aquatic environment. J. Polym. Res. 2016, 23, 79. [Google Scholar] [CrossRef]
- Akl, Z.F.; El-Saeed, S.M.; Atta, A.M. In-situ synthesis of magnetite acrylamide amino-amidoxime nanocomposite adsorbent for highly efficient sorption of U(VI) ions. J. Ind. Eng. Chem. 2016, 34, 105–116. [Google Scholar] [CrossRef]
- Tran, M.T.; Nguyen, T.H.T.; Vu, Q.T.; Nguyen, M.V. Properties of poly (1-naphthylamine)/Fe3O4 composites and arsenic adsorption capacity in wastewater. Front. Mater. Sci. 2016, 10, 56–65. [Google Scholar] [CrossRef]
- Musico, Y.L.F.; Santos, C.M.; Dalida, M.L.P.; Rodrigues, D.F. Improved removal of lead(ii) from water using a polymer-based graphene oxide nanocomposite. J. Mater. Chem. A 2013, 1, 3789–3796. [Google Scholar] [CrossRef]
- Bagheripour, E.; Moghadassi, A.; Hosseini, S.; Nemati, M. Fabrication and Characterization of Novel Mixed Matrix Polyethersulfone Nanofiltration Membrane Modified by Iron-Nickel Oxide Nanoparticles. J. Membr. Sci. Res 2016, 2, 14–19. [Google Scholar]
- Mittal, A.; Ahmad, R.; Hasan, I. Poly (methyl methacrylate)-grafted alginate/Fe3O4 nanocomposite: Synthesis and its application for the removal of heavy metal ions. Desalin. Water Treat. 2015, 57, 19820–19833. [Google Scholar] [CrossRef]
- Saad, A.H.A.; Azzam, A.M.; El-Wakeel, S.T.; Mostafa, B.B.; El-latif, M.B.A. Removal of toxic metal ions from wastewater using ZnO@ Chitosan core-shell nanocomposite. Environ. Nanotechnol. Monit. Manag. 2018, 9, 67–75. [Google Scholar] [CrossRef]
- Zhao, G.; Huang, X.; Tang, Z.; Huang, Q.; Niu, F.; Wang, X.-K. Polymer-based nanocomposites for heavy metal ions removal from aqueous solution: A review. Polym. Chem. 2018, 9, 3562–3582. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.-H.; Wu, J. Organic-Inorganic Hybrid Polymers as Adsorbents for Removal of Heavy Metal Ions from Solutions: A Review. Materials 2014, 7, 673–726. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, L.; Yang, F. Preparation of polyaniline/reduced graphene oxide nanocomposite and its application in adsorption of aqueous Hg (II). Chem. Eng. J. 2013, 229, 460–468. [Google Scholar] [CrossRef]
- Samuel, M.S.; Shah, S.S.; Subramaniyan, V.; Qureshi, T.; Bhattacharya, J.; Singh, N.P. Preparation of graphene oxide/chitosan/ferrite nanocomposite for Chromium (VI) removal from aqueous solution. Int. J. Biol. Macromol. 2018, 119, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, R.; Arsalani, N.; Panahian, Y. One-pot synthesis of novel magnetic three-dimensional graphene/chitosan/nickel ferrite nanocomposite for lead ions removal from aqueous solution: RSM modelling design. J. Clean. Prod. 2018, 201, 507–515. [Google Scholar] [CrossRef]
- Shoukat, A.; Wahid, F.; Khan, T.; Siddique, M.; Nasreen, S.; Yang, G.; Ullah, M.W.; Khan, R. Titanium oxide-bacterial cellulose bioadsorbent for the removal of lead ions from aqueous solution. Int. J. Biol. Macromol. 2019, 129, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Fallah, Z.; Isfahani, H.N.; Tajbakhsh, M.; Tashakkorian, H.; Amouei, A. TiO2-grafted cellulose via click reaction: An efficient heavy metal ions bioadsorbent from aqueous solutions. Cellulose 2017, 25, 639–660. [Google Scholar] [CrossRef]
- Moreno-Sader, K.; García-Padilla, A.; Realpe, A.; Acevedo-Morantes, M.; Soares, J.B.P. Removal of heavy metal water pollutants (Co2+ and Ni2+) using polyacrylamide/sodium montmorillonite (PAM/Na-MMT) nanocomposites. ACS Omega 2019, 4, 10834–10844. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Siddiqui, M.F.; Alam Khan, T. Ultrasonic-assisted synthesis of polyacrylamide/bentonite hydrogel nanocomposite for the sequestration of lead and cadmium from aqueous phase: Equilibrium, kinetics and thermodynamic studies. Ultrason. Sonochem. 2019, 60, 104761. [Google Scholar] [CrossRef] [PubMed]
- Mohammadnezhad, G.; Moshiri, P.; Dinari, M.; Steiniger, F. In situ synthesis of nanocomposite materials based on modified-mesoporous silica MCM-41 and methyl methacrylate for copper (II) adsorption from aqueous solution. J. Iran. Chem. Soc. 2019, 16, 1491–1500. [Google Scholar] [CrossRef]
- Ahmad, R.; Hasan, I. Efficient remediation of an aquatic environment contaminated by Cr (VI) and 2, 4-dinitrophenol by XG-g-polyaniline@ ZnO nanocomposite. J. Chem. Eng. Data 2017, 62, 1594–1607. [Google Scholar] [CrossRef]
- Chen, L.; Peng, X. Silver nanoparticle decorated cellulose nanofibrous membrane with good antibacterial ability and high water permeability. Appl. Mater. Today 2017, 9, 130–135. [Google Scholar] [CrossRef]
- Sarkandi, A.F.; Montazer, M.; Harifi, T.; Rad, M.M. Innovative preparation of bacterial cellulose/silver nanocomposite hydrogels: In situ green synthesis, characterization, and antibacterial properties. J. Appl. Polym. Sci. 2021, 138, 49824. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Li, C.; Cao, J.; He, E.; Wu, X.; Wang, F.; Wang, L. Facile preparation PCL/ modified nano ZnO organic-inorganic composite and its application in antibacterial materials. J. Polym. Res. 2020, 27, 78. [Google Scholar] [CrossRef]
- Al-Naamani, L.; Dobretsov, S.; Dutta, J.; Burgess, J.G. Chitosan-zinc oxide nanocomposite coatings for the prevention of marine biofouling. Chemosphere 2017, 168, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Obaid, A.Y.; El-Shishtawy, R.M.; Mohamed, S.A. Synthesis of nanocomposites of polypyrrole/carbon nanotubes/silver nano particles and their application in water disinfection. RSC Adv. 2017, 7, 16878–16884. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics Integrated Biosensors: A Leading Technology towards Lab-on-a-Chip and Sensing Applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef]
- Gupta, S.; Sharma, A.; Verma, R.S. Polymers in biosensor devices for cardiovascular applications. Curr. Opin. Biomed. Eng. 2020, 13, 69–75. [Google Scholar] [CrossRef]
- Olowu, R.A.; Arotiba, O.; Mailu, S.N.; Waryo, T.T.; Baker, P.; Iwuoha, E. Electrochemical Aptasensor for Endocrine Disrupting 17β-Estradiol Based on a Poly(3,4-ethylenedioxylthiopene)-Gold Nanocomposite Platform. Sensors 2010, 10, 9872–9890. [Google Scholar] [CrossRef] [PubMed]
- Narang, J.; Chauhan, N.; Pundir, C. Construction of triglyceride biosensor based on nickel oxide–chitosan/zinc oxide/zinc hexacyanoferrate film. Int. J. Biol. Macromol. 2013, 60, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Demircan, D.; Ilk, S.; Zhang, B. Cellulose-Organic Montmorillonite Nanocomposites as Biomacromolecular Quorum-Sensing Inhibitor. Biomacromolecules 2017, 18, 3439–3446. [Google Scholar] [CrossRef] [PubMed]
- Manno, D.; Filippo, E.; Di Giulio, M.; Serra, A. Synthesis and characterization of starch-stabilized Ag nanostructures for sensors applications. J. Non-Cryst. Solids 2008, 354, 5515–5520. [Google Scholar] [CrossRef]
- Singh, K.K.; Solanki, P.R.; Basu, T.; Malhotra, B.D. Polypyrrole/multiwalled carbon nanotubes-based biosensor for cholesterol estimation. Polym. Adv. Technol. 2012, 23, 1084–1091. [Google Scholar] [CrossRef]
- Kumar, B.; Castro, M.; Feller, J. Poly(lactic acid)–multi-wall carbon nanotube conductive biopolymer nanocomposite vapour sensors. Sens. Actuators B Chem. 2012, 161, 621–628. [Google Scholar] [CrossRef]
- Zhu, P.; Liu, Y.; Fang, Z.; Kuang, Y.; Zhang, Y.; Peng, C.; Chen, G. Flexible and Highly Sensitive Humidity Sensor Based on Cellulose Nanofibers and Carbon Nanotube Composite Film. Langmuir 2019, 35, 4834–4842. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, L.; Huo, J.; Yu, H. A novel polycaprolactone-grafted-carbon black nanocomposite-based sensor for detecting solvent vapors. J. Appl. Polym. Sci. 2011, 121, 3277–3282. [Google Scholar] [CrossRef]
- Pandey, S.; Goswami, G.; Nanda, K.K. Green synthesis of biopolymer–silver nanoparticle nanocomposite: An optical sensor for ammonia detection. Int. J. Biol. Macromol. 2012, 51, 583–589. [Google Scholar] [CrossRef]
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sens. Actuators B Chem. 2019, 283, 786–792. [Google Scholar] [CrossRef]
- Chakraborty, G.; Pugazhenthi, G.; Katiyar, V. Exfoliated graphene-dispersed poly (lactic acid)-based nanocomposite sensors for ethanol detection. Polym. Bull. 2018, 76, 2367–2386. [Google Scholar] [CrossRef]
- Khachatryan, G.; Khachatryan, K. Starch based nanocomposites as sensors for heavy metals—detection of Cu2+ and Pb2+ ions. Int. Agrophys. 2019, 33, 121–126. [Google Scholar] [CrossRef]
- Nie, D.; Han, Z.; Yu, Y.; Shi, G. Composites of multiwalled carbon nanotubes/polyethyleneimine (MWCNTs/PEI) and molecularly imprinted polymers for dinitrotoluene recognition. Sens. Actuators B Chem. 2016, 224, 584–591. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, E.; Gu, D.; Wang, Y. Glassy carbon electrode coated with polyaniline-functionalized carbon nanotubes for detection of trace lead in acetate solution. Thin Solid Films 2011, 519, 5280–5284. [Google Scholar] [CrossRef]
- Shao, Y.; Dong, Y.; Bin, L.; Fan, L.; Wang, L.; Yuan, X.; Li, D.; Liu, X.; Zhao, S. Application of gold nanoparticles/polyaniline-multi-walled carbon nanotubes modified screen-printed carbon electrode for electrochemical sensing of zinc, lead, and copper. Microchem. J. 2021, 170, 106726. [Google Scholar] [CrossRef]
- Sharma, A.L.; Kumar, P.; Deep, A. Highly Sensitive Glucose Sensing With Multi-Walled Carbon Nanotubes—Polyaniline Composite. Polym. Technol. Eng. 2012, 51, 1382–1387. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Du, X.; Li, Y.; Tang, W. A highly sensitive glucose sensor based on a gold nanoparticles/polyaniline/multi-walled carbon nanotubes composite modified glassy carbon electrode. New J. Chem. 2018, 42, 11944–11953. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Li, S.-C.; Liao, S.-W. Electrodeposition of polypyrrole–multiwalled carbon nanotube–glucose oxidase nanobiocomposite film for the detection of glucose. Biosens. Bioelectron. 2006, 22, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Alagappan, M.; Immanuel, S.; Sivasubramanian, R.; Kandaswamy, A. Development of cholesterol biosensor using Au nanoparticles decorated f-MWCNT covered with polypyrrole network. Arab. J. Chem. 2020, 13, 2001–2010. [Google Scholar] [CrossRef]
- Maity, D.; Kumar, R.T.R. Polyaniline Anchored MWCNTs on Fabric for High Performance Wearable Ammonia Sensor. ACS Sens. 2018, 3, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, X.; Yuan, Z.; Jiang, Y.; Su, Y.; Ma, J.; Tai, H. A flexible NO2 gas sensor based on polypyrrole/nitrogen-doped multiwall carbon nanotube operating at room temperature. Sens. Actuators B Chem. 2019, 295, 86–92. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, X.; Pan, P.; Liu, J.; Yang, Z.; Wei, J.; Xu, W.; Bao, Q.; Zhang, H.; Liao, Z. All-Printed Flexible Electrochemical Sensor Based on Polyaniline Electronic Ink for Copper (II), Lead (II) and Mercury (II) Ion Determination. J. Electron. Mater. 2020, 49, 6695–6705. [Google Scholar] [CrossRef]
- Lo, M.; Seydou, M.; Bensghaïer, A.; Pires, R.; Gningue-Sall, D.; Aaron, J.-J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole-Wrapped Carbon Nanotube Composite Films Coated on Diazonium-Modified Flexible ITO Sheets for the Electroanalysis of Heavy Metal Ions. Sensors 2020, 20, 580. [Google Scholar] [CrossRef]
- Alruwais, R.S.; Adeosun, W.A.; Alsafrani, A.E.; Marwani, H.M.; Asiri, A.M.; Khan, I.; Jawaid, M.; Khan, A. Development of Cd (II) Ion Probe Based on Novel Polyaniline-Multiwalled Carbon Nanotube-3-aminopropyltriethoxylsilane Composite. Membranes 2021, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Al-Refai, H.H.; Ganash, A.A.; Hussein, M.A. Polythiophene-based MWCNTCOOH@RGO nanocomposites as a modified glassy carbon electrode for the electrochemical detection of Hg (II) ions. Chem. Pap. 2021, 1–16. [Google Scholar] [CrossRef]
- Shukla, P.; Saxena, P. Polymer Nanocomposites in Sensor Applications: A Review on Present Trends and Future Scope. Chin. J. Polym. Sci. 2021, 39, 665–691. [Google Scholar] [CrossRef]
- He, P.; Cao, M.-S.; Cao, W.-Q.; Yuan, J. Developing MXenes from Wireless Communication to Electromagnetic Attenuation. Nano-Micro Lett. 2021, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Kumar, K.K.S.; Rao, C.R.; Vijayan, M.; Trivedi, D.C.K. EMI shielding: Methods and materials—A review. J. Appl. Polym. Sci. 2009, 112, 2073–2086. [Google Scholar] [CrossRef]
- Thomassin, J.-M.; Jérôme, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R Rep. 2013, 74, 211–232. [Google Scholar] [CrossRef]
- Chung, D.D.L. Materials for electromagnetic interference shielding. Mater. Chem. Phys. 2020, 255, 123587. [Google Scholar] [CrossRef]
- Deeraj, B.D.S.; Mathew, M.S.; Parameswaranpillai, J.; Joseph, K. EMI shielding materials based on thermosetting polymers. In Materials for Potential EMI Shielding Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–110. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M. Fabrication of magnetite nanoparticles/polyvinylpyrrolidone composite nanofibers and their application as electromagnetic interference shielding material. J. Thermoplast. Compos. Mater. 2017, 31, 431–446. [Google Scholar] [CrossRef]
- Khan, S.U.D.; Arora, M.; Wahab, M.A.; Saini, P. Permittivity and Electromagnetic Interference Shielding Investigations of Activated Charcoal Loaded Acrylic Coating Compositions. J. Polym. 2014, 2014, 193058. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Guo, J.; Hu, Z.; Wang, Z.; Wang, B.; Liu, L.; Huang, Y.; Guo, Z. Flexible, conductive, porous, fibrillar polymer–gold nanocomposites with enhanced electromagnetic interference shielding and mechanical properties. J. Mater. Chem. C 2017, 5, 1095–1105. [Google Scholar] [CrossRef]
- Joseph, N.; Varghese, J.; Sebastian, M.T. In situ polymerized polyaniline nanofiber-based functional cotton and nylon fabrics as millimeter-wave absorbers. Polym. J. 2017, 49, 391–399. [Google Scholar] [CrossRef]
- Villacorta, B.S.; Ogale, A.A.; Hubing, T.H. Effect of heat treatment of carbon nanofibers on the electromagnetic shielding effectiveness of linear low density polyethylene nanocomposites. Polym. Eng. Sci. 2013, 53, 417–423. [Google Scholar] [CrossRef]
- Huang, Y.; Li, N.; Ma, Y.; Du, F.; Li, F.; He, X.; Lin, X.; Gao, H.; Chen, Y. The influence of single-walled carbon nanotube structure on the electromagnetic interference shielding efficiency of its epoxy composites. Carbon 2007, 45, 1614–1621. [Google Scholar] [CrossRef]
- Wu, Z.P.; Li, M.M.; Hu, Y.Y.; Li, Y.S.; Wang, Z.X.; Yin, Y.H.; Chen, Y.S.; Zhou, X. Electromagnetic interference shielding of carbon nanotube macrofilms. Scr. Mater. 2011, 64, 809–812. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, H.; Wang, S.; Li, M.; Gu, Y. Electromagnetic characteristics of carbon nanotube film materials. Chin. J. Aeronaut. 2015, 28, 1245–1254. [Google Scholar] [CrossRef]
- Gouda, M.; Hebeish, A.; Aljaafari, A. New route for development of electromagnetic shielding based on cellulosic nanofibers. J. Ind. Text. 2017, 46, 1598–1615. [Google Scholar] [CrossRef]
- Pande, S.; Singh, B.; Mathur, R.; Dhami, T.; Saini, P.; Dhawan, S. Improved Electromagnetic Interference Shielding Properties of MWCNT–PMMA Composites Using Layered Structures. Nanoscale Res. Lett. 2009, 4, 327–334. [Google Scholar] [CrossRef]
- Al-Ghamdi, A.A.; Al-Hartomy, O.A.; Al-Salamy, F.; El-Mossalamy, E.H.; Daiem, A.M.A.; El-Tantawy, F. Novel electromagnetic interference shielding effectiveness in the microwave band of magnetic nitrile butadiene rubber/magnetite nanocomposites. J. Appl. Polym. Sci. 2012, 125, 2604–2613. [Google Scholar] [CrossRef]
- Chiscan, O.; Dumitru, I.; Postolache, P.; Tura, V.; Stancu, A. Electrospun PVC/Fe3O4 composite nanofibers for microwave absorption applications. Mater. Lett. 2012, 68, 251–254. [Google Scholar] [CrossRef]
- Gupta, T.K.; Singh, B.P.; Dhakate, S.R.; Singh, V.N.; Mathur, R.B. Improved nanoindentation and microwave shielding properties of modified MWCNT reinforced polyurethane composites. J. Mater. Chem. A 2013, 1, 9138–9149. [Google Scholar] [CrossRef]
- Hoang, A.S. Electrical conductivity and electromagnetic interference shielding characteristics of multiwalled carbon nanotube filled polyurethane composite films. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 25007. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Zhang, S.; Ni, Y.; Huang, J. Electrical conductivity and electromagnetic interference shielding characteristics of multiwalled carbon nanotube filled polyacrylate composite films. Appl. Surf. Sci. 2008, 254, 5766–5771. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. X-band EMI shielding mechanisms and shielding effectiveness of high structure carbon black/polypropylene composites. J. Phys. D. Appl. Phys. 2012, 46, 35304. [Google Scholar] [CrossRef]
- Nayak, L.; Khastgir, D.; Chaki, T.K. A mechanistic study on electromagnetic shielding effectiveness of polysulfone/carbon nanofibers nanocomposites. J. Mater. Sci. 2013, 48, 1492–1502. [Google Scholar] [CrossRef]
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Morphology, electromagnetic properties and electromagnetic interference shielding performance of poly lactide/graphene nanoplatelet nanocomposites. Mater. Des. 2016, 95, 119–126. [Google Scholar] [CrossRef]
- Yu, H.; Wang, T.; Wen, B.; Lu, M.; Xu, Z.; Zhu, C.; Chen, Y.; Xue, X.; Sun, C.; Cao, M. Graphene/polyaniline nanorod arrays: Synthesis and excellent electromagnetic absorption properties. J. Mater. Chem. 2012, 22, 21679–21685. [Google Scholar] [CrossRef]
- Ling, J.; Zhai, W.; Feng, W.; Shen, B.; Zhang, J.; Zheng, W.G. Facile Preparation of Lightweight Microcellular Polyetherimide/Graphene Composite Foams for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2013, 5, 2677–2684. [Google Scholar] [CrossRef]
- Das, N.C.; Liu, Y.; Yang, K.; Peng, W.; Maiti, S.; Wang, H. Single-walled carbon nanotube/poly(methyl methacrylate) composites for electromagnetic interference shielding. Polym. Eng. Sci. 2009, 49, 1627–1634. [Google Scholar] [CrossRef]
- Ganguly, S.; Bhawal, P.; Ravindren, R.; Das, N.C. Polymer Nanocomposites for Electromagnetic Interference Shielding: A Review. J. Nanosci. Nanotechnol. 2018, 18, 7641–7669. [Google Scholar] [CrossRef]
- Lyu, L.; Liu, J.; Liu, H.; Liu, C.; Lu, Y.; Sun, K.; Fan, R.; Wang, N.; Lu, N.; Guo, Z.; et al. An Overview of Electrically Conductive Polymer Nanocomposites toward Electromagnetic Interference Shielding. Eng. Sci. 2018 2, 26–42. [CrossRef]
- Vas, J.V.; Thomas, M.J. Electromagnetic Shielding Effectiveness of Layered Polymer Nanocomposites. IEEE Trans. Electromagn. Compat. 2017, 60, 376–384. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J.-W. Polymer Nanocomposites for Food Packaging Applications. In Functional and Physical Properties of Polymer Nanocomposites; Dasari, A., Njuguna, J., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2016; Volume 29, pp. 29–55. [Google Scholar] [CrossRef]
- Rahman, P.M.; Mujeeb, V.A.; Muraleedharan, K. Flexible chitosan-nano ZnO antimicrobial pouches as a new material for extending the shelf life of raw meat. Int. J. Biol. Macromol. 2017, 97, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, A.; Ghodrati, S.; Khorasani, M. High-Strength, Low-Permeable, and Light-Protective Nanocomposite Films Based on a Hybrid Nanopigment and Biodegradable PLA for Food Packaging Applications. ACS Omega 2019, 4, 14947–14954. [Google Scholar] [CrossRef]
- Pina, H.D.V.; De Farias, A.J.A.; Barbosa, F.C.; Souza, J.W.D.L.; Barros, A.B.D.S.; Cardoso, M.J.B.; Fook, M.V.L.; Wellen, R.M.R. Microbiological and cytotoxic perspectives of active PCL/ZnO film for food packaging. Mater. Res. Express 2020, 7, 025312. [Google Scholar] [CrossRef]
- Pirsa, S.; Shamusi, T.; Kia, E.M. Smart films based on bacterial cellulose nanofibers modified by conductive polypyrrole and zinc oxide nanoparticles. J. Appl. Polym. Sci. 2018, 135, 46617. [Google Scholar] [CrossRef]
- Yu, H.-Y.; Qin, Z.-Y.; Sun, B.; Yang, X.-G.; Yao, J.-M. Reinforcement of transparent poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by incorporation of functionalized carbon nanotubes as a novel bionanocomposite for food packaging. Compos. Sci. Technol. 2014, 94, 96–104. [Google Scholar] [CrossRef]
- Kumar, P.; Gautam, S. Developing ZnO Nanoparticle embedded antimicrobial starch biofilm for food packaging. arXiv 2019, arXiv:1909.05083. [Google Scholar]
- Fernández, A.; Picouet, P.; Lloret, E. Cellulose-silver nanoparticle hybrid materials to control spoilage-related microflora in absorbent pads located in trays of fresh-cut melon. Int. J. Food Microbiol. 2010, 142, 222–228. [Google Scholar] [CrossRef]
- Malik, N. Thermally exfoliated graphene oxide reinforced polycaprolactone-based bactericidal nanocomposites for food packaging applications. Mater. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Ahmadzadeh, S.; Keramat, J.; Nasirpour, A.; Hamdami, N.; Behzad, T.; Aranda, L.; Vilasi, M.; Desobry, S. Structural and mechanical properties of clay nanocomposite foams based on cellulose for the food-packaging industry. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Grande, C.D.; Mangadlao, J.; Fan, J.; De Leon, A.; Delgado-Ospina, J.; Rojas, J.G.; Rodrigues, D.F.; Advincula, R. Chitosan Cross-Linked Graphene Oxide Nanocomposite Films with Antimicrobial Activity for Application in Food Industry. Macromol. Symp. 2017, 374, 1600114. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwish, M.S.A.; Mostafa, M.H.; Al-Harbi, L.M. Polymeric Nanocomposites for Environmental and Industrial Applications. Int. J. Mol. Sci. 2022, 23, 1023. https://doi.org/10.3390/ijms23031023
Darwish MSA, Mostafa MH, Al-Harbi LM. Polymeric Nanocomposites for Environmental and Industrial Applications. International Journal of Molecular Sciences. 2022; 23(3):1023. https://doi.org/10.3390/ijms23031023
Chicago/Turabian StyleDarwish, Mohamed S. A., Mohamed H. Mostafa, and Laila M. Al-Harbi. 2022. "Polymeric Nanocomposites for Environmental and Industrial Applications" International Journal of Molecular Sciences 23, no. 3: 1023. https://doi.org/10.3390/ijms23031023
APA StyleDarwish, M. S. A., Mostafa, M. H., & Al-Harbi, L. M. (2022). Polymeric Nanocomposites for Environmental and Industrial Applications. International Journal of Molecular Sciences, 23(3), 1023. https://doi.org/10.3390/ijms23031023








