Impaired Pain Processing at a Brainstem Level Is Involved in Maladaptive Neuroplasticity in Patients with Chronic Complex Regional Pain Syndrome
Abstract
1. Introduction
2. Results
2.1. Demographics and Clinical Data
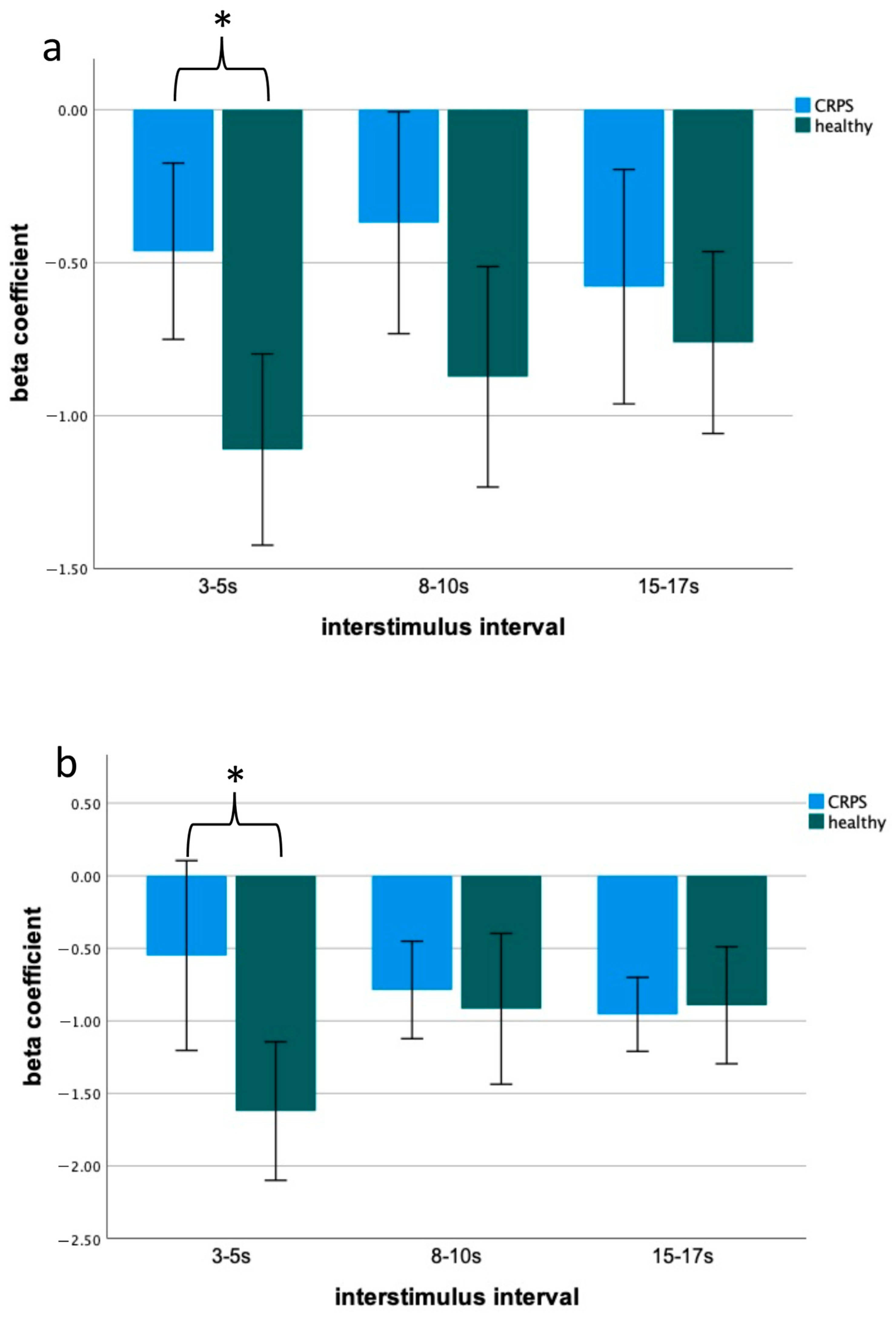
2.2. Blink Reflex Assessment—Differences between Patients with CRPS and Controls
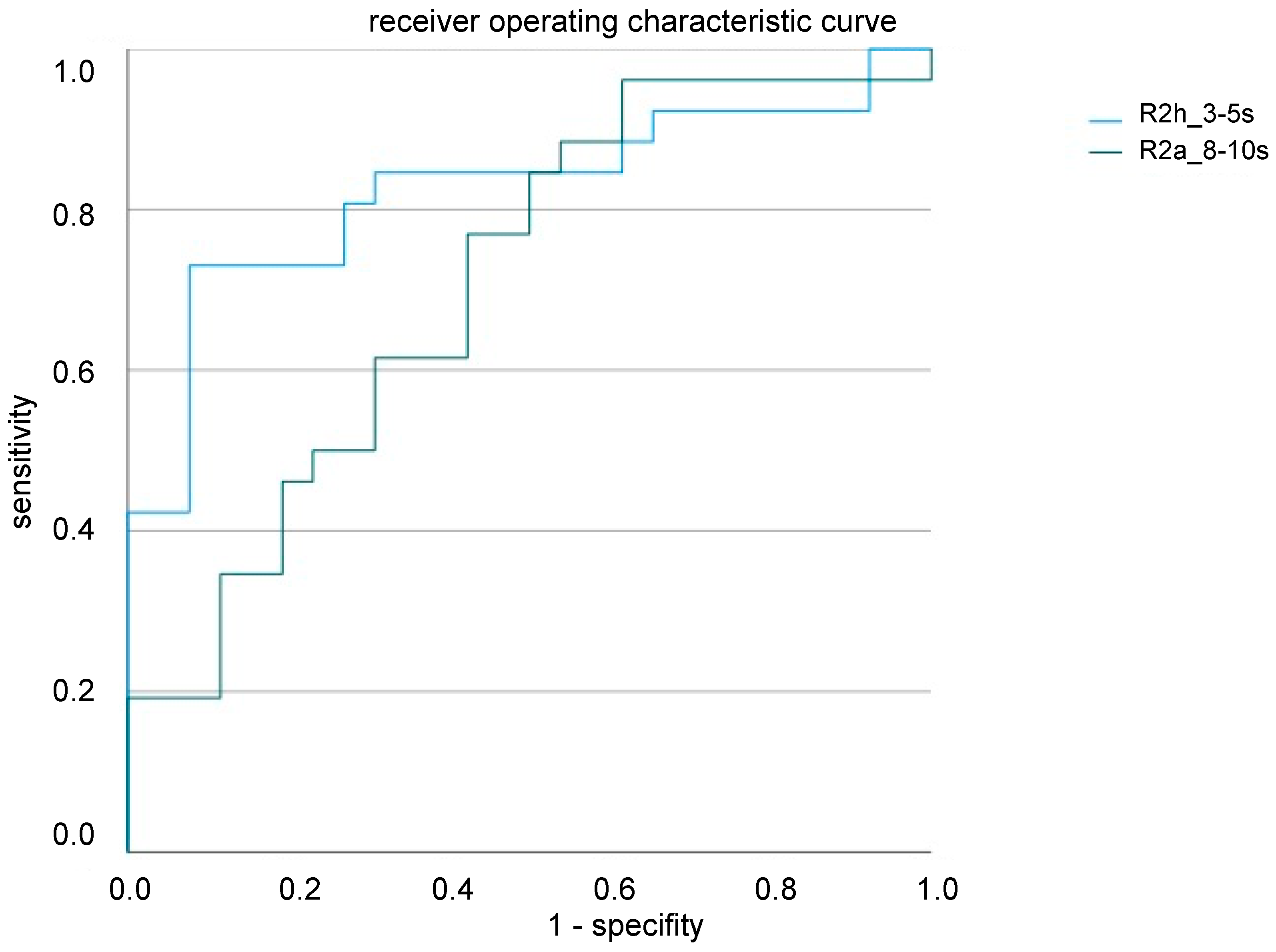
2.3. Blink Reflex as a Potential Biomarker for CRPS
3. Discussion
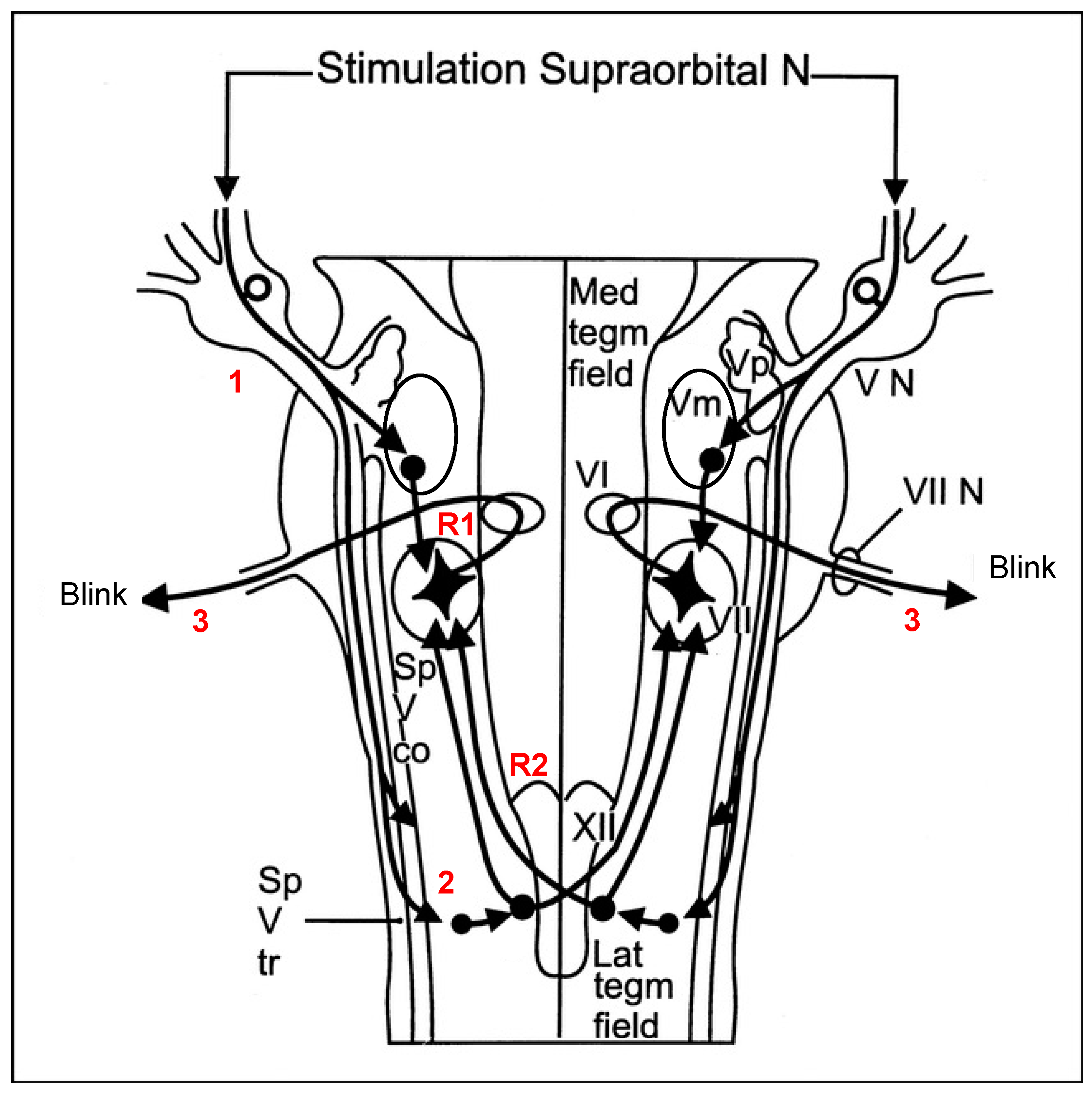
3.1. Blink Reflex and Brainstem Involvement in CRPS Pathophysiology
3.2. Blink Reflex as a Potential Biomarker of CRPS
3.3. Limitations
4. Materials and Methods
4.1. Participants
4.2. Assessment of Demographics and Clinical Characteristics
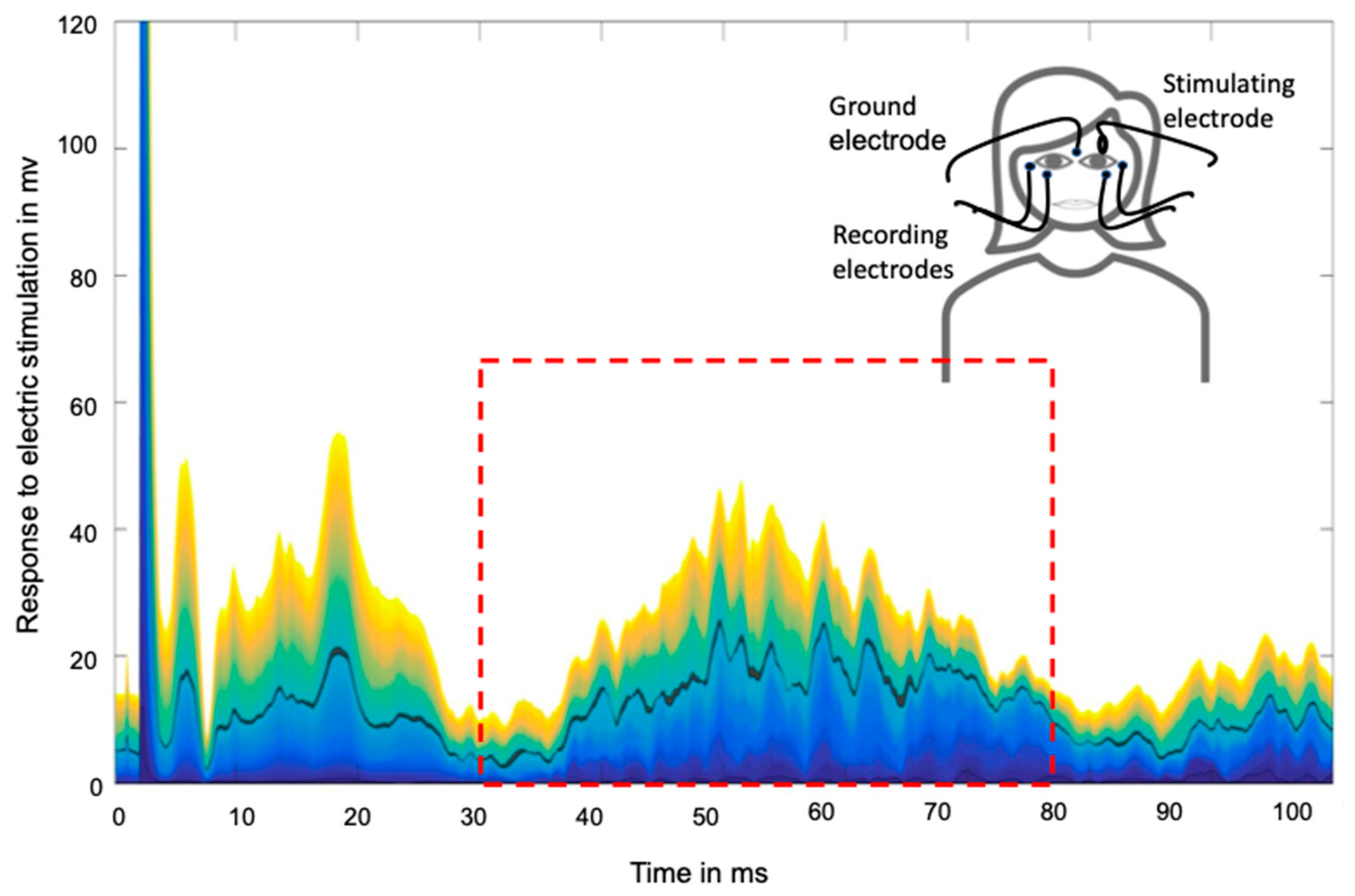
4.3. Assessment of Nociceptive Blink Reflex Habituation and Sensitization
4.4. Data Evaluation
4.5. Sample Size Estimation and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beerthuizen, A.; van ‘t Spijker, A.; Huygen, F.J.; Klein, J.; de Wit, R. Is there an association between psychological factors and the complex regional pain syndrome type 1 (crps1) in adults? A systematic review. Pain 2009, 145, 52–59. [Google Scholar] [CrossRef]
- Veldman, P.; Reynen, H.; Arntz, I.; Goris, R. Signs and symptoms of reflex sympathetic dystrophy: Prospective study of 829 patients. Lancet 1993, 342, 1012–1016. [Google Scholar] [CrossRef]
- Marinus, J.; Moseley, G.L.; Birklein, F.; Baron, R.; Maihöfner, C.; Kingery, W.S.; van Hilten, J.J. Clinical features and pathophysiology of complex regional pain syndrome. Lancet Neurol. 2011, 10, 637–648. [Google Scholar] [CrossRef]
- Eldufani, J.; Elahmer, N.; Blaise, G. A medical mystery of complex regional pain syndrome. Heliyon 2020, 6, e03329. [Google Scholar] [CrossRef]
- Vera-Portocarrero, L.; Zhang, E.-T.; Ossipov, M.; Xie, J.; King, T.; Lai, J.; Porreca, F. Descending facilitation from the rostral ventromedial medulla maintains nerve injury-induced central sensitization. Neuroscience 2006, 140, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.H.; Scheel-Krüger, J.; Blackburn-Munro, G. Amygdala gaba-a receptor involvement in mediating sensory-discriminative and affective-motivational pain responses in a rat model of peripheral nerve injury. Pain 2007, 127, 17–26. [Google Scholar] [CrossRef]
- Baron, R.; Hans, G.; Dickenson, A.H. Peripheral input and its importance for central sensitization. Ann. Neurol. 2013, 74, 630–636. [Google Scholar] [CrossRef]
- Mills, E.P.; Akhter, R.; Di Pietro, F.; Murray, G.M.; Peck, C.C.; Macey, P.M.; Henderson, L.A. Altered brainstem pain modulating circuitry functional connectivity in chronic painful temporomandibular disorder. J. Pain 2020, 22, 219–232. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Fields, H.L. Endogenous pain control systems: Brainstem spinal pathways and endorphin circuitry. Annu. Rev. Neurosci. 1984, 7, 309–338. [Google Scholar] [CrossRef]
- Thiele, A.; Klehr, L.; Strauß, S.; Angermaier, A.; Schminke, U.; Kronenbuerger, M.; Naegel, S.; Fleischmann, R. Preventive treatment with cgrp monoclonal antibodies restores brain stem habituation deficits and excitability to painful stimuli in migraine: Results from a prospective case-control study. J. Headache Pain 2021, 22, 149. [Google Scholar] [CrossRef]
- Ellrich, J.; Bromm, B.; Hopf, H.C. Pain-evoked blink reflex. Muscle Nerve 1997, 20, 265–270. [Google Scholar] [CrossRef]
- Martins, I.; Tavares, I. Reticular formation and pain: The past and the future. Front. Neuroanat. 2017, 11, 51. [Google Scholar] [CrossRef]
- Esteban, A. A neurophysiological approach to brainstem reflexes. Blink reflex. Neurophysiol. Clin. 1999, 29, 7–38. [Google Scholar] [CrossRef]
- Cooper, M.S.; Clark, V.P. Neuroinflammation, neuroautoimmunity, and the co-morbidities of complex regional pain syndrome. J. Neuroimmune Pharmacol. 2012, 8, 452–469. [Google Scholar] [CrossRef]
- Majoie, C.B.L.M.; Aramideh, M.; Hulsmans, F.-J.H.; Castelijns, J.A.; Van Beek, E.J.R.; De Visser, B.W.O. Correlation between electromyographic reflex and mr imaging examinations of the trigeminal. Am. J. Neuroradiol. 1999, 20, 1119–1125. Available online: http://www.ajnr.org/content/ajnr/20/6/1119.full.pdf (accessed on 28 October 2022).
- De Tommaso, M.; Delussi, M. Nociceptive blink reflex habituation biofeedback in migraine. Funct. Neurol. 2017, 32, 123–130. [Google Scholar] [CrossRef]
- Kaube, H.; Katsarava, Z.; Käufer, T.; Diener, H.-C.; Ellrich, J. A new method to increase nociception specificity of the human blink reflex. Clin. Neurophysiol. 2000, 111, 413–416. [Google Scholar] [CrossRef]
- Coppola, G.; Di Lorenzo, C.; Schoenen, J.; Pierelli, F. Habituation and sensitization in primary headaches. J. Headache Pain 2013, 14, 65. [Google Scholar] [CrossRef]
- Ziegeler, C.; Schulte, L.H.; May, A. Altered trigeminal pain processing on brainstem level in persistent idiopathic facial pain. Pain 2020, 162, 1374–1378. [Google Scholar] [CrossRef]
- Drummond, P.D.; Vo, L.; Finch, P.M. The source of hemisensory disturbances in complex regional pain syndrome. Clin. J. Pain 2020, 37, 79–85. [Google Scholar] [CrossRef]
- Taylor, S.-S.; Noor, N.; Urits, I.; Paladini, A.; Sandhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex regional pain syndrome: A comprehensive review. Pain Ther. 2021, 10, 875–892. [Google Scholar] [CrossRef]
- Strauss, S.; Barby, S.; Härtner, J.; Pfannmöller, J.P.; Neumann, N.; Moseley, G.L.; Lotze, M. Graded motor imagery modi-fies movement pain, cortical excitability and sensorimotor function in complex regional pain syndrome. Brain Commun. 2021, 3, fcab216. [Google Scholar] [CrossRef] [PubMed]
- Ekuttikat, A.; Noreika, V.; Eshenker, N.; Chennu, S.; Bekinschtein, T.; Brown, C.A. Neurocognitive and neuroplastic mecha-nisms of novel clinical signs in crps. Front. Hum. Neurosci. 2016, 10, 16. [Google Scholar] [CrossRef]
- De Marinis, M.; Pujia, A.; Natale, L.; D’Arcangelo, E.; Accornero, N. Decreased habituation of the r2 component of the blink reflex in migraine patients. Clin. Neurophysiol. 2003, 114, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, A.; Anastasio, M.G.; De Icco, R.; Coppola, G.; Ambrosini, A.; Serrao, M.; Sandrini, G.; Pierelli, F. Frequency-dependent habituation deficit of the nociceptive blink reflex in aura with migraine headache. Can migraine aura modulate trigeminal excitability? Headache J. Head Face Pain 2017, 57, 887–898. [Google Scholar] [CrossRef]
- Desmedt, J.E. (Ed.) Human Reflexes, Pathophysiology of Motor Systems, Methodology of Human Reflexes; Karger: Basel, Switzerland, 1973; Volume 3, pp. 682–691. [Google Scholar] [CrossRef]
- Ellrich, J. Brain stem reflexes: Probing human trigeminal nociception. Physiology 2000, 15, 94–97. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; Food and Drug Administration (US): Silver Spring, MD, USA, 2016.
- Holle, D.; Gaul, C.; Krebs, S.; Naegel, S.; Diener, H.-C.; Kaube, H.; Katsarava, Z.; Obermann, M. Nociceptive blink reflex and pain-related evoked potentials in hypnic headache. Cephalalgia 2011, 31, 1181–1188. [Google Scholar] [CrossRef]
- Harden, R.N.; Maihofner, C.; Abousaad, E.; Vatine, J.-J.; Kirsling, A.; Perez, R.S.; Kuroda, M.; Brunner, F.; Stanton-Hicks, M.; Marinus, J.; et al. A prospective, multisite, international validation of the complex regional pain syndrome severity score. Pain 2017, 158, 1430–1436. [Google Scholar] [CrossRef]
- Varenna, M.; Crotti, C.; Ughi, N.; Zucchi, F.; Caporali, R. Determinants of diagnostic delay in complex regional pain syndrome type 1: An observational study of 180 consecutive new cases. JCR J. Clin. Rheumatol. 2020, 27, e491–e495. [Google Scholar] [CrossRef]
- Marin, J.C.; Gantenbein, A.R.; Paemeleire, K.; Kaube, H.; Goadsby, P.J. Nociception-specific blink reflex: Pharmacology in healthy volunteers. J. Headache Pain 2015, 16, 1–8. [Google Scholar] [CrossRef][Green Version]
- Harden, R.N.; Bruehl, S.; Stanton-Hicks, M.M.; Wilson, M.P.R. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007, 8, 326–331. [Google Scholar] [CrossRef]
- Di Clemente, L.; Coppola, G.; Magis, D.; Fumal, A.; De Pasqua, V.; Di Piero, V.; Schoenen, J. Interictal habituation deficit of the nociceptive blink reflex: An endophenotypic marker for presymptomatic migraine? Brain 2007, 130, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Di Clemente, L.; Coppola, G.; Magis, D.; Fumal, A.; De Pasqua, V.; Schoenen, J. Nociceptive blink reflex and visual evoked potential habituations are correlated in migraine. Headache J. Head Face Pain 2005, 45, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
| Patient No | Age | Gender 1 | Affected Limb 2 | Inciting Event 3 | Time Onset 4 | Rpain 5 | Mpain 6 | CSS 7 | Medication 8 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | f | R | O | 58 | 6 | 7 | 15 | ABCD |
| 2 | 67 | f | R | CT | 120 | 5 | 6 | 13 | A |
| 3 | 50 | m | R | WF | 144 | 6 | 7 | 15 | ABD |
| 4 | 54 | f | L | CT | 132 | 5 | 6 | 13 | CD |
| 5 | 69 | m | L | WF | 84 | 2 | 2 | 7 | |
| 6 | 66 | f | L | O | 50 | 5 | 6 | 10 | ABD |
| 7 | 49 | m | L | O | 96 | 6 | 9 | 15 | ABCD |
| 8 | 82 | f | R | WF | 36 | 2 | 9 | 7 | |
| 9 | 21 | f | R | AR | 84 | 6 | 10 | 14 | BD |
| 10 | 66 | m | R | WF | 60 | 3 | 5 | 8 | ACD |
| 11 | 49 | f | L | RF | 36 | 2 | 10 | 9 | AC |
| 12 | 61 | f | R | CT | 96 | 7 | 8 | 10 | BCD |
| 13 | 43 | f | R | RF | 12 | 7 | 10 | 11 | ABCD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoma, P.; Drämel, N.; Grothe, M.; Lotze, M.; Fleischmann, R.; Strauss, S. Impaired Pain Processing at a Brainstem Level Is Involved in Maladaptive Neuroplasticity in Patients with Chronic Complex Regional Pain Syndrome. Int. J. Mol. Sci. 2022, 23, 15368. https://doi.org/10.3390/ijms232315368
Thoma P, Drämel N, Grothe M, Lotze M, Fleischmann R, Strauss S. Impaired Pain Processing at a Brainstem Level Is Involved in Maladaptive Neuroplasticity in Patients with Chronic Complex Regional Pain Syndrome. International Journal of Molecular Sciences. 2022; 23(23):15368. https://doi.org/10.3390/ijms232315368
Chicago/Turabian StyleThoma, Pauline, Nina Drämel, Matthias Grothe, Martin Lotze, Robert Fleischmann, and Sebastian Strauss. 2022. "Impaired Pain Processing at a Brainstem Level Is Involved in Maladaptive Neuroplasticity in Patients with Chronic Complex Regional Pain Syndrome" International Journal of Molecular Sciences 23, no. 23: 15368. https://doi.org/10.3390/ijms232315368
APA StyleThoma, P., Drämel, N., Grothe, M., Lotze, M., Fleischmann, R., & Strauss, S. (2022). Impaired Pain Processing at a Brainstem Level Is Involved in Maladaptive Neuroplasticity in Patients with Chronic Complex Regional Pain Syndrome. International Journal of Molecular Sciences, 23(23), 15368. https://doi.org/10.3390/ijms232315368





