Nutrition Alters the Stiffness of Adipose Tissue and Cell Signaling
Abstract
:1. Introduction
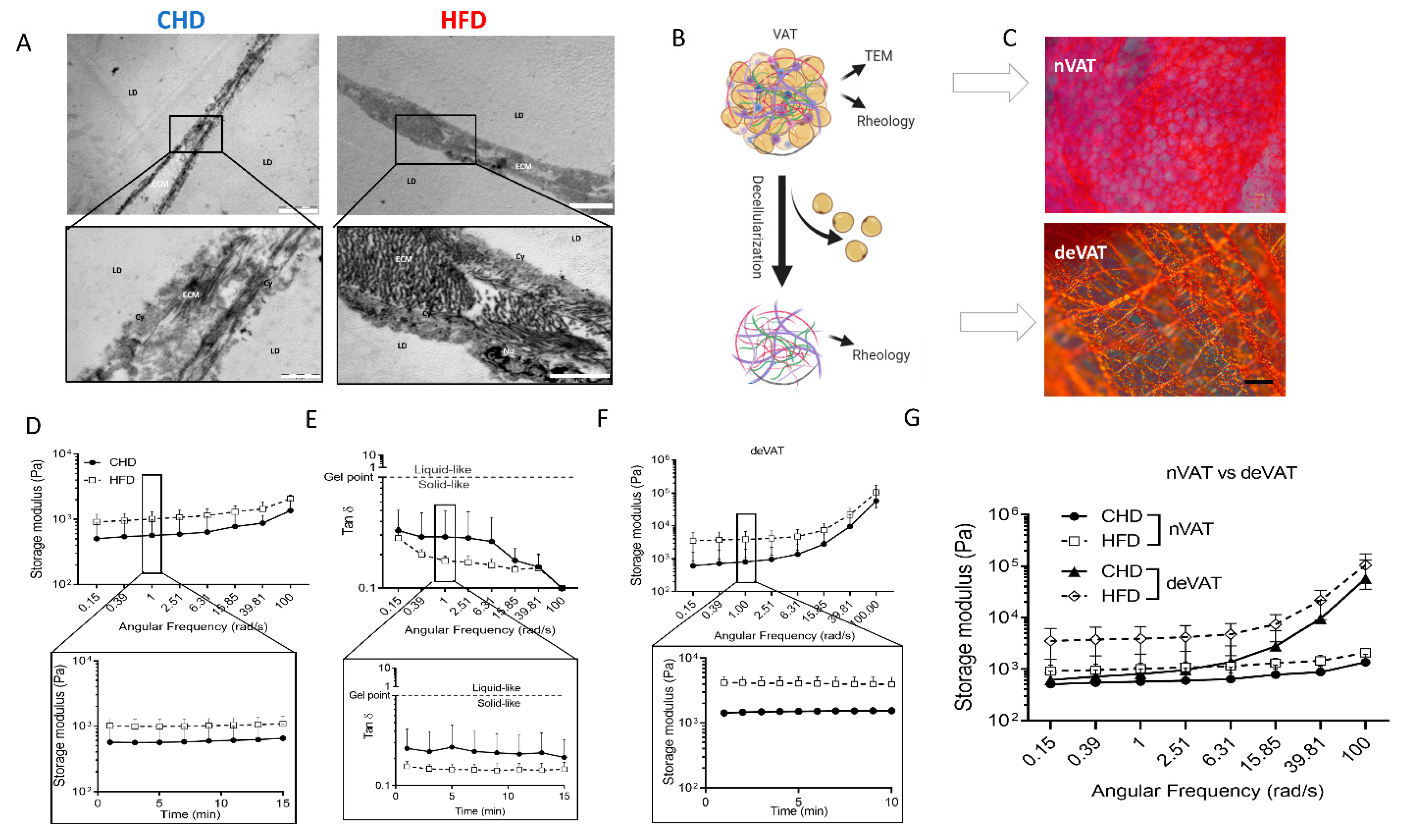
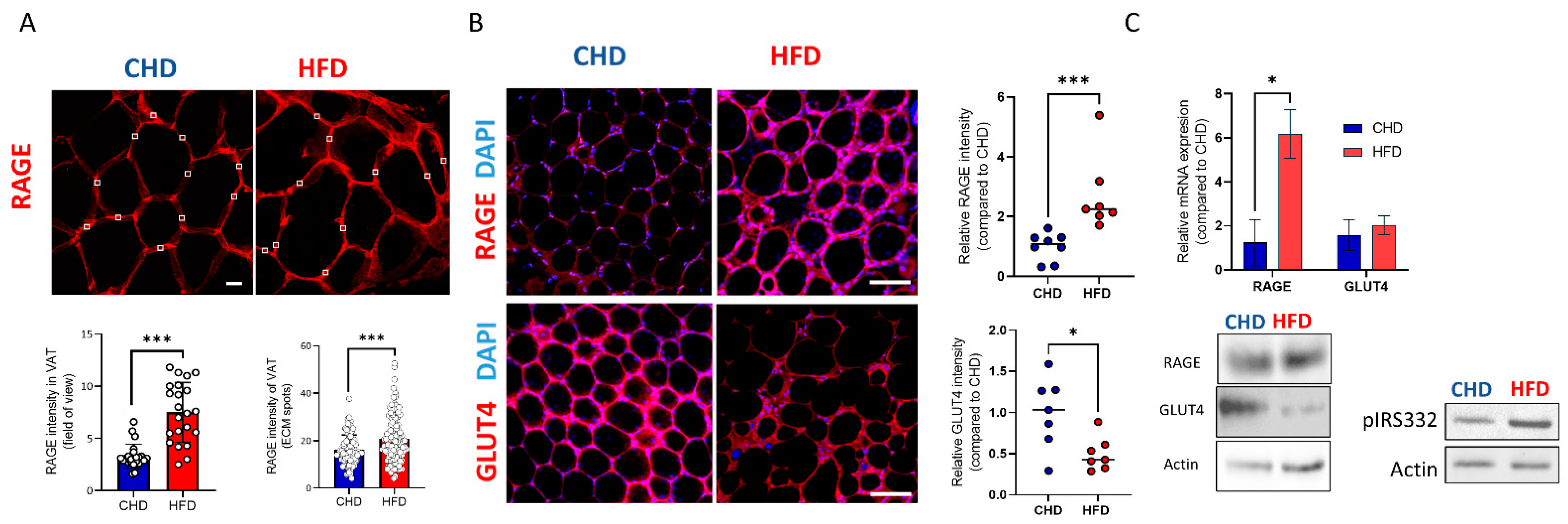
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Histology and Immunofluorecence Staining and Analysis
4.3. Picrosirius Red Staining for Collagen Fibers
4.4. Transmission Electron Microscopy (TEM)
4.5. Tissue Extracellular Matrix (ECM) and Decellularization
4.6. Rheological Measurements
4.7. Whole-Mount immunofluorescence staining
4.8. Biochemistry and Protein Identification Western Blot (WB)
4.9. RNA Isolation and qPCR
| Gene | Forward Primer | Reverse Primer |
| Actin | CATCGTGGGCCGCCCTAGGCACCA | CGGTTGGCCTTAGGGTTCAGGGGG |
| Rage | AACACAGCCCCCATCCAA | GCTCA CCAACAGCTGAATG |
| Glut4 | TTCACGTTGGTCTCGGTGCT | TAGCTCATGGCTGGAACCCG |
4.10. Statistical Analysis
4.11. Schematic Illustrations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [Green Version]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Kislev, N.; Izgilov, R.; Adler, R.; Benayahu, D. Exploring the Cell Stemness and the Complexity of the Adipose Tissue Niche. Biomolecules 2021, 11, 1906. [Google Scholar] [CrossRef] [PubMed]
- Torres, N.; Vargas-Castillo, A.E.; Tovar, A.R. Adipose Tissue: White Adipose Tissue Structure and Function. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 35–42. [Google Scholar] [CrossRef]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, T.; Lamendola, C.; Liu, A.; Abbasi, F. Preferential Fat Deposition in Subcutaneous versus Visceral Depots Is Associated with Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2011, 96, E1756–E1760. [Google Scholar] [CrossRef] [Green Version]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jørgensen, A.M.; Dashti, H.; Stefek, A.; et al. A Single-Cell Atlas of Human and Mouse White Adipose Tissue. Nature 2022, 603, 926–933. [Google Scholar] [CrossRef]
- Mor-Yossef Moldovan, L.; Lustig, M.; Naftaly, A.; Mardamshina, M.; Geiger, T.; Gefen, A.; Benayahu, D. Cell Shape Alteration during Adipogenesis Is Associated with Coordinated Matrix Cues. J. Cell. Physiol. 2019, 234, 3850–3863. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Lackey, D.E.; Burk, D.H.; Ali, M.R.; Mostaedi, R.; Smith, W.H.; Park, J.; Scherer, P.E.; Seay, S.A.; McCoin, C.S.; Bonaldo, P.; et al. Contributions of Adipose Tissue Architectural and Tensile Properties toward Defining Healthy and Unhealthy Obesity. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E233–E246. [Google Scholar] [CrossRef]
- Strieder-Barboza, C.; Baker, N.A.; Flesher, C.G.; Karmakar, M.; Neeley, C.K.; Polsinelli, D.; Dimick, J.B.; Finks, J.F.; Ghaferi, A.A.; Varban, O.A.; et al. Advanced Glycation End-Products Regulate Extracellular Matrix-Adipocyte Metabolic Crosstalk in Diabetes. Sci. Rep. 2019, 9, 19748. [Google Scholar] [CrossRef] [Green Version]
- Naftaly, A.; Izgilov, R.; Omari, E.; Benayahu, D. Revealing Advanced Glycation End Products Associated Structural Changes in Serum Albumin. ACS Biomater. Sci. Eng. 2021, 7, 3179–3189. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Jacobs, K.; Haucke, E.; Navarrete Santos, A.; Grune, T.; Simm, A. Role of Advanced Glycation End Products in Cellular Signaling. Redox Biol. 2014, 2, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, A.; McGraw, T.E.; Kahn, B.B. Insulin Action in Adipocytes, Adipose Remodeling, and Systemic Effects. Cell Metab. 2021, 33, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Schmidt, A.M. Glycation and Insulin Resistance: Novel Mechanisms and Unique Targets? Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1760–1765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, L.R.; Cho, S.; Discher, D.E. Stem Cell Differentiation Is Regulated by Extracellular Matrix Mechanics. Physiology 2018, 33, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth Factors, Matrices, and Forces Combine and Control Stem Cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [Green Version]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [Green Version]
- Mor-Yossef Moldovan, L.; Kislev, N.; Lustig, M.; Pomeraniec, L.; Benayahu, D. Biomechanical Stimulation Effects on the Metabolism of Adipocyte. J. Cell. Physiol. 2020, 235, 8702–8713. [Google Scholar] [CrossRef]
- Gefen, A.; Benayahu, D. The Mechanobiology of Obesity and Related Diseases; Springer International Publishing: London, UK, 2015; ISBN 3319093363. [Google Scholar] [CrossRef]
- Ge, H.; Tian, M.; Pei, Q.; Tan, F.; Pei, H. Extracellular Matrix Stiffness: New Areas Affecting Cell Metabolism. Front. Oncol. 2021, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Fletcher, D.A.; Reinhart-King, C.A. Stiffness Sensing by Cells. Physiol. Rev. 2020, 100, 695. [Google Scholar] [CrossRef]
- Copps, K.D.; White, M.F. Regulation of Insulin Sensitivity by Serine/Threonine Phosphorylation of Insulin Receptor Substrate Proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silber, M.; Miller, I.; Bar-Joseph, H.; Ben-Ami, I.; Shalgi, R. Elucidating the Role of Pigment Epithelium-Derived Factor (PEDF) in Metabolic PCOS Models. J. Endocrinol. 2020, 244, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Liberman, Z.; Eldar-Finkelman, H. Serine 332 Phosphorylation of Insulin Receptor Substrate-1 by Glycogen Synthase Kinase-3 Attenuates Insulin Signaling. J. Biol. Chem. 2005, 280, 4422–4428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deedwania, P. Hypertension, Dyslipidemia, and Insulin Resistance in Patients With Diabetes Mellitus or the Cardiometabolic Syndrome: Benefits of Vasodilating β-Blockers. J. Clin. Hypertens. 2011, 13, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, P.R.; Gnudi, L.; Tozzo, E.; Yang, H.; Leach, F.; Kahn, B.B. Adipose Cell Hyperplasia and Enhanced Glucose Disposal in Transgenic Mice Overexpressing GLUT4 Selectively in Adipose Tissue. J. Biol. Chem. 1993, 268, 22243–22246. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.C.; Seeley, R.J.; Rushing, P.A.; D’Alessio, D.; Tso, P. A Controlled High-Fat Diet Induces an Obese Syndrome in Rats. J. Nutr. 2003, 133, 1081–1087. [Google Scholar] [CrossRef]
- Vessby, B. Dietary Fat and Insulin Action in Humans. Br. J. Nutr. 2000, 83, S91–S96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poret, J.M.; Souza-Smith, F.; Marcell, S.J.; Gaudet, D.A.; Tzeng, T.H.; Braymer, H.D.; Harrison-Bernard, L.M.; Primeaux, S.D. High Fat Diet Consumption Differentially Affects Adipose Tissue Inflammation and Adipocyte Size in Obesity-Prone and Obesity-Resistant Rats. Int. J. Obes. 2018, 42, 525–541. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The Extracellular Matrix and Insulin Resistance. Trends Endocrinol. Metab. 2015, 26, 357. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.E.C.; Rabhi, N.; Orofino, J.; Gamini, R.; Perissi, V.; Vernochet, C.; Farmer, S.R. The Adipocyte Acquires a Fibroblast-Like Transcriptional Signature in Response to a High Fat Diet. Sci. Rep. 2020, 10, 2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.; Muise, E.S.; Iyengar, P.; Wang, Z.V.; Chandalia, M.; Abate, N.; Zhang, B.B.; Bonaldo, P.; Chua, S.; Scherer, P.E. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Mol. Cell. Biol. 2009, 29, 1575–1591. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Ojeda, F.J.; Méndez-Gutiérrez, A.; Aguilera, C.M.; Plaza-Díaz, J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int. J. Mol. Sci. 2019, 20, 4888. [Google Scholar] [CrossRef] [Green Version]
- Pasarica, M.; Gowronska-Kozak, B.; Burk, D.; Remedios, I.; Hymel, D.; Gimble, J.; Ravussin, E.; Bray, G.A.; Smith, S.R. Adipose Tissue Collagen VI in Obesity. J. Clin. Endocrinol. Metab. 2009, 94, 5155–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, J.; Löffler, M.; Bilban, M.; Reimers, M.; Kadl, A.; Todoric, J.; Zeyda, M.; Geyeregger, R.; Schreiner, M.; Weichhart, T.; et al. Prevention of High-Fat Diet-Induced Adipose Tissue Remodeling in Obese Diabetic Mice by n-3 Polyunsaturated Fatty Acids. Int. J. Obes. 2007, 31, 1004–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in Tissue Homeostasis and Dysregulation—Lessons Learned from Model Organisms and Human Patients. Matrix Biol. 2017, 57–58, 55–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petäistö, T.; Vicente, D.; Mäkelä, K.A.; Finnilä, M.A.; Miinalainen, I.; Koivunen, J.; Izzi, V.; Aikio, M.; Karppinen, S.M.; Devarajan, R.; et al. Lack of Collagen XVIII Leads to Lipodystrophy and Perturbs Hepatic Glucose and Lipid Homeostasis. J. Physiol. 2020, 598, 3373–3393. [Google Scholar] [CrossRef]
- DeMarsilis, J.; Elastin, A. Insufficiency Predisposes Mice to Impaired Glucose Metabolism. J. Mol. Genet. Med. 2014, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Blaise, S.; Romier, B.; Kawecki, C.; Ghirardi, M.; Rabenoelina, F.; Baud, S.; Duca, L.; Maurice, P.; Heinz, A.; Schmelzer, C.E.H.; et al. Elastin-Derived Peptides Are New Regulators of Insulin Resistance Development in Mice. Diabetes 2013, 62, 3807–3816. [Google Scholar] [CrossRef]
- Shoham, N.; Girshovitz, P.; Katzengold, R.; Shaked, N.T.; Benayahu, D.; Gefen, A. Adipocyte 43Stiffness Increases with Accumulation of Lipid Droplets. Biophys. J. 2014, 106, 1421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoham, N.; Gefen, A. The Influence of Mechanical Stretching on Mitosis, Growth, and Adipose Conversion in Adipocyte Cultures. Biomech. Model. Mechanobiol. 2012, 11, 1029–1045. [Google Scholar] [CrossRef] [PubMed]
- Shoham, N.; Levy, A.; Shabshin, N.; Benayahu, D.; Gefen, A. A Multiscale Modeling Framework for Studying the Mechanobiology of Sarcopenic Obesity. Biomech. Model. Mechanobiol. 2017, 16, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Gefen, A.; Benayahu, D. Adipogenesis and Lipid Production in Adipocytes Subjected to Sustained Tensile Deformations and Elevated Glucose Concentration: A Living Cell-Scale Model System of Diabesity. Biomech. Model. Mechanobiol. 2018, 17, 903–913. [Google Scholar] [CrossRef]
- Ben-Or Frank, M.; Shoham, N.; Benayahu, D.; Gefen, A. Effects of Accumulation of Lipid Droplets on Load Transfer between and within Adipocytes. Biomech. Model. Mechanobiol. 2015, 14, 15–28. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced Glycation End Products in Extracellular Matrix Proteins Contribute to the Failure of Sensory Nerve Regeneration in Diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef] [Green Version]
- Bansode, S.; Bashtanova, U.; Li, R.; Clark, J.; Müller, K.H.; Puszkarska, A.; Goldberga, I.; Chetwood, H.H.; Reid, D.G.; Colwell, L.J.; et al. Glycation Changes Molecular Organization and Charge Distribution in Type I Collagen Fibrils. Sci. Rep. 2020, 10, 3397. [Google Scholar] [CrossRef]
- Paul, R.G.; Bailey, A.J. Glycation of Collagen: The Basis of Its Central Role in the Late Complications of Ageing and Diabetes. Int. J. Biochem. Cell Biol. 1996, 28, 1297–1310. [Google Scholar] [CrossRef]
- Hutchinson, K.R.; Lord, C.K.; West, T.A.; Stewart, J.A. Cardiac Fibroblast-Dependent Extracellular Matrix Accumulation Is Associated with Diastolic Stiffness in Type 2 Diabetes. PLoS ONE 2013, 8, e72080. [Google Scholar] [CrossRef] [Green Version]
- Van Heerebeek, L.; Hamdani, N.; Handoko, M.L.; Falcao-Pires, I.; Musters, R.J.; Kupreishvili, K.; Ijsselmuiden, A.J.J.; Schalkwijk, C.G.; Bronzwaer, J.G.F.; Diamant, M.; et al. Diastolic Stiffness of the Failing Diabetic Heart: Importance of Fibrosis, Advanced Glycation End Products, and Myocyte Resting Tension. Circulation 2008, 117, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansode, S.B.; Gacche, R.N. Glycation-Induced Modification of Tissue-Specific ECM Proteins: A Pathophysiological Mechanism in Degenerative Diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129411. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I. Role of Advanced Glycation End Products (AGEs) and Receptor for AGEs (RAGE) in Vascular Damage in Diabetes. Exp. Gerontol. 2011, 46, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Matsui, T.; Takeuchi, M.; Yoshida, Y.; Yamakawa, R.; Fukami, K.; Yamagishi, S.I. Pigment Epithelium-Derived Factor (PEDF) Inhibits Proximal Tubular Cell Injury in Early Diabetic Nephropathy by Suppressing Advanced Glycation End Products (AGEs)-Receptor (RAGE) Axis. Pharmacol. Res. 2011, 63, 241–248. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Maeda, S.; Higashimoto, Y.; Yamagishi, S.I. Advanced Glycation End Products Evoke Endothelial Cell Damage by Stimulating Soluble Dipeptidyl Peptidase-4 Production and Its Interaction with Mannose 6-Phosphate/Insulin-like Growth Factor II Receptor. Cardiovasc. Diabetol. 2013, 12, 125. [Google Scholar] [CrossRef] [Green Version]
- Gaens, K.H.J.; Goossens, G.H.; Niessen, P.M.; Van Greevenbroek, M.M.; Van Der Kallen, C.J.H.; Niessen, H.W.; Rensen, S.S.; Buurman, W.A.; Greve, J.W.M.; Blaak, E.E.; et al. Nε-(Carboxymethyl)Lysine-Receptor for Advanced Glycation End Product Axis Is a Key Modulator of Obesity-Induced Dysregulation of Adipokine Expression and Insulin Resistance. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Du, Z.; Shu, X.; Zhu, L.; Wu, J.; Gao, Q.; Wang, L.; Chen, N.; Li, Y.; Luo, M.; et al. Role of RAGE in Obesity-Induced Adipose Tissue Inflammation and Insulin Resistance. Cell Death Discov. 2021, 7, 305. [Google Scholar] [CrossRef]
- Werner, E.D.; Lee, J.; Hansen, L.; Yuan, M.; Shoelson, S.E. Insulin Resistance Due to Phosphorylation of Insulin Receptor Substrate-1 at Serine 302. J. Biol. Chem. 2004, 279, 35298–35305. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Johnson, J.A.; Zhang, Q.; Beahm, E.K. Combining Decellularized Human Adipose Tissue Extracellular Matrix and Adipose-Derived Stem Cells for Adipose Tissue Engineering. Acta Biomater. 2013, 9, 8921–8931. [Google Scholar] [CrossRef] [Green Version]
- Kislev, N.; Moldovan, L.M.Y.; Barak, R.; Egozi, M.; Benayahu, D. MYH10 Governs Adipocyte Function and Adipogenesis through Its Interaction with GLUT4. Int. J. Mol. Sci. 2022, 23, 2367. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naftaly, A.; Kislev, N.; Izgilov, R.; Adler, R.; Silber, M.; Shalgi, R.; Benayahu, D. Nutrition Alters the Stiffness of Adipose Tissue and Cell Signaling. Int. J. Mol. Sci. 2022, 23, 15237. https://doi.org/10.3390/ijms232315237
Naftaly A, Kislev N, Izgilov R, Adler R, Silber M, Shalgi R, Benayahu D. Nutrition Alters the Stiffness of Adipose Tissue and Cell Signaling. International Journal of Molecular Sciences. 2022; 23(23):15237. https://doi.org/10.3390/ijms232315237
Chicago/Turabian StyleNaftaly, Alex, Nadav Kislev, Roza Izgilov, Raizel Adler, Michal Silber, Ruth Shalgi, and Dafna Benayahu. 2022. "Nutrition Alters the Stiffness of Adipose Tissue and Cell Signaling" International Journal of Molecular Sciences 23, no. 23: 15237. https://doi.org/10.3390/ijms232315237
APA StyleNaftaly, A., Kislev, N., Izgilov, R., Adler, R., Silber, M., Shalgi, R., & Benayahu, D. (2022). Nutrition Alters the Stiffness of Adipose Tissue and Cell Signaling. International Journal of Molecular Sciences, 23(23), 15237. https://doi.org/10.3390/ijms232315237






