Integral Algorithms to Evaluate TiO2 and N-TiO2 Thin Films’ Cytocompatibility
Abstract
:1. Introduction
2. Results
2.1. Sample Characteristics
2.2. Surface Characteristics
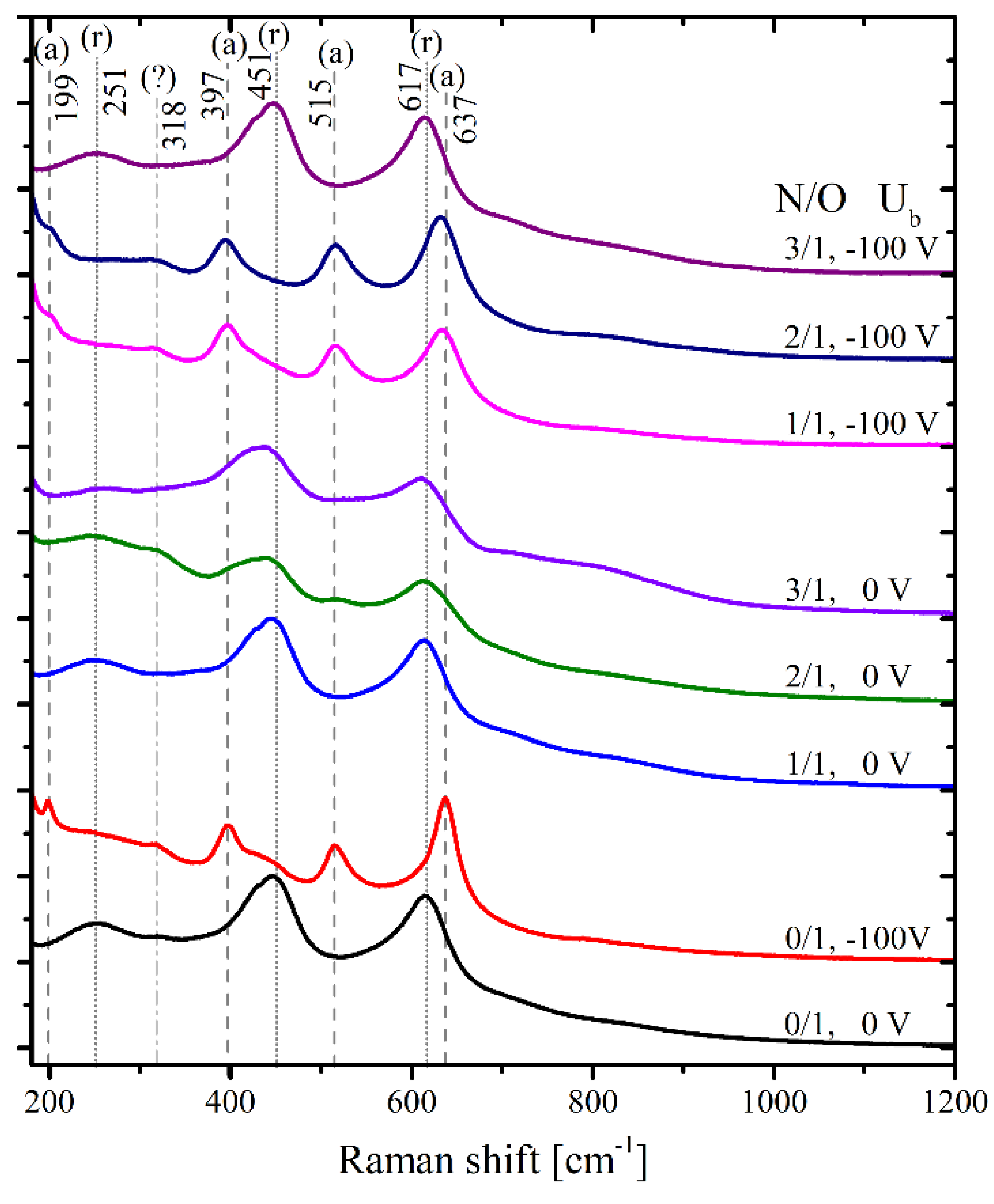
2.3. Raman Scattering
2.4. TiO2 and TiOxNy Coatings’ Cytocompatibility
3. Discussion
4. Materials and Methods
4.1. Sample Coatings
4.2. Scanning Electron Microscopy (SEM)
4.3. Raman Scattering
4.4. Cytocompatibility Evaluation
4.4.1. Materials
4.4.2. Direct Cytotoxicity Evaluation
4.4.3. Evaluation of Cell Adhesion
4.4.4. Cell Viability Assay
4.4.5. NO Production Assay
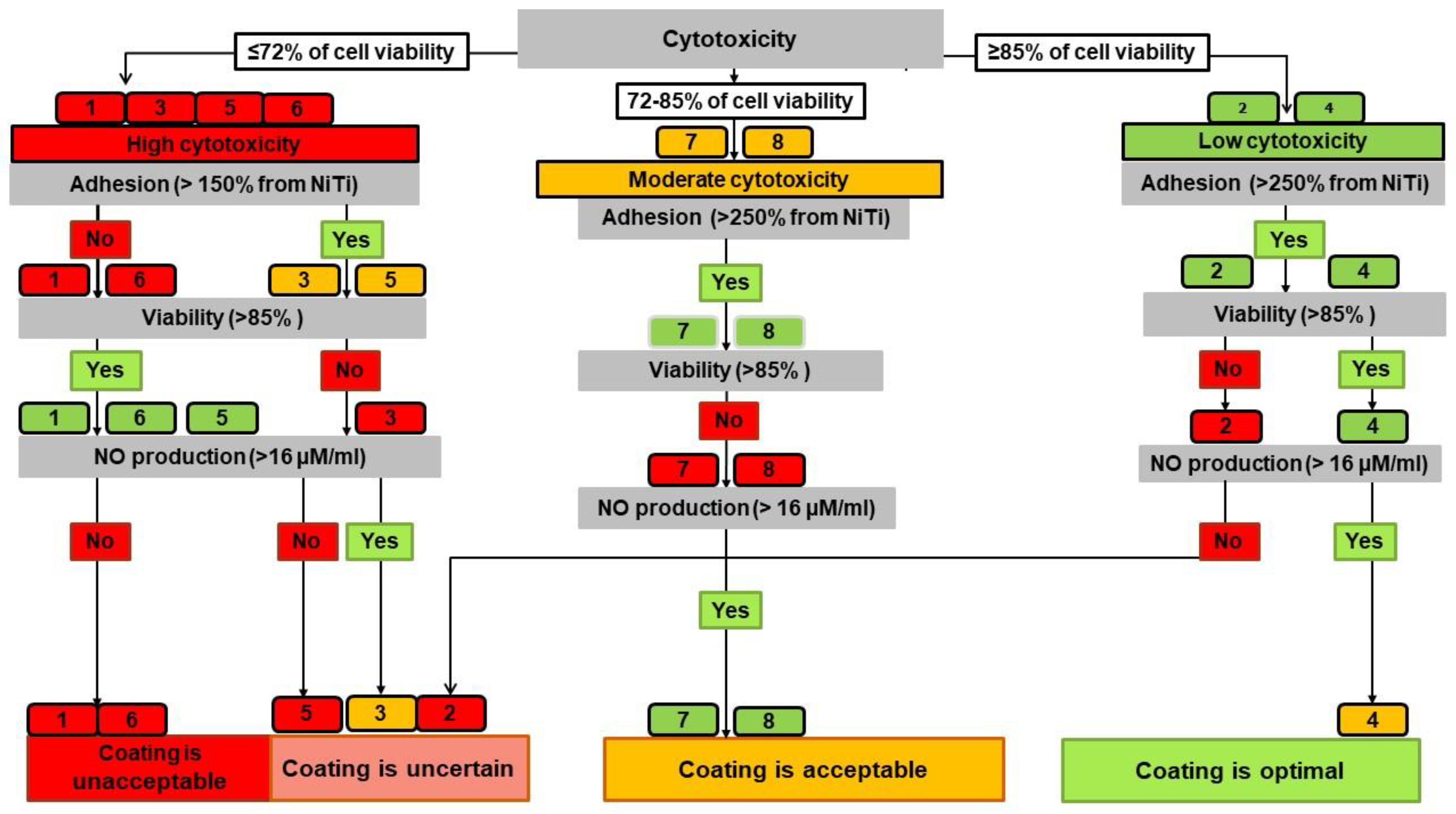
4.5. Development of an Algorithm for Assessing the Cytocompatibility of Coatings
4.5.1. Integrated Assessment of the Studied Indicators
- The direct cytotoxicity of thin films (by the MTT test) to assess the toxic effect of thin films which can prevent cell-to-surface contacts in living systems;
- The adhesion of cells on the samples’ surface to assess the probability of cells seeding after contact;
- Cell viability to assess the relative survival of cells on the samples’ surface, indicating the formation of a proliferating cell biofilm;
- NO production to assess the functional activity of endothelial cells.
4.5.2. Decision Tree Algorithm
- -
- Optimal cytocompatibility had a surface with no red leaf and at least two green leaves;
- -
- A surface was considered acceptable with three green or yellow leaves;
- -
- The cytocompatibility was classified as uncertain when two leaves were red;
- -
- The surface was determined to be unacceptable when three leaves were red.
4.6. Statistical Analysis
5. Conclusions
- By comparing two algorithms for assessing the thin films’ cytocompatibility, we have shown that DTA provides more detailed information on the changes in the properties of the studied coatings, which depend on the sputtering mode, the surface morphology, and the crystalline phase.
- N-TiO2 coatings sputtered in modes providing a predominance of the rutile crystalline phase make it possible to obtain the most cytocompatible thin films.
- The evaluation of thin films using both algorithms demonstrated the best cytocompatibility of Sample No. 4 sputtered at 0 V and N:O = 1:1. The surfaces of Samples No. 7 (−100 V; N:O = 3:1) and No. 8 (0 V; N:O = 3:1) had acceptable cytocompatibility.
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, W.J. Evolution of Optical Thin Films by Sputtering. Appl. Opt. 1974, 13, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Suketa, N.; Sawase, T.; Kitaura, H.; Naito, M.; Baba, K.; Nakayama, K.; Wennerberg, A.; Atsuta, M. An Antibacterial Surface on Dental Implants, Based on the Photocatalytic Bactericidal Effect. Clin. Implant Dent. Relat. Res. 2005, 7, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Healy, K.; Ducheyne, P. Hydration and preferential molecular adsorption on titanium in vitro. Biomaterials 1992, 13, 553–561. [Google Scholar] [CrossRef]
- Pan, J.; Leygraf, C.; Thierry, D.; Ektessabi, A.M. Corrosion resistance for biomaterial applications of TiO2 films deposited on titanium and stainless steel by ion-beam-assisted sputtering. J. Biomed. Mater. Res. 1997, 35, 309–318. [Google Scholar] [CrossRef]
- Sunny, M.; Sharma, C. Titanium-Protein Interaction: Changes with Oxide Layer Thickness. J. Biomater. Appl. 1991, 6, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Yuanru, C.; Xianghuai, L. In vitro investigation of blood compatibility of Ti with oxide layer of rutile structure. J. Biomater. Appl. 1994, 8, 404–412. [Google Scholar]
- Nan, H.; Ping, Y.; Xuan, C.; Yongxang, L.; Xiaolan, Z.; Guangjun, C.; Zihong, Z.; Feng, Z.; Yuanru, C.; Xianghuai, L.; et al. Blood compatibility of amorphous titanium oxide films synthesized by ion beam enhanced deposition. Biomaterials 1998, 19, 771–776. [Google Scholar] [CrossRef]
- Huang, N.; Yang, P.; Leng, Y.X.; Chen, J.Y.; Sun, H.; Wang, J.; Wang, G.J.; Ding, P.D.; Xi, T.F.; Leng, Y. Hemocompatibility of titanium oxide films. Biomaterials 2003, 24, 2177–2187. [Google Scholar] [CrossRef]
- Achneck, H.E.; Jamiolkowski, R.M.; Jantzen, A.E.; Haseltine, J.M.; Lane, W.O.; Huang, J.K.; Galinat, L.J.; Serpe, M.J.; Lin, F.-H.; Li, M.; et al. The biocompatibility of titanium cardiovascular devices seeded with autologous blood-derived endothelial progenitor cells: EPC-seeded antithrombotic Ti implants. Biomaterials 2011, 32, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Lee, I.-S.; Choi, Y.-J.; Noh, I.-S.; Chung, S.-M. Characterizations of the TiO2−x films synthesized by e-beam evaporation for endovascular applications. Biomed. Mater. 2008, 4, 015013. [Google Scholar] [CrossRef]
- Mohan, C.C.; Cherian, A.M.; Kurup, S.; Joseph, J.; Nair, M.B.; Vijayakumar, M.; Nair, S.V.; Menon, D. Stable Titania Nanostructures on Stainless Steel Coronary Stent Surface for Enhanced Corrosion Resistance and Endothelialization. Adv. Health Mater. 2017, 6, 1601353. [Google Scholar] [CrossRef]
- Windecker, S.; Mayer, I.; De Pasquale, G.; Maier, W.; Dirsch, O.; De Groot, P.; Wu, Y.-P.; Noll, G.; Leskosek, B.; Meier, B.; et al. Stent Coating with Titanium-Nitride-Oxide for Reduction of Neointimal Hyperplasia. Circulation 2001, 104, 928–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karjalainen, P.P.; Niemelä, M.; Airaksinen, J.K.; Rivero-Crespo, F.; Romppanen, H.; Sia, J.; Lalmand, J.; de Bruyne, B.; DeBelder, A.; Carlier, M.; et al. A prospective randomised comparison of titanium-nitride-oxide-coated bioactive stents with everolimus-eluting stents in acute coronary syndrome: The BASE-ACS trial. EuroIntervention 2012, 8, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilgrim, T.; Räber, L.; Limacher, A.; Wenaweser, P.; Cook, S.; Stauffer, J.-C.; Garachemani, A.; Moschovitis, A.; Meier, B.; Jüni, P.; et al. Five-year results of a randomised comparison of titanium-nitride-oxide-coated stents with zotarolimus-eluting stents for coronary revascularisation. EuroIntervention 2015, 10, 1284–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nammas, W.; Airaksinen, J.K.E.; Romppanen, H.; Sia, J.; De Belder, A.; Karjalainen, P.P. Impact of Preexisting Vascular Disease on the Outcome of Patients with Acute Coronary Syndrome: Insights from the Comparison of Bioactive Stent to the Everolimus-Eluting Stent in Acute Coronary Syndrome Trial. Angiology 2017, 68, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Tonino, P.A.; Pijls, N.H.; Collet, C.; Nammas, W.; Van der Heyden, J.; Romppanen, H.; Kervinen, K.; Airaksinen, J.K.; Sia, J.; Lalmand, J.; et al. Titanium-Nitride-Oxide–Coated Versus Everolimus-Eluting Stents in Acute Coronary Syndrome: The Randomized TIDES-ACS Trial. JACC Cardiovasc. Interv. 2020, 13, 1697–1705. [Google Scholar] [CrossRef]
- Karjalainen, P. Neointimal coverage and vasodilator response to titanium-nitride-oxide-coated bioactive stents and everolimus-eluting stents in patients with acute coronary syndrome: Insights from the BASE-ACS trial. Int. J. Cardiovasc. Imaging 2013, 29, 1693–1703. [Google Scholar] [CrossRef]
- Park, D.S.; Bae, I.-H.; Jeong, M.H.; Lim, K.S.; Sim, D.S.; Hong, Y.J.; Lee, S.-Y.; Jang, E.J.; Shim, J.-W.; Park, J.-K.; et al. In vitro and in vivo evaluation of a novel polymer-free everolimus-eluting stent by nitrogen-doped titanium dioxide film deposition. Mater. Sci. Eng. C 2018, 91, 615–623. [Google Scholar] [CrossRef]
- Sun, Z.; Khlusov, I.A.; Evdokimov, K.E.; Konishchev, M.E.; Kuzmin, O.S.; Khaziakhmatova, O.G.; Malashchenko, V.V.; Litvinova, L.S.; Rutkowski, S.; Frueh, J.; et al. Nitrogen-doped titanium dioxide films fabricated via magnetron sputtering for vascular stent biocompatibility improvement. J. Colloid Interface Sci. 2022, 626, 101–112. [Google Scholar] [CrossRef]
- Surovtseva, M.A.; Poveschenko, O.V.; Kuzmin, O.S.; Kim, I.I.; Kozhukhov, A.S.; Bondarenko, N.A.; Chepeleva, E.V.; Kolodin, A.N.; Lykov, A.P.; Shcheglov, D.V.; et al. Titanium oxide– and oxynitride–coated nitinol: Effects of surface structure and composition on interactions with endothelial cells. Appl. Surf. Sci. 2022, 578, 152059. [Google Scholar] [CrossRef]
- Pustovalova, A.; Boytsova, E.; Aubakirova, D.; Bruns, M.; Tverdokhlebov, S.; Pichugin, V. Formation and structural features of nitrogen-doped titanium dioxide thin films grown by reactive magnetron sputtering. Appl. Surf. Sci. 2020, 534, 147572. [Google Scholar] [CrossRef]
- Parker, J.C.; Siegel, R.W. Calibration of the Raman spectrum to the oxygen stoichiometry of nanophase TiO2. Appl. Phys. Lett. 1990, 57, 943–945. [Google Scholar] [CrossRef]
- Swamy, V.; Muddle, B.C.; Dai, Q. Size-dependent modifications of the Raman spectrum of rutile TiO2. Appl. Phys. Lett. 2006, 89, 163118. [Google Scholar] [CrossRef]
- Swamy, V.; Kuznetsov, A.; Dubrovinsky, L.S.; Caruso, R.A.; Shchukin, D.G.; Muddle, B.C. Finite-size and pressure effects on the Raman spectrum of nanocrystalline anatase Ti O 2. Phys. Rev. B 2005, 71, 184302. [Google Scholar] [CrossRef]
- Mengel, M.; Reeve, J.; Bunnag, S.; Einecke, G.; Jhangri, G.S.; Sis, B.; Famulski, K.; Guembes-Hidalgo, L.; Halloran, P. Scoring Total Inflammation Is Superior to the Current Banff Inflammation Score in Predicting Outcome and the Degree of Molecular Disturbance in Renal Allografts. Am. J. Transplant. 2009, 9, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Holden, S.; Bernard, B.; Ouyang, B.; Goetz, C.G.; Stebbins, G.T. Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2013, 28, 1972–1979. [Google Scholar] [CrossRef] [Green Version]
- García, M.I.M.; Jian, Z.; Hatib, F.; Settels, J.J.; Cecconi, M.; Pinsky, M.R. Dynamic Arterial Elastance as a Ventriculo-Arterial Coupling Index: An Experimental Animal Study. Front. Physiol. 2020, 11, 284. [Google Scholar] [CrossRef]
- Barrios, J.M.; Romero, P.E. Decision Tree Methods for Predicting Surface Roughness in Fused Deposition Modeling Parts. Materials 2019, 12, 2574. [Google Scholar] [CrossRef] [Green Version]
- Witten, I.H.; Frank, E.; Hall, M.A. Data Mining: Practical Machine Learning Tools and Techniques, 3rd ed.; Morgan Kaufmann: Burlington, NJ, USA, 2011; ISBN 978-0-12-374856-0. [Google Scholar]
- Tong, J.C.; Ranganathan, S. Computer-Aided Vaccine Design || Computational T Cell Vaccine Design; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 59–86. [Google Scholar] [CrossRef]
- Eynde, J.; Bennett, J.; McCutcheon, K.; Adriaenssens, T.; Desmet, W.; Dubois, C.; Sinnaeve, P.; Verbelen, T.; Jacobs, S.; Oosterlinck, W. Heart team 2.0: A decision tree for minimally invasive and hybrid myocardial revascularization. Trends Cardiovasc. Med. 2021, 31, 382–391. [Google Scholar] [CrossRef]
- Tefft, B.J.; Uthamaraj, S.; Harbuzariu, A.; Harburn, J.J.; Witt, T.A.; Newman, B.; Psaltis, P.J.; Hlinomaz, O.; Holmes, D.R.; Gulati, R.; et al. Nanoparticle-Mediated Cell Capture Enables Rapid Endothelialization of a Novel Bare Metal Stent. Tissue Eng. Part A 2018, 24, 1157–1166. [Google Scholar] [CrossRef]
- Strohbach, A.; Busch, R. Polymers for Cardiovascular Stent Coatings. Int. J. Polym. Sci. 2015, 2015, 782653. [Google Scholar] [CrossRef] [Green Version]
- Pichugin, V.F.; Pustovalova, A.A.; Evdokimov, E.K.; Konishchev, E.M.; Kuzmin, O.S.; Boytsova, E.L.; Beshchasna, N.; Ficai, A.; Aubakirova, D.M.; Sun, Z. Structural features and nitrogen positions in titanium oxynitride films grown in plasma of magnetron discharge. J. Phys. Conf. Ser. 2019, 1281, 012062. [Google Scholar] [CrossRef]
- Liao, M.; Niu, H.; Chen, G. Effect of sputtering pressure and post-annealing on hydrophilicity of TiO2 thin films deposited by reactive magnetron sputtering. Thin Solid Films 2010, 518, 7258–7262. [Google Scholar] [CrossRef]
- Nolan, R.P.; Langer, A.M.; Weisman, I.; Herson, G.B. Surface character and membranolytic activity of rutile and anatase: Two titanium dioxide polymorphs. Br. J. Ind. Med. 1987, 44, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Cihlar, J.; Navarro, L.K.T.; Kasparek, V.; Michalicka, J.; Kastyl, J.; Castkova, K.; Celko, L. Influence of LA/Ti molar ratio on the complex synthesis of anatase/brookite nanoparticles and their hydrogen production. Int. J. Hydrogen Energy 2021, 46, 8578–8593. [Google Scholar] [CrossRef]
- Arias, L.F.; Kleiman, A.; Heredia, E.; Márquez, A. Rutile titanium dioxide films deposited with a vacuum arc at different temperatures. J. Phys. Conf. Ser. 2012, 370, 012027. [Google Scholar] [CrossRef]
- López-Huerta, F.; Cervantes, B.; González, O.; Hernández-Torres, J.; García-González, L.; Vega, R.; Herrera-May, A.L.; Soto, E. Biocompatibility and Surface Properties of TiO2 Thin Films Deposited by DC Magnetron Sputtering. Materials 2014, 7, 4105–4117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Braz, J.K.F.S.; Martins, G.M.; Sabino, V.; Vitoriano, J.O.; Barboza, C.A.G.; Soares, A.K.M.C.; Rocha, H.A.O.; Oliveira, M.F.; Alves Júnior, C.; Moura, C.E.B. Plasma nitriding under low temperature improves the endothelial cell biocompatibility of 316L stainless steel. Biotechnol. Lett. 2019, 41, 503–510. [Google Scholar] [CrossRef]
- Czarnowska, E.; Borowski, T.; Sowińska, A.; Lelątko, J.; Oleksiak, J.; Kamiński, J.; Tarnowski, M.; Wierzchoń, T. Structure and properties of nitrided surface layer produced on NiTi shape memory alloy by low temperature plasma nitriding. Appl. Surf. Sci. 2015, 334, 24–31. [Google Scholar] [CrossRef]
- Dong, J.; Pacella, M.; Liu, Y.; Zhao, L. Surface engineering and the application of laser-based processes to stents—A review of the latest development. Bioact. Mater. 2022, 10, 159–184. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.D.; Ridnour, L.A.; Isenberg, J.S.; Flores-Santana, W.; Switzer, C.H.; Donzelli, S.; Hussain, P.; Vecoli, C.; Paolocci, N.; Ambs, S.; et al. The chemical biology of nitric oxide: Implications in cellular signaling. Free. Radic. Biol. Med. 2008, 45, 18–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2009.
- Martin-Piedra, M.A.; Garzon, I.; Oliveira, A.C.; Alfonso-Rodriguez, C.A.; Carriel, V.; Scionti, G.; Alaminos, M. Cell viability and proliferation capability of long-term human dental pulp stem cell cultures. Cytotherapy 2014, 16, 266–277. [Google Scholar] [CrossRef] [PubMed]

| Sample No. | O2/N2 Ratio | Bias Voltage, V | Film | N (wt%) | O (wt%) | O/N AR |
|---|---|---|---|---|---|---|
| (While Sputtering) | In the Thin Film | |||||
| 1 | - | −100 | TiO2 | - | 22.07 ± 0.09 | - |
| 2 | - | 0 | TiO2 | - | 22.27 ± 0.12 | - |
| 3 | 1 | −100 | Ti-O-N | 5.42 ± 0.28 | 24.36 ± 0.61 | 3.94 ± 0.14 |
| 4 | 1 | 0 | Ti-O-N | 5.13 ± 0.06 | 23.97 ± 0.23 | 4.08 ± 0.01 |
| 5 | 0.5 | −100 | Ti-O-N | 4.87 ± 0.12 | 21.01 ± 0.20 | 3.79 ± 0.12 |
| 6 | 0.5 | 0 | Ti-O-N | 5.31 ± 0.08 | 22.95 ± 0.14 | 3.79 ± 0.04 |
| 7 | 0.33 | −100 | Ti-O-N | 4.77 ± 0.05 | 20.08 ± 0.29 | 3.68 ± 0.09 |
| 8 | 0.33 | 0 | Ti-O-N | 4.85 ± 0.05 | 21.55 ± 0.05 | 3.89 ± 0.05 |
| C | - | - | - | - | - | - |
| Sample No. | Direct Cytotoxicity, % | Adhesion and Spreading of Cells’ Relative Content from NiTi, % | Viability of Cells, % | NO Production, µM/mL |
|---|---|---|---|---|
| No. 1 | 63.9 ± 2.9 | 114.5 ± 23.3 | 87.4 ± 7.5 | 14.6 ± 3.1 |
| No. 2 | 87.1 ± 3.4 | 261.9 ± 67.7 | 75.8 ± 9.2 | 13.5 ± 2.6 |
| No. 3 | 66.9 ± 4.2 | 180.6 ± 60.9 | 83.0 ± 5.8 | 17.7 ± 0.5 |
| No. 4 | 86.6 ± 5.3 | 279.5 ± 20.8 | 88.7 ± 3.3 | 22.1 ± 5.4 |
| No. 5 | 68.8 ± 3.6 | 180.2 ± 19.0 | 94.4 ± 2.3 | 9.8 ± 3.2 |
| No. 6 | 70.2 ± 4.5 | 126.4 ±34.2 | 87.5 ± 2.7 | 12.5 ± 2.4 |
| No. 7 | 79.1 ± 3.1 | 312.1 ± 38.2 | 84.1 ± 4.9 | 56.2 ± 31.4 |
| No. 8 | 79.0 ± 3.5 | 259.0 ± 39.1 | 83.1 ± 6.2 | 52.2 ± 13.7 |
| Control (NiTi) | 71.7 ± 3.6 | 100 ± 0 | 64.6 ± 29.7 | 16.1 ± 2.4 |
| Criterion | Parameter | ||
|---|---|---|---|
| Unacceptable Level (Score = 0) | Moderate Level (Score = 1) | Optimal Level (Score = 2) | |
| Direct cytotoxicity, % (relative cell viability in the MTT test) | Bare NiTi control’s level or less (≤72%), high surface cytotoxicity | > Bare NiTi control ≤85% (73–85%), moderate cytotoxicity | >85%, low cytotoxicity |
| Cell adhesion, % (390 cells/mm2 = 100% adhesion on the bare NiTi surface) | <150% of the bare NiTi control’s level, low adhesion | 150–250% of the bare NiTi control’s level, moderate adhesion | >250% of the bare NiTi control’s level, high adhesion |
| Cell viability, % (relative content of living cells on the sample’s surface) | <85%, non-cytocompatible surface | - | ≥85%, cytocompatible surface |
| NO production, μM/mL, (16 μM/mL- NO production on the bare NiTi surface) | Bare NiTi control’s level or less (<16 μM/mL), low functional activity | >Bare NiTi control, but <150% of the bare NiTi control’s level (16–24 μM/mL), moderate functional activity | >150% of the bare NiTi control’s level (>24 μM/mL). high functional activity |
| Criterion | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 | No. 6 | No. 7 | No. 8 | Control (NiTi) |
|---|---|---|---|---|---|---|---|---|---|
| Direct cytotoxicity | 0 | 1.8 ± 0.41 | 0.3 ± 0.52 | 1.5 ± 0.55 | 0.2 ± 0.41 | 0.3 ± 0.52 | 1 ± 0 | 1.2 ± 0.41 | 0.3 ± 0.52 |
| Adhesion and spreading of cells | 0 | 1.6 ± 0.55 | 0.8 ± 0.50 | 2 ± 0 | 1 ± 0 | 0.3 ± 0.50 | 2 ± 0 | 1.3 ± 0.50 | 0 |
| Viability of cells | 1.3 ± 0.82 | 0.5 ± 0.55 | 0.8 + 0.45 | 1.4 ± 0.55 | 2 ± 0 | 1.2 ± 0.45 | 0.8 ± 0.45 | 0.6 ± 0.89 | 0.7 ± 1.0 |
| NO production | 0.3 ± 0.52 | 0 ± 0 | 1 ± 0 | 1.3 ± 0.52 | 0 ± 0 | 0 ± 0 | 2 ± 0 | 2 ± 0 | 0.5 ± 0.55 |
| ICI (ICImax = 8) | 1.6 | 3.9 | 2.9 | 6.2 | 3.2 | 1.8 | 5.8 | 5.1 | 1.5 |
| Sample No. | Partial Gas Pressure, Pa, N2/O2 | Negative Bias Voltage | Films | N2/O2 Ratio |
|---|---|---|---|---|
| control (NiTi) | - | - | - | - |
| 1 | 0/0.130 | U = −100 V | TiO2 | - |
| 2 | 0/0.130 | U = 0 | TiO2 | - |
| 3 | 0.065/0.065 | U = −100 V | Ti-O-N | 1/1 |
| 4 | 0.065/0.065 | U = 0 | Ti-O-N | 1/1 |
| 5 | 0.087/0.046 | U = −100 V | Ti-O-N | 2/1 |
| 6 | 0.087/0.046 | U = 0 | Ti-O-N | 2/1 |
| 7 | 0.102/0.033 | U = −100 V | Ti-O-N | 3/1 |
| 8 | 0.102/0.033 | U = 0 | Ti-O-N | 3/1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuravleva, I.Y.; Surovtseva, M.A.; Alshevskaya, A.A.; Surovtsev, N.V.; Okotrub, K.A.; Kim, I.I.; Nasimov, D.A.; Bondarenko, N.A.; Kuzmin, O.S.; Poveshchenko, O.V. Integral Algorithms to Evaluate TiO2 and N-TiO2 Thin Films’ Cytocompatibility. Int. J. Mol. Sci. 2022, 23, 15183. https://doi.org/10.3390/ijms232315183
Zhuravleva IY, Surovtseva MA, Alshevskaya AA, Surovtsev NV, Okotrub KA, Kim II, Nasimov DA, Bondarenko NA, Kuzmin OS, Poveshchenko OV. Integral Algorithms to Evaluate TiO2 and N-TiO2 Thin Films’ Cytocompatibility. International Journal of Molecular Sciences. 2022; 23(23):15183. https://doi.org/10.3390/ijms232315183
Chicago/Turabian StyleZhuravleva, Irina Yu., Maria A. Surovtseva, Alina A. Alshevskaya, Nikolay V. Surovtsev, Konstantin A. Okotrub, Irina I. Kim, Dmitriy A. Nasimov, Natalia A. Bondarenko, Oleg S. Kuzmin, and Olga V. Poveshchenko. 2022. "Integral Algorithms to Evaluate TiO2 and N-TiO2 Thin Films’ Cytocompatibility" International Journal of Molecular Sciences 23, no. 23: 15183. https://doi.org/10.3390/ijms232315183






