A Method for the Immobilization of Chitosan onto Urinary Catheters
Abstract
:1. Introduction
2. Results and Discussion
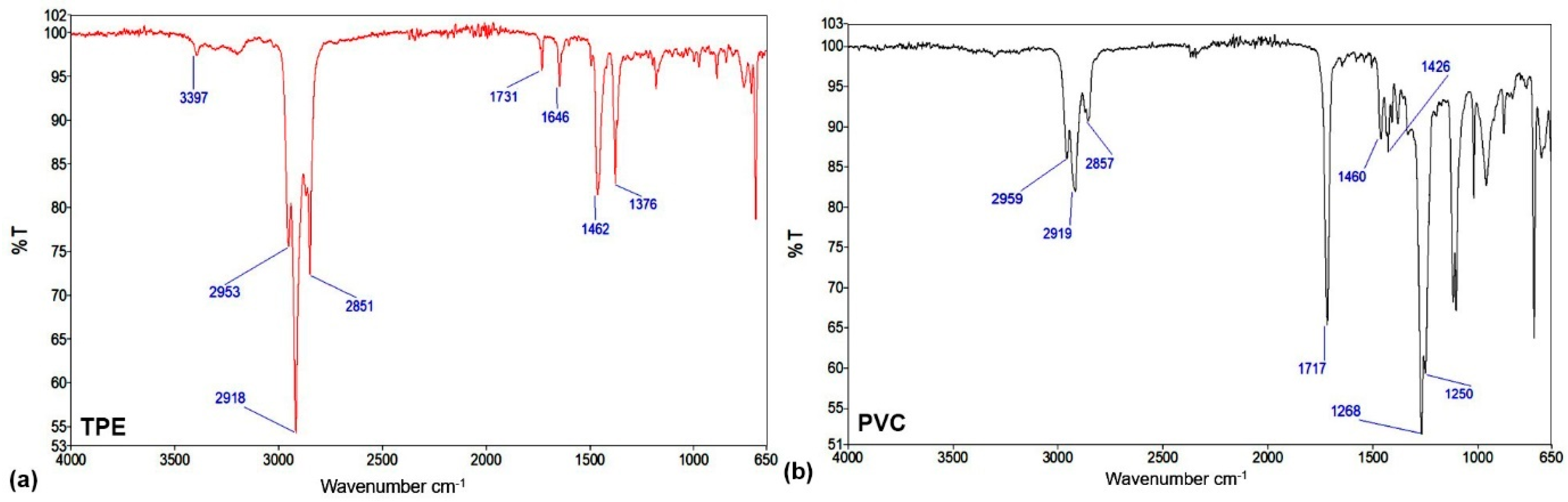
2.1. Characterization of Reference Materials for Urinary Catheters
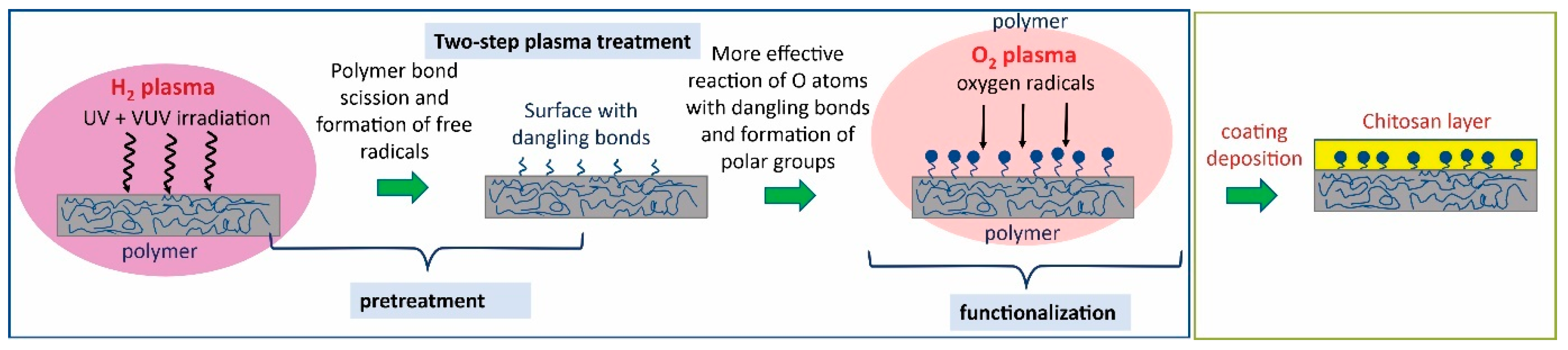
2.2. Effect of Plasma Treatment
2.2.1. Wettability of Plasma-Treated Catheters
2.2.2. XPS Characterization of Plasma-Treated Catheters
2.3. Analysis of Plasma-Treated Catheters with the Chitosan Coating
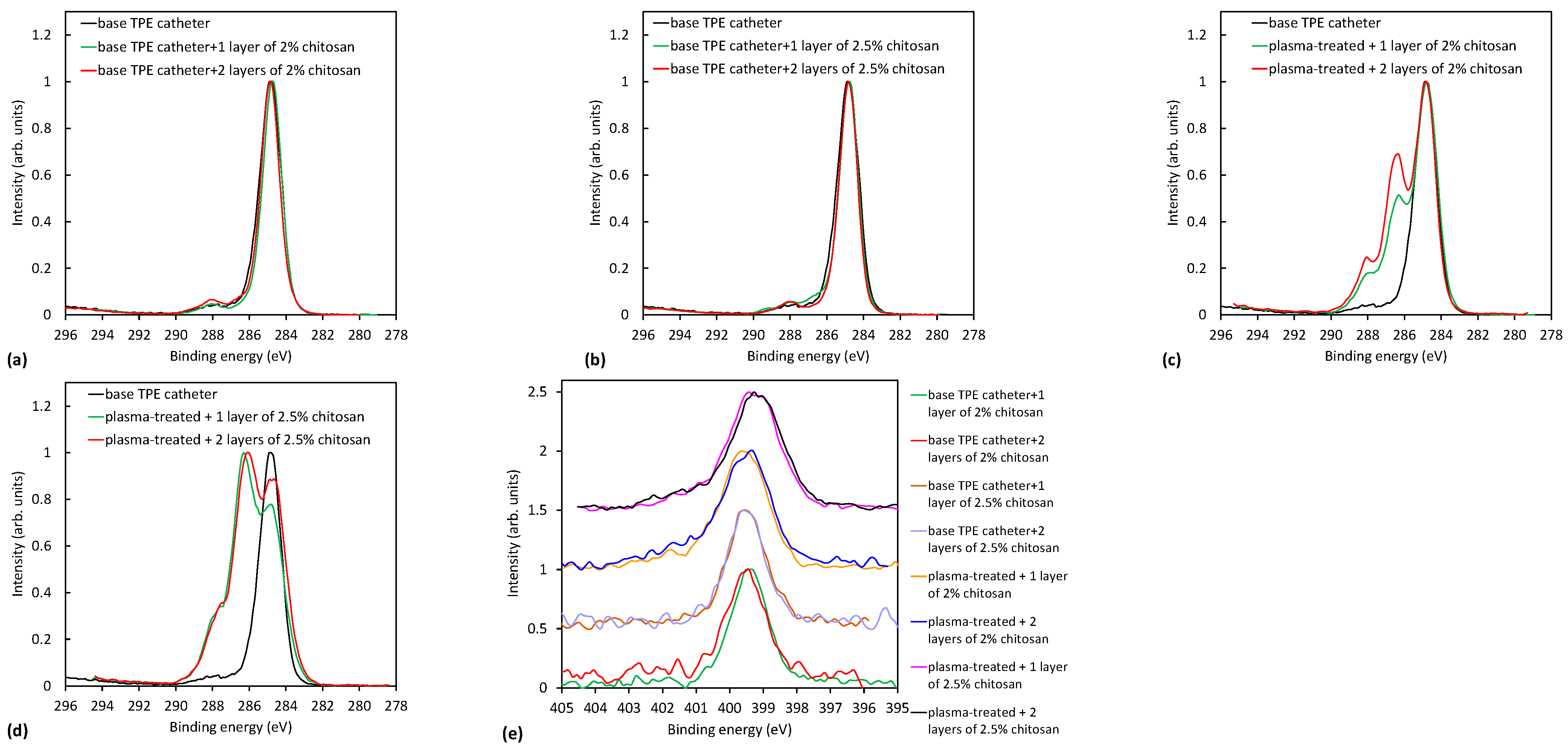
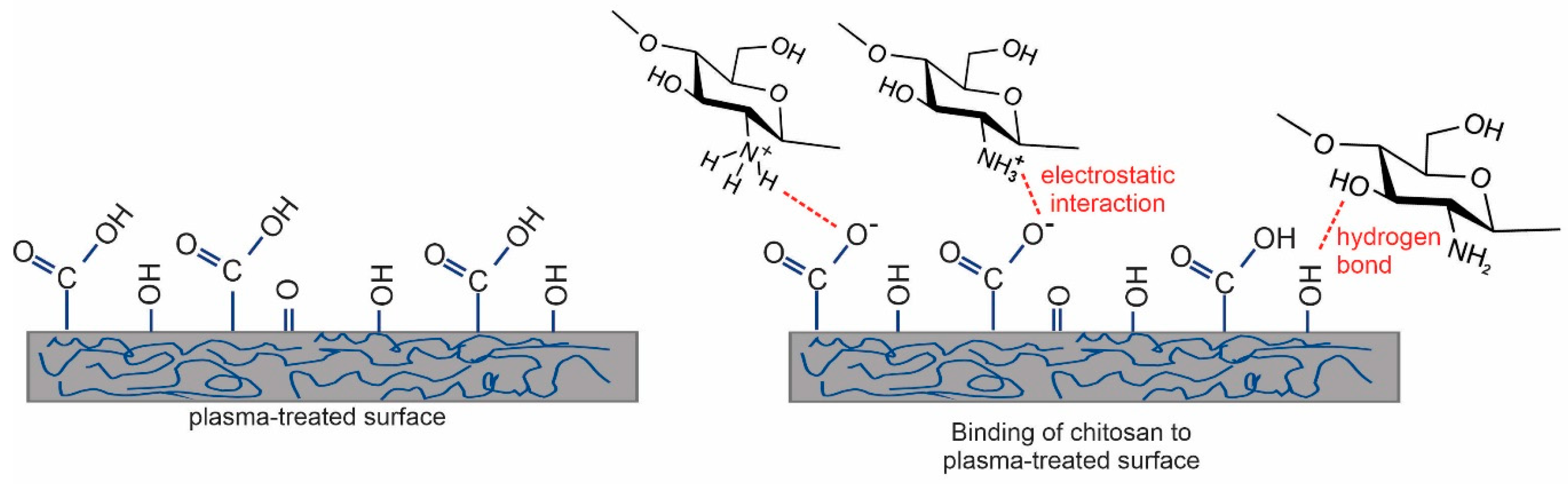
2.3.1. XPS Characterization of Plasma-Treated Catheters
2.3.2. FTIR Characterization of Plasma-Treated Catheters
2.3.3. Wettability of the Coated Catheters
3. Materials and Methods
3.1. Materials and Chemical Reagents
3.2. Preparation of Coated Urinary Catheters
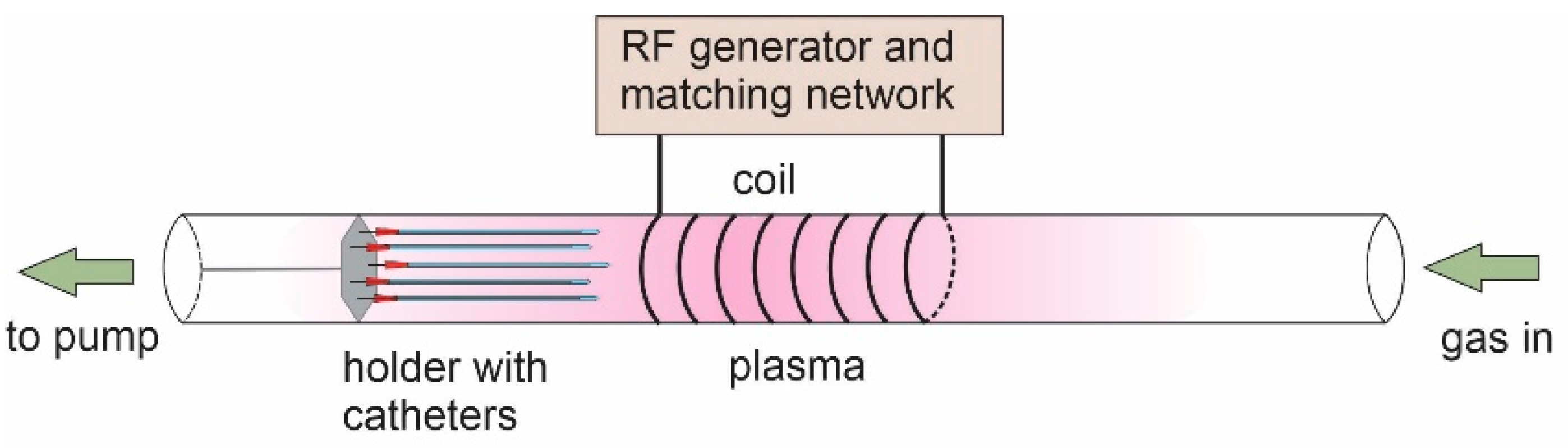
3.2.1. Plasma Treatment of Catheters
3.2.2. Deposition of Chitosan Coating
3.3. Surface Characterization
3.3.1. Chemical Composition by XPS and FTIR
3.3.2. Surface Wettability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef] [PubMed]
- Másson, M. Antimicrobial Properties of Chitosan and Its Derivatives. In Chitosan for Biomaterials III: Structure-Property Relationships; Jayakumar, R., Prabaharan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 131–168. [Google Scholar]
- Kucharska, M.; Sikora, M.; Brzoza-Malczewska, K.; Owczarek, M. Antimicrobial Properties of Chitin and Chitosan. In Chitin and Chitosan; Wiley: New York, NY, USA, 2019; pp. 169–187. [Google Scholar]
- Chung, Y.M.; Jung, M.J.; Han, J.G.; Lee, M.W.; Kim, Y.M. Atmospheric RF plasma effects on the film adhesion property. Thin Solid Films 2004, 447–448, 354–358. [Google Scholar] [CrossRef]
- Deshmukh, R.R.; Bhat, N.V. The mechanism of adhesion and printability of plasma processed PET films. Mater. Res. Innov. 2003, 7, 283–290. [Google Scholar] [CrossRef]
- Pâslaru, E.; Fras Zemljic, L.; Bračič, M.; Vesel, A.; Petrinić, I.; Vasile, C. Stability of a chitosan layer deposited onto a polyethylene surface. J. Appl. Polym. Sci. 2013, 130, 2444–2457. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Novák, I.; Lehocký, M.; Bílek, F.; Vesel, A.; Junkar, I.; Sáha, P.; Popelka, A. Polysaccharides Coatings on Medical-Grade PVC: A Probe into Surface Characteristics and the Extent of Bacterial Adhesion. Molecules 2010, 15, 1007–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, N.; Nouri Khorasani, S.; Mahdavi, H.; Ghiaci, M.; Mokhtari, R. Low-pressure plasma surface modification of polyurethane films with chitosan and collagen biomolecules. J. Appl. Polym. Sci. 2019, 136, 47567. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Wiącek, A.E.; Jurak, M. Influence of nitrogen plasma treatment on the wettability of polyetheretherketone and deposited chitosan layers. Adv. Polym. Technol. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Wiącek, A.E.; Terpiłowski, K.; Jurak, M.; Worzakowska, M. Effect of low-temperature plasma on chitosan-coated PEEK polymer characteristics. Eur. Polym. J. 2016, 78, 1–13. [Google Scholar] [CrossRef]
- Carette, X.; Mincheva, R.; Herbin, M.; Cabecas Segura, P.; Wattiez, R.; Noirfalise, X.; Thai, C.; Leclere, P.; Godfroid, T.; Boudifa, M.; et al. Microwave Atmospheric Plasma: A Versatile and Fast Way to Confer Antimicrobial Activity toward Direct Chitosan Immobilization onto Poly(lactic acid) Substrate. ACS Appl. Bio Mater. 2021, 4, 7445–7455. [Google Scholar] [CrossRef] [PubMed]
- Demina, T.S.; Piskarev, M.S.; Romanova, O.A.; Gatin, A.K.; Senatulin, B.R.; Skryleva, E.A.; Zharikova, T.M.; Gilman, A.B.; Kuznetsov, A.A.; Akopova, T.A.; et al. Plasma Treatment of Poly(ethylene terephthalate) Films and Chitosan Deposition: DC- vs. AC-Discharge. Materials 2020, 13, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suganya, A.; Shanmugvelayutham, G.; Hidalgo-Carrillo, J. Plasma surface modified polystyrene and grafted with chitosan coating for improving the shelf lifetime of postharvest grapes. Plasma Chem. Plasma Process. 2018, 38, 1151–1168. [Google Scholar] [CrossRef]
- Tkavc, T.; Petrinič, I.; Luxbacher, T.; Vesel, A.; Ristić, T.; Zemljič, L.F. Influence of O2 and CO2 plasma treatment on the deposition of chitosan onto polyethylene terephthalate (PET) surfaces. Int. J. Adhes. Adhes. 2014, 48, 168–176. [Google Scholar] [CrossRef]
- Ghobeira, R.; Esbah Tabaei, P.S.; Morent, R.; De Geyter, N. Chemical characterization of plasma-activated polymeric surfaces via XPS analyses: A review. Surf. Interfaces 2022, 31, 102087. [Google Scholar] [CrossRef]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666–685. [Google Scholar] [CrossRef] [Green Version]
- Lojen, D.; Zaplotnik, R.; Primc, G.; Mozetič, M.; Vesel, A. Optimization of surface wettability of polytetrafluoroethylene (PTFE) by precise dosing of oxygen atoms. Appl. Surf. Sci. 2022, 598, 153817. [Google Scholar] [CrossRef]
- Fantz, U.; Briefi, S.; Rauner, D.; Wünderlich, D. Quantification of the VUV radiation in low pressure hydrogen and nitrogen plasmas. Plasma Sources Sci. Technol. 2016, 25, 045006. [Google Scholar] [CrossRef]
- Kehrer, M.; Duchoslav, J.; Hinterreiter, A.; Mehic, A.; Stehrer, T.; Stifter, D. Surface functionalization of polypropylene using a cold atmospheric pressure plasma jet with gas water mixtures. Surf. Coat. Technol. 2020, 384, 125170. [Google Scholar] [CrossRef]
- Polášková, K.; Klíma, M.; Jeníková, Z.; Blahová, L.; Zajíčková, L. Effect of low molecular weight oxidized materials and nitrogen groups on adhesive joints of polypropylene treated by a cold atmospheric plasma jet. Polymers 2021, 13, 4396. [Google Scholar] [CrossRef] [PubMed]
- Strobel, M.; Lyons, C.S. The role of low-molecular-weight oxidized materials in the adhesion properties of corona-treated polypropylene film. J. Adhes. Sci. Technol. 2003, 17, 15–23. [Google Scholar] [CrossRef]
- Primc, G.; Mozetič, M. Hydrophobic recovery of plasma-hydrophilized polyethylene terephthalate polymers. Polymers 2022, 14, 2496. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.-P.; Mozetič, M.; Nikiforov, A.; Oehr, C. Foundations of plasma surface functionalization of polymers for industrial and biological applications. Plasma Sources Sci. Technol. 2022, 31, 103001. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Fassihi, A.; Emami, J.; Davies, N.M.; Dorkoosh, F. Physicochemical, pharmaceutical and biological approaches toward designing optimized and efficient hydrophobically modified chitosan-based polymeric micelles as a nanocarrier system for targeted delivery of anticancer drugs. J. Drug Target. 2013, 21, 693–709. [Google Scholar] [CrossRef] [PubMed]
- Pemble, O.J.; Bardosova, M.; Povey, I.M.; Pemble, M.E. A slot-die technique for the preparation of continuous, high-area, chitosan-based thin films. Polymers 2021, 13, 1566. [Google Scholar] [CrossRef]
- Cunha, A.G.; Fernandes, S.C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. What Is the Real Value of Chitosan’s Surface Energy? Biomacromolecules 2008, 9, 610–614. [Google Scholar] [CrossRef]
- Lauren, S. White Paper: Surface Free Energy—What Is It and How to Measure It? Biolin Scientific Manchaster, UK. 2020. Available online: https://content.biolinscientific.com/wp-surface-free-energy?hsCtaTracking=466a8519-3bc2-43d7-b304-5695aee8cb10%7C7c3243db-452c-4c1b-8f14-238db32d992c (accessed on 1 November 2022).
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owen–Wendt method. Int. J. Adhes. Adhes. 2009, 29, 451–457. [Google Scholar] [CrossRef]



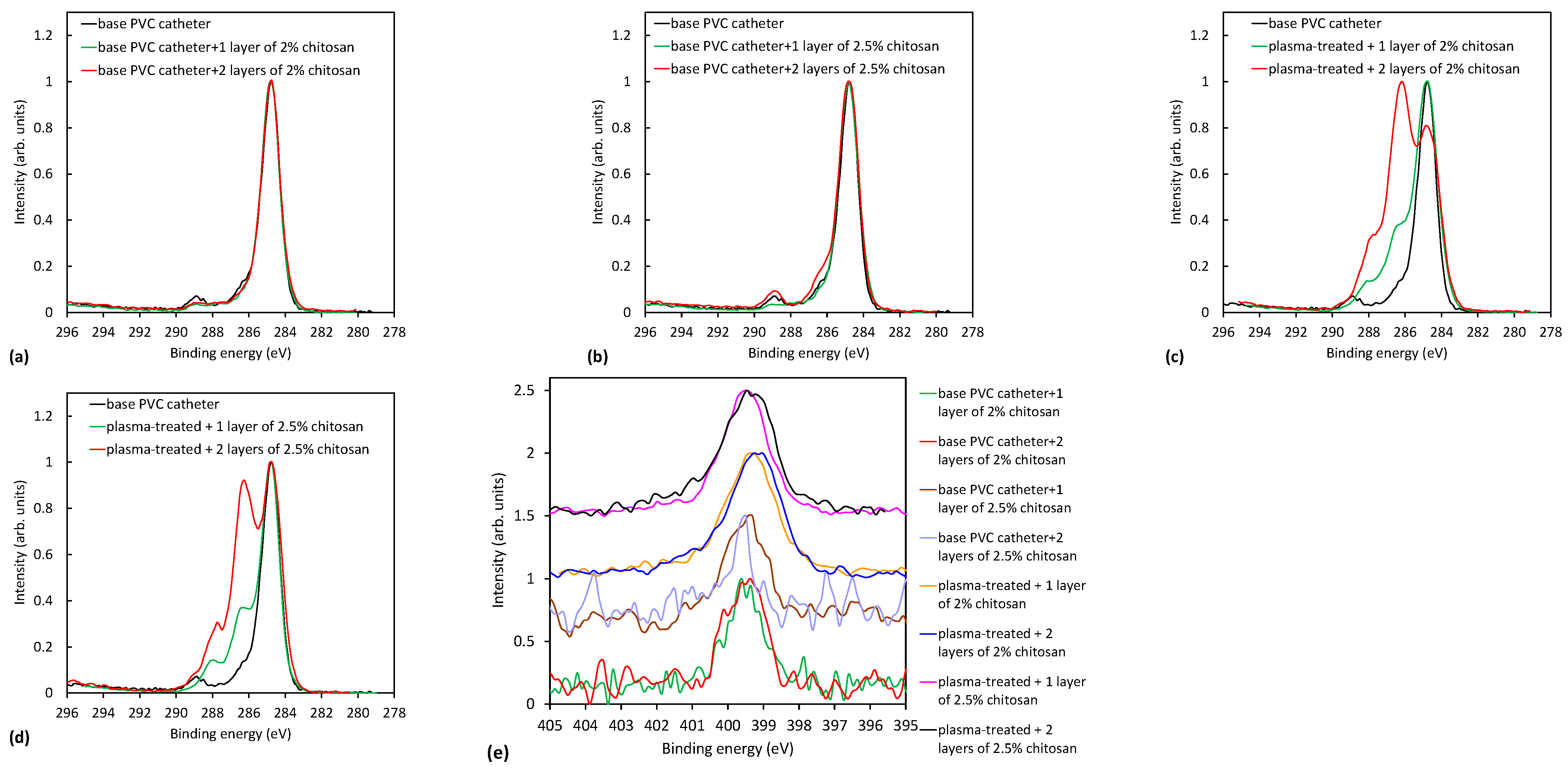
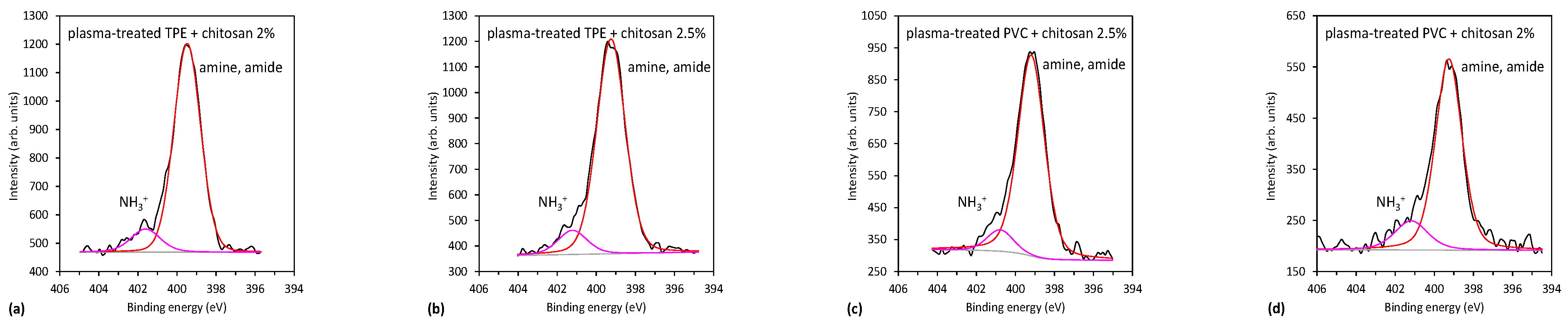


| Type of Catheter | C | N | O | Si | Cl |
|---|---|---|---|---|---|
| Base TPE catheter | 88.7 | 2.4 | 7.6 | 1.4 | / |
| Base PVC catheter | 84.1 | / | 11.4 | 2.4 | 2.1 |
| Catheter Material | Treatment Condition | Water Contact Angle (°) When Treating Small Samples | Water Contact Angle (°) When Treating the Whole Catheter |
|---|---|---|---|
| PVC | Untreated | 96° | / |
| TPE | 103° | ||
| PVC | 2 s O2 | 74° | / |
| TPE | 77° | ||
| PVC | 30 s O2 | 60° | / |
| TPE | 56° | ||
| PVC | 60 s O2 | 52° | / |
| TPE | 48° | ||
| PVC | 30 s H2 + 2 s O2 | 34° | 46° |
| TPE | 11° | 37° |
| PVC Catheter | Untreated | Two-Step Plasma Treatment |
|---|---|---|
| Water contact angle: | 96.1° ± 1.7° | 46.3° ± 3.3° |
| Surface free energy: | 46.7 mN/m | 59.0 mN/m |
| Dispersive component: | 45.6 mN/m | 37.1 mN/m |
| Polar component: | 0.1 mN/m | 21.4 mN/m |
| TPE Catheter | Untreated | Two-Step Plasma Treatment |
|---|---|---|
| Water contact angle: | 103.1° ± 1.5° | 37.2° ± 8.0° |
| Surface free energy: | 25.1 mN/m | 64.2 mN/m |
| Dispersive component: | 25.3 mN/m | 37.9 mN/m |
| Polar component: | 1.7 mN/m | 26.4 mN/m |
| Sample | C | N | O | Si | Cl | O/C |
|---|---|---|---|---|---|---|
| Untreated TPE | 88.7 | 2.4 | 7.6 | 1.4 | 0.09 | |
| Two-step plasma-treated TPE | 83.0 | 1.9 | 15.0 | 0.1 | 0.18 |
| Treatment Condition for TPE Catheters | C | N | O | Si | S |
|---|---|---|---|---|---|
| untreated TPE + chitosan 2% (one layer) | 86.7 | 2.9 | 9.4 | 1.1 | |
| two-step plasma-treated TPE + chitosan 2% (one layer) | 71.8 | 5.1 | 20.9 | 2.0 | 0.1 |
| untreated TPE + chitosan 2% (two layers) | 86.1 | 2.6 | 10.5 | 0.8 | |
| two-step plasma-treated TPE + chitosan 2% (two layers) | 71.3 | 4.7 | 22.6 | 1.2 | 0.2 |
| untreated TPE + chitosan 2.5% (one layer) | 84.7 | 3.5 | 10.7 | 1.1 | |
| two-step plasma-treated TPE + chitosan 2.5% (one layer) | 65.8 | 6.3 | 27.8 | 0 | 0.1 |
| untreated TPE + chitosan 2.5% (two layers) | 86.8 | 2.5 | 10.2 | 0.4 | |
| two-step plasma-treated TPE + chitosan 2.5% (two layers) | 66.9 | 5.6 | 26.9 | 0.4 | 0.1 |
| Treatment Condition for PVC Catheters | C | N | O | Si | Cl |
|---|---|---|---|---|---|
| untreated PVC + chitosan 2% (one layer) | 85.2 | 3.1 | 8.9 | 1.6 | 1.2 |
| two-step plasma-treated PVC + chitosan 2% (one layer) | 76.3 | 4.7 | 17.5 | 1.5 | |
| untreated PVC + chitosan 2% (two layers) | 86.6 | 1.6 | 8.6 | 1.9 | 1.3 |
| two-step plasma-treated PVC + chitosan 2% (two layers) | 63.8 | 5.9 | 28.9 | 1.4 | |
| untreated PVC + chitosan 2.5% (one layer) | 85.3 | 2.3 | 9.0 | 1.9 | 1.5 |
| two-step plasma-treated PVC + chitosan 2.5% (one layer) | 76.5 | 4.5 | 17.6 | 1.4 | |
| untreated PVC + chitosan 2.5% (two layers) | 82.8 | 1.7 | 12.0 | 1.5 | 2.0 |
| two-step plasma-treated PVC + chitosan 2.5% (two layers) | 67.6 | 5.0 | 26.8 | 0.5 |
| Treatment Condition for TPE Catheters | WCAdry (°) | WCAwet (°) |
|---|---|---|
| Reference TPE | 103.1 | 107.3 |
| untreated TPE + chitosan 2% (one layer) | 100.3 | 75.7 |
| two-step plasma-treated TPE + chitosan 2% (one layer) | 107.3 | 0 |
| untreated TPE + chitosan 2% (two layers) | 82.8 | 62.7 |
| two-step plasma-treated TPE + chitosan 2% (two layers) | 82.3 | 0 |
| untreated TPE + chitosan 2.5% (one layer) | 100.9 | 82.0 |
| two-step plasma-treated TPE + chitosan 2.5% (one layer) | 105.9 | 0 |
| untreated TPE + chitosan 2.5% (two layers) | 96.4 | 83.6 |
| two-step plasma-treated TPE + chitosan 2.5% (two layers) | 90.2 | 0 |
| Treatment Condition for PVC Catheters | WCAdry (°) | WCAwet (°) |
|---|---|---|
| Reference PVC | 96.1 | 104.6 |
| untreated PVC + chitosan 2% (one layer) | 102.3 | 97.7 |
| two-step plasma-treated PVC + chitosan 2% (one layer) | 77.1 | 0 |
| untreated PVC + chitosan 2% (two layers) | 99.7 | 89.3 |
| two-step plasma-treated PVC + chitosan 2% (two layers) | 81.2 | 0 |
| untreated PVC + chitosan 2.5% (one layer) | 98.6 | 95.7 |
| two-step plasma-treated PVC + chitosan 2.5% (one layer) | 91.7 | 0 |
| untreated PVC + chitosan 2.5% (two layers) | 96.7 | 95.0 |
| two-step plasma-treated PVC + chitosan 2.5% (two layers) | 78.7 | 0 |
| Liquid | γLtot (mN/m) | γLD (mN/m) | γLP (mN/m) |
|---|---|---|---|
| water | 72.8 | 21.8 | 51 |
| ethanol | 22.4 | 18.8 | 3.6 |
| ethylene glycol | 48 | 29 | 19 |
| diiodomethane | 50.8 | 50.8 | 0 |
| glycerol | 64 | 34 | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vesel, A.; Recek, N.; Zaplotnik, R.; Kurinčič, A.; Kuzmič, K.; Zemljič, L.F. A Method for the Immobilization of Chitosan onto Urinary Catheters. Int. J. Mol. Sci. 2022, 23, 15075. https://doi.org/10.3390/ijms232315075
Vesel A, Recek N, Zaplotnik R, Kurinčič A, Kuzmič K, Zemljič LF. A Method for the Immobilization of Chitosan onto Urinary Catheters. International Journal of Molecular Sciences. 2022; 23(23):15075. https://doi.org/10.3390/ijms232315075
Chicago/Turabian StyleVesel, Alenka, Nina Recek, Rok Zaplotnik, Albert Kurinčič, Katja Kuzmič, and Lidija Fras Zemljič. 2022. "A Method for the Immobilization of Chitosan onto Urinary Catheters" International Journal of Molecular Sciences 23, no. 23: 15075. https://doi.org/10.3390/ijms232315075
APA StyleVesel, A., Recek, N., Zaplotnik, R., Kurinčič, A., Kuzmič, K., & Zemljič, L. F. (2022). A Method for the Immobilization of Chitosan onto Urinary Catheters. International Journal of Molecular Sciences, 23(23), 15075. https://doi.org/10.3390/ijms232315075









