Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System
Abstract
:1. Introduction
2. Results and Analysis
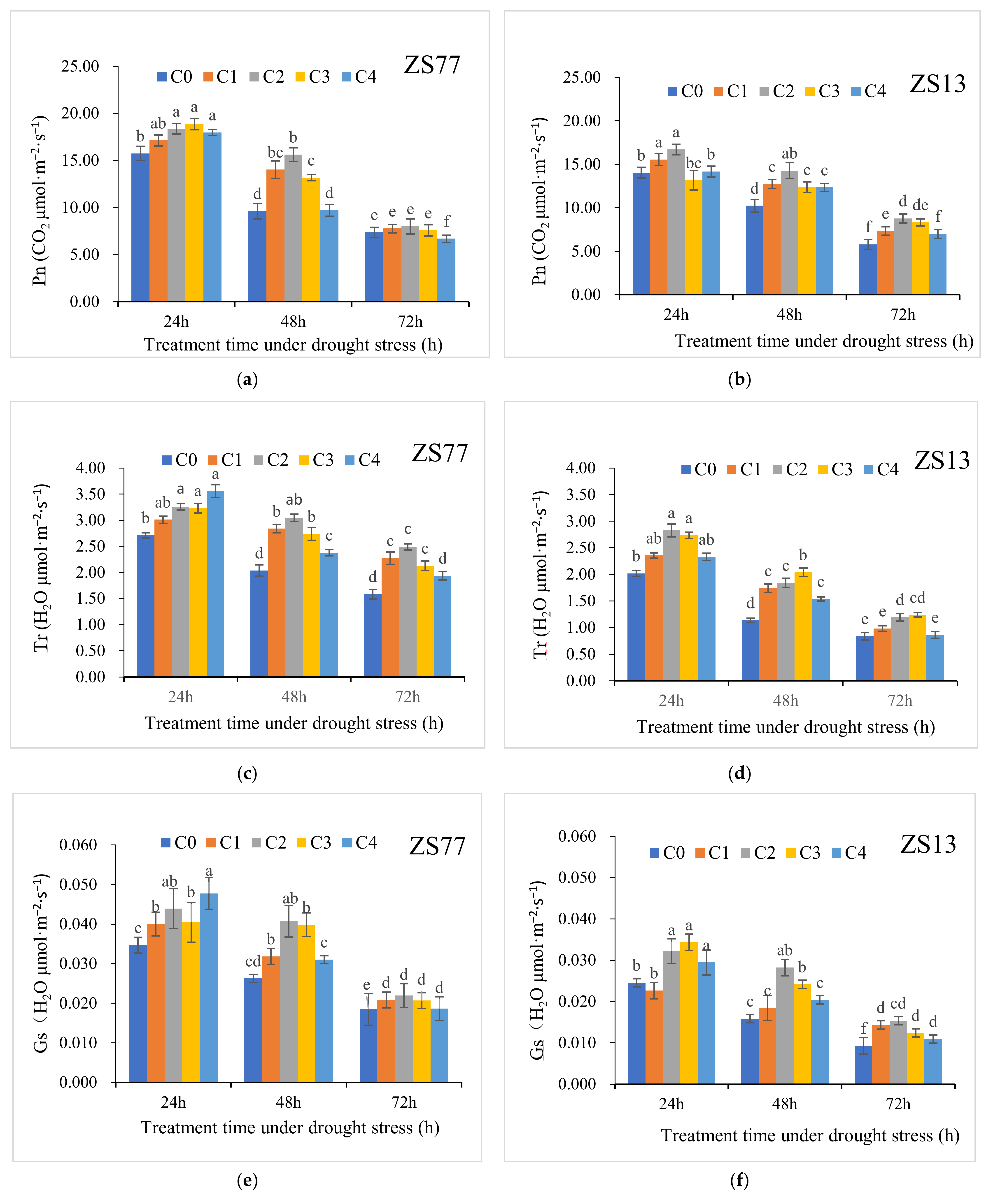
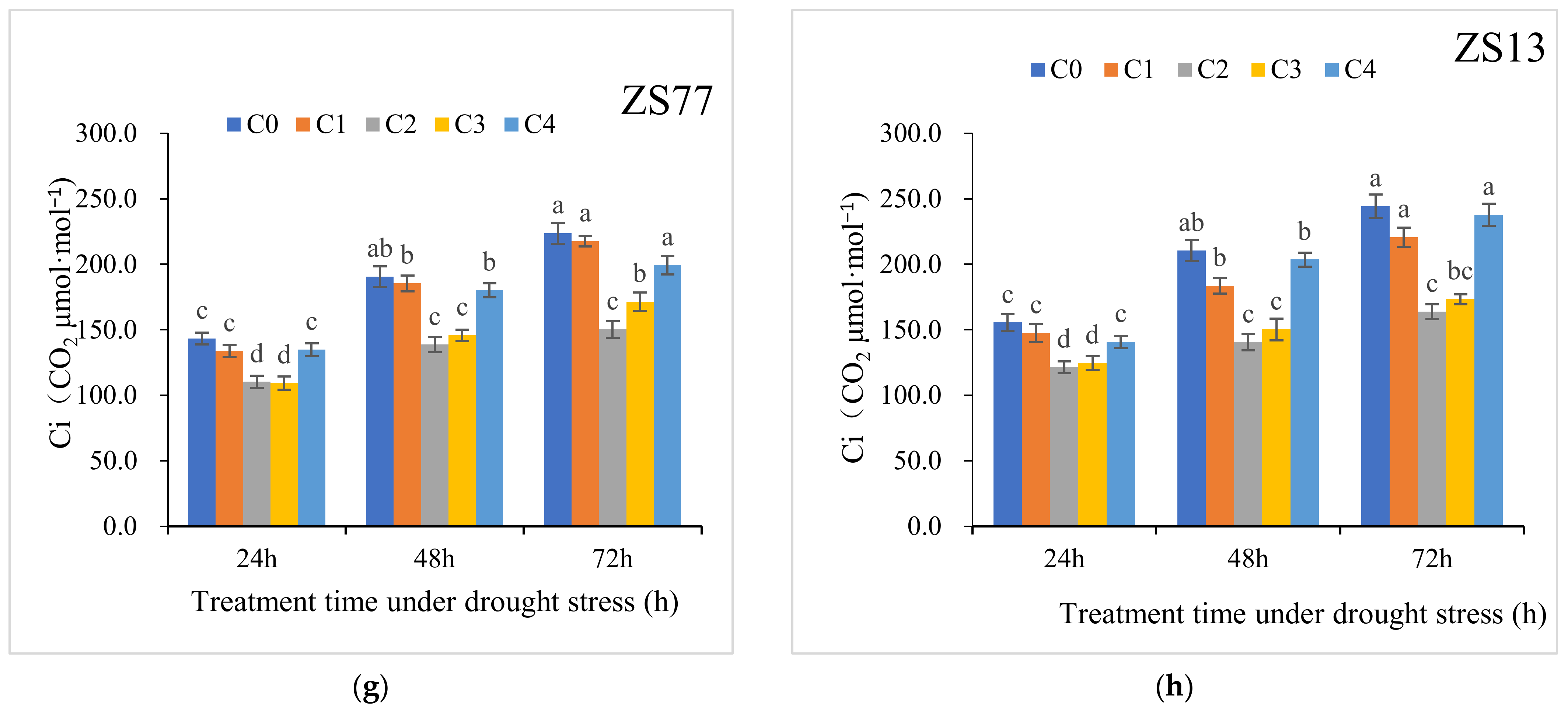
2.1. SA Protects Photosynthetic Pigments and Increases the Photosynthetic Rate
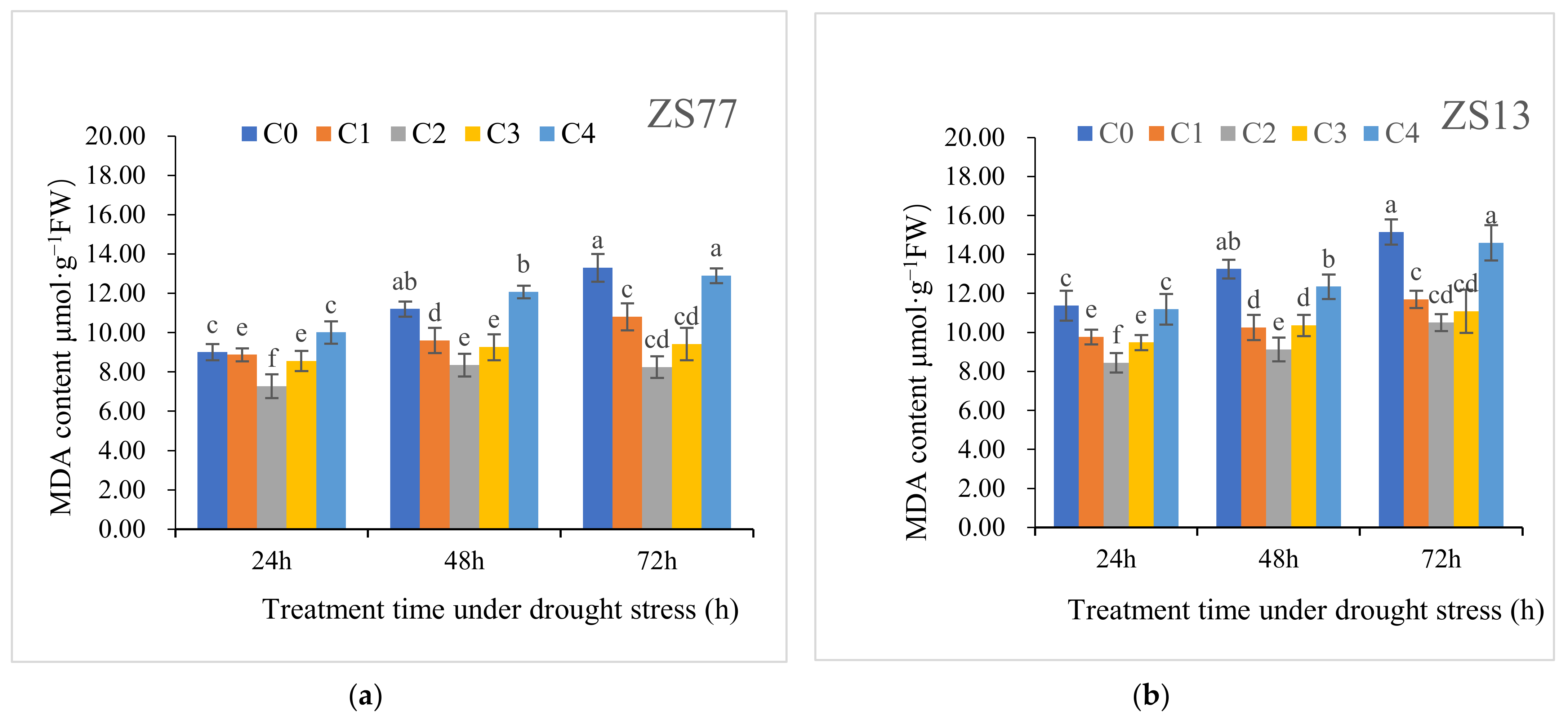
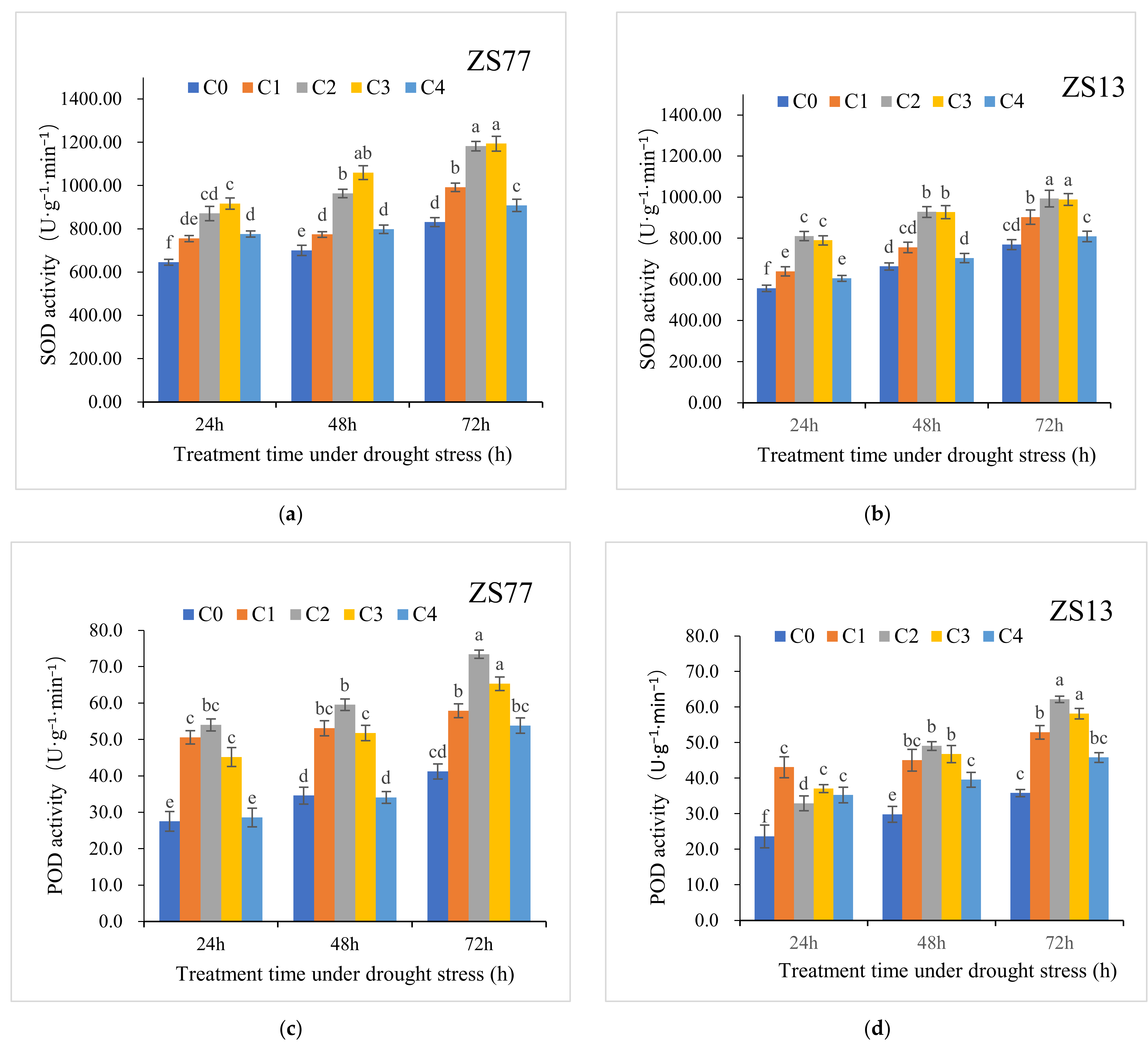
2.2. SA Reduces Oxidative Stress and Enhances Antioxidant Enzyme Activities
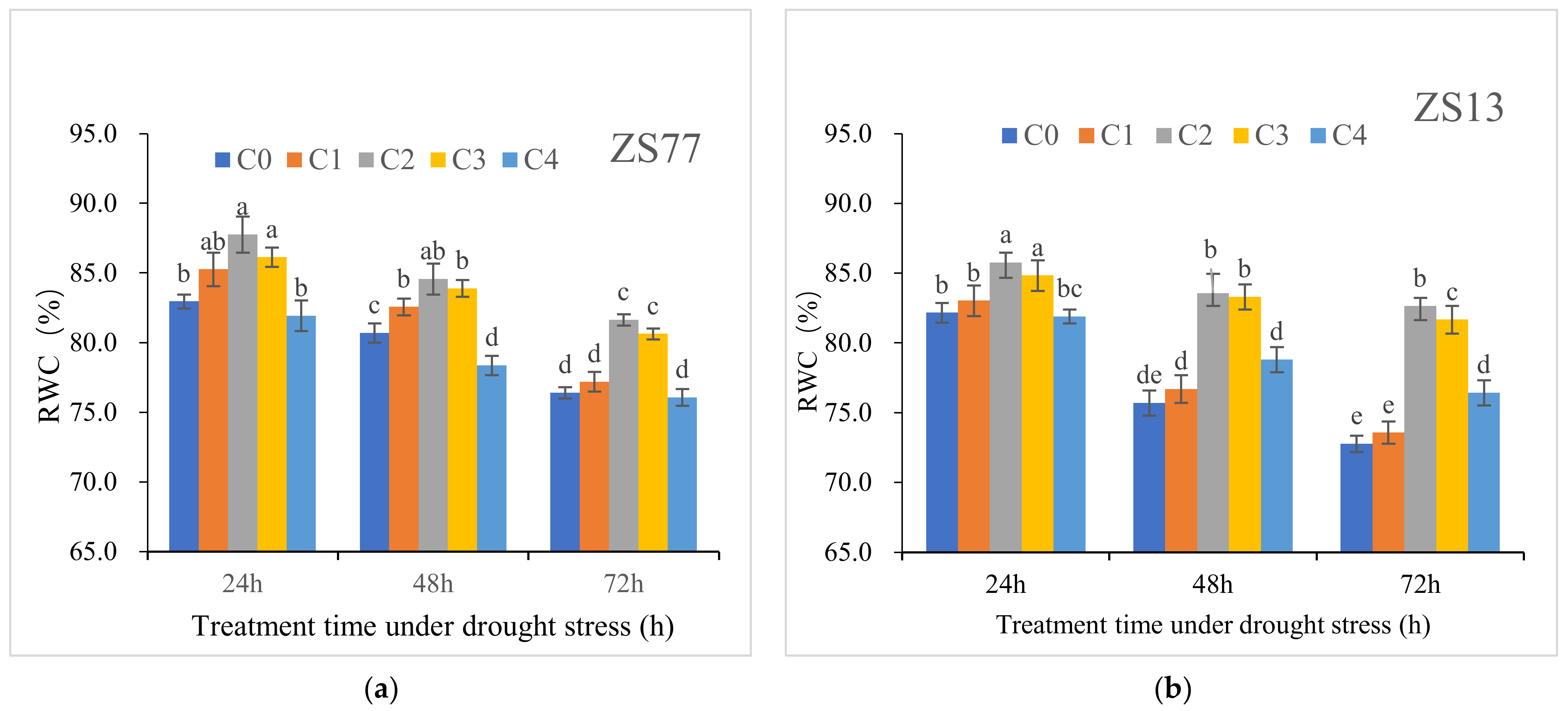
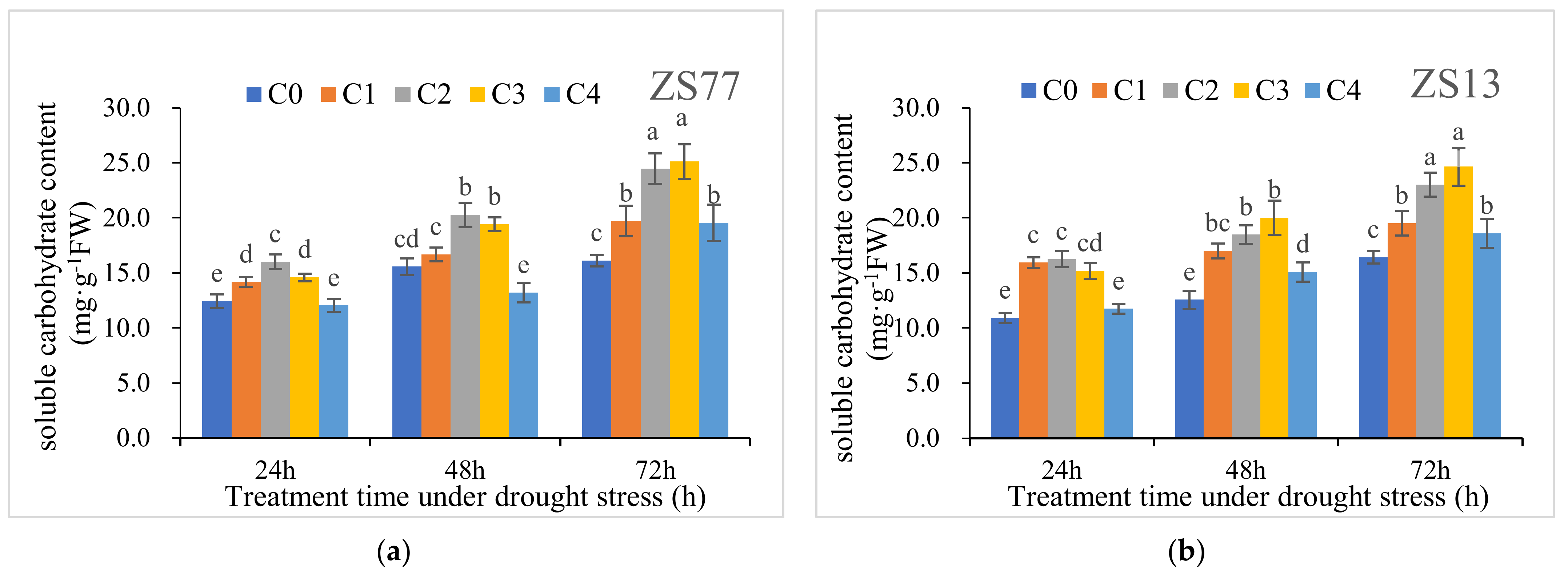
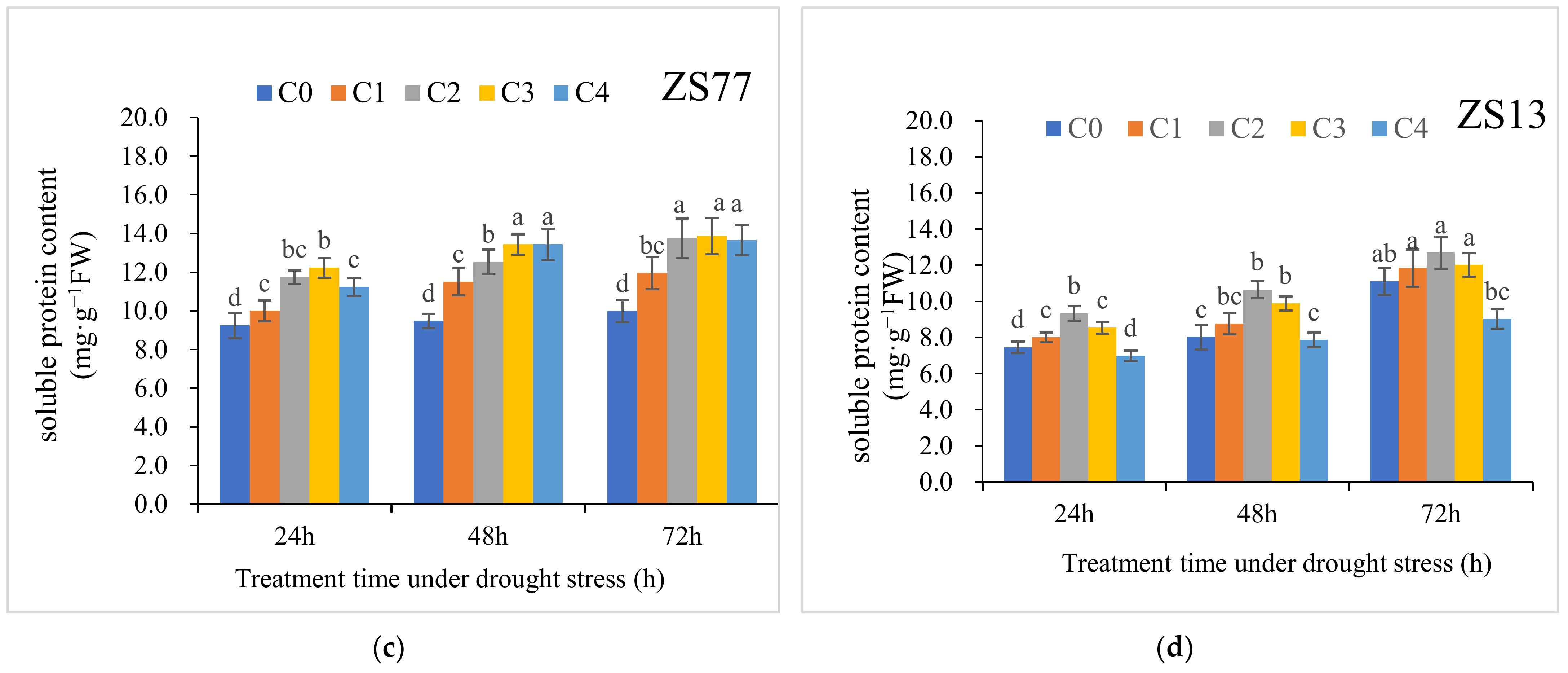
2.3. SA Mediates the Plant Osmotic Status
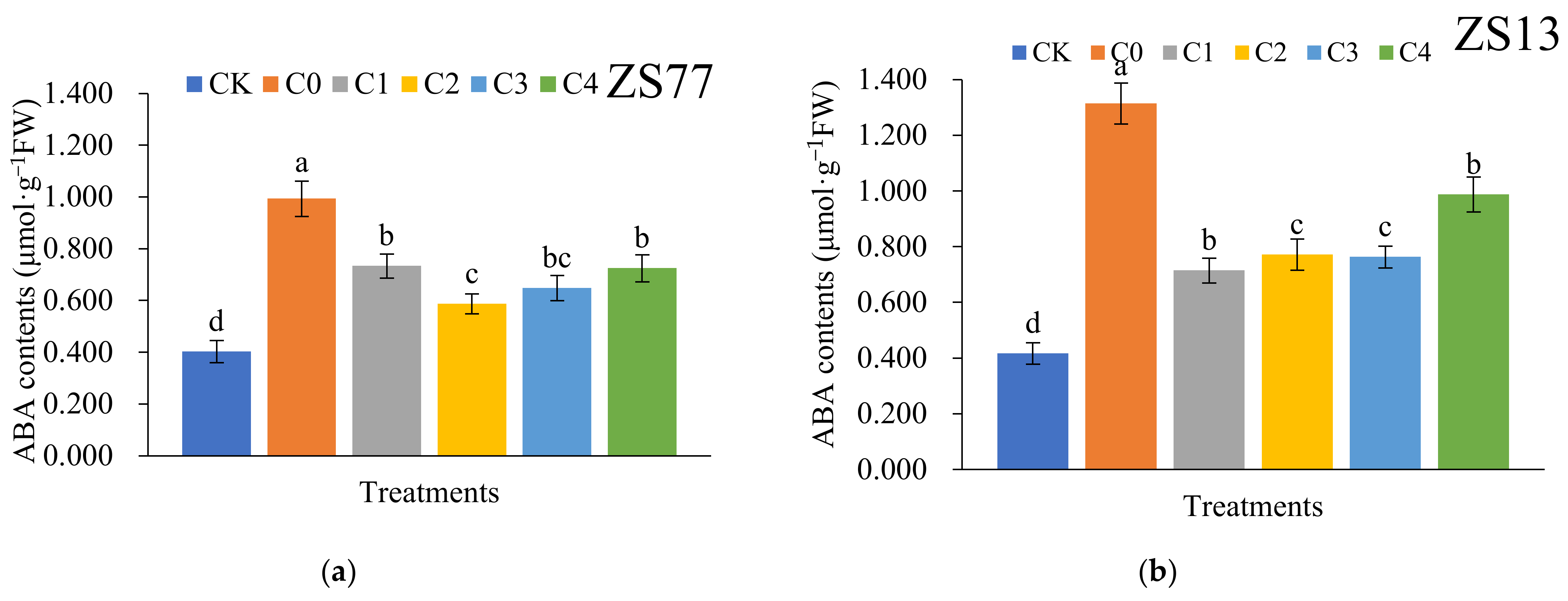
2.4. SA Effects on Abscisic Acid (ABA) Content and NCED3-Like Gene Express
2.5. SA Improves Growth Traits under Drought-Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growing Conditions
4.2. SA Treatment and Drought Stress
4.3. Determination of the Agronomy Traits
4.4. Determination of Chlorophyll Content and Photosynthetic Parameters
4.5. Determination of H2O2 and Malondialdehyde (MDA) Content
4.6. Determination of Antioxidant Enzyme Activities
4.7. Determination of Relative Water Content (RWC) and Soluble Carbohydrate and Protein Contents
4.8. Determination of the ABA Content and Semi-Quantitative RT-PCR Analysis of NCED3-Like Genes
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Munson, S.M.; Bradford, J.B.; Hultine, K.R. An integrative ecological drought framework to span plant stress to ecosystem transformation. Ecosystems 2021, 24, 739–754. [Google Scholar] [CrossRef]
- Huang, C.P.; Yu, M.Y.; Sun, L.; Wei, L. Physiological responses of sweet potato seedlings under drought-stress conditions with selenium applications. J. Agric. Crop Res. 2020, 8, 98–112. [Google Scholar] [CrossRef]
- Rao, D.E.; Chaitanya, K.V. Photosynthesis and antioxidative defense mechanisms in deciphering drought stress tolerance of crop plants. Biol. Plant. 2016, 60, 201–218. [Google Scholar] [CrossRef]
- Huang, C.P.; Qin, N.N.; Sun, L.; Yu, M.Y.; Hu, W.Z.; Qi, Z.Y. Selenium improves physiological parameters and alleviates oxidative stress in strawberry seedlings under low-temperature stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.P.; Huang, W.J.; Liao, J.L. Selenium- and nano-selenium-mediated cold-stress tolerance in crop plants. In Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement; Sustainable Plant Nutrition in a Changing World; Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, M.T., Schiavon, M., Eds.; Springer: Cham, Switzerland, 2022; pp. 173–190. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, Z.Q.; Zhao, J.; Zhang, G.P.; Wu, F.B. Physiological and biochemical responses to drought stress in cultivated and Tibetan wild barley. Plant Growth Regul. 2015, 75, 567–574. [Google Scholar] [CrossRef]
- Ding, P.T.; Ding, Y.L. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Huang, C.P.; Wang, D.; Sun, L.; Wei, L. Effects of exogenous salicylic acid on the physiological characteristics of Dendrobium officinale under chilling stress. Plant Growth Regul. 2016, 79, 199–208. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Lan, M.F.; Han, X.Y.; Wu, J.H.; Wang-Pruski, G. Response of ornamental pepper to high-temperature stress and role of exogenous salicylic acid in mitigating high temperature. J. Plant Growth Regul. 2020, 39, 133–146. [Google Scholar] [CrossRef]
- Osama, S.; Sherei, M.E.; Al-Mahdy, D.A.; Bishr, M.; Salama, O. Effect of salicylic acid foliar spraying on growth parameters, γ-pyrones, phenolic content and radical scavenging activity of drought stressed Ammi visnaga L. plant. Ind. Crop Prod. 2019, 134, 1–10. [Google Scholar] [CrossRef]
- Guo, J.K.; Zhou, R.; Ren, X.H.; Jia, H.L.; Hua, L.; Xu, H.H.; Lv, X.; Zhao, J.; Wei, T. Effects of salicylic acid, Epi-brassinolide and calcium on stress alleviation and Cd accumulation in tomato plants. Ecotoxicol. Environ. Saf. 2018, 157, 491–496. [Google Scholar] [CrossRef]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.A.; Kouzmanova, M.; Apostolova, E.L. Improvement of the rice photosynthetic apparatus defence under cadmium stress modulated by salicylic acid supply to roots. Theor. Exp. Plant Physiol. 2018, 30, 57–70. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Growth Regul. 2018, 37, 1318–1330. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, T.T.; Zhang, L.Y.; Li, H.L.; Liu, S.K.; Yang, S.; An, Q.S.; Pan, C.P.; Zou, N. Exogenous salicylic acid alleviates the accumulation of pesticides and mitigates pesticide-induced oxidative stress in cucumber plants (Cucumis sativus L.). Ecotoxicol. Environ. Saf. 2021, 208, 111654. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Cao, Q.H.; Ma, D.F. Current status and future prospective of sweet potato production and seed industry in China. Sci. Agric. Sin. 2021, 54, 483–492. (In Chinese) [Google Scholar] [CrossRef]
- Lu, J.Z.; Yi, Z.Y.; Xu, X.G.; Qing, J.J.; Wang, X. Evaluation of international competitiveness of China sweet potato industry. J. Jiangsu Nor. Uni. Nat. Sci. Ed. 2021, 39, 35–39. (In Chinese) [Google Scholar]
- Zhang, H.Y.; Duan, W.X.; Xie, B.T.; Dong, S.X.; Wang, B.Q.; Shi, C.Y.; Zhang, L.M. Effects of drought stress at different growth stages on endogenous hormones and its relationship with storage root yield in sweet potato. Acta Agron. Sin. 2018, 44, 126–136. [Google Scholar] [CrossRef]
- Li, C.Z.; Li, H.; Liu, Q.; Shi, Y.X. Comparison of root development and fluorescent physiological characteristics of sweet potato exposure to drought stress in different growth stages. J. Plant Nutr. Fertil. 2016, 22, 511–517. (In Chinese) [Google Scholar] [CrossRef]
- Fan, Y.H.; Li, Y.X.; Ma, L.L.; Lyu, Z.Y.; Wu, Q.Q.; Zhang, W.J.; Ma, S.Y.; Huang, Z.L. Effects of exogenous salicylic acid on antioxidant physiological characteristics of wheat flag leaves under high temperature stress at grain filling stage. Acta Agric. Nucl. Sin. 2022, 36, 1878–1886. (In Chinese) [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Zaid, A.; Latef, A.A.H.A. Salicylic acid spraying-induced resilience strategies against the damaging impacts of drought and/or salinity stress in two varieties of Vicia faba L. seedlings. J. Plant Growth Regul. 2022, 41, 1919–1942. [Google Scholar] [CrossRef]
- Kareem, F.; Rihan, H.; Fuller, M.P. The Effect of exogenous applications of salicylic acid on drought tolerance and up-regulation of the drought response regulon of Iraqi wheat. J. Crop Sci. Biotech. 2019, 22, 37–45. [Google Scholar] [CrossRef]
- Wang, H.L. Protective Effect of SA on Photosynthetic Apparatus of Wheat Leaves under Heat and High Irradiance Stress. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2011. (In Chinese). [Google Scholar]
- Rai, K.K. Revisiting the critical role of ROS and RNS in plant defense. J. Plant Growth Regul. 2022. [Google Scholar] [CrossRef]
- Chen, Q.H.; Yang, G.W. Signal function studies of ROS, especially RBOH-dependent ROS, in plant growth, development and environmental stress. J. Plant Growth Regul. 2020, 39, 157–171. [Google Scholar] [CrossRef]
- Yaqoob, U.; Jan, N.; Raman, P.V.; Siddique, K.H.M.; John, R. Crosstalk between brassinosteroid signaling, ROS signaling and phenylpropanoid pathway during abiotic stress in plants: Does it exist? Plant Stress 2022, 4, 100075. [Google Scholar] [CrossRef]
- Ma, Y.H.; Li, J.; Ke, J. Cloning and identification of LrPIP1 gene of Lycium ruthenicum and effect of exogenous SA on its expression under drought stress. Acta Agric. Boreali-Occident. Sin. 2022, 31, 310–319. (In Chinese) [Google Scholar] [CrossRef]
- Peng, D.L.; Zhang, Y.; Li, Q.; Song, Y.; Li, X. Exogenous application and endogenous elevation of salicylic acid levels by overexpressing a salicylic acid-binding protein 2 gene enhance nZnO tolerance of tobacco plants. Plant Soil 2020, 450, 443–461. [Google Scholar] [CrossRef]
- Shu, P.; Zhang, S.J.; Li, Y.J.; Wang, X.Y.; Yao, L.; Sheng, J.P.; Shen, L. Over-expression of SlWRKY46 in tomato plants increases susceptibility to Botrytis cinerea by modulating ROS homeostasis and SA and JA signaling pathways. Plant Physiol. Biochem. 2021, 166, 1–9. [Google Scholar] [CrossRef]
- Torun, H. Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol. Plant 2019, 165, 169–182. [Google Scholar] [CrossRef]
- Haghighi, S.R.; Hosseininaveh, V.; Maali-Amiri, R.; Talebi, K.; Irani, S. Improving the drought tolerance in pistachio (Pistacia vera) seedlings by foliar application of salicylic acid. Gesunde Pflanz. 2021, 73, 495–507. [Google Scholar] [CrossRef]
- Mehrasa, H.; Farnia, A.; Kenarsari, M.J.; Nakhjavan, S. Endophytic bacteria and SA application improve growth, biochemical properties, and nutrient uptake in white beans under drought stress. J. Soil Sci. Plant Nutr. 2022, 22, 3268–3279. [Google Scholar] [CrossRef]
- Munsif, F.; Shah, T.; Arif, M.; Jehangir, M.; Afridi, M.Z.; Ahmad, I.; Jan, B.L.; Alansi, S. Combined effect of salicylic acid and potassium mitigates drought stress through the modulation of physio-biochemical attributes and key antioxidants in wheat. Saudi J. Biol. Sci. 2022, 29, 103294. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, C.Q.; Huang, J.; Xu, D.A.; Zhang, C.H.; Chen, J. Effect of exogenous abscisic acid and salicylic acid on germination and physiological characteristics of wheat seed. Chin. J. Appl. Environ. Biol. 2014, 20, 139–143. (In Chinese) [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Lin, Z.F.; Li, S.S.; Lin, G.Z.; Guo, J.Y. The accumulation of hydrogen peroxide in senescing leaves and chloroplasts in relation to lipid peroxidation. Acta Photophysiol. Sin. 1988, 14, 16–22. (In Chinese) [Google Scholar]
- Wu, F.B.; Zhang, G.P.; Dominy, P. Four barley genotypes respond differently to cadmium: Lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003, 50, 67–78. [Google Scholar] [CrossRef]
- Prochazkova, D.; Sairam, R.K.; Srivastava, G.C.; Singh, D.V. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001, 161, 765–771. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Kong, X.J. Effects of foliar application of nitric oxide and salicylic acid on salt-induced changes in photosynthesis and antioxidative metabolism of cotton seedlings. Plant Growth Regul. 2014, 73, 67–78. [Google Scholar] [CrossRef]
- Lin, A.; Wang, Y.; Tang, J.; Xue, P.; Li, C.; Liu, L.; Hu, B.; Loake, Y.G.; Chu, C.C. Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol. 2012, 158, 451–464. [Google Scholar] [CrossRef]




| Total Chlorophyll Content (mg·g−1FW) | ||||||
|---|---|---|---|---|---|---|
| Cultivars | Treatment Duration (h) | C0 | C1 | C2 | C3 | C4 |
| 0.0 | 1.00 | 2.00 | 4.00 | 8.00 | ||
| ZS77 | 24 | 3.41 ± 0.08 b | 3.68 ± 0.14 a | 3.54 ± 0.16 a | 3.43 ± 0.09 ab | 3.26 ± 0.11 b |
| 48 | 2.78 ± 0.11 d | 3.43 ± 0.11 ab | 3.31 ± 0.09 b | 3.11 ± 0.14 c | 2.79 ± 0.07 d | |
| 72 | 2.38 ± 0.07 e | 2.74 ± 0.14 d | 2.92 ± 0.13 c | 2.91 ± 0.09 c | 2.60 ± 0.07 d | |
| ZS13 | 24 | 3.50 ± 0.08 b | 3.62 ± 0.13 a | 3.76 ± 0.09 a | 3.52 ± 0.11 b | 3.47 ± 0.15 b |
| 48 | 3.02 ± 0.10 c | 3.42 ± 0.09 b | 3.38 ± 0.13 b | 3.27 ± 0.17 c | 2.75 ± 0.12 d | |
| 72 | 2.62 ± 0.14 d | 2.62 ± 0.09 d | 2.89 ± 0.08 c | 3.07 ± 0.11 c | 2.31 ± 0.13 e | |
| Item | Treatments | Duration of Drought Stress (h) | |||||
|---|---|---|---|---|---|---|---|
| ZS77 | ZS13 | ||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| Pn (CO2 μmol·m−2 s−1) | CK | 18.34 ± 0.57 | 19.05 ± 0.82 | 18.74 ± 0.77 | 16.58 ± 0.92 | 16.37 ± 1.11 | 17.05 ± 0.98 |
| C0 | 15.74 ± 0.77 | 9.61 ± 0.82 | 7.37 ± 0.54 | 14.04 ± 0.62 | 10.24 ± 0.73 | 5.78 ± 0.59 | |
| Tr (H2O mmol·m−2 s−1) | CK | 5.38 ± 0.10 | 5.62 ± 0.09 | 5.55 ± 0.07 | 3.77 ± 0.08 | 3.69 ± 0.11 | 3.73 ± 0.09 |
| C0 | 2.71 ± 0.05 | 2.04 ± 0.11 | 1.58 ± 0.09 | 2.02 ± 0.06 | 1.14 ± 0.04 | 0.84 ± 0.07 | |
| Gs (H2O mol·m−2 s−1) | CK | 0.214 ± 0.007 | 0.228 ± 0.005 | 0.237 ± 0.08 | 0.188 ± 0.04 | 0.170 ± 0.07 | 0.183 ± 0.10 |
| C0 | 0.035 ± 0.002 | 0.026 ± 0.001 | 0.018 ± 0.004 | 0.025 ± 0.001 | 0.016 ± 0.001 | 0.009 ± 0.002 | |
| Ci (CO2 μmol·mol−1) | CK | 250.4 ± 4.8 | 256.8 ± 8.5 | 255.6 ± 10.1 | 223.9 ± 7.3 | 240.3 ± 5.2 | 239.2 ± 6.7 |
| C0 | 143.4 ± 4.6 | 190.6 ± 7.9 | 223.7 ± 8.1 | 155.6 ± 6.4 | 210.4 ± 8.0 | 244.3 ± 9.0 | |
| Item | Treatments | Duration of Drought Stress | |||||
|---|---|---|---|---|---|---|---|
| ZS77 | ZS13 | ||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||
| H2O2 content (μmol·g−1 FW) | CK | 6.43 ± 0.38 | 6.71 ± 0.44 | 6.59 ± 0.51 | 7.55 ± 0.49 | 7.63 ± 0.33 | 7.59 ± 0.50 |
| C0 | 8.52 ± 0.49 | 11.74 ± 0.35 | 12.91 ± 0.66 | 8.58 ± 0.32 | 12.45 ± 0.43 | 13.07 ± 0.47 | |
| MDA content (μmol·g−1 FW) | CK | 7.77 ± 0.31 | 7.81 ± 0.47 | 7.69 ± 0.62 | 8.40 ± 0.69 | 8.77 ± 0.63 | 8.64 ± 0.29 |
| C0 | 9.01 ± 0.41 | 11.20 ± 0.39 | 13.30 ± 0.70 | 11.38 ± 0.77 | 13.26 ± 0.48 | 15.16 ± 0.65 | |
| Vine Length (cm) | Dry Matter Weight (g) | Leaf Area (cm2) | ||||
|---|---|---|---|---|---|---|
| ZS77 | ZS13 | ZS77 | ZS13 | ZS77 | ZS13 | |
| C0 | 14.81 ± 1.67 b | 15.46 ± 1.42 b | 15.35 ± 1.78 b | 16.43 ± 1.03 b | 19.58 ± 1.35 c | 20.71 ± 1.22 c |
| C1 | 15.62 ± 0.64 b | 16.74 ± 0.67 ab | 16.94 ± 2.37 ab | 17.85 ± 2.78 ab | 20.66 ± 1.67 cb | 21.95 ± 1.54 bc |
| C2 | 17.82 ± 0.55 a | 18.08 ± 0.85 a | 19.36 ± 1.08 a | 19.94 ± 1.47 a | 22.82 ± 0.89 ba | 24.44 ± 1.04 ab |
| C3 | 17.64 ± 0.61 a | 18.18 ± 0.65 a | 18.93 ± 0.78 a | 19.81 ± 0.95 a | 23.91 ± 0.89 a | 25.35 ± 1.08 a |
| C4 | 16.44 ± 0.59 ab | 16.76 ± 0.58 ab | 17.75 ± 1.57 ab | 17.6 ± 1.63 ab | 21.41 ± 2.41 abc | 22.45 ± 2.47 abc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Liao, J.; Huang, W.; Qin, N. Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System. Int. J. Mol. Sci. 2022, 23, 14819. https://doi.org/10.3390/ijms232314819
Huang C, Liao J, Huang W, Qin N. Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System. International Journal of Molecular Sciences. 2022; 23(23):14819. https://doi.org/10.3390/ijms232314819
Chicago/Turabian StyleHuang, Chongping, Junlin Liao, Wenjie Huang, and Nannan Qin. 2022. "Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System" International Journal of Molecular Sciences 23, no. 23: 14819. https://doi.org/10.3390/ijms232314819
APA StyleHuang, C., Liao, J., Huang, W., & Qin, N. (2022). Salicylic Acid Protects Sweet Potato Seedlings from Drought Stress by Mediating Abscisic Acid-Related Gene Expression and Enhancing the Antioxidant Defense System. International Journal of Molecular Sciences, 23(23), 14819. https://doi.org/10.3390/ijms232314819







