Down-Regulation of Neogenin Decreases Proliferation and Differentiation of Spermatogonia during the Early Phase of Spermatogenesis
Abstract
:1. Introduction
2. Results
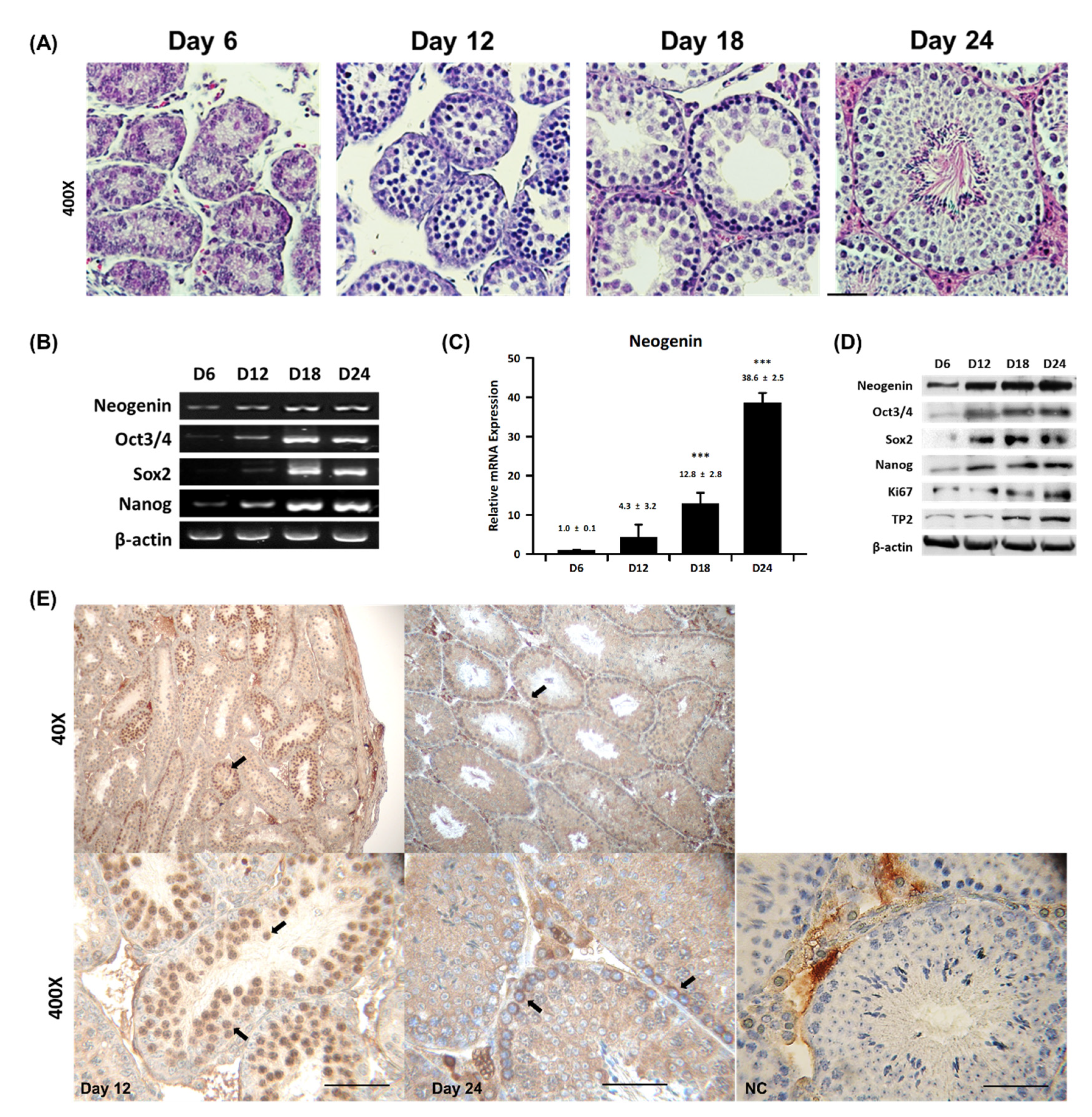
2.1. Neogenin Expression Varies during Spermatogenesis
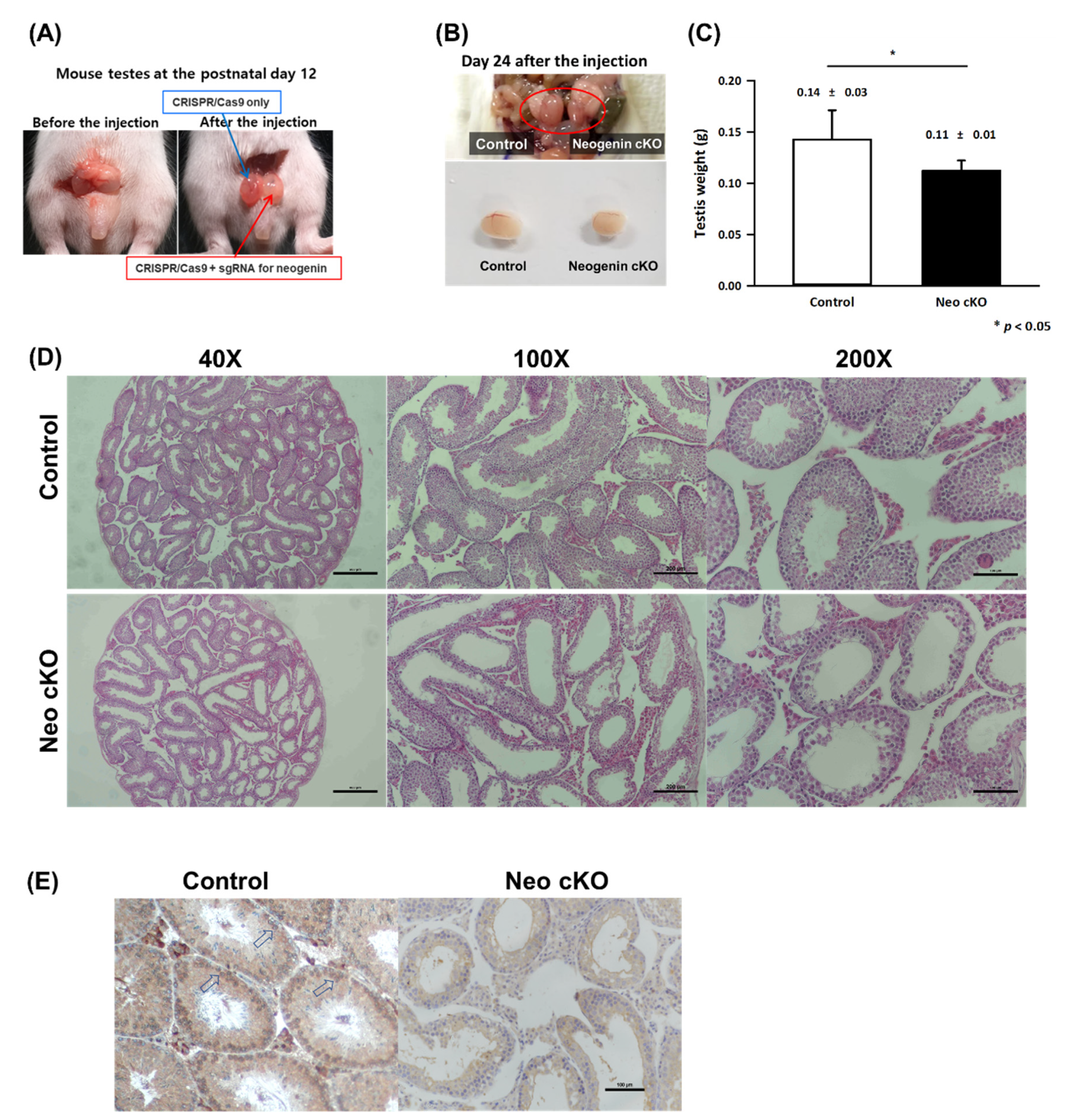
2.2. Neogenin Down-Regulation Hampers Differentiation of Primary Spermatocytes into Secondary Spermatocytes
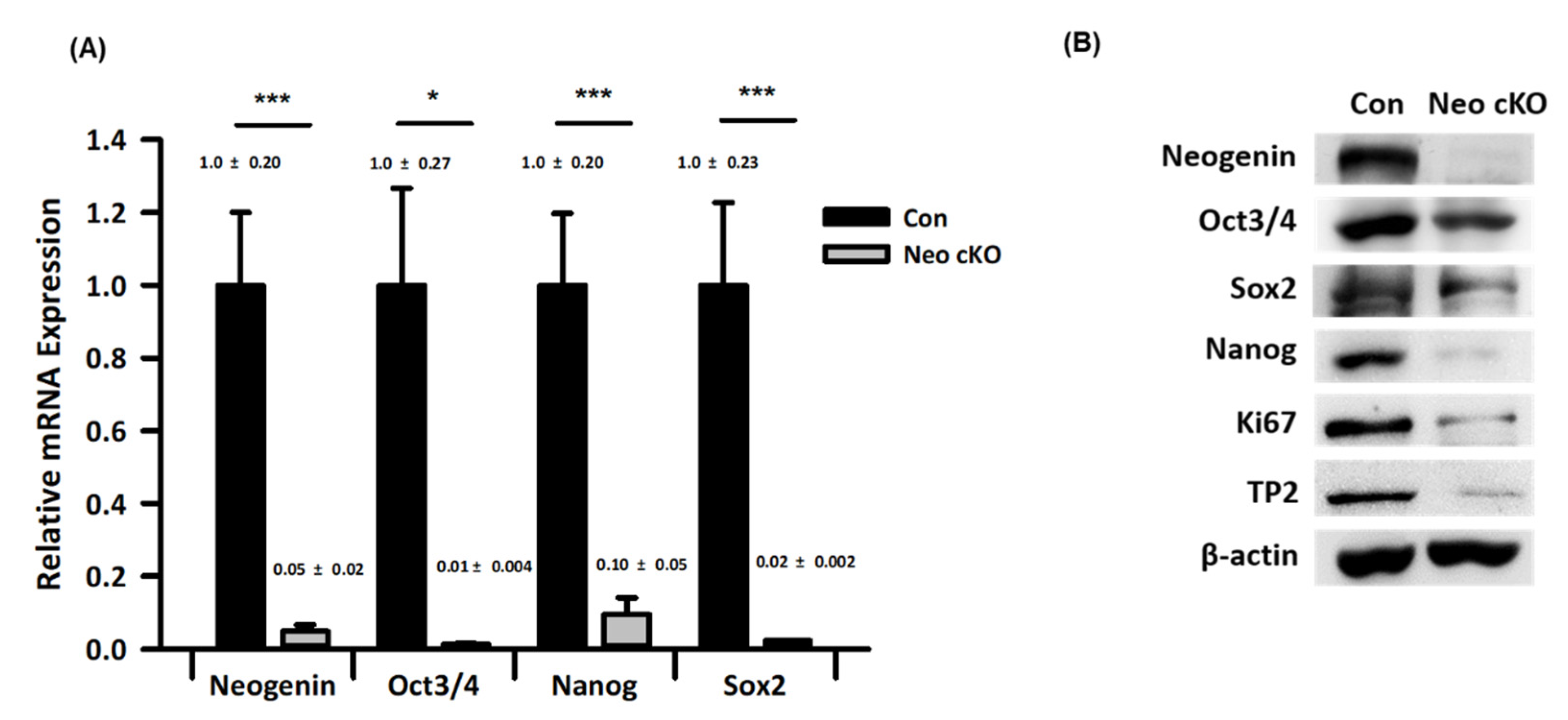
2.3. Expression Profiles of Spermatogonial Stem Cell Markers in Neogenin-cKO Testes
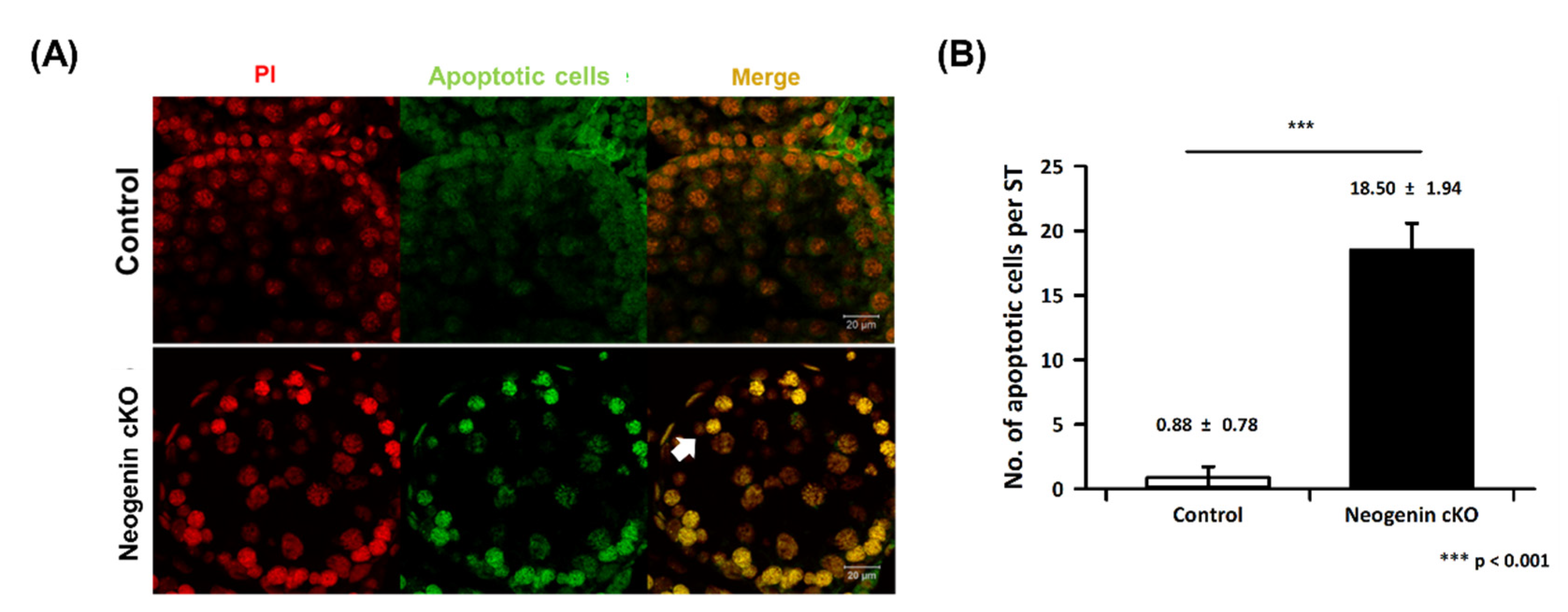
2.4. Apoptosis in Neogenin-cKO Testes
3. Discussion
4. Materials and Methods
4.1. Preparation of Mouse Testes
4.2. Histochemical Examination of Testes
4.3. PCR for Gene Expression Analysis
4.4. Western Blotting for Protein Expression Analysis
4.5. cKO of Neogenin in the Testis
4.6. Analysis of Apoptosis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chiba, K.; Enatsu, N.; Fujisawa, M. Management of non-obstructive azoospermia. Reprod. Med. Biol. 2016, 15, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.S.; Shakes, D.C. Spermatogenesis. Adv. Exp. Med. Biol. 2013, 757, 171–203. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, B.T.; Gassei, K.; Orwig, K.E. Spermatogonial stem cell regulation and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1663–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wosnitzer, M.; Goldstein, M.; Hardy, M.P. Review of Azoospermia. Spermatogenesis 2014, 4, e28218. [Google Scholar] [CrossRef] [Green Version]
- Jan, S.Z.; Hamer, G.; Repping, S.; de Rooij, D.G.; van Pelt, A.M.; Vormer, T.L. Molecular control of rodent spermatogenesis. Biochim. Biophys. Acta 2012, 1822, 1838–1850. [Google Scholar] [CrossRef] [Green Version]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, H.; L’Hernault, S.W. Spermatogenesis. Curr. Biol. 2017, 27, R988–R994. [Google Scholar] [CrossRef] [Green Version]
- Oatley, J.M.; Brinster, R.L. Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef] [Green Version]
- Wilson, N.H.; Key, B. Neogenin: One receptor, many functions. Int. J. Biochem. Cell Biol. 2007, 39, 874–878. [Google Scholar] [CrossRef]
- Rodriguez, A.; Pan, P.; Parkkila, S. Expression studies of neogenin and its ligand hemojuvelin in mouse tissues. J. Histochem. Cytochem. 2007, 55, 85–96. [Google Scholar] [CrossRef]
- Cole, S.J.; Bradford, D.; Cooper, H.M. Neogenin: A multi-functional receptor regulating diverse developmental processes. Int. J. Biochem. Cell Biol. 2007, 39, 1569–1575. [Google Scholar] [CrossRef]
- Fitzgerald, D.P.; Cole, S.J.; Hammond, A.; Seaman, C.; Cooper, H.M. Characterization of neogenin-expressing neural progenitor populations and migrating neuroblasts in the embryonic mouse forebrain. Neuroscience 2006, 142, 703–716. [Google Scholar] [CrossRef]
- Healey, E.G.; Bishop, B.; Elegheert, J.; Bell, C.H.; Padilla-Parra, S.; Siebold, C. Repulsive guidance molecule is a structural bridge between neogenin and bone morphogenetic protein. Nat. Struct. Mol. Biol. 2015, 22, 458–465. [Google Scholar] [CrossRef]
- Huang, Z.; Sun, D.; Hu, J.X.; Tang, F.L.; Lee, D.H.; Wang, Y.; Hu, G.; Zhu, X.J.; Zhou, J.; Mei, L.; et al. Neogenin Promotes BMP2 Activation of YAP and Smad1 and Enhances Astrocytic Differentiation in Developing Mouse Neocortex. J. Neurosci. 2016, 36, 5833–5849. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Kim, H.J.; Bae, J.Y.; Kim, S.W.; Park, J.S.; Shin, H.J.; Han, W.; Kim, S.W.; Kang, K.S.; Noh, D.Y. Neogenin expression may be inversely correlated to the tumorigenicity of human breast cancer. BMC Cancer 2005, 5, 154. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, E.; Tauszig-Delamasure, S.; Monnier, P.P.; Mueller, B.K.; Strittmatter, S.M.; Mehlen, P.; Chedotal, A. RGM and its receptor neogenin regulate neuronal survival. Nat. Cell Biol. 2004, 6, 749–755. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Deitinghoff, L.; Davis, D.; Conrad, S.; Skutella, T.; Chedotal, A.; Mueller, B.K.; Strittmatter, S.M. Neogenin mediates the action of repulsive guidance molecule. Nat. Cell Biol. 2004, 6, 756–762. [Google Scholar] [CrossRef]
- Sun, D.; Sun, X.D.; Zhao, L.; Lee, D.H.; Hu, J.X.; Tang, F.L.; Pan, J.X.; Mei, L.; Zhu, X.J.; Xiong, W.C. Neogenin, a regulator of adult hippocampal neurogenesis, prevents depressive-like behavior. Cell Death Dis. 2018, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Zhong, S.; Zou, L.; Zhao, Y.; Hu, B.; Xie, H. Effect of different concentrations of neogenin on proliferation, apoptosis and related proliferative factors in human trophoblasts. J. Huazhong Univ. Sci. Technol. Med. Sci. 2010, 30, 500–504. [Google Scholar] [CrossRef]
- Dann, C.T.; Alvarado, A.L.; Molyneux, L.A.; Denard, B.S.; Garbers, D.L.; Porteus, M.H. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem Cells 2008, 26, 2928–2937. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seandel, M.; Falciatori, I.; Wen, D.; Rafii, S. CD34+ testicular stromal cells support long-term expansion of embryonic and adult stem and progenitor cells. Stem Cells 2008, 26, 2516–2522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Choi, S.S.; Kim, H.W.; Xiong, W.C.; Min, C.K.; Lee, S.J. Neogenin as a receptor for early cell fate determination in preimplantation mouse embryos. PLoS ONE 2014, 9, e101989. [Google Scholar] [CrossRef] [Green Version]
- Pradeepa, M.M.; Rao, M.R. Chromatin remodeling during mammalian spermatogenesis: Role of testis specific histone variants and transition proteins. Soc. Reprod. Fertil. Suppl. 2007, 63, 1–10. [Google Scholar] [PubMed]
- Steger, K.; Aleithe, I.; Behre, H.; Bergmann, M. The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: An immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol. Hum. Reprod. 1998, 4, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Niederberger, C.S.; Shubhada, S.; Kim, S.J.; Lamb, D.J. Paracrine factors and the regulation of spermatogenesis. World J. Urol. 1993, 11, 120–128. [Google Scholar] [CrossRef]
- Buaas, F.W.; Kirsh, A.L.; Sharma, M.; McLean, D.J.; Morris, J.L.; Griswold, M.D.; de Rooij, D.G.; Braun, R.E. Plzf is required in adult male germ cells for stem cell self-renewal. Nat. Genet. 2004, 36, 647–652. [Google Scholar] [CrossRef]
- Giampietri, C.; Petrungaro, S.; Coluccia, P.; D’Alessio, A.; Starace, D.; Riccioli, A.; Padula, F.; Palombi, F.; Ziparo, E.; Filippini, A.; et al. Germ cell apoptosis control during spermatogenesis. Contraception 2005, 72, 298–302. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.W.; Kim, Y.J.; Lee, S.J.; Ko, J.J.; Kim, D.K.; Lee, J.H. Down-Regulation of Neogenin Decreases Proliferation and Differentiation of Spermatogonia during the Early Phase of Spermatogenesis. Int. J. Mol. Sci. 2022, 23, 14761. https://doi.org/10.3390/ijms232314761
Park JW, Kim YJ, Lee SJ, Ko JJ, Kim DK, Lee JH. Down-Regulation of Neogenin Decreases Proliferation and Differentiation of Spermatogonia during the Early Phase of Spermatogenesis. International Journal of Molecular Sciences. 2022; 23(23):14761. https://doi.org/10.3390/ijms232314761
Chicago/Turabian StylePark, Jin Woo, Yu Jin Kim, Sang Jin Lee, Jung Jae Ko, Dae Keun Kim, and Jae Ho Lee. 2022. "Down-Regulation of Neogenin Decreases Proliferation and Differentiation of Spermatogonia during the Early Phase of Spermatogenesis" International Journal of Molecular Sciences 23, no. 23: 14761. https://doi.org/10.3390/ijms232314761




