Cerebral Blood Flow in Predator Stress-Resilient and -Susceptible Rats and Mechanisms of Resilience
Abstract
:1. Introduction
2. Results
2.1. Effect of PS on Behavior in an EPM
2.2. Effect of PS on Cerebral Blood Flow and on Factors That Affect Cerebral Blood Flow
2.3. Effect of PS on Cerebral eNOS mRNA
2.4. Effect of PS on Dopamine in the Cerebral Parietal Cortex
2.5. Effect of PS on Plasma Corticosterone
2.6. Effect of PS on Hemostatic Parameters
3. Discussion
4. Material and Methods
4.1. Animals and Experimental Procedure
4.2. Exposure to PS
4.3. Behavioral Testing
4.4. Measurement of Regional CBF
4.5. Blood and Tissue Collection and Storage
4.6. Measurement of eNOS mRNA
4.6.1. RNA Isolation
4.6.2. cDNA Synthesis and Real-Time RT–PCR
4.7. Dopamine Measurement
4.8. Corticosterone Measurement
4.9. Measurement of Hemostatic Parameters
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, H.; Kozlovsky, N.; Alona, C.; Matar, M.A.; Joseph, Z. Animal model for PTSD: From clinical concept to translational research. Neuropharmacology 2012, 62, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Zohar, J. Animal models of post-traumatic stress disorder: The use of cut off behavioral criteria. Ann. N. Y. Acad. Sci. 2004, 1032, 167–178. [Google Scholar] [CrossRef]
- Gupta, M.A. Review of somatic symptoms in post-traumatic stress disorder. Int. Rev. Psychiatry 2013, 25, 86–99. [Google Scholar] [CrossRef]
- Pietrzak, R.H.; Goldstein, R.B.; Southwick, S.M.; Grant, B.F. Medical comorbidity of full and partial posttraumatic stress disorder in US adults: Results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom. Med. 2011, 73, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Edmondson, D.; Kronish, I.M.; Shaffer, J.A.; Falzon, L.; Burg, M.M. Posttraumatic stress disorder and risk for coronary heart disease: A meta-analytic review. Am. Heart J. 2013, 166, 806–814. [Google Scholar] [CrossRef] [Green Version]
- Seligowski, A.V.; Webber, T.K.; Marvar, P.J.; Ressler, K.J.; Philip, N.S. Involvement of the brain-heart axis in the link between PTSD and cardiovascular disease. Depress. Anxiety 2022, 39, 663–674, Epub ahead of print. [Google Scholar] [CrossRef]
- Ogoh, S. Relationship between cognitive function and regulation of cerebral blood flow. J. Physiol. Sci. 2017, 67, 345–351. [Google Scholar] [CrossRef]
- Remch, M.; Laskaris, Z.; Flory, J.; Mora-McLaughlin, C.; Morabia, A. Post-traumatic stress disorder and cardiovascular diseases: A cohort study of men and women involved in cleaning the debris of the World Trade Center Complex. Circ. Cardiovasc. Qual. Outcomes. 2018, 11, e004572. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Feng, N.; Pu, H.; Zhang, X.; Lu, H.; Yin, H. Perfusion deficits and functional connectivity alterations in memory-related regions of patients with post-traumatic stress disorder. PLoS ONE 2016, 11, e0156016. [Google Scholar] [CrossRef] [Green Version]
- Ogoh, S.; Yoo, J.K.; Badrov, M.B.; Parker, R.S.; Anderson, E.H.; Wiblin, J.L.; North, C.S.; Suris, A.; Fu, Q. Cerebral blood flow regulation and cognitive function in women with posttraumatic stress disorder. J. Appl. Physiol. 2018, 125, 627–1635. [Google Scholar] [CrossRef]
- Scott, J.C.; Matt, G.E.; Wrocklage, K.M.; Crnich, C.; Jordan, J.; Southwick, S.M.; Krystal, J.H.; Schweinsburg, B.C. A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol. Bull. 2015, 141, 105–140. [Google Scholar] [CrossRef] [Green Version]
- Elias, A.; Rowe, C.; Hopwood, M. Risk of dementia in posttraumatic stress disorder. J. Geriatr. Psychiatry Neurol. 2021, 34, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Von Känel, R.; Hepp, U.; Traber, R.; Kraemer, B.; Mica, L.; Keel, M.; Mausbach, B.T.; Schnyder, U. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Res. 2008, 158, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Grenon, S.M.; Owens, C.D.; Alley, H.; Perez, S.; Whooley, M.A.; Neylan, T.C.; Aschbacher, K.; Gasper, W.J.; Hilton, J.F.; Cohen, B.E. Posttraumatic stress disorder is associated with worse endothelial function among veterans. J. Am. Heart Assoc. 2016, 5, e003010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleveland, S.; Reed, K.; Thomas, J.L.; Ajijola, O.A.; Ebrahimi, R.; Hsia, T.; Lazarov, A.; Montoya, A.K.; Neria, Y.; Shimbo, D.; et al. Key dimensions of post-traumatic stress disorder and endothelial dysfunction: A protocol for a mechanism-focused cohort study. BMJ Open 2021, 11, e043060. [Google Scholar] [CrossRef]
- Breslau, N.; Chilcoat, H.D.; Kessler, R.C.; Davis, G.C. Previous exposure to trauma and PTSD effects of subsequent trauma: Results from the Detroit Area Survey of Trauma. Am. J. Psychiatry 1999, 156, 902–907. [Google Scholar] [CrossRef]
- Horn, S.R.; Charney, D.S.; Feder, A. Understanding resilience: New approaches for preventing and treating PTSD. Exp. Neurol. 2016, 284, 119–132. [Google Scholar] [CrossRef]
- Osorio, C.; Probert, T.; Jones, E.; Young, A.H.; Robbins, I. Adapting to stress: Understanding the neurobiology of resilience. Behav. Med. 2017, 43, 307–322. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.; Ryznar, R. The molecular basis of resilience: A narrative review. Front. Psychiatry 2022, 13, 856998. [Google Scholar] [CrossRef]
- Manukhina, E.B.; Tseilikman, V.E.; Komelkova, M.V.; Lapshin, M.S.; Goryacheva, A.V.; Kondashevskaya, M.V.; Mkhitarov, V.A.; Lazuko, S.S.; Tseilikman, O.B.; Sarapultsev, A.P.; et al. Cardiac injury in rats with experimental posttraumatic stress disorder and mechanisms of its limitation in experimental posttraumatic stress disorder-resistant rats. J. Appl. Physiol. (1985) 2021, 130, 759–771. [Google Scholar] [CrossRef]
- Lazuko, S.S.; Kuzhel, O.P.; Belyaeva, L.E.; Manukhina, E.B.; Downey, H.F.; Tseilikman, O.B.; Komelkova, M.V.; Tseilikman, V.E. Posttraumatic stress disorder disturbs coronary tone and its regulatory mechanisms. Cell Mol. Neurobiol. 2018, 38, 209–217. [Google Scholar] [CrossRef]
- Manukhina, E.B.; Tseilikman, V.E.; Tseilikman, O.B.; Komelkova, M.V.; Kondashevskaya, M.V.; Goryacheva, A.V.; Lapshin, M.S.; Platkovskii, P.O.; Alliluev, A.V.; Downey, H.F. Intermittent hypoxia improves behavioral and adrenal gland dysfunction induced by post-traumatic stress disorder in rats. J. Appl. Physiol. 2018, 125, 931–937. [Google Scholar] [CrossRef]
- Manukhina, E.B.; Tseilikman, V.E.; Karpenko, M.N.; Pestereva, N.S.; Tseilikman, O.B.; Komelkova, M.V.; Kondashevskaya, M.V.; Goryacheva, A.V.; Lapshin, M.S.; Platkovskii, P.O.; et al. Intermittent hypoxic conditioning alleviates post-traumatic stress disorder-induced damage and dysfunction of rat visceral organs and brain. Int. J. Mol. Sci. 2020, 21, 345. [Google Scholar] [CrossRef] [Green Version]
- Dremencov, E.; Lapshin, M.; Komelkova, M.; Alliluev, A.; Tseilikman, O.; Karpenko, M.; Pestereva, N.; Manukhina, E.; Downey, H.F.; Tseilikman, V. Chronic predator scent stress alters serotonin and dopamine levels in the rat thalamus and hypothalamus, respectively. Gen. Physiol. Biophys. 2019, 38, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Tseilikman, V.; Dremencov, E.; Maslennikova, E.; Ishmatova, A.; Manukhina, E.; Downey, H.F.; Klebanov, I.; Tseilikman, O.; Komelkova, M.; Lapshin, M.S.; et al. Post-traumatic stress disorder chronification via monoaminoxidase and cortisol metabolism. Horm. Metab. Res. 2019, 51, 618–622. [Google Scholar]
- Tseilikman, V.; Komelkova, M.; Kondashevskaya, M.V.; Manukhina, E.; Downey, H.F.; Chereshnev, V.; Chereshneva, M.; Platkovskii, P.; Goryacheva, A.; Pashkov, A.; et al. A rat model of post-traumatic stress syndrome causes phenotype-associated morphological changes and hypofunction of the adrenal gland. Int. J. Mol. Sci. 2021, 22, 13235. [Google Scholar] [CrossRef]
- Tseilikman, V.; Lapshin, M.; Klebanov, I.; Chrousos, G.; Vasilieva, M.; Pashkov, A.; Fedotova, J.; Tseilikman, D.; Shatilov, V.; Manukhina, E.; et al. The link between activities of hepatic 11beta-hydroxysteroid dehydrogenase-1 and monoamine oxidase-A in the brain following repeated predator stress: Focus on heightened anxiety. Int. J. Mol. Sci. 2022, 23, 4881. [Google Scholar] [CrossRef] [PubMed]
- Pyne-Geithman, G.J.; Caudell, D.N.; Cooper, M.; Clark, J.F.; Shutter, L.A. Dopamine D2-receptor-mediated increase in vascular and endothelial NOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in vitro. Neurocrit. Care 2009, 10, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Pressman, P.; Simuni, T.; Parrish, T.B.; Gitelman, D.R. Effects of acute levodopa challenge on resting cerebral blood flow in Parkinson’s Disease patients assessed using pseudo-continuous arterial spin labeling. Peer J. 2015, 3, e1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [PubMed] [Green Version]
- Baker, D.G.; Ekhator, N.N.; Kasckow, J.W.; Hill, K.K.; Zoumakis, E.; Dashevsky, B.A.; Chrousos, G.P.; Geracioti, T.D., Jr. Plasma and cerebrospinal fluid Interleukin-6 concentrations in posttraumatic stress disorder. Neuroimmunomodulation 2001, 9, 209–217. [Google Scholar] [CrossRef]
- De Berardis, D.; Vellante, F.; Fornaro, M.; Anastasia, A.; Olivieri, L.; Rapini, G.; Serroni, N.; Orsolini, L.; Valchera, A.; Carano, A.; et al. Alexithymia, suicide ideation, affective temperaments and homocysteine levels in drug naïve patients with post-traumatic stress disorder: An exploratory study in the everyday ‘real world’ clinical practice. Int. J. Psychiatry Clin. Pract. 2020, 24, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Belozertseva, I.V.; Tamkovich, N.V.; Baranov, K.O.; Kudryavtseva, N.N. Chronic lithium treatment affects anxious behaviors and the expression of serotonergic genes in midbrain raphe nuclei of defeated male mice. Biomedicines 2021, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, F.; Rubattu, S.; Savoia, C.; Venturelli, V.; Pagannonne, E.; Volpe, M. Endothelial dysfunction and stroke. J. Cardiovasc. Pharmacol. 2001, 38 (Suppl. 2), S75–S78. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Rahman, L.; Zapata-Martín Del Campo, C.M.; Maldonado, J.C.; Jafri, N.; Cummings, M.A.; Maurer, S.; Kozlakidis, Z. PTSD as an endothelial disease: Insights from COVID-19. Front. Cell Neurosci. 2021, 15, 770387. [Google Scholar] [CrossRef]
- Benincasa, G.; Coscioni, E.; Napoli, C. Cardiovascular risk factors and molecular routes underlying endothelial dysfunction: Novel opportunities for primary prevention. Biochem. Pharmacol. 2022, 202, 115108. [Google Scholar] [CrossRef] [PubMed]
- Atochin, D.N.; Huang, P.L. Role of endothelial nitric oxide in cerebrovascular regulation. Curr. Pharm. Biotechnol. 2011, 12, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, W.I. Endothelium-dependent responses in the microcirculation observed in vivo. Acta Physiol. 2018, 224, e13111. [Google Scholar] [CrossRef]
- Thurston, R.C.; Barinas-Mitchell, E.; von Känel, R.; Chang, Y.; Koenen, K.C.; Matthews, K.A. Trauma exposure and endothelial function among midlife women. Menopause 2018, 25, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Celano, C.M.; Daunis, D.J.; Lokko, H.N.; Campbell, K.A.; Huffman, J.C. Anxiety disorders and cardiovascular disease. Curr. Psychiatry Rep. 2016, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Felice, F.; Di Stefano, R.; Pini, S.; Mazzotta, G.; Bovenzi, F.M.; Bertoli, D.; Abelli, M.; Borelli, L.; Cardini, A.; Lari, L.; et al. Influence of depression and anxiety on circulating endothelial progenitor cells in patients with acute coronary syndromes. Hum. Psychopharmacol. 2015, 30, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Violanti, J.M.; Andrew, M.; Burchfiel, C.M.; Hartley, T.A.; Charles, L.R.; Miller, D.B. Post-traumatic stress symptoms and cortisol patterns among police officers. Policing An Intl. J. Police Strateg. Mgmt. 2007, 30, 189–202. [Google Scholar] [CrossRef]
- Jesmin, S.; Togashi, H.; Mowa, C.N.; Ueno, K.; Yamaguchi, T.; Shibayama, A.; Miyauchi, T.; Sakuma, I.; Yoshioka, M. Characterization of regional cerebral blood flow and expression of angiogenic growth factors in the frontal cortex of juvenile male SHRSP and SHR. Brain Res. 2004, 1030, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Daiber, A.; Kröller-Schön, S.; Oelze, M.; Hahad, O.; Li, H.; Schulz, R.; Steven, S.; Münzel, T. Oxidative stress and inflammation contribute to traffic noise-induced vascular and cerebral dysfunction via uncoupling of nitric oxide synthases. Redox Biol. 2020, 34, 101506. [Google Scholar] [CrossRef] [PubMed]
- Skantze, H.B.; Kaplan, J.; Pettersson, K.; Manuck, S.; Blomqvist, N.; Kyes, R.; Williams, K.; Bondjers, G. Psychosocial stress causes endothelial injury in cynomolgus monkeys via β1- adrenoceptor activation. Atherosclerosis 1998, 136, 153–161. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, Y.M.; Yoo, M.H.; Shin, M.K.; Kim, C.K.; Suh, S.H. Immobilization stress induces endothelial dysfunction by oxidative stress via the activation of angiotensin II/its type I receptor pathway. Atherosclerosis 2010, 213, 109–114. [Google Scholar] [CrossRef]
- López-Figueroa, M.O.; Day, H.E.; Akil, H.; Watson, S.J. Nitric oxide in the stress axis. Histol. Histopath. 1998, 13, 1243–1252. [Google Scholar]
- Toda, N.; Nakanishi-Toda, M. How mental stress affects endothelial function. Pflugers Arch. 2011, 462, 779–794. [Google Scholar] [CrossRef]
- Liu, Y.; Mladinov, D.; Pietrusz, J.L.; Usa, K.; Liang, M. Glucocorticoid response elements and 11 β-hydroxysteroid dehydrogenases in the regulation of endothelial nitric oxide synthase expression. Cardiovasc. Res. 2009, 81, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Wallerath, T.; Whited, K.; Schäfer, S.C.; Schwarz, P.M.; Wohlfart, P.; Kleinert, H.; Lehr, H.A.; Lemmer, B.; Förstermann, U. Down–regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. Proc. Natl. Acad. Sci. USA 1999, 96, 13357–13362. [Google Scholar] [CrossRef] [Green Version]
- Blum, K.; Gondré-Lewis, M.C.; Modestino, E.J.; Lott, L.; Baron, D.; Siwicki, D.; McLaughlin, T.; Howeedy, A.; Krengel, M.H.; Oscar-Berman, M.; et al. Understanding the scientific basis of post-traumatic stress disorder (PTSD): Precision behavioral management overrides stigmatization. Mol. Neurobiol. 2019, 56, 7836–7850. [Google Scholar] [CrossRef]
- Blum, K.; Giordano, J.; Oscar-Berman, M.; Bowirrat, A.; Simpatico, T.; Barh, D. Diagnosis and healing in veterans suspected of suffering from post-traumatic stress disorder (PTSD) using reward gene testing and reward circuitry natural dopaminergic activation. J. Genet. Syndr. Gene Ther. 2012, 3, 1000116. [Google Scholar] [CrossRef]
- Roy-Byrne, P.; Arguelles, L.; Vitek, M.E.; Goldberg, J.; Keane, T.M.; True, W.R.; Pitman, R.K. Persistence and change of PTSD symptomatology—A longitudinal co-twin control analysis of the Vietnam Era Twin Registry. Soc. Psychiatry Psychiatr. Epidemiol. 2004, 39, 681–685. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Blum, K.; Oscar-Berman, M.; Febo, M.; Agan, G.; Fratantonio, J.L.; Simpatico, T.; Gold, M.S. Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients: Role of enhanced brain reward functional connectivity and homeostasis redeeming joy. J. Behav. Addict. 2015, 4, 106–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLaughlin, T.; Blum, K.; Oscar-Berman, M.; Febo, M.; Demetrovics, Z.; Agan, G.; Fratantonio, J.; Gold, M.S. Using the neuroadaptagen KB200z to ameliorate terrifying, lucid nightmares in RDS patients: The role of enhanced, brain-reward, functional connectivity and dopaminergic homeostasis. J. Reward. Defic. Syndr. 2015, 1, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Curvello, V.; Hekierski, H.; Pastor, P.; Vavilala, M.S.; Armstead, W.M. Dopamine protects cerebral autoregulation and prevents hippocampal necrosis after traumatic brain injury via block of ERK MAPK in juvenile pigs. Brain Res. 2017, 1670, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Afonso-Oramas, D.; Cruz-Muros, I.; Castro-Hernández, J.; Salas-Hernández, J.; Barroso-Chinea, P.; García-Hernández, S.; Lanciego, J.L.; González-Hernández, T. Striatal vessels receive phosphorylated tyrosine hydroxylase-rich innervation from midbrain dopaminergic neurons. Front. Neuroanat. 2014, 26, 84. [Google Scholar] [CrossRef] [Green Version]
- Melamed, E.; Lavy, S.; Cooper, G.; Bentin, S. Regional cerebral blood flow in parkinsonism. Measurement before and after levodopa. J. Neurol. Sci. 1978, 38, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, Y.; Liu, J.; Cao, Y.; Si, C.; Zheng, R.; Zeng, C.; Guan, H.; Li, L. Dopamine D4 receptor protected against hyperglycemia-induced endothelial dysfunction via PI3K/eNOS pathway. Biochem. Biophys. Res. Commun. 2019, 518, 554–559. [Google Scholar] [CrossRef]
- von Essen, C.; Zervas, N.T.; Brown, D.R.; Koltun, W.A.; Pickren, K.S. Local cerebral blood flow in the dog during intravenous infusion of dopamine. Surg. Neurol. 1980, 13, 181–188. [Google Scholar]
- Martens, M.; McConnell, F.K.; Filippini, N.; Mackay, C.E.; Harrison, P.J.; Tunbridge, E.M. Dopaminergic modulation of regional cerebral blood flow: An arterial spin labelling study of genetic and pharmacological manipulation of COMT activity. Neuroimage 2021, 234, 117999. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R. Post-traumatic stress disorder. N. Engl. J. Med. 2002, 346, 108–114. [Google Scholar] [CrossRef]
- Skórzewska, A.; Lehner, M.; Wisłowska-Stanek, A.; Turzyńska, D.; Sobolewska, A.; Krząścik, P.; Szyndler, J.; Maciejak, P.; Chmielewska, N.; Kołosowska, K.; et al. Individual susceptibility or resistance to posttraumatic stress disorder-like behaviours. Behav. Brain Res. 2020, 386, 112591. [Google Scholar] [CrossRef]
- Miller, M.W.; Lin, A.P.; Wolf, E.J.; Miller, D.R. Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harv. Rev. Psychiatry 2018, 26, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, R.L.; Levy-Carrick, N.; Reibman, J.; Xu, N.; Shao, Y.; Liu, M.; Ferri, L.; Kazeros, A.; Caplan-Shaw, C.E.; Pradhan, D.R.; et al. Elevated C-reactive protein and posttraumatic stress pathology among survivors of the 9/11 World Trade Center attacks. J. Psychiatr. Res. 2017, 16, 14–21. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 7, 137–162. [Google Scholar] [CrossRef]
- Michopoulos, V.; Jovanovic, T. Chronic inflammation: A new therapeutic target for post-traumatic stress disorder? Lancet Psychiatry 2015, 2, 954–955. [Google Scholar] [CrossRef]
- Boscarino, J.A. Posttraumatic stress disorder, exposure to combat, and lower plasma cortisol among Vietnam veterans: Findings and clinical implications. J. Consult. Clin. Psychol. 1996, 64, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Heim, C.; Ehlert, U.; Hellhammer, D.H. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology 2000, 25, 1–35. [Google Scholar] [CrossRef]
- Maes, M.; Lin, A.H.; Delmeire, L.; Van Gastel, A.; Kenis, G.; De Jongh, R.; Bosmans, E. Elevated serum interleukin-6 (IL-6) and IL-6 receptor concentrations in posttraumatic stress disorder following accidental man-made traumatic events. Biol. Psychiatry 1999, 45, 833–839. [Google Scholar] [CrossRef]
- Szotowski, B.; Antoniak, S.; Poller, W.; Schultheiss, H.P.; Rauch, U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ. Res. 2005, 96, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- von Känel, R.; Hepp, U.; Kraemer, B.; Traber, R.; Keel, M.; Mica, L.; Schnyder, U. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007, 41, 744–752. [Google Scholar] [CrossRef]
- Kubzansky, L.D.; Koenen, K.C.; Spiro, A., 3rd; Vokonas, P.S.; Sparrow, D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch. Gen. Psychiatry 2007, 64, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Känel, R.; Dimsdale, J.E.; Patterson, T.L.; Grant, I. Association of negative life event stress with coagulation activity in elderly Alzheimer caregivers. Psychosom. Med. 2003, 65, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Robicsek, O.; Makhoul, B.; Klein, E.; Brenner, B.; Sarig, G. Hypercoagulation in chronic post-traumatic stress disorder. Isr. Med. Assoc. J. 2011, 13, 548–552. [Google Scholar] [PubMed]
- Austin, A.W.; Wirtz, P.H.; Patterson, S.M.; Stutz, M.; von Känel, R. Stress-induced alterations in coagulation: Assessment of a new hemoconcentration correction technique. Psychosom. Med. 2012, 74, 288–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Känel, R.; Kudielka, B.M.; Haeberli, A.; Stutz, M.; Fischer, J.E.; Patterson, S.M. Prothrombotic changes with acute psychological stress: Combined effect of hemoconcentration and genuine coagulation activation. Thromb. Res. 2009, 123, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Von Känel, R.; Hepp, U.; Buddeberg, C.; Keel, M.; Mica, L.; Aschbacher, K.; Schnyder, U. Altered blood coagulation in patients with posttraumatic stress disorder. Psychosom. Med. 2006, 68, 598–604. [Google Scholar] [CrossRef]
- Austin, A.W.; Wissmann, T.; von Känel, R. Stress and hemostasis: An update. Semin. Thromb. Hemost. 2013, 39, 902–912. [Google Scholar] [CrossRef] [Green Version]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory Mechanisms Contributing to Endothelial Dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, D.M.; Berke, E.T. Gender- and sex-based contributors to sex differences in PTSD. Curr. Psychiatry Rep. 2020, 22, 19. [Google Scholar] [CrossRef]
- Breslau, N. Gender differences in trauma and posttraumatic stress disorder. J. Gend. Specif. Med. 2002, 5, 34–40. [Google Scholar]
- Zlotnick, C.; Zimmerman, M.; Wolfsdorf, B.A.; Mattia, J.I. Gender differences in patients with posttraumatic stress disorder in a general psychiatric practice. Am. J. Psychiatry 2001, 158, 1923–1925. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Matar, M.A.; Joseph, Z. Animal models of post-traumatic stress disorder. Curr. Protoc. Neurosci. 2013, 64, 9–45. [Google Scholar] [CrossRef] [PubMed]
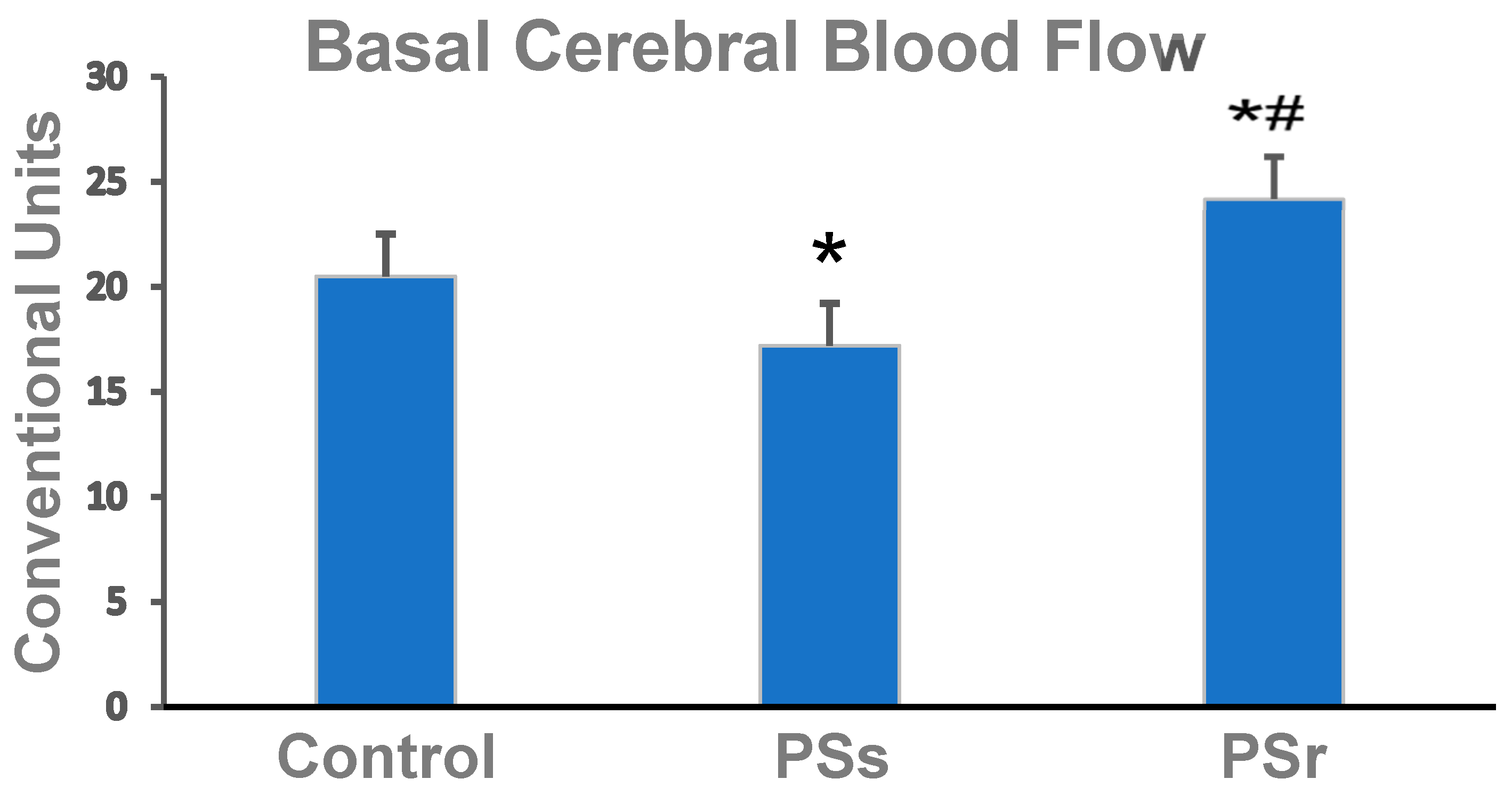
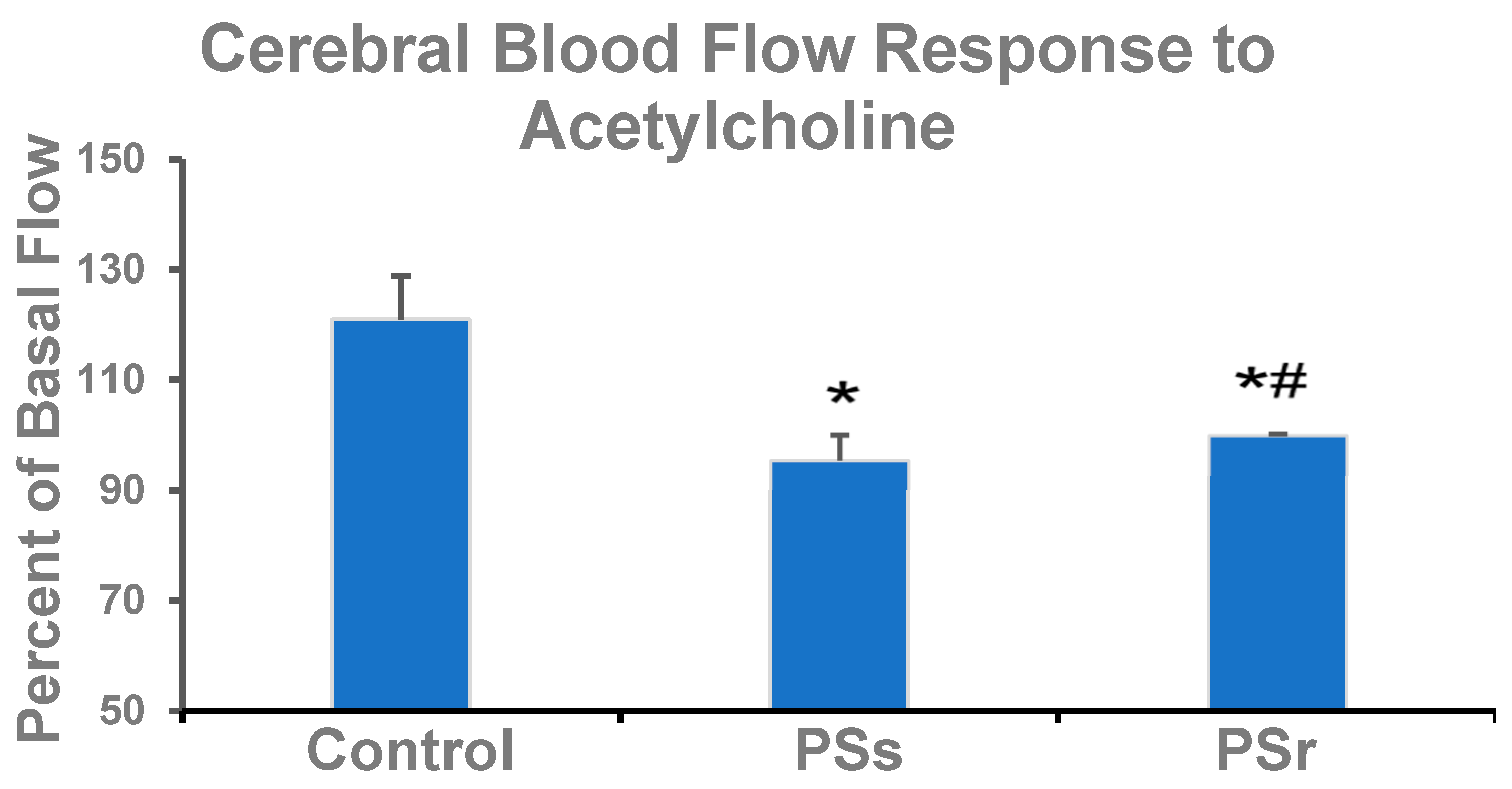
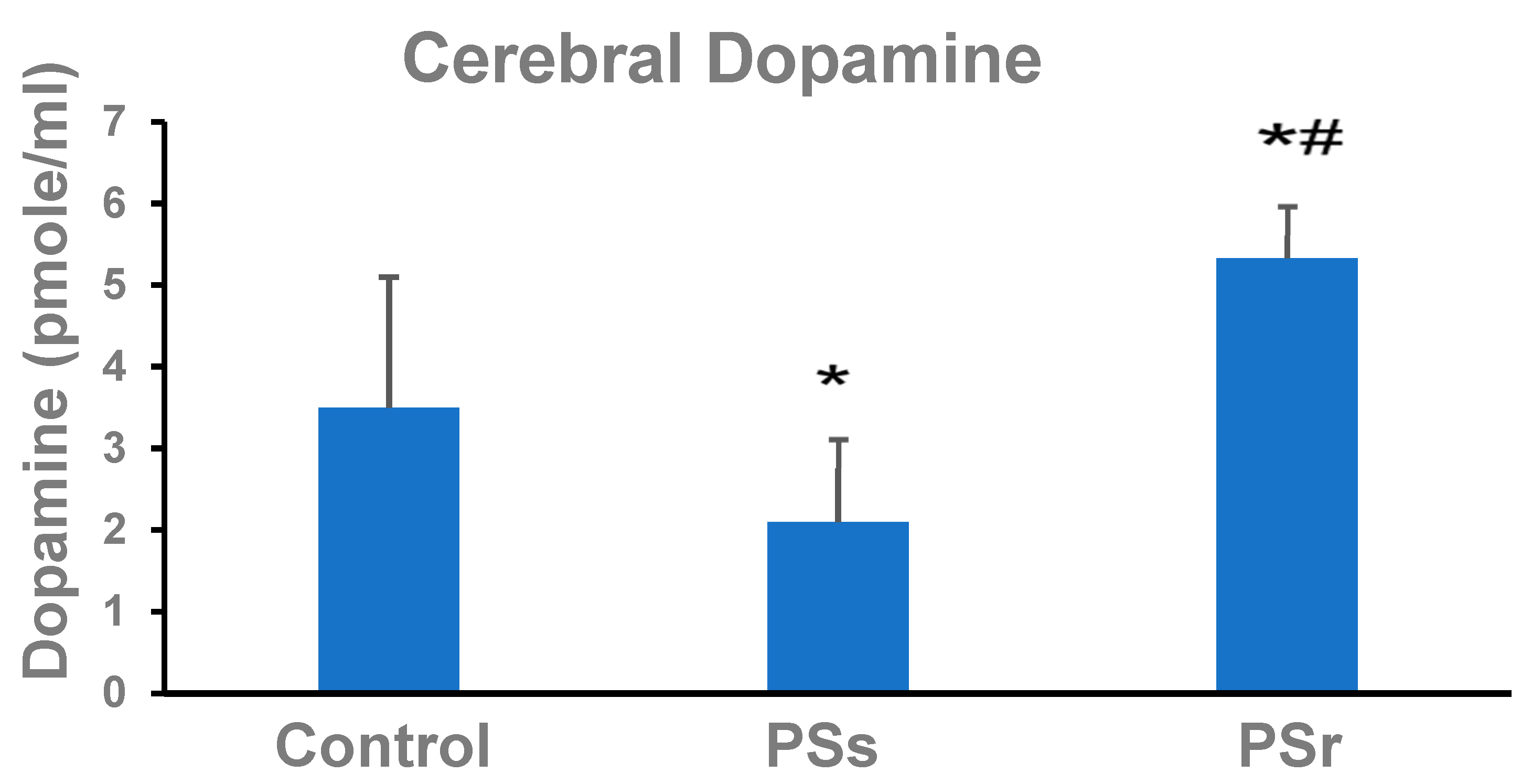

| Variable | Control Rats (n = 30) | PSs Rats (n = 18) | PSr Rats (n = 22) |
|---|---|---|---|
| Number of entries into closed arms | 10.3 ± 0.6 | 6.1 ± 0.3 * | 9.4 ± 0.5 # |
| Number of entries into open arms | 6.3 ± 0.4 | 0.9 ± 0.1 * | 5.5 ± 0.3 # |
| Time spent in closed arms, s | 346 ± 20 | 589 ± 32 * | 431± 26 # |
| Time spent in open arms, s | 255 ± 12 | 10.2 ± 0.6 * | 170 ± 10 # |
| Anxiety index | 0.61 ± 0.04 | 0.92 ± 0.05 * | 0.67 ± 0.04 # |
| Groups | mRNA eNOS |
|---|---|
| Control (n = 5) | 3.44 ± 2.63 |
| PSs (n = 5) | 0.5 ± 0.01 * |
| PSr (n = 10) | 3.3 ± 0.25 # |
| Variable | Control Rats (n = 10) | PSs Rats (n = 10) | PSr Rats (n = 10) |
|---|---|---|---|
| APTT, s | 23.6 ± 1.4 | 17.3 ± 0.7 *,# | 22.9 ± 1.2 |
| PT, s | 13.9 ± 0.7 | 10.1 ± 0.6 *,# | 13.6 ± 0.6 |
| Fibrinogen concentration, g/L | 2.7 ± 0.1 | 3.6 ± 0.4 *,# | 2.8 ± 0.2 |
| Platelet aggregation, % | 48 ± 2 | 69 ± 2 *,# | 50 ± 3 |
| Name of the Gene | Primer Sequence  | Annealing Temperature, °C |
|---|---|---|
| eNOS | F GATCCTAACTTGCCTTGCATCCT R TGTAATCGGTCTTGCCAGAATCC | 58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondashevskaya, M.V.; Downey, H.F.; Tseilikman, V.E.; Alexandrin, V.V.; Artem’yeva, K.A.; Aleksankina, V.V.; Tseilikman, O.B.; Pashkov, A.A.; Goryacheva, A.V.; Ivleva, I.S.; et al. Cerebral Blood Flow in Predator Stress-Resilient and -Susceptible Rats and Mechanisms of Resilience. Int. J. Mol. Sci. 2022, 23, 14729. https://doi.org/10.3390/ijms232314729
Kondashevskaya MV, Downey HF, Tseilikman VE, Alexandrin VV, Artem’yeva KA, Aleksankina VV, Tseilikman OB, Pashkov AA, Goryacheva AV, Ivleva IS, et al. Cerebral Blood Flow in Predator Stress-Resilient and -Susceptible Rats and Mechanisms of Resilience. International Journal of Molecular Sciences. 2022; 23(23):14729. https://doi.org/10.3390/ijms232314729
Chicago/Turabian StyleKondashevskaya, Marina V., H. Fred Downey, Vadim E. Tseilikman, Valery V. Alexandrin, Kseniya A. Artem’yeva, Valentina V. Aleksankina, Olga B. Tseilikman, Anton A. Pashkov, Anna V. Goryacheva, Irina S. Ivleva, and et al. 2022. "Cerebral Blood Flow in Predator Stress-Resilient and -Susceptible Rats and Mechanisms of Resilience" International Journal of Molecular Sciences 23, no. 23: 14729. https://doi.org/10.3390/ijms232314729





