Knockdown of YY1 Inhibits XIST Expression and Enhances Cloned Pig Embryo Development
Abstract
1. Introduction
2. Results
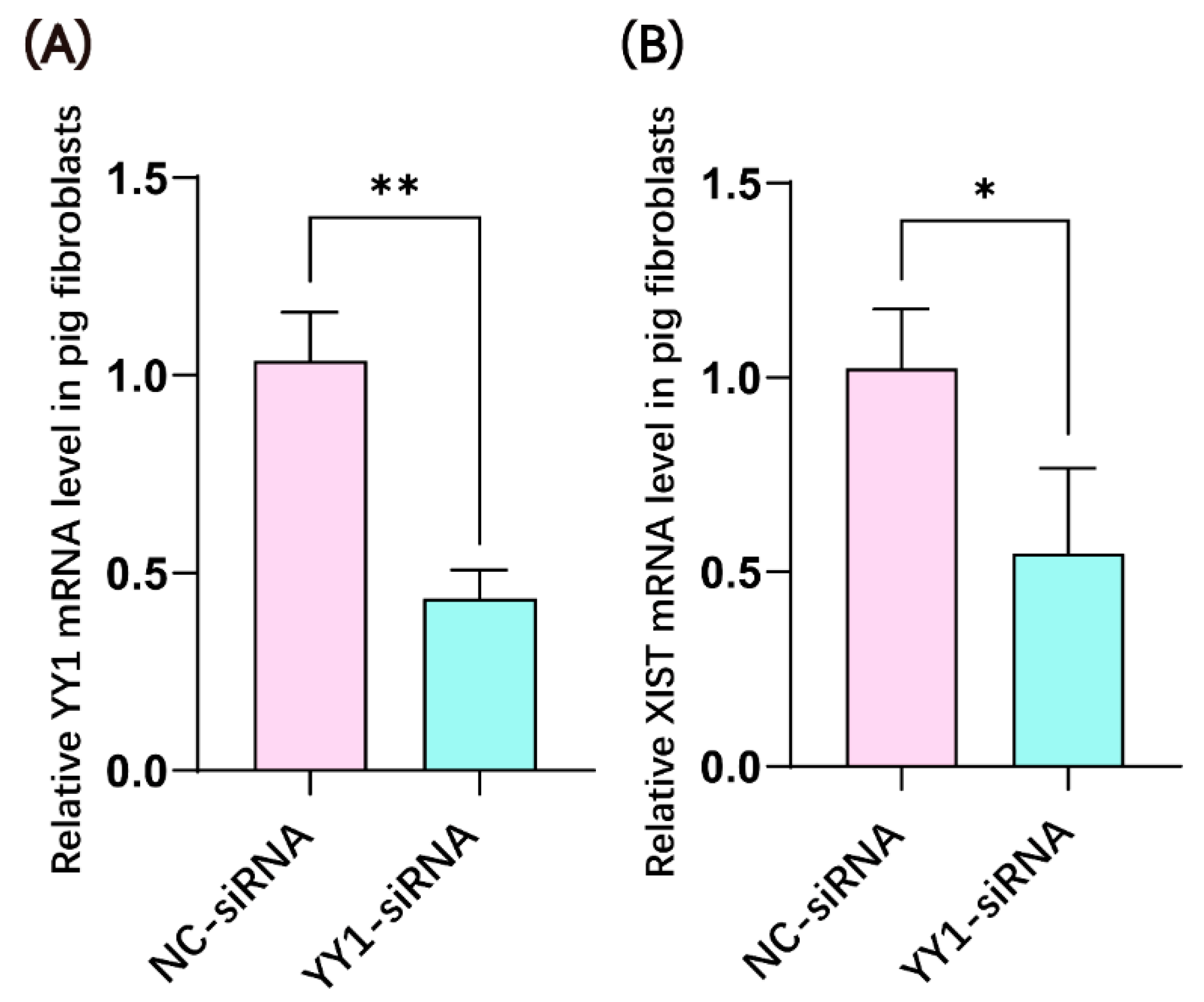
2.1. YY1 siRNA Transfection Inhibited the mRNA Expression of YY1 and XIST in Porcine Fibroblasts
2.2. YY1 Can Bind to the 5′ Regulatory Region of the Porcine XIST Gene
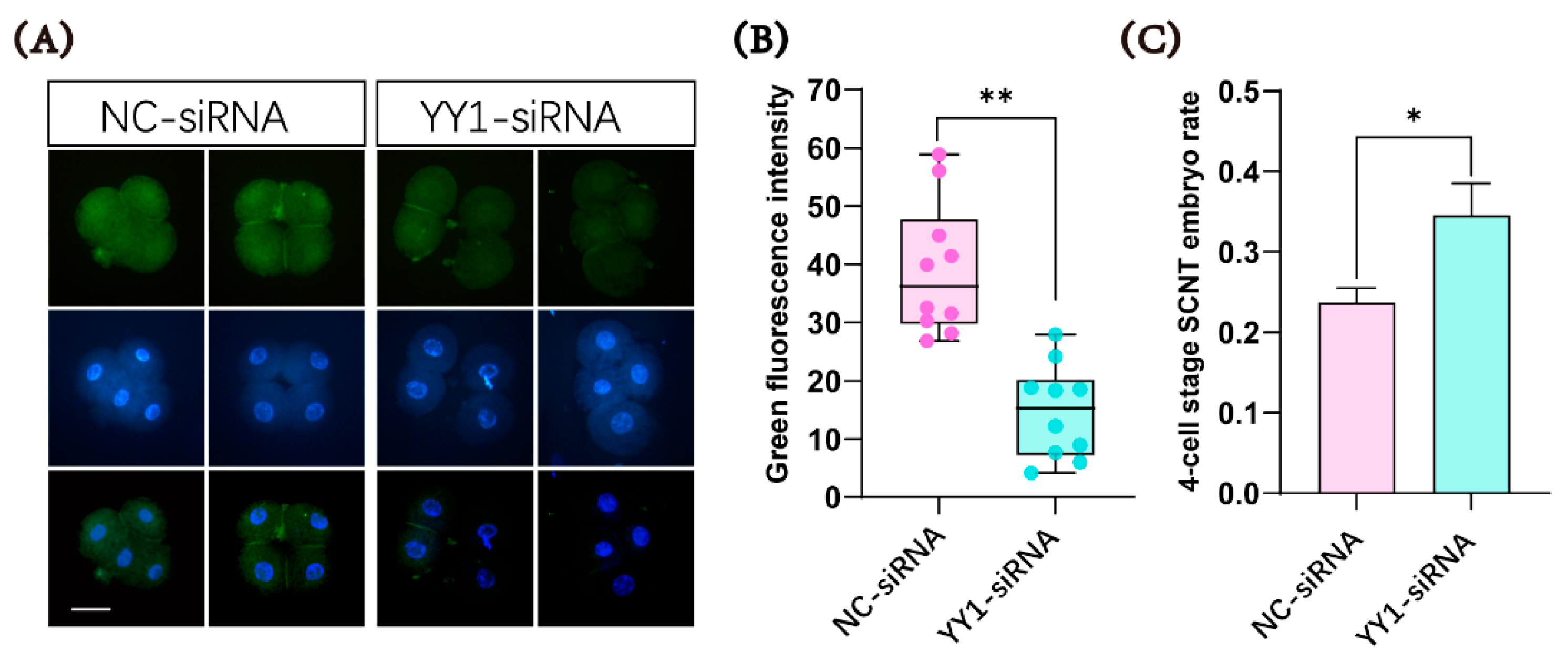
2.3. Microinjection of YY1 siRNA Inhibited the Expression of the YY1 Protein and Improved the Developmental Ability of Cloned Pig Embryos
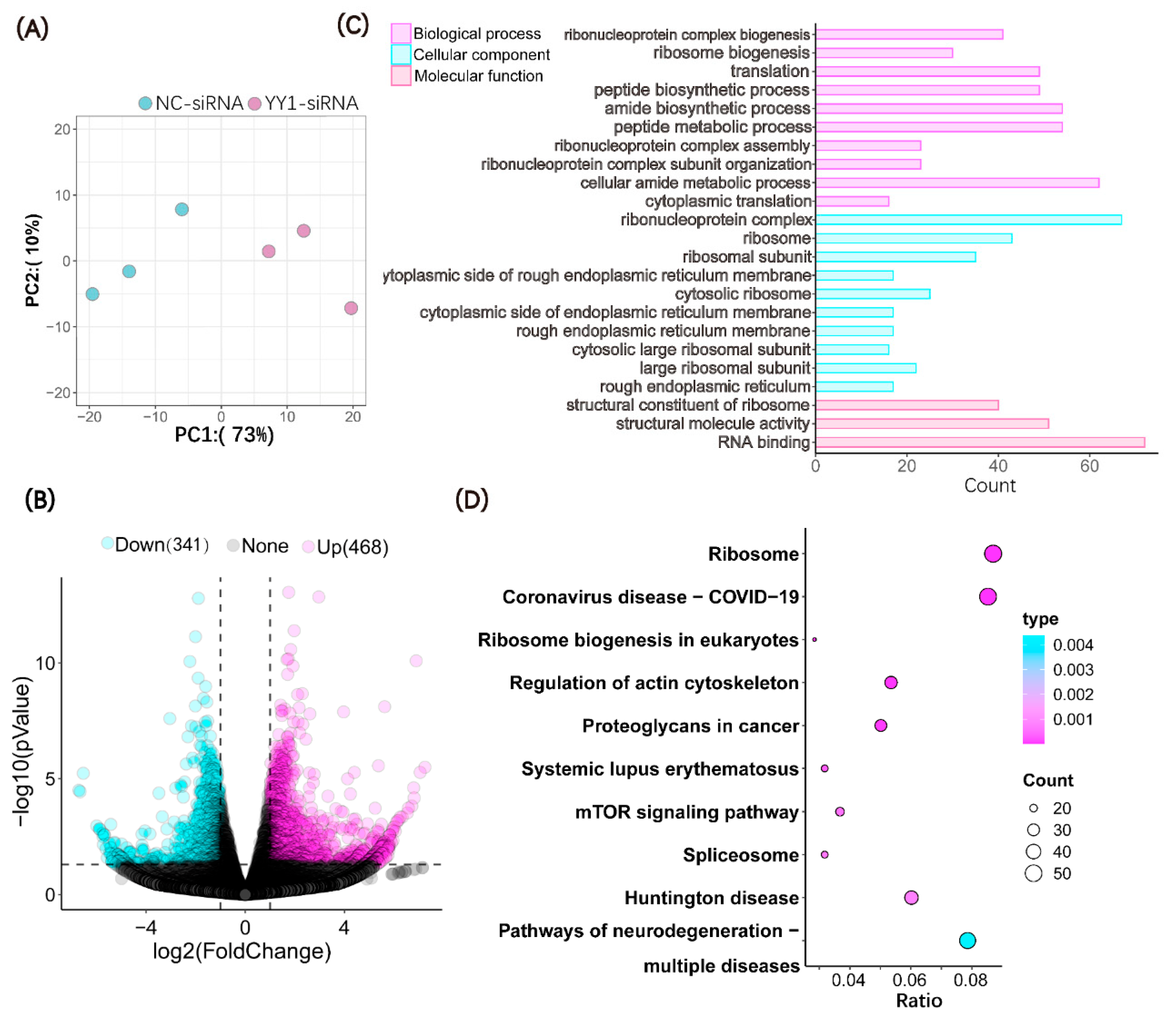
2.4. Microinjection of YY1 siRNA Altered the Transcriptome of Cloned Pig Embryos
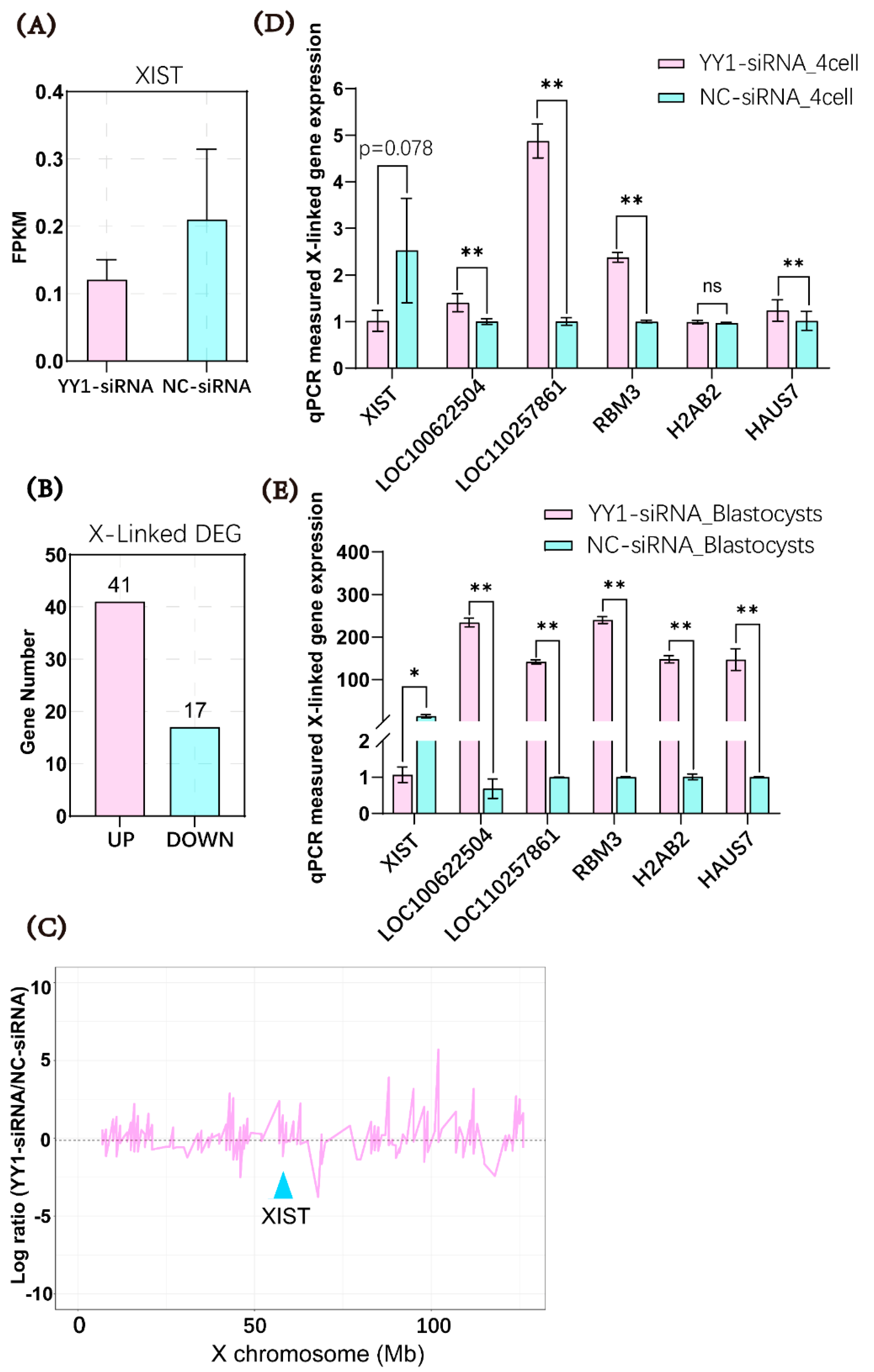
2.5. Microinjection of YY1 siRNA Inhibited XIST Expression and Upregulated the mRNA Levels of X-Linked Genes in Cloned Pig Embryos
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Design, Synthesis, and Transfection of siRNA
- YY1-siRNA (sense): CCGAGUACAUGACAGGAAATT
- YY1-siRNA (antisense): UUUCCUGUCAUGUACUCGGTT
- NC-siRNA (sense): UUCUCCGAACGUGUCACGUTT
- NC-siRNA (antisense): ACGUGACACGUUCGGAGAATT
4.3. Real-Time Quantitative Polymerase Chain Reaction (qPCR) Analysis
4.4. Chromatin Immunoprecipitation-Sequencing (ChIP-Seq) and Data Analysis
4.5. Analysis of the Binding Site of YY1 on the XIST Gene
4.6. Production of Cloned Embryos
4.7. Microinjection of siRNA into Cloned Embryos
4.8. Immunofluorescence Assay
4.9. Embryo Transfer
4.10. RNA Sequencing and Bioinformatics Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| bp | Base pair |
| BP | Biological process |
| CC | Cellular component |
| cDNA | Complementary DNA |
| ChIP-seq | Chromatin immunoprecipitation sequence |
| COCs | Cumulus oocyte complexes |
| DEGs | Differentially expressed genes |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DNA | Deoxyribonucleic acid |
| DPBS | Dulbecco’s Phosphate Buffered Saline |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| mRNA | Messenger RNA |
| MF | Molecular function |
| PCA | Principal component analysis |
| PEF | Porcine embryonic fibroblast |
| PZM-3 | Porcine zygote medium 3 |
| qPCR | Quantitative Real-time PCR |
| RLIM | Ring finger protein, LIM domain interacting |
| RNA | Ribonucleic acid |
| RPKM | Reads per kilobase per million mapped reads |
| SCNT | Somatic cell nuclear transfer |
| sgRNA | Single guide RNA |
| siRNA | Small interfering RNA |
| TES | Transcription termination site |
| TSS | Transcriptional start site |
| XCI | X-chromosome inactivation X |
| XIST | X-inactive specific transcript |
| YY1 | Yin yang 1 |
References
- Lee, E.; Estrada, J.; Piedrahita, J.A. A comparative study on the efficiency of two enucleation methods in pig somatic cell nuclear transfer: Effects of the squeezing and the aspiration methods. Anim. Biotechnol. 2008, 19, 71–79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lagutina, I.; Fulka, H.; Lazzari, G.; Galli, C. Interspecies somatic cell nuclear transfer: Advancements and problems. Cell. Reprogram. 2013, 15, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Wani, N.A.; Vettical, B.S.; Hong, S.B. First cloned Bactrian camel (Camelus bactrianus) calf produced by interspecies somatic cell nuclear transfer: A step towards preserving the critically endangered wild Bactrian camels. PLoS ONE 2017, 12, e177800. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Nam, Y.H.; Cheong, S.A.; Kwak, S.S.; Lee, E.; Hyun, S.H. Absence of nucleolus formation in raccoon dog-porcine interspecies somatic cell nuclear transfer embryos results in embryonic developmental failure. J. Reprod. Dev. 2016, 62, 345–350. [Google Scholar] [CrossRef]
- Tunstall, T.; Kock, R.; Vahala, J.; Diekhans, M.; Fiddes, I.; Armstrong, J.; Paten, B.; Ryder, O.A.; Steiner, C.C. Evaluating recovery potential of the northern white rhinoceros from cryopreserved somatic cells. Genome Res. 2018, 28, 780–788. [Google Scholar] [CrossRef]
- Loi, P.; Modlinski, J.A.; Ptak, G. Interspecies somatic cell nuclear transfer: A salvage tool seeking first aid. Theriogenology 2011, 76, 217–228. [Google Scholar] [CrossRef]
- Matoba, S.; Zhang, Y. Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell Stem Cell 2018, 23, 471–485. [Google Scholar] [CrossRef]
- Loi, P.; Iuso, D.; Czernik, M.; Ogura, A. A New, Dynamic Era for Somatic Cell Nuclear Transfer? Trends Biotechnol. 2016, 34, 791–797. [Google Scholar] [CrossRef]
- Loi, P.; Beaujean, N.; Khochbin, S.; Fulka, J.J.; Ptak, G. Asymmetric nuclear reprogramming in somatic cell nuclear transfer? Bioessays 2008, 30, 66–74. [Google Scholar] [CrossRef]
- Lomax, G.P.; DeWitt, N.D. Somatic cell nuclear transfer in Oregon: Expanding the pluripotent space and informing research ethics. Stem Cells Dev. 2013, 22 (Suppl. 1), 25–28. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Liao, S.; Kuang, Z.; Zhu, Q.; Wei, H.; Shi, J.; Zheng, E.; Xu, Z.; Huang, S.; Hong, L.; et al. Genetically Engineered Pigs as Efficient Salivary Gland Bioreactors for Production of Therapeutically Valuable Human Nerve Growth Factor. Cells 2022, 11, 2378. [Google Scholar] [CrossRef] [PubMed]
- Skrzyszowska, M.; Samiec, M. Generating Cloned Goats by Somatic Cell Nuclear Transfer-Molecular Determinants and Application to Transgenics and Biomedicine. Int. J. Mol. Sci. 2021, 22, 7490. [Google Scholar] [CrossRef] [PubMed]
- Hodges, C.A.; Stice, S.L. Generation of bovine transgenics using somatic cell nuclear transfer. Reprod. Biol. Endocrinol. 2003, 1, 81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogura, A.; Inoue, K.; Wakayama, T. Recent advancements in cloning by somatic cell nuclear transfer. Philos. Trans. R. Soc. Lond B Biol. Sci. 2013, 368, 20110329. [Google Scholar] [CrossRef]
- Thuan, N.V.; Kishigami, S.; Wakayama, T. How to improve the success rate of mouse cloning technology. J. Reprod. Dev. 2010, 56, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, X.; Zhang, N.; Shi, J.; Zhou, R.; Su, Q.; Zheng, E.; Huang, S.; Xu, Z.; Hong, L.; et al. Knockdown of RLIM inhibits XIST expression and improves developmental competence of cloned male pig embryos. Mol. Reprod. Dev. 2021, 88, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Kohda, T.; Sugimoto, M.; Sado, T.; Ogonuki, N.; Matoba, S.; Shiura, H.; Ikeda, R.; Mochida, K.; Fujii, T.; et al. Impeding Xist expression from the active X chromosome improves mouse somatic cell nuclear transfer. Science 2010, 330, 496–499. [Google Scholar] [CrossRef]
- Matoba, S.; Inoue, K.; Kohda, T.; Sugimoto, M.; Mizutani, E.; Ogonuki, N.; Nakamura, T.; Abe, K.; Nakano, T.; Ishino, F.; et al. RNAi-mediated knockdown of Xist can rescue the impaired postimplantation development of cloned mouse embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 20621–20626. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Huang, Z.; Yuan, Y.; Shi, J.; Cai, G.; Liu, D.; Wu, Z.; Li, Z. Effects of RNAi-mediated knockdown of Xist on the developmental efficiency of cloned male porcine embryos. J. Reprod. Dev. 2016, 62, 591–597. [Google Scholar] [CrossRef]
- Yang, X.; Wu, X.; Yang, Y.; Gu, T.; Hong, L.; Zheng, E.; Xu, Z.; Zeng, F.; Shi, J.; Zhou, R.; et al. Improvement of developmental competence of cloned male pig embryos by short hairpin ribonucleic acid (shRNA) vector-based but not small interfering RNA (siRNA)-mediated RNA interference (RNAi) of Xist expression. J. Reprod. Dev. 2019, 65, 533–539. [Google Scholar] [CrossRef]
- Ruan, D.; Peng, J.; Wang, X.; Ouyang, Z.; Zou, Q.; Yang, Y.; Chen, F.; Ge, W.; Wu, H.; Liu, Z.; et al. XIST Derepression in Active X Chromosome Hinders Pig Somatic Cell Nuclear Transfer. Stem Cell Rep. 2018, 10, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Makhlouf, M.; Ouimette, J.F.; Oldfield, A.; Navarro, P.; Neuillet, D.; Rougeulle, C. A prominent and conserved role for YY1 in Xist transcriptional activation. Nat. Commun. 2014, 5, 4878. [Google Scholar] [CrossRef]
- Chapman, A.G.; Cotton, A.M.; Kelsey, A.D.; Brown, C.J. Differentially methylated CpG island within human XIST mediates alternative P2 transcription and YY1 binding. BMC Genet. 2014, 15, 89. [Google Scholar] [CrossRef]
- Syrett, C.M.; Sindhava, V.; Hodawadekar, S.; Myles, A.; Liang, G.; Zhang, Y.; Nandi, S.; Cancro, M.; Atchison, M.; Anguera, M.C. Loss of Xist RNA from the inactive X during B cell development is restored in a dynamic YY1-dependent two-step process in activated B cells. PLoS Genet. 2017, 13, e1007050. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Lee, J.T. YY1 tethers Xist RNA to the inactive X nucleation center. Cell 2011, 146, 119–133. [Google Scholar] [CrossRef]
- Perry, R.P. The architecture of mammalian ribosomal protein promoters. BMC Evol. Biol. 2005, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shioda, T.; Coser, K.R.; Lynch, M.C.; Yang, C.; Schmidt, E.V. Genome-wide analysis of YY2 versus YY1 target genes. Nucleic Acids Res. 2010, 38, 4011–4026. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Meyer, C.A.; Eeckhoute, J.; Johnson, D.S.; Bernstein, B.E.; Nusbaum, C.; Myers, R.M.; Brown, M.; Li, W.; et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008, 9, R137. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; He, Q.Y. ChIPseeker: An R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics 2015, 31, 2382–2383. [Google Scholar] [CrossRef]
- Ramirez, F.; Ryan, D.P.; Gruning, B.; Bhardwaj, V.; Kilpert, F.; Richter, A.S.; Heyne, S.; Dundar, F.; Manke, T. deepTools2: A next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016, 44, W160–W165. [Google Scholar] [CrossRef] [PubMed]
- Heinz, S.; Benner, C.; Spann, N.; Bertolino, E.; Lin, Y.C.; Laslo, P.; Cheng, J.X.; Murre, C.; Singh, H.; Glass, C.K. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 2010, 38, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef]

| Groups | No. of Repetition | No. of Cultured SCNT Embryos | No. of Cleaved Embryos (%, Mean) | No. of Blastocysts (%, Mean) |
|---|---|---|---|---|
| YY1-siRNA (10 μM) | 2 | 83 | 67 (80.75 ± 3.08) ab | 24 (28.92 ± 0.49) ab |
| YY1-siRNA (50 μM) | 2 | 65 | 56 (86.08 ± 6.83) b | 21 (32.24 ± 5.83) b |
| NC-siRNA (50 μM) | 2 | 75 | 49 (65.33 ± 0.65) a | 17 (22.65 ± 1.46) a |
| Groups | No. of Repetition | No. of Cultured SCNT Embryos | No. of Cleaved Embryos (%, Mean) | No. of Blastocysts (%, Mean) |
|---|---|---|---|---|
| YY1-siRNA (50 μM) | 8 | 305 | 257 (84.29 ± 5.05) b | 81 (26.85 ± 6.76) b |
| NC-siRNA (50 μM) | 8 | 318 | 229 (71.74 ± 5.21) a | 60 (18.58 ± 6.54) a |
| Groups | No. of Total/Farrowed Recipients | No. of Transferred Cloned Embryos | No. of Total Born Cloned Piglets | No. of Live-Born Cloned Piglets |
|---|---|---|---|---|
| YY1-siRNA (50 μM) | 6/2 | 1271 | 8 | 3 |
| NC-siRNA (50 μM) | 5/0 | 971 | 0 | 0 |
| Gene | GenBank Accession No. | Primer Sequence |
|---|---|---|
| YY1 | XM_021099699 | (forward): ATACCCGGCATCGACCTCTC |
| (reverse): AACATCTTTGTGCAGCCTTTGT | ||
| XIST | KC753465 | (forward): CTTGCCGCAATCGAAAACAT |
| (reverse): ACCAATTCCACCACCCTTTC | ||
| LOC100622504 | XM_003360396 | (forward): GTGAGACAACGTGCTCTGAGA |
| (reverse): ATTCCATGGGTTCCTGAGCTG | ||
| LOC110257861 | XR_002340890 | (forward): GCACAGTACCTTCTCCCCTTC |
| (reverse): CCCTGTTGCTTTGGTTTCGAG | ||
| RBM3 | NM_001243419 | (forward): GCTTCAAATGCAATGAGAGCCA |
| (reverse): TCTGCCATTTCCATAGCCCTG | ||
| H2AB2 | XM_021080443 | (forward): AAAAGGAGCCGTCGAAAGTCA |
| (reverse): GTCAGGTACTCGATGGTGGC | ||
| HAUS7 | XM_021079955 | (forward): CTCTGACTTCCACCAGCTCATC |
| (reverse): CTCTTCCATTTTGCTGGTTGGC | ||
| GAPDH | NM_001206359 | (forward): TGACCCCTTCATTGACCTCC |
| (reverse): CTCCGCCTTGACTGTGCC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, Y.; Wu, X.; Peng, X.; Yang, L.; Tan, B.; Zhao, H.; Zheng, E.; Hong, L.; Cai, G.; Wu, Z.; et al. Knockdown of YY1 Inhibits XIST Expression and Enhances Cloned Pig Embryo Development. Int. J. Mol. Sci. 2022, 23, 14572. https://doi.org/10.3390/ijms232314572
Dong Y, Wu X, Peng X, Yang L, Tan B, Zhao H, Zheng E, Hong L, Cai G, Wu Z, et al. Knockdown of YY1 Inhibits XIST Expression and Enhances Cloned Pig Embryo Development. International Journal of Molecular Sciences. 2022; 23(23):14572. https://doi.org/10.3390/ijms232314572
Chicago/Turabian StyleDong, Yazheng, Xiao Wu, Xitong Peng, Liusong Yang, Baohua Tan, Huaxing Zhao, Enqin Zheng, Linjun Hong, Gengyuan Cai, Zhenfang Wu, and et al. 2022. "Knockdown of YY1 Inhibits XIST Expression and Enhances Cloned Pig Embryo Development" International Journal of Molecular Sciences 23, no. 23: 14572. https://doi.org/10.3390/ijms232314572
APA StyleDong, Y., Wu, X., Peng, X., Yang, L., Tan, B., Zhao, H., Zheng, E., Hong, L., Cai, G., Wu, Z., & Li, Z. (2022). Knockdown of YY1 Inhibits XIST Expression and Enhances Cloned Pig Embryo Development. International Journal of Molecular Sciences, 23(23), 14572. https://doi.org/10.3390/ijms232314572





