Natural Compound 2,2′,4′-Trihydroxychalcone Suppresses T Helper 17 Cell Differentiation and Disease Progression by Inhibiting Retinoid-Related Orphan Receptor Gamma T
Abstract
:1. Introduction
2. Results
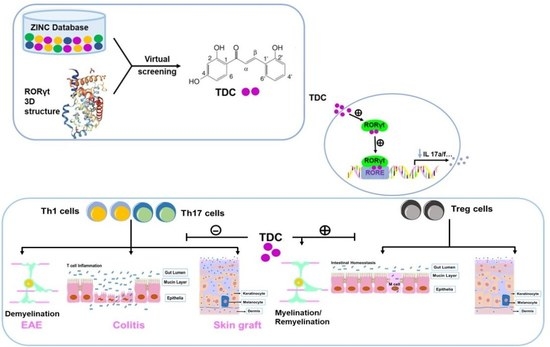
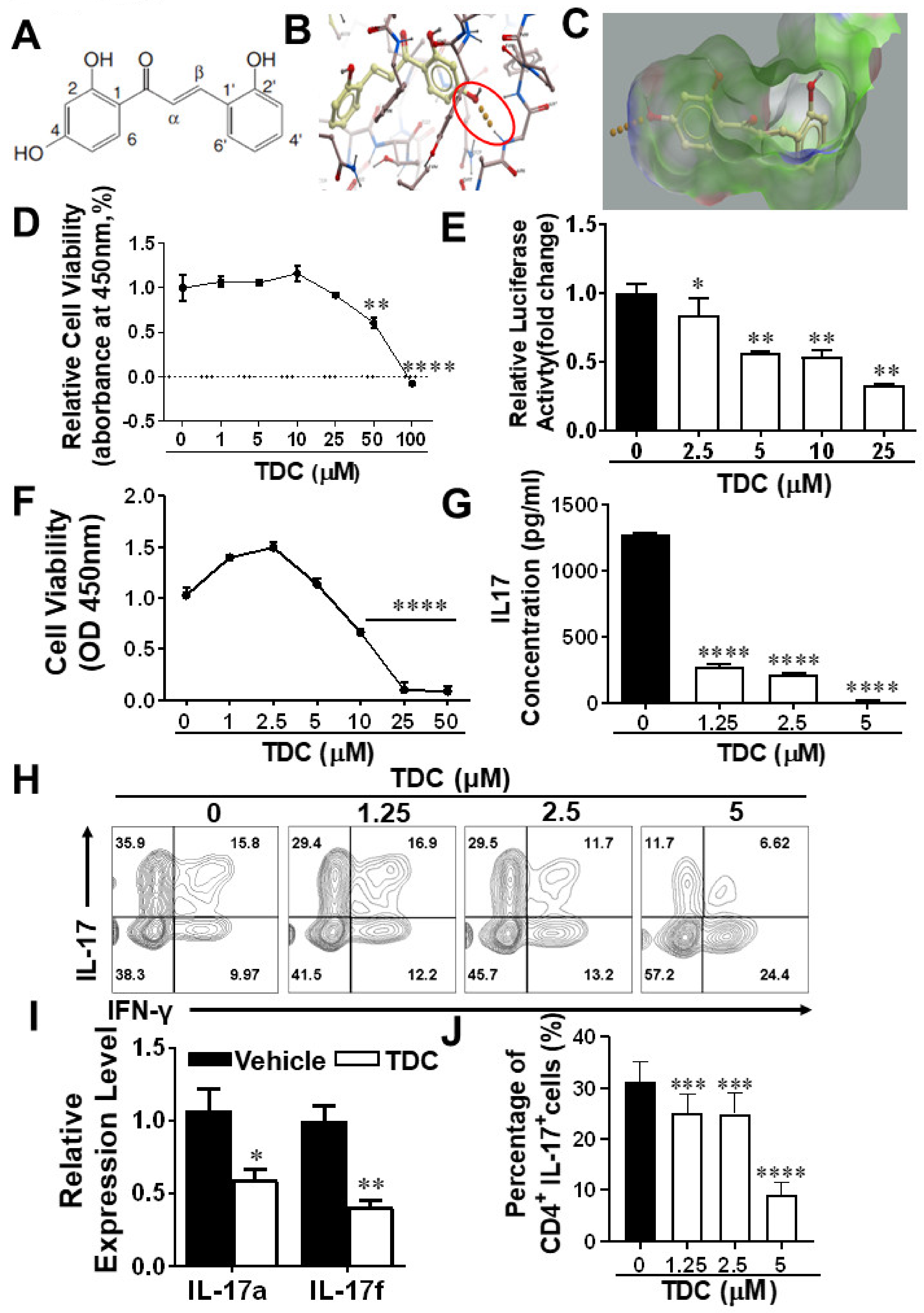
2.1. Structure-Based Virtual Screening of Small Molecules Targeting RORγt
2.2. TDC Suppressed RORγt Transcription Activity In Vitro
2.3. TDC Inhibited Th17 Cell Differentiation In Vitro
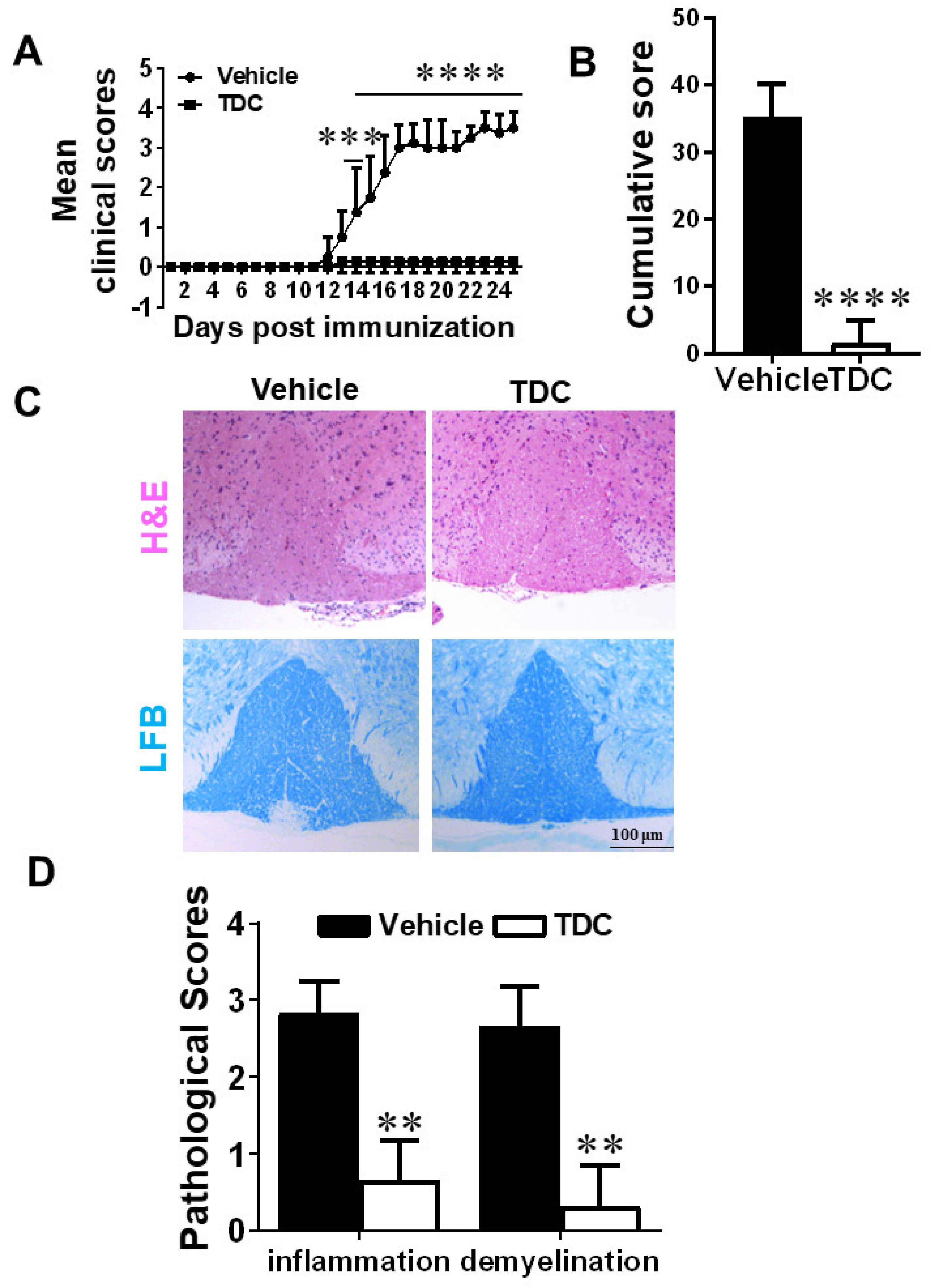
2.4. TDC Efficiently Ameliorated the Onset of EAE and Reduced CNS Inflammation
2.5. TDC Treatment Blocked the Activation of Debdritic Cells (DCs) and MOG-Reactive T Cells in the CNS
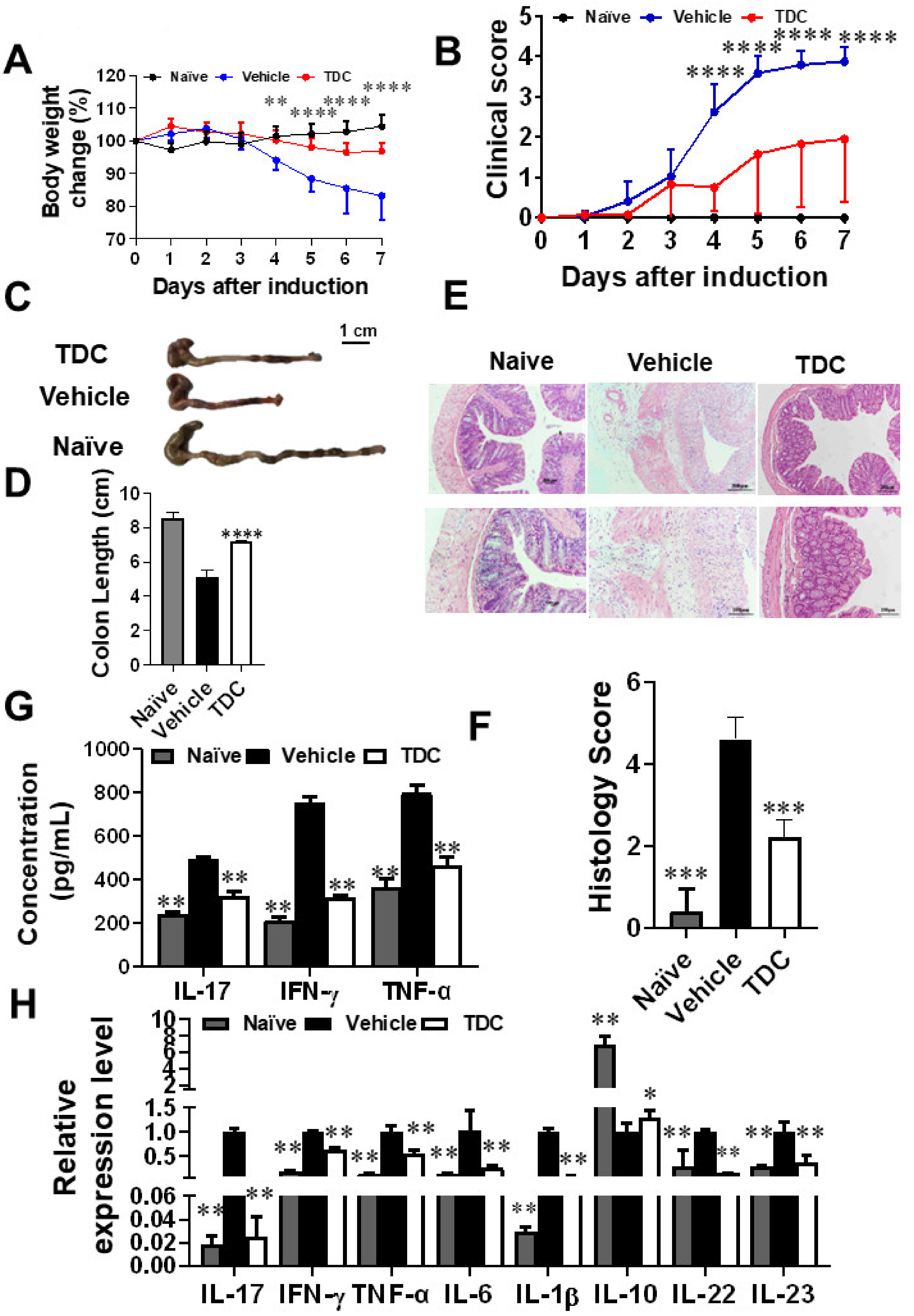
2.6. TDC Alleviated DSS-Induced Colitis
2.7. TDC Treatment Decreased the Secretion of Pro-Inflammatory Cytokines and Preserved the Proportion of Th17/Treg Cells
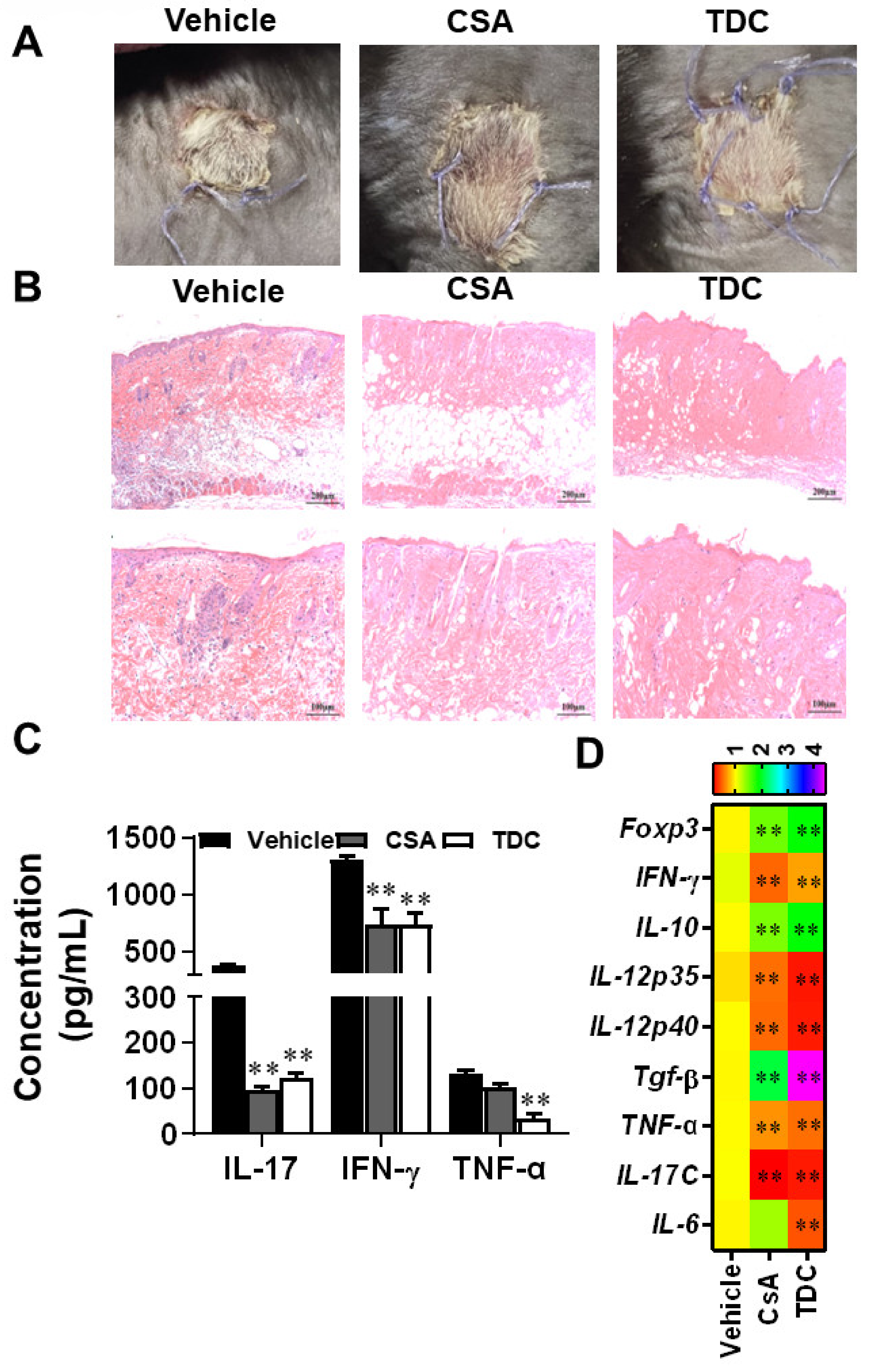
2.8. TDC Alleviated the Transplantation Rejection Responses of Skin Graft In Vivo
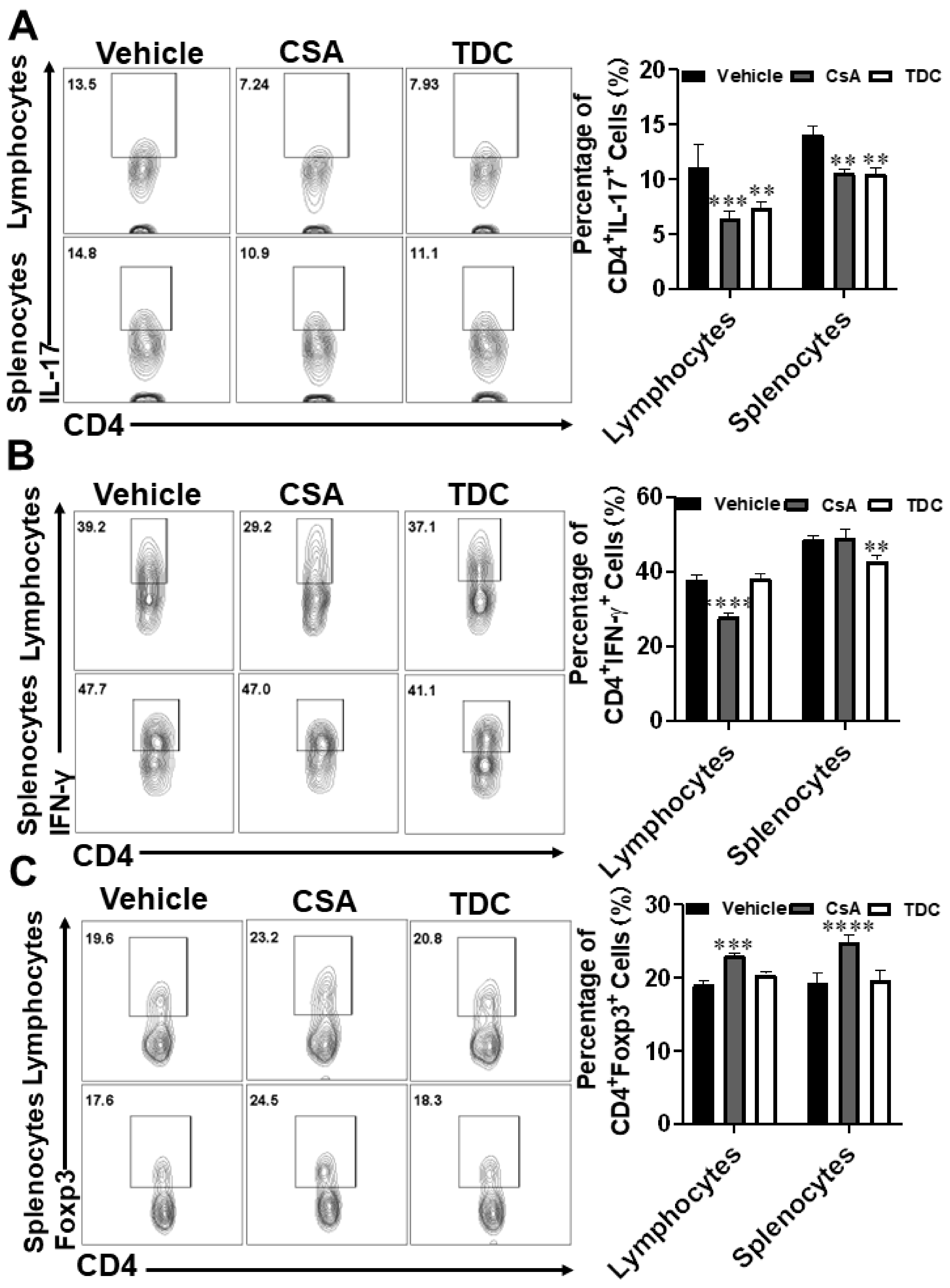
2.9. TDC Inhibited Skin Graft Rejection in Allogeneic Mice by Maintaining a Balance between Th17 Cells and Treg Cells
3. Discussion
4. Material and Methods
4.1. Virtual Screening and Molecular Docking
4.2. Luciferase Reporter Assays
4.3. EAE Induction and Treatment
4.4. DSS-Induced Colitis
4.5. Skin Grafting
4.6. Histopathological Analysis
4.7. Th17 Cell Polarization
4.8. Cytokine Measurement by Flow Cytometry and ELISA
4.9. Real-Time Quantitative PCR
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Ethics Approval
Abbreviations
References
- Park, H.; Li, Z.; Yang, X.O.; Chang, S.H.; Nurieva, R.; Wang, Y.-H.; Wang, Y.; Hood, L.; Zhu, Z.; Tian, Q.; et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005, 6, 1133–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, S.; Yosef, N.; Yang, J.; Wang, Y.; Zhou, L.; Zhu, C.; Wu, C.; Baloglu, E.; Schmidt, D.; Ramesh, R.; et al. Small-molecule RORγt antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity 2014, 40, 477–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noack, M.; Miossec, P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun. Rev. 2014, 13, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Zhao, L.; Wu, L.; Palanichamy, A.; Ergun, A.; Peng, L.; Quigley, C.; Hamann, S.; Dunstan, R.; Cullen, P.; et al. Small molecule mediated inhibition of RORγ-dependent gene expression and autoimmune disease pathology in vivo. Immunology 2016, 147, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Ciric, B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013, 333, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Ding, Q.; Zhao, M.; Bai, C.; Yu, B.; Huang, Z. Inhibition of RORγt activity and Th17 differentiation by a set of novel compounds. BMC Immunol. 2015, 16, 32. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Bolin, S.; Miller, H.; Ng, H.L. RORγ Structural Plasticity and Druggability. Int. J. Mol. Sci. 2020, 21, 5329. [Google Scholar] [CrossRef]
- Zapadka, T.E.; Lindstrom, S.I.; Taylor, B.E.; Lee, C.A.; Tang, J.; Taylor, Z.R.R.; Howell, S.J.; Taylor, P.R. RORγt Inhibitor-SR1001 Halts Retinal Inflammation, Capillary Degeneration, and the Progression of Diabetic Retinopathy. Int. J. Mol. Sci. 2020, 21, 3547. [Google Scholar] [CrossRef]
- Huh, J.R.; Leung, M.W.L.; Huang, P.; Ryan, D.A.; Krout, M.R.; Malapaka, R.R.V.; Chow, J.; Manel, N.; Ciofani, M.; Kim, S.V.; et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 2011, 472, 486–490. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Wang, X.; Zhong, B.; Nurieva, R.I.; Ding, S.; Dong, C. Ursolic acid suppresses interleukin-17 (IL-17) production by selectively antagonizing the function of RORgamma t protein. J. Biol. Chem. 2011, 286, 22707–22710. [Google Scholar] [CrossRef]
- Ghoshal, S.; Stevens, J.R.; Billon, C.; Girardet, C.; Sitaula, S.; Leon, A.S.; Rao, D.C.; Skinner, J.S.; Rankinen, T.; Bouchard, C.; et al. Adropin: An endocrine link between the biological clock and cholesterol homeostasis. Mol. Metab. 2018, 8, 51–64. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Vitali, A.; Giardina, B.; Mordente, A. Chalcone inhibition of anthracycline secondary alcohol metabolite formation in rabbit and human heart cytosol. Chem. Res. Toxicol. 2006, 19, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, J.C.; Pouget, C.; Fagnere, C.; Basly, J.P.; Chulia, A.J.; Habrioux, G. Chalcones are potent inhibitors of aromatase and 17beta-hydroxysteroid dehydrogenase activities. Life Sci. 2001, 68, 751–761. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2020, 11, 592654. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, C.; Wang, X.; Yang, Z.; Chen, J.; Hu, L.; Jiang, H.; Shen, X. 2,2′,4′-trihydroxychalcone from Glycyrrhiza glabra as a new specific BACE1 inhibitor efficiently ameliorates memory impairment in mice. J. Neurochem. 2010, 114, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.-H.; Zhang, Y.-Y.; Xing, K.; Hao, D.-X.; Zhang, F.; Wang, R.-N.; Bao, M.-Y.; Tian, M.-Y.; Yang, Y.-N.; Li, X.; et al. 2′, 4′-Dihydroxy-2,3-dimethoxychalcone: A pharmacological inverse agonist of RORγt ameliorating Th17-driven inflammatory diseases by regulating Th17/Treg. Int. Immunopharmacol. 2022, 108, 108769. [Google Scholar] [CrossRef]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef]
- Lee, E.; Eo, J.-C.; Lee, C.; Yu, J.-W. Distinct Features of Brain-Resident Macrophages: Microglia and Non-Parenchymal Brain Macrophages. Mol. Cells 2021, 44, 281–291. [Google Scholar] [CrossRef]
- Nam, J.-H.; Lee, J.-H.; Choi, S.-Y.; Jung, N.-C.; Song, J.-Y.; Seo, H.-G.; Lim, D.-S. Functional Ambivalence of Dendritic Cells: Tolerogenicity and Immunogenicity. Int. J. Mol. Sci. 2021, 22, 4430. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhou, C.; Chen, W.; Xie, A.; Li, J.; Wang, S.; Ye, P.; Wang, W.; Xia, J. Digoxin attenuates acute cardiac allograft rejection by antagonizing RORγt activity. Transplantation 2013, 95, 434–441. [Google Scholar] [CrossRef]
- Tian, Y.; Han, C.; Wei, Z.; Dong, H.; Shen, X.; Cui, Y.; Fu, X.; Tian, Z.; Wang, S.; Zhou, J.; et al. SOX-5 activates a novel RORγt enhancer to facilitate experimental autoimmune encephalomyelitis by promoting Th17 cell differentiation. Nat. Commun. 2021, 12, 481. [Google Scholar] [CrossRef]
- Cha, H.J.; Park, M.T.; Chung, H.Y.; Kim, N.D.; Sato, H.; Seiki, M.; Kim, K.W. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene 1998, 16, 771–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manel, N.; Unutmaz, D.; Littman, D.R. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008, 9, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Elson, C.O.; Cong, Y.; Weaver, C.T.; Schoeb, T.R.; McClanahan, T.K.; Fick, R.B.; Kastelein, R.A. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology 2007, 132, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Becher, B.; Durell, B.G.; Noelle, R.J. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J. Clin. Investig. 2002, 110, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Hafler, D.A. The plasticity of human Treg and Th17 cells and its role in autoimmunity. Semin. Immunol. 2013, 25, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Tan, J.; Liu, H.; Huang, M.; Li, N.; Tang, S.; Meng, J.; Tang, S.; Zhou, H.; Kijlstra, A.; Yang, P.; et al. Small molecules targeting RORγt inhibit autoimmune disease by suppressing Th17 cell differentiation. Cell Death Dis. 2020, 11, 697. [Google Scholar] [CrossRef]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and therapeutic targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef]
- Hohenberger, M.; Cardwell, L.A.; Oussedik, E.; Feldman, S.R. Interleukin-17 inhibition: Role in psoriasis and inflammatory bowel disease. J. Dermatol. Treat. 2018, 29, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Hall, J.A.; Kroehling, L.; Wu, L.; Najar, T.; Nguyen, H.H.; Lin, W.-Y.; Yeung, S.T.; Silva, H.M.; Li, D.; et al. Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell 2020, 180, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Mickael, M.E.; Bhaumik, S.; Basu, R. Retinoid-Related Orphan Receptor RORγt in CD4 T-Cell-Mediated Intestinal Homeostasis and Inflammation. Am. J. Pathol. 2020, 190, 1984–1999. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Blanco, M.; Lozano-Ojalvo, D.; Pérez-Rodríguez, L.; Benedé, S.; Molina, E.; López-Fandiño, R. Retinoic Acid Induces Functionally Suppressive Foxp3RORγt T Cells. Front. Immunol. 2021, 12, 675733. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shen, H.; Zhang, Y.; Wang, C.; Li, Q.; Zhang, C.; Zhuang, X.; Li, C.; Shi, Y.; Xing, Y.; et al. Discovery and Characterization of Benzimidazole Derivative XY123 as a Potent, Selective, and Orally Available RORγ Inverse Agonist. J. Med. Chem. 2021, 64, 8775–8797. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Li, X.; Yu, J.; Deng, X.; Shen, P.-X.; Jiang, Y.-B.; Zhu, L.; Wang, Z.-Z.; Zhang, Y. Eriodictyol suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis. Food Funct. 2020, 11, 6875–6888. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Ciric, B.; Ma, C.-G.; Gran, B.; Rostami, A.; Zhang, G.-X. Therapeutic effect of baicalin on experimental autoimmune encephalomyelitis is mediated by SOCS3 regulatory pathway. Sci. Rep. 2015, 5, 17407. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.-I.; Namba, T.; Arai, Y.; Fujimoto, M.; Adachi, H.; Sobue, G.; Takeuchi, K.; Nakai, A.; Mizushima, T. Genetic evidence for a protective role for heat shock factor 1 and heat shock protein 70 against colitis. J. Biol. Chem. 2007, 282, 23240–23252. [Google Scholar] [CrossRef] [Green Version]
- Murano, M.; Maemura, K.; Hirata, I.; Toshina, K.; Nishikawa, T.; Hamamoto, N.; Sasaki, S.; Saitoh, O.; Katsu, K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin. Exp. Immunol. 2000, 120, 51–58. [Google Scholar] [CrossRef]
- Qiu, R.; Wang, Y. Retinoic Acid Receptor-Related Orphan Receptor γt (RORγt) Agonists as Potential Small Molecule Therapeutics for Cancer Immunotherapy. J. Med. Chem. 2018, 61, 5794–5804. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Qi, W.; Zhang, Y.; Wang, R.; Bao, M.; Tian, M.; Li, X.; Zhang, Y. Natural Compound 2,2′,4′-Trihydroxychalcone Suppresses T Helper 17 Cell Differentiation and Disease Progression by Inhibiting Retinoid-Related Orphan Receptor Gamma T. Int. J. Mol. Sci. 2022, 23, 14547. https://doi.org/10.3390/ijms232314547
Yang Y, Qi W, Zhang Y, Wang R, Bao M, Tian M, Li X, Zhang Y. Natural Compound 2,2′,4′-Trihydroxychalcone Suppresses T Helper 17 Cell Differentiation and Disease Progression by Inhibiting Retinoid-Related Orphan Receptor Gamma T. International Journal of Molecular Sciences. 2022; 23(23):14547. https://doi.org/10.3390/ijms232314547
Chicago/Turabian StyleYang, Yana, Wenhui Qi, Yanyan Zhang, Ruining Wang, Mingyue Bao, Mengyuan Tian, Xing Li, and Yuan Zhang. 2022. "Natural Compound 2,2′,4′-Trihydroxychalcone Suppresses T Helper 17 Cell Differentiation and Disease Progression by Inhibiting Retinoid-Related Orphan Receptor Gamma T" International Journal of Molecular Sciences 23, no. 23: 14547. https://doi.org/10.3390/ijms232314547
APA StyleYang, Y., Qi, W., Zhang, Y., Wang, R., Bao, M., Tian, M., Li, X., & Zhang, Y. (2022). Natural Compound 2,2′,4′-Trihydroxychalcone Suppresses T Helper 17 Cell Differentiation and Disease Progression by Inhibiting Retinoid-Related Orphan Receptor Gamma T. International Journal of Molecular Sciences, 23(23), 14547. https://doi.org/10.3390/ijms232314547