Triacetyl Resveratrol Inhibits PEDV by Inducing the Early Apoptosis In Vitro
Abstract
:1. Introduction
2. Results
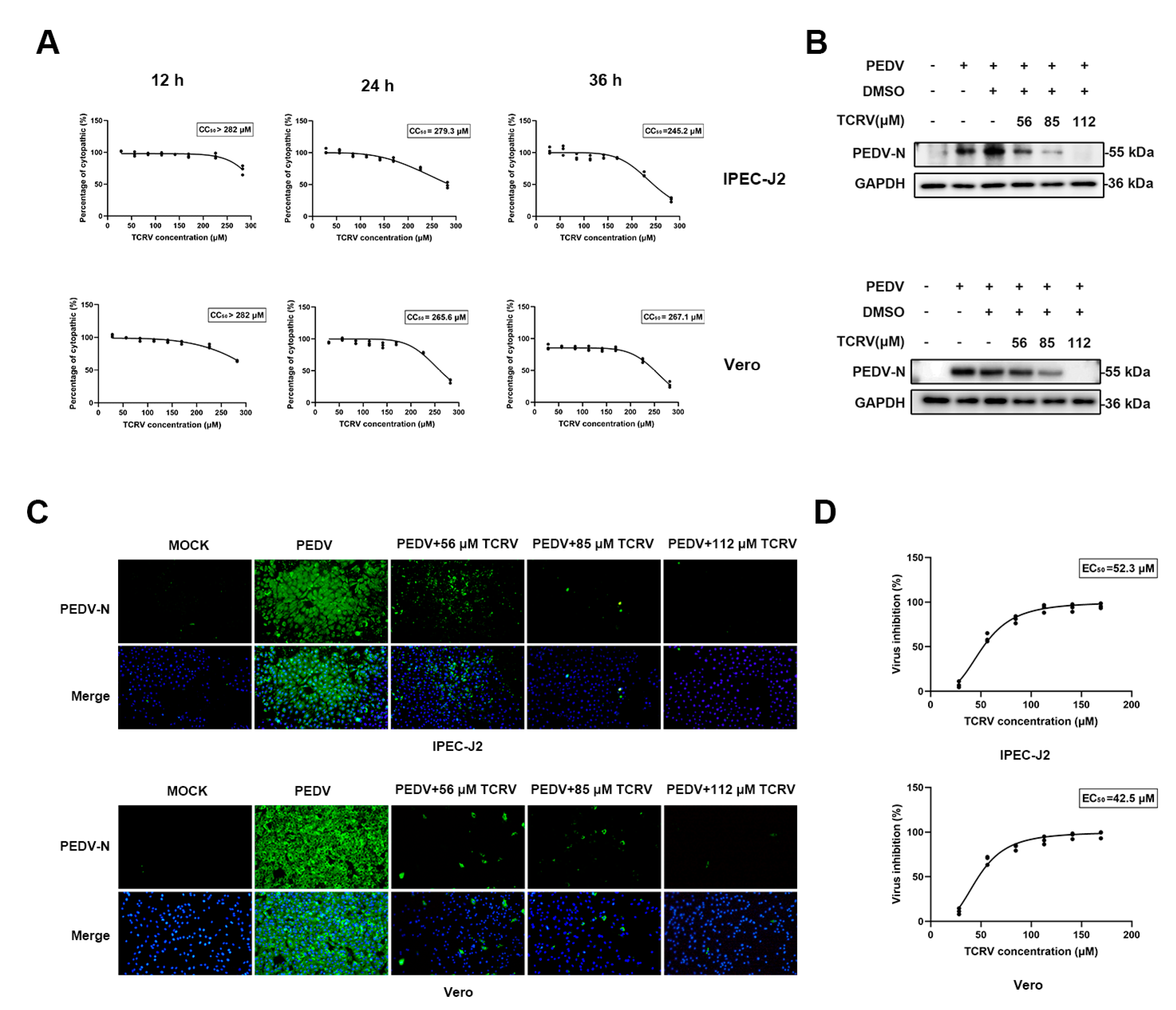
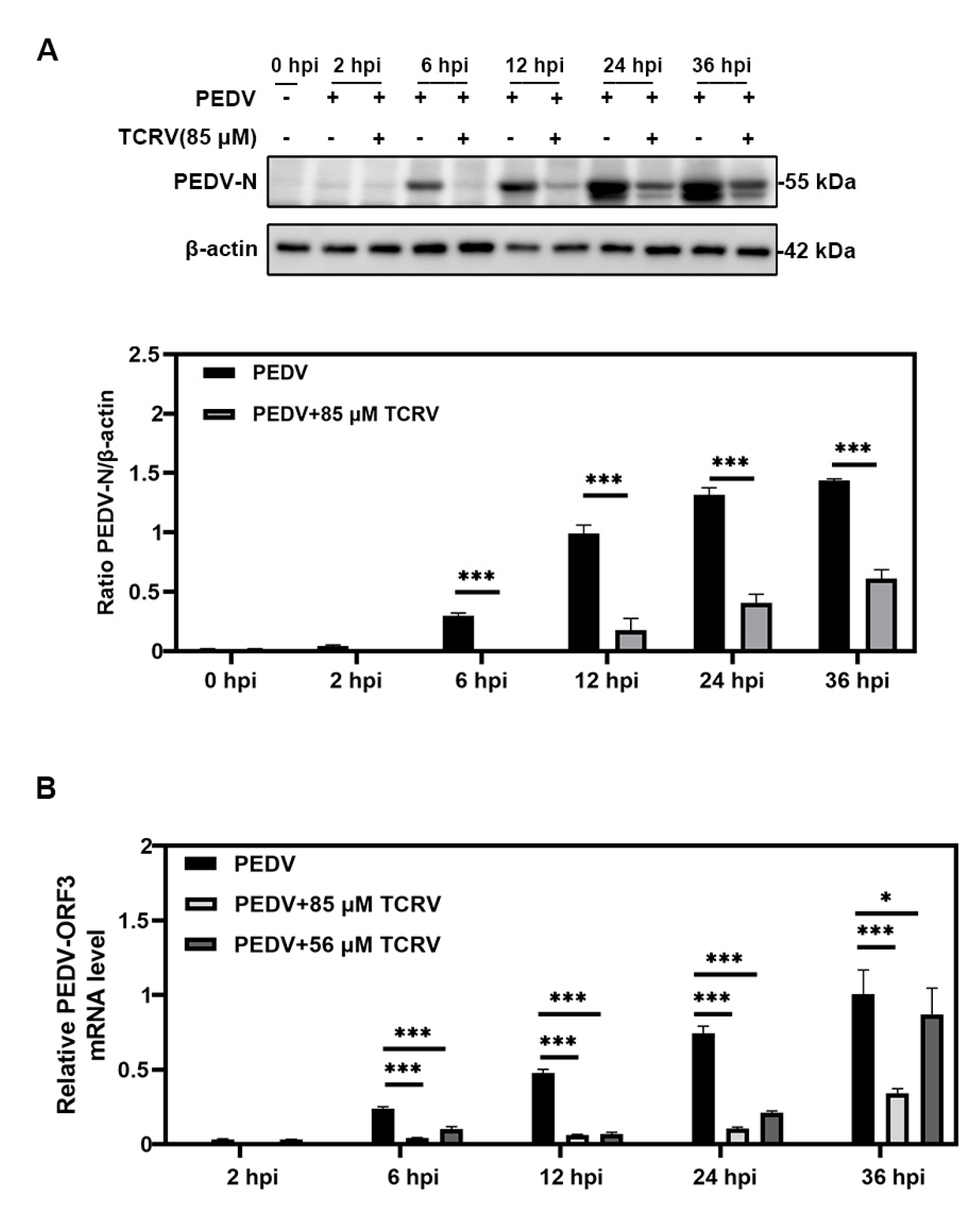
2.1. TCRV Inhibits PEDV Infection In Vitro
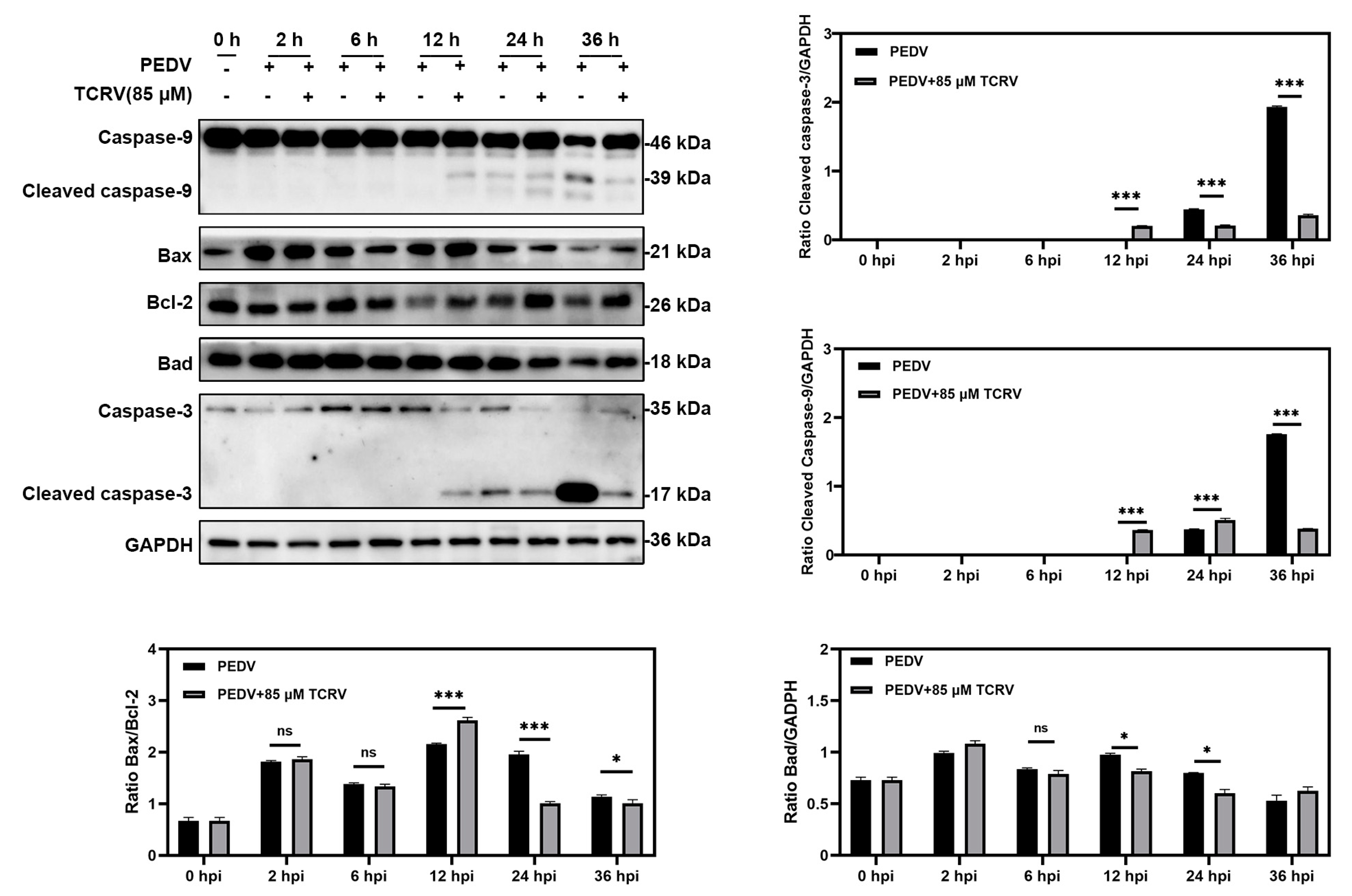
2.2. TCRV Promotes PEDV-Induced Apoptosis In Vitro
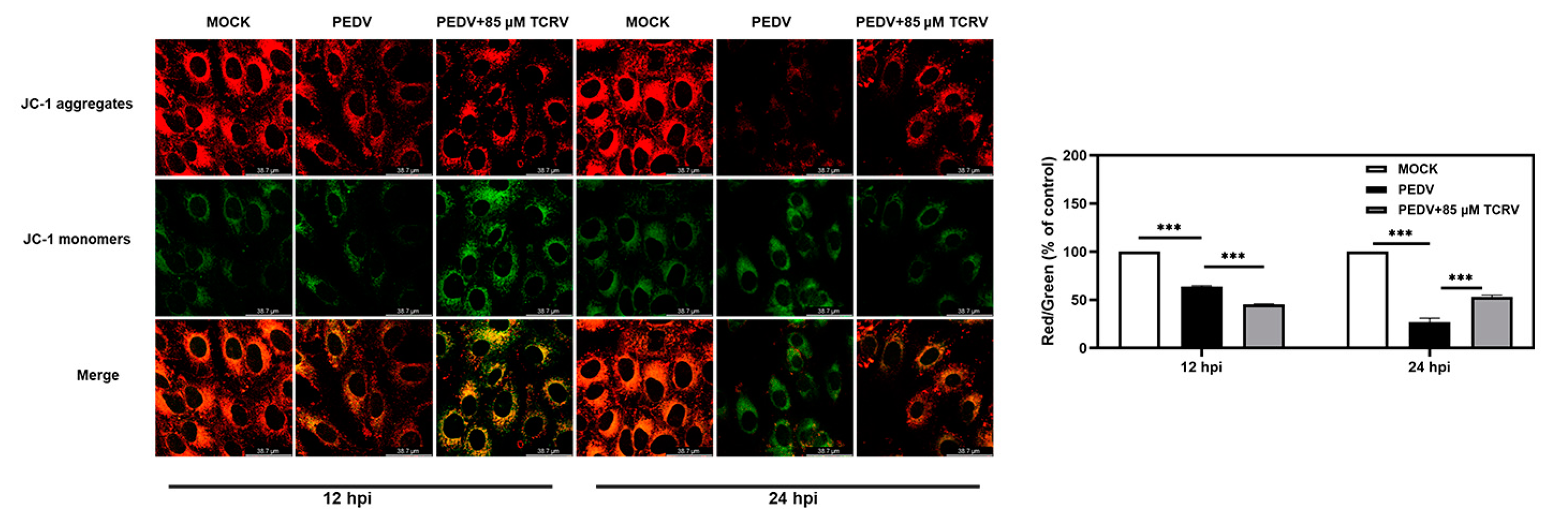
2.3. TCRV-Induced Mitochondrial Membrane Potential (MMP) Decreased in PEDV-Infected Cells
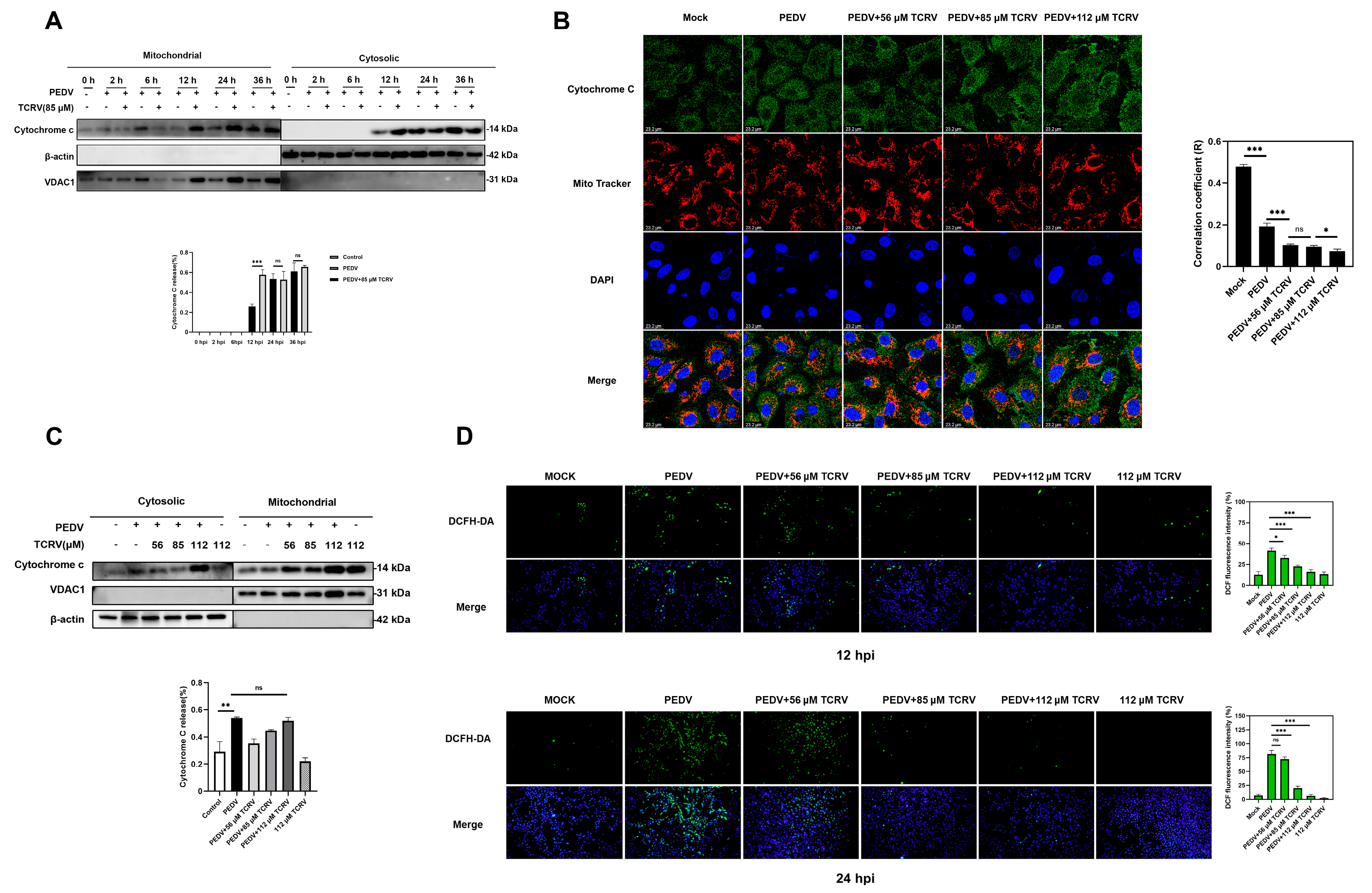
2.4. TCRV-Induced Cytochrome C Translocation after PEDV Infection
2.5. TCRV Inhibits PEDV Infection by the Regulation of Caspase-3
3. Discussion
4. Materials and Methods
4.1. Cytotoxicity Assay
4.2. Cells and Virus
4.3. Medicine Treatment
4.4. Indirect Immunofluorescence Assay (IFA)
4.5. Western Blotting (WB) Assay
4.6. RNA Extraction and Quantitative RT-PCR(RT-qPCR)
4.7. Annexin V-PE and 7-Amino-Actinomycin (7-AAD) Double-Staining Assay
4.8. Determination of Mitochondrial Membrane Potential (MMP)
4.9. Determination of the Levels of Intracellular Reactive Oxygen Species (ROS)
4.10. Mitochondria Isolation and Cytochrome C Release Assays
4.11. Indirect Immunofluorescence Assay (IFA) to Analyze Cytochrome C Subcellular Localization
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int. J. Biol. Sci. 2020, 16, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Busceti, C.L.; Biagioni, F.; Lazzeri, G.; Forte, M.; Schiavon, S.; Sciarretta, S.; Frati, G.; Fornai, F. Cell Clearing Systems as Targets of Polyphenols in Viral Infections: Potential Implications for COVID-19 Pathogenesis. Antioxidants 2020, 9, 1105. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, Y.; Peng, P.; Liu, Y.; Huang, M.; Ma, Y.; Xue, C.; Cao, Y. Aloe extract inhibits porcine epidemic diarrhea virus in vitro and in vivo. Vet. Microbiol. 2020, 249, 108849–108849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, G.; Wang, T.; Cao, W.; Zhang, L.; Chen, X. Nrf2-Keap1 pathway-mediated effects of resveratrol on oxidative stress and apoptosis in hydrogen peroxide-treated rheumatoid arthritis fibroblast-like synoviocytes. Ann. N. Y. Acad. Sci. 2019, 1457, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Campagna, M.; Rivas, C. Antiviral activity of resveratrol. Biochem. Soc. Trans. 2010, 38, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Shrivastava, A.; Shrivastava, S.K.; Srivastava, R.K.; Shankar, S. Triacetyl resveratrol upregulates miRNA-200 and suppresses the Shh pathway in pancreatic cancer: A potential therapeutic agent. Int. J. Oncol. 2019, 54, 1306–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.; Shi, D.; Xing, X.; Qi, S.; Yang, D.; Zhang, J.; Han, Y.; Zhu, Q.; Sun, H.; Wang, X.; et al. Coronavirus Porcine Epidemic Diarrhea Virus Nucleocapsid Protein Interacts with p53 To Induce Cell Cycle Arrest in S-Phase and Promotes Viral Replication. J. Virol. 2021, 95, e0018721. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sun, Y.; Lan, T.; Wu, R.; Chen, J.; Wu, Z.; Xie, Q.; Zhang, X.; Ma, J. Retrospective detection and phylogenetic analysis of swine acute diarrhoea syndrome coronavirus in pigs in southern China. Transbound. Emerg. Dis. 2019, 66, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Yang, X.; Li, H.; Ma, B.; Guan, R.; Yang, J.; Chen, D.; Han, X.; Zhou, L.; Song, Z.; et al. Molecular characterization of porcine epidemic diarrhea virus associated with outbreaks in southwest China during 2014–2018. Transbound. Emerg. Dis. 2021, 68, 3482–3497. [Google Scholar] [CrossRef]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.E.; Polo, J.; Meeker, D. The Canadian 2014 porcine epidemic diarrhoea virus outbreak: Important risk factors that were not considered in the epidemiological investigation could change the conclusions. Transbound. Emerg. Dis. 2020, 67, 1101–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reveles-Félix, S.; Carreón-Nápoles, R.; Mendoza-Elvira, S.; Quintero-Ramírez, V.; García-Sánchez, J.; Martínez-Bautista, R.; Saavedra-Montañez, M.; Mosqueda Gualito, J.J.; Sánchez-Betancourt, J.I. Emerging strains of porcine epidemic diarrhoea virus (PEDv) in Mexico. Transbound. Emerg. Dis. 2020, 67, 1035–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, M.; Zambrano-Moreno, C.; Pineda, P.; Calderón, C.; Rincón-Monroy, M.A.; Diaz, A.; Marthaler, D.G. Several lineages of porcine epidemic diarrhea virus in Colombia during the 2014 and 2016 epidemic. Transbound. Emerg. Dis. 2021, 68, 2465–2476. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Lee, K.K.; Kim, S.H.; Lee, C. Prevalence, complete genome sequencing and phylogenetic analysis of porcine deltacoronavirus in South Korea, 2014–2016. Transbound. Emerg. Dis. 2017, 64, 1364–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garrido-Mantilla, J.; Lara, A.; Guardado, E.; Lopez, J.; Nerem, J.; Pizarro, G.; Paul Cano, J. Feed or feed transport as a potential route for a porcine epidemic diarrhea outbreak in a 10,000-sow breeding herd in Mexico. Transbound. Emerg. Dis. 2021, 69, 66–71. [Google Scholar] [CrossRef]
- Jang, G.; Lee, S.; Lee, C. Assessing the risk of recurrence of porcine epidemic diarrhea virus in affected farms on Jeju Island, South Korea. J. Vet. Sci. 2021, 22, e48. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, H.Y. Porcine enteric coronaviruses: An updated overview of the pathogenesis, prevalence, and diagnosis. Vet. Res. Commun. 2021, 45, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zheng, Z.; Wang, H.; Yi, S.; Zhang, G.; Gong, L. The New Porcine Epidemic Diarrhea Virus Outbreak May Mean That Existing Commercial Vaccines Are Not Enough to Fully Protect Against the Epidemic Strains. Front. Vet. Sci. 2021, 8, 697839. [Google Scholar] [CrossRef]
- Díaz, I.; Pujols, J.; Cano, E.; Cortey, M.; Navarro, N.; Vidal, A.; Mateu, E.; Martín, M. Immune response does not prevent homologous Porcine epidemic diarrhoea virus reinfection five months after the initial challenge. Transbound. Emerg. Dis. 2021, 69, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.X.; Liang, J.Q.; Zhu, Q.C.; Dai, G.; Li, S.; Fung, T.S.; Liu, D.X. Gammacoronavirus Avian Infectious Bronchitis Virus and Alphacoronavirus Porcine Epidemic Diarrhea Virus Exploit a Cell-Survival Strategy via Upregulation of cFOS to Promote Viral Replication. J. Virol. 2020, 95, e02107–20. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiol. Rev. 2022, 102, 411–454. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.; Green, D.R. Mitochondria and cell death: Outer membrane permeabilization and beyond. Nature reviews. Mol. Cell Biol. 2010, 11, 621–632. [Google Scholar] [CrossRef]
- Araya, L.E.; Soni, I.V.; Hardy, J.A.; Julien, O. Deorphanizing Caspase-3 and Caspase-9 Substrates In and Out of Apoptosis with Deep Substrate Profiling. ACS Chem. Biol. 2021, 16, 2280–2296. [Google Scholar] [CrossRef]
- Mootha, V.K.; Wei, M.C.; Buttle, K.F.; Scorrano, L.; Panoutsakopoulou, V.; Mannella, C.A.; Korsmeyer, S.J. A reversible component of mitochondrial respiratory dysfunction in apoptosis can be rescued by exogenous cytochrome c. EMBO J. 2001, 20, 661–671. [Google Scholar] [CrossRef]
- Kroemer, G.; Reed, J.C. Mitochondrial control of cell death. Nat. Med. 2000, 6, 513–519. [Google Scholar] [CrossRef]
- Si, F.; Hu, X.; Wang, C.; Chen, B.; Wang, R.; Dong, S.; Yu, R.; Li, Z. Porcine Epidemic Diarrhea Virus (PEDV) ORF3 Enhances Viral Proliferation by Inhibiting Apoptosis of Infected Cells. Viruses 2020, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnan, M.A. Determination of 50% endpoint titer using a simple formula. World J. Virol. 2016, 5, 85–86. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Li, J.; Gao, Y.; Zhou, L.; Ge, X.; Han, J.; Guo, X.; Yang, H. Porcine epidemic diarrhea virus S1 protein is the critical inducer of apoptosis. Virol. J. 2018, 15, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Xu, J.; Song, X.; Jia, R.; Yin, Z.; Cheng, A.; Jia, R.; Zou, Y.; Li, L.; Yin, L.; et al. Antiviral effect of resveratrol in ducklings infected with virulent duck enteritis virus. Antivir. Res. 2016, 130, 93–100. [Google Scholar] [CrossRef]
- Duan, J.; Yue, W.; Jian, E.; Malhotra, J.; Lu, S.E.; Gu, J.; Xu, F.; Tan, X.L. In vitro comparative studies of resveratrol and triacetylresveratrol on cell proliferation, apoptosis, and STAT3 and NFκB signaling in pancreatic cancer cells. Sci. Rep. 2016, 6, 31672. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Pan, X.; Xu, X.; Lin, J.; Que, F.; Tian, Y.; Li, L.; Liu, S. Resveratrol Reactivates Latent HIV through Increasing Histone Acetylation and Activating Heat Shock Factor 1. J. Agric. Food Chem. 2017, 65, 4384–4394. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.A.; Norris, P.S.; Ware, C.F. To kill or be killed: Viral evasion of apoptosis. Nat. Immunol. 2002, 3, 1013–1018. [Google Scholar] [CrossRef]
- Brokatzky, D.; Dörflinger, B.; Haimovici, A.; Weber, A.; Kirschnek, S.; Vier, J.; Metz, A.; Henschel, J.; Steinfeldt, T.; Gentle, I.E.; et al. A non-death function of the mitochondrial apoptosis apparatus in immunity. EMBO J. 2019, 38, e100907. [Google Scholar] [CrossRef]
- Oh, C.; Kim, Y.; Chang, K.O. Caspase-mediated cleavage of nucleocapsid protein of a protease-independent porcine epidemic diarrhea virus strain. Virus Res. 2020, 285, 198026. [Google Scholar] [CrossRef]
- Guo, L.; Tan, K.; Wang, H.; Zhang, X. Pterostilbene inhibits hepatocellular carcinoma through p53/SOD2/ROS-mediated mitochondrial apoptosis. Oncol. Rep. 2016, 36, 3233–3240. [Google Scholar] [CrossRef] [Green Version]
- Mahyar-Roemer, M.; Katsen, A.; Mestres, P.; Roemer, K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int. J. Cancer 2001, 94, 615–622. [Google Scholar] [CrossRef]
- Yang, L.; Wang, C.; Shu, J.; Feng, H.; He, Y.; Chen, J.; Shu, J. Porcine Epidemic Diarrhea Virus Induces Vero Cell Apoptosis via the p53-PUMA Signaling Pathway. Viruses 2021, 13, 1218. [Google Scholar] [CrossRef]



| Genes | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| PEDV ORF3 | TAGACAAGCTTCAAATGTGAC | GTATTAAAGATAATAAGGAGCGC |
| GAPDH | AGGTCGGAGTCAACGGATTT | TAGTTGAGGTCAATGAAGGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Y.; Li, K.; Yang, M.; Wang, Q.; Hao, Z. Triacetyl Resveratrol Inhibits PEDV by Inducing the Early Apoptosis In Vitro. Int. J. Mol. Sci. 2022, 23, 14499. https://doi.org/10.3390/ijms232314499
Wang X, Liu Y, Li K, Yang M, Wang Q, Hao Z. Triacetyl Resveratrol Inhibits PEDV by Inducing the Early Apoptosis In Vitro. International Journal of Molecular Sciences. 2022; 23(23):14499. https://doi.org/10.3390/ijms232314499
Chicago/Turabian StyleWang, Xue, Yi Liu, Kaiyuan Li, Ming Yang, Qingtao Wang, and Zhihui Hao. 2022. "Triacetyl Resveratrol Inhibits PEDV by Inducing the Early Apoptosis In Vitro" International Journal of Molecular Sciences 23, no. 23: 14499. https://doi.org/10.3390/ijms232314499
APA StyleWang, X., Liu, Y., Li, K., Yang, M., Wang, Q., & Hao, Z. (2022). Triacetyl Resveratrol Inhibits PEDV by Inducing the Early Apoptosis In Vitro. International Journal of Molecular Sciences, 23(23), 14499. https://doi.org/10.3390/ijms232314499






