Abstract
Conventional antidepressants are widely employed in several psychiatric and neurologic disorders, yet the mechanisms underlying their delayed and partial therapeutic effects are only gradually being understood. This narrative review provides an up-to-date overview of the interplay between antidepressant treatment and Brain-Derived Neurotrophic Factor (BDNF) signaling. In addition, the impact of nutritional, environmental and physiological factors on BDNF and the antidepressant response is outlined. This review underlines the necessity to include information on lifestyle choices in testing and developing antidepressant treatments in the future.
1. The Paradigm Shift in Antidepressant Mechanism of Action
Antidepressants are widely used drugs indicated in several psychiatric and neurologic disorders, including major depressive disorder (MDD), anxiety, obsessive compulsive disorder and neuropathic pain. These drugs facilitate the signaling of serotonin and/or norepinephrine either by blocking their reuptake to presynaptic terminals, by inhibiting their catabolism or by binding to monoamine autoreceptors. Increased monoamine synaptic availability occurs soon after drug administration. However, the clinical antidepressant effect is delayed and typically requires several weeks of chronic treatment before an antidepressant response is obtained [1]. Thus, the enhancement of extracellular monoamine levels alone cannot explain the antidepressant effect [2]. In addition, a recent review on the link between serotonin and depression found no convincing evidence that MDD is associated with, or is caused by, low serotonin concentrations or abnormal serotonin system activity [3]. Instead, starting from the work of Duman and collaborators [4], novel molecular and cellular mechanisms were identified, highlighting the role of second messenger systems, which are involved in gene expression regulation [5]. In detail, the signaling of the neurotrophin Brain-Derived Neurotrophic Factor (BDNF), activated by the second messenger cyclic AMP system, accounts for adaptive changes in intracellular signal transduction and synaptic connectivity, which emerged as the relevant mechanism of action of antidepressant medications [6]. The delayed therapeutic effect of chronic antidepressant treatment has been traditionally attributed to the progressive neuroplastic changes involving monoaminergic transmissions, including the progressive desensitization of upregulated serotonin receptors [7]. However, progressive neuronal plasticity resulting from the recruitment of BDNF signaling, which is indicated as central to the antidepressant response, is also consistent with the delayed therapeutic effect observed after chronic antidepressant treatment [8]. As a game changer in this scenario, the dissociative anesthetic and N-methyl-D-aspartate (NMDA) receptor inhibitor ketamine showed rapid and sustained antidepressant action in clinical trials [9] and the (S)-ketamine nasal spray has been developed and approved in the United States and Europe for the employment in treatment-resistant depression [10]. Interestingly, it becomes increasingly clear that antidepressants with different molecular targets, including monoaminergic drugs and ketamine share BDNF-mediated neuroanatomical effects [11,12].
BDNF binds with a high affinity to the tropomyosin receptor kinase B (TrkB), a tyrosine kinase receptor expressed both pre- and post-synaptically [13]. The binding of BDNF to TrkB induces the rapid receptor autophosphorylation, which promotes at least three intracellular cascades [14]. Remarkably, TrkB activation is able to modulate BDNF expression levels in neurons, forming a transcriptional positive feedback loop, involving CREB family transcription factors as the main regulators of Bdnf gene expression after TrkB signaling [15].
TrkB is crucial to antidepressant responses. When direct BDNF infusions were administered in rodents, the inhibition of TrkB signaling prevented the antidepressant-like effects of BDNF [16,17]. Antidepressants have been shown to promote TrkB autophosphorylation and downstream CREB-signaling after both acute and repeated administration as soon as 30 min after antidepressant administration. However, this effect vanished after at 6 or 24 h [17], supporting the need for a chronic regimen. Interestingly, recent findings demonstrated that antidepressants activate TrkB independently of BDNF binding as well. The tricyclic antidepressant imipramine readily induced the phosphorylation of TrkB in conditional Bdnf−/− knock-out mice, indicating that BDNF is not required for TrkB activation. Moreover, TrkB phosphorylation induced by either fluoxetine, citalopram, and reboxetine was also observed in serotonin transporter-deficient mice, suggesting that antidepressant drugs transactivate TrkB independently of BDNF and monoamine transporter blockade [18,19].
Overall, the activation of TrkB triggers activity-dependent synaptic plasticity [20]. Thus, it has been postulated that antidepressant-induced TrkB signaling reactivates a state of juvenile-like plasticity in the adult brain, restructuring neural circuits and, in consequence, mood and behavior [8,21]. In particular, the chronic administration of antidepressants is able to stimulate adult hippocampal neurogenesis, interacting with TrkB on the neural progenitor cells of the dentate gyrus, inducing a continuous proliferation and neuronal differentiation, which is the source of such functional plasticity [22]. In addition, the deletion of TrkB in neural stem/progenitor cells, or the pharmacological inhibition of ERK signaling also abolished ketamine-induced behavioral responses in depression- and anxiety-like paradigms [23], suggesting that dysfunctional TrkB in the neurogenic niche can be an etiological factor for refractory depression. Experimental data are also corroborated by consistent postmortem findings of decreased prefrontal and hippocampal TrkB signaling in MDD patients who committed suicide [24,25,26,27].
The effects of monoaminergic antidepressants and ketamine on TrkB signaling have long been considered indirect, caused by the inhibition of monoamine transporters and NMDA receptors, respectively, and mediated by the increase in BDNF expression and release [19]. However, early evidence and more recent data indicate that antidepressants directly bind to TrkB, [28,29]. The work of the Castrén group elegantly showed that antidepressants of various classes directly bind to a site formed by a dimer of TrkB transmembrane domains, thereby facilitating cell surface expression of the receptor, increasing the sensitivity of the dimerized receptor for BDNF, and promoting BDNF-mediated TrkB signaling [29].
Although the affinity of antidepressants for TrkB is lower than those for monoaminergic transporters, under chronic regimen they accumulate in the brain, and the concentration achieved upon long-term use allows for direct binding to TrkB [29]. As recently highlighted by a proximity ligation assay, acute imipramine administration increased the physical interaction between BDNF and TrkB in the rat cingulate cortex 3 h later, although this effect was not long-lasting. In contrast, repeated imipramine administration exerted a durable effect, which decreased slowly and vanished only after 21 drug-free days [30].
In addition, recent evidence reveals a physical interaction between TrkB and 5-HT2A receptors, in a heterodimeric complex which prevents TrkB activation in brain regions including the hippocampus, prefrontal cortex and striatum [7]. The monoaminergic antidepressant-induced neuronal events, including the progressive 5-HT receptor downregulation, thus decreased functional inhibition of TrkB, and the intracellular events triggered by direct TrkB modulation, are in accordance with the slow development of the clinical antidepressant response.
Overall, TrkB is emerging as the sly target for antidepressants (Figure 1). As such, the paradigm of the antidepressant mechanism of action shifts from the modulation of monoamine signaling to the direct promotion of BDNF transmission. Thus, alterations in upstream or downstream components of BDNF signaling can undermine the response to antidepressants. On the other hand, environmental factors which modulate BDNF signaling, or activate its downstream pathways, may provide additional tools for amplifying the antidepressant response.
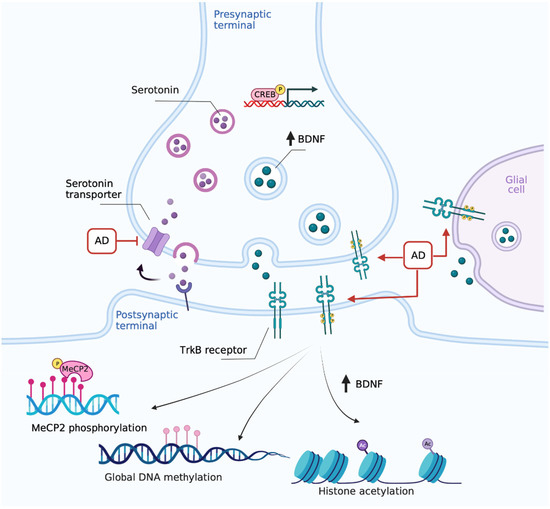
Figure 1.
The paradigm shift in antidepressants’ mechanism of action: focus on TrkB. Chronic monoaminergic antidepressants (AD) increase hippocampal and cortical BDNF levels promoting autocrine/paracrine mechanisms, which include the direct interaction with TrkB and CREB-mediated upregulation in serotonergic terminals. Created with BioRender.com.
2. BDNF as a Critical Antidepressant Mediator
2.1. BDNF Exerts Distinct Effects in MDD-Linked Brain Regions
BDNF is a crucial regulator of neurite outgrowth, synaptic plasticity, and the selection of functional neuronal connections in the central nervous system [20,31,32], that mediates the plastic changes induced by antidepressants [6].
The first studies unveiling the involvement of BDNF in antidepressant response showed that chronic administration of conventional antidepressant drugs, including tranylcypromine, sertraline, desipramine, and mianserin, significantly increased BDNF mRNA expression in the hippocampus and cortical regions, counteracting the downregulation of Bdnf mRNA in the hippocampus observed in response to restraint stress [4,33].
When heterozygous Bdnf null (Bdnf+/−) mice were employed to assess BDNF’s role in the antidepressant response, a ~50% reduction in BDNF levels did not impact depression-related behavior per se, but the antidepressant imipramine was ineffective in the forced swim test [17]. In addition, when BDNF was selectively deleted in the forebrain of inducible knockout mice, no basal alterations in depression-related behavior were highlighted. Still, an attenuated response to the tricyclic antidepressant desipramine was observed [34], further indicating that forebrain BDNF was required for antidepressant efficacy.
To directly examine the causal involvement of BDNF in antidepressant responses, the BDNF protein was infused directly into the midbrain, and an antidepressant-like effect was observed in two animal models of MDD [35]. Subsequent work showed that a single bilateral infusion of a low dose of BDNF into the dentate gyrus or CA3, but not CA1, region of the hippocampus was sufficient to exert an antidepressant-like effect in forced swim test and learned helplessness paradigms, without affecting locomotor activity or passive avoidance [36]. This suggested that BDNF may act as a region-specific effector of the antidepressant action. Notably, the effects of the hippocampal BDNF infusion were rapid (within three days), long-lasting (at least ten days) and recapitulated the therapeutic effects of repeated imipramine treatment [36]. In addition, the involvement of hippocampal BDNF, specifically in the dentate gyrus, in antidepressant efficacy was demonstrated by the employment of a viral-mediated gene transfer approach, which allowed for selectively deleting BDNF in discrete hippocampal subregions. The Cre-dependent deletion of BDNF in the dentate gyrus or CA1 subregion of the hippocampus in floxed Bdnf mice was not sufficient to induce depression-like behavior. However, the loss of BDNF in the dentate gyrus, but not the CA1, was crucial for the antidepressant-like effect of conventional antidepressants such as citalopram and desipramine in the forced swim test [37].
Although the role of BDNF in antidepressant effects has been consistently shown, the mechanisms through which antidepressants activate BDNF signaling remain unclear. BDNF is anterogradely transported to nerve terminals and released in response to depolarization [38,39]. Antidepressant-induced increases in synaptic availability of serotonin and norepinephrine levels were suggested to locally promote BDNF release [40,41]. In detail, incubation of cultured raphe neurons with serotonin increased BDNF release, which in turn enhanced serotonergic differentiation of the neurons [41]. Moreover, both preclinical and clinical research show a close relationship between antidepressants and alterations in Bdnf expression, indicating that the Bdnf gene is a critical epigenetic target [42].
Nevertheless, the role of BDNF in depression pathogenesis is not univocal. Rather, it depends on the brain region and individual circuits. As such, while BDNF exerts an antidepressant effect in the prefrontal cortex [43] and hippocampus [36], an opposite role has been highlighted in the mesolimbic circuit, including dopamine projections from the ventral tegmental area to the nucleus accumbens [44,45]. Interestingly, in situ hybridization of Bdnf mRNA highlighted that chronic fluoxetine administration increased Bdnf expression not only in hippocampal cell bodies, but also in the mesocorticolimbic circuit, including ventral tegmental area, prefrontal cortex, and nucleus accumbens shell, whereas no changes were detected in the substantia nigra and dorsal striatum [46]. This study did not report behavioral correlates of antidepressant response, but a strong BDNF activity in the mesocorticolimbic pathway may be a source of treatment resistance.
Indeed, the inhibition of BDNF activity in the mesolimbic pathway exerts antidepressant-like effects in rodents and reverses the social avoidance behavior caused by chronic social defeat stress paradigm [47,48]. In addition, a recent study by Furuse and colleagues (2019) further implicates the changes in BDNF levels in the nucleus accumbens in the pathophysiology and treatment of treatment-resistant depression [49]. Specifically, the antidepressant response to escitalopram in the resistant-depression model, which combines the synergic pro-depressant effects of early life adversity, such as prenatal ethanol exposure [50], and adolescent corticosterone exposure, was associated with decreased BDNF levels in the serum and the nucleus accumbens of rats.
2.2. Multiple Cell Types Contribute to Increased BDNF Following Antidepressant Treatment
While significant work has implicated hippocampal and cortical BDNF in antidepressant responses, only a few reports looked at the contribution of specific cell types in the brain, which produce BDNF following antidepressant treatment. In this regard, BDNF is predominantly expressed by neurons and it is conceivable that cortical and hippocampal interneurons are involved in the actions of antidepressants, contributing to the storage, production, and release of BDNF [51,52].
However, the antidepressant-induced increase in BDNF levels might also result from the upregulation in neurons targeting the prefrontal and hippocampal regions. For instance, the selective ablation of the BDNF-promoting transcription factor cAMP response element binding protein (CREB) in serotonergic neurons counteracted the upregulation of BDNF in the hippocampus and prefrontal cortex, evoked by chronic fluoxetine administration [53]. In addition, the selective BDNF overexpression in serotonergic terminals, effective in inducing antidepressant-like behavioral responses, serotonergic axonal sprouting and upregulated neurogenesis in the dentate gyrus, occluded further antidepressant-like action of fluoxetine [54], suggesting a ceiling effect in BDNF receptor-mediated fluoxetine-response. This evidence highlights that serotonergic and BDNF signaling in the hippocampus might influence each other in an auto-paracrine loop.
Other studies suggest that antidepressants may increase BDNF expression in cells other than neurons, albeit at lower levels. The incubation of rat primary cultured neurons with amitriptyline, at therapeutically relevant concentrations, did not affect BDNF expression [55]. This was in accordance with previous findings, showing monoamines presence is required for hippocampal and cortical neuronal cultures to show antidepressant-induced activation of BDNF promoters [56]. However, amitriptyline incubation, as well as clomipramine, fluvoxamine or duloxetine, but not cocaine, produced a rapid increase in Bdnf mRNA expression in astrocytes and microglia through an ERK-dependent pathway [55]. This evidence suggests a glial expression of BDNF, not dependent on the presence of monoamines that could contribute to the therapeutic effect of antidepressants. Furthermore, BDNF exerts a positive feedback effect on its transcription, through an autocrine/paracrine mechanism [14].
2.3. Epigenetic Regulation of the Bdnf Gene
The increased Bdnf mRNA variant expression induced by antidepressant treatment is mediated through epigenetic modifications, including DNA methylation and histone modifications [42,57]. DNA methylation can directly silence genes through the formation of methylcytosine in CpG dinucleotide-rich regions known as CpG islands, which subsequently perturbs gene transcription near transcriptional start sites [58].
Although recent work examining the link between Bdnf gene methylation and antidepressant medication in depressed patients did not provide consistent results [59,60,61,62,63], the variable-controlled environment of preclinical research showed decreased expression of the DNA demethylation machinery, able to target Bdnf transcripts, in mice exposed to unpredictable chronic mild stress [64]. Additionally, when DNA methylation was prevented by the systemic or hippocampal administration of DNMT1 inhibitor 5-azacytidine (decitabine), an antidepressant-like effect in the forced swim test was observed in rats, accompanied by decreased global DNA cytidine methylation and increased Bdnf levels in the hippocampus [65].
Apart from altering the degree of DNA methylation, antidepressants, including fluoxetine and escitalopram, modulate the phosphorylation of methyl-CpG binding protein 2 (MeCP2), a protein that decreases Bdnf transcription by occluding the Bdnf exon IV promoter activity [66,67]. Notably, knock-in mice with a non-functional MeCP2 phosphorylation site at Ser421 displayed depressive-like behavior and antidepressant resistance. When mice were exposed to chronic social defeat stress, they showed social avoidance, which was not rescued by chronic imipramine administration [68].
In addition, the ever-unfolding epigenetic modifications at Bdnf promoters revealed a functional role of histone modifications in antidepressant action. Early life stress and chronic restraint stress decreased histone H3 acetylation at Bdnf promoters, and downregulated expression of hippocampal BDNF, which were normalized by escitalopram treatment [67,69].
3. Lifestyle Interactions with Antidepressants in Rodent Models and Humans
Lifestyle involves a great diversity of factors such as nutrition, life events and the time that passes while we progress through our lives. All these factors have determinant effects on depression pharmacotherapy efficacy. Thus, although current antidepressants usually alleviate symptoms, delays in the onset, decrease, and lack of effects have been observed. Besides individual genetic and epigenetic propensities, the efficacy of antidepressants may therefore be, at least in part, explained by lifestyle interactions. Moreover, such lifestyle interactions induce changes in Bdnf gene expression regulated by epigenetic modifications such as DNA methylations and histone modifications.
3.1. Nutrition
A fundamental and well-studied aspect of lifestyle is nutrition, in which BDNF has an important role in the food intake control via reward learning [70]. A considerable effort has been made to understand the role of nutrition on antidepressant efficacy and disease management in addition to the MDD symptoms themselves [71,72,73,74]. Table 1 summarizes the clinical trial data addressing the impact of adjunctive nutrients on antidepressant therapies.
A deficiency in long-chain omega-3 fatty acids (omega-3 FA) is generally associated with mood disorders including major depressive disorder (MDD) and it also influences the treatment response. Dietary omega-3 FA themselves support Bdnf expression in the hippocampus of rats [75], through calmodulin kinase II and activated Akt [76], and increase Bdnf regulators such as CREB [77]. Supplementation with omega-3 FA increased serum BDNF levels and improved depressive symptoms in a randomized controlled trial with children and adolescents with depressive disorder [78]. Omega-3 FA such as Eicosapentaenoic acid and Docosahexaenoic acid used in combination with various antidepressants generally improved the antidepressant response [79,80,81,82,83,84,85,86,87]. Consistently, a randomized, double-blind, placebo-controlled study of a combination therapy with citalopram and omega-3 FA revealed a higher efficacy of antidepressant treatment when both components were administered together [88]. Moreover, in a randomized, double-blind, placebo-controlled dose-escalation study with the SSRI paroxetine, treated patients showed higher FA-chain length, unsaturation, and peroxidation. Negative relationships between FA and cortisol were associated with paroxetine nonresponse, which was also associated with low docosahexaenoic acid and low fatty fish intake [89]. In a 6-week double-blind, randomized, and placebo-controlled trial, therapy with Palmitoylethanolamide, a FA, and citalopram exerted a higher and faster antidepressant effect than in the absence of this FA [90]. Accordingly, in an animal model of FA insufficiency, rats fed without the omega-3 FA precursor alpha-linolenic acid and chronically treated with fluoxetine (FLX) exhibited greater climbing behavior, alpha2A adrenergic receptor mRNA expression, and lower 5-HT1A mRNA expression compared with rats fed with normal food [91]. However, omega-3 FA deficiency did not significantly reduce the effects of chronic FLX treatment on serotonin turnover or behavior in the forced swim test in female rats [92].
In line with an altered fatty acid metabolism, abnormalities in serum cholesterol levels in patients with mood disorders have been epidemiologically identified (reviewed in [93]). In rats fed with a cholesterol-enriched diet and subjected to chronic stress and anxiety paradigms, diazepam and clomipramine effects were not altered [94].
The relationship between caloric intake and the etiology of mood disorders has also gained attention in recent decades (reviewed in [95]). Intermittent fasting itself increased levels of BDNF and ameliorated anxiety and depressive-like behaviors in a type 2 diabetes mellitus model in rats [96]. However, differential effects were reported in case of MDD patients with mild to severe symptoms [97]. In mice, 9 h of fasting and imipramine treatment potentiated antidepressant-like effects and increased the ratio of phospho-CREB/CREB. These effects were partially reversed by treatment with the 5-HT2A/2C receptor agonist, (±)-1-(2, 5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride. Furthermore, the effect of DOI hydrochloride was stronger compared to the non-fasting control group [98,99]. Moreover, mice fed with a high-fat diet, and exposed to social defeat stress showed a diminished antidepressant response to FLX [100].
The phosphocreatine pathway is vital for replenishing the energy donor ATP in the brain and is involved in MDD pathophysiology [101]. Subchronic administration of creatine alone showed antidepressant-like effects that were dependent on Akt activation and increased expression of BDNF in the hippocampus of mice [102]. In a randomized, double-blind placebo controlled trial an increase in creatine as adjunctive treatment to escitalopram enhanced the efficacy of the antidepressant in women [103]. In female adolescent patients with FLX-resistant depression, the Children’s Depression Rating Scale-Revised (CDRS-R) raw score decreased by 56% following adjunctive creatine treatment [104]. Similarly, only female rats supplemented with creatine showed a significant increase in the FLX antidepressant-like behavior compared to FLX alone [105].
Alterations in serotonergic neurotransmission have been considered one of the main causes of neuropsychiatric disorders including MDD during the 1960s [106]. However, more reliable evidence came from experiments using tryptophan depletion approaches [107]. A decrease in the essential amino acid tryptophan, the main metabolite in serotonin synthesis, may disturb serotonin synthesis [108]. In rats fed with a low tryptophan chow, fluvoxamine and fenfluramine diminished 5-HT release and, in rats fed with a high tryptophan diet, fenfluramine enhanced serotonin release [109]. Clinical data show an improvement in response to fluoxetine, chlorimipramine and phenelzine when adjunctive tryptophan to these antidepressants is used [110,111,112].
Methionine is another essential amino acid crucial to mental health. The methionine-derivative S-adenosyl methionine, cofactor in the single carbon cycle is involved in the synthesis of serotonin, noradrenalin and dopamine as well as in providing methyl donors for DNA- and histone methylation [113]. Clinical data demonstrated the efficacy of adjunctive S-adenosyl methionine use to improve antidepressant response [73,114,115], even in case of selective serotonin reuptake inhibitor (SSRI)-treatment resistant patients [116]. Moreover, N-acetylcysteine, a derivative of the amino acid cysteine, typically prescribed for bronchopulmonary disorders, improved antidepressant response when used as a treatment adjunctive for MDD [114]. However, the beneficial effects of S-adenosyl methionine and N-acetylcysteine and were not moderated by BDNF [117,118]. The dietary supplement N,N-dimethylglycine, a derivative of the amino acid glycine, acts at the glycine binding site of the NMDA receptor. Therefore, it enhances antidepressant-like effects of ketamine, but does not affect ketamine-induced anesthesia in mice [119]. Similarly, the dietary supplement betaine, a methyl derivative of glycine, increases the antidepressant-like effects but blocks the psychotomimetic effects of ketamine in mice [120].
Vitamins are also essential to our health. Evidence has suggested that folic acid, a water-soluble vitamin of the B complex group, can be used for enhancing antidepressant therapy in patients with deficient folic acid levels [121], and usually this deficiency is related to loss of responsiveness to conventional antidepressants [122,123,124]. Folic acid itself affected antidepressant-like behaviors and modulated BDNF in a chronic unpredictable mild stress rat model [125]. Clinical data show an improvement in the SSRI/SNRI antidepressant response in the presence of folic acid or its’ biological active form L-methylfolate [126,127,128,129,130]. However, this effect could not be demonstrated in the response of patients treated with other antidepressants, suggesting a role in serotonin and norepinephrine reuptake [131]. Furthermore, vitamin B12, like folate a member of the one carbon metabolism, improved the response to SSRI and tricyclic antidepressants in MDD-patients [132]. In a mouse model of combined chronic and acute stress, vitamin B12 increased stress resilience and restored TrkB levels [133]. However, no clear potentiation of antidepressant medication by B12 supplementation in combination with other vitamins on depressive symptomatology was reported in two clinical trials with patients treated with diverse antidepressant therapies [134,135]. Other vitamins including vitamin C and vitamin D3 also showed promising effects on the response to SSRIs fluoxetine and citalopram [136,137,138,139]. The effect of vitamin D3 in particular might be mediated by BDNF [140]. However, more research is needed in this direction.
Minerals are very important in several cellular processes. More than 300 enzymes and around 2000 transcription factors require zinc for their function [141,142]. The effect of zinc itself on depression has been broadly studied, and an interaction between zinc, BDNF and neuropeptides is emerging [143]. In mice fed with a zinc-deficient diet, BDNF levels and the antidepressant-like effects of both imipramine and escitalopram were reduced [144,145], with a reduction in the serum zinc levels and an increase in the corticosterone level [146]. Moreover, FLX evoked antidepressant-like effects and blocked the zinc restriction-induced changes in hippocampal p-CREB but not BDNF protein levels in rats fed with a zinc-deficient diet [147]. Clinical data show an improvement in the antidepressant response to fluoxetine, citalopram and imipramine in the presence of zinc as adjunctive compound [148,149,150,151]. Magnesium, the second most abundant intracellular cation [152], which in itself may have antidepressant properties [153], did not affect the efficacy of FLX in MDD-patients at any stage of treatment [154].
3.2. Environment
In addition to each person’s unique genetic heritage, the individual’s environment plays a crucial role in leaving a distinct epigenetic footprint that may predispose to or provide resilience against mental illnesses such as MDD. Studies on chromatin marks are thus necessary to understand the etiology and pathology of MDD [155]. Accordingly, changes in the epigenome have been related to antidepressant response as well. Altered DNA methylation on the promoter region of the serotonin transporter SLC6A4 has been associated with impaired antidepressant treatment response [156,157]. Furthermore, DNA methylation and related enzymes have been suggested to affect the therapeutic effects of tricyclic antidepressants, SSRIs and valproate [158,159,160]. Moreover, an association in the DNA methylation state from MDD patients with paroxetine response has been reported [161].
Great efforts have been made to find environmental predictors for better antidepressant responses. Studies on gene-environment interactions may not only help to understand the pathophysiology of MDD but could also provide predictive factors for personalized antidepressant therapy [162,163]. However, a number of studies have not found consistent associations and a more complex relationship between environment and treatment is likely [164,165,166,167]. Hence, individualized therapy is gaining importance, as the sum of genetic risk and environmental factors results in a differential and individual response to antidepressants [168,169].
Environmental conditions, including stressful or traumatic events are known risk factors to depression [170]. Accordingly, chronic stress alters the expression of neurotrophic factors including BDNF and could thereby inhibit signaling pathways that mediate antidepressant effects [171]. During stressful situations, hypothalamic corticotropin-releasing hormone (CRH) is released, which elevates cortisol levels. Cortisol is increased in patients suffering from MDD [172] and is thought to impair serotonin transmission [173]. In a study of CRH polymorphisms and stressful life events in patients with MDD, authors suggested an association between CRH ht1 haplotype, which is moderated by stressful events, and the antidepressant treatment outcomes [174]. In chronically stressed mice, treatment with ketamine increased antidepressant-like effects in stressed animals but had the opposite effect in unstressed controls that were housed in an enriched environment [175].
One of the most useful environmental predictors of antidepressant response in MDD are traumatic childhood experiences [176]. It has been reported that traumatized patients respond better to psychotherapy than to pharmacological treatments [177]. Moreover, individuals with traumatic childhood experiences showed generally lower treatment responses across various treatment approaches [178]. In addition, an association between higher levels of childhood abuse and depression severity has been reported in patients with a weak affinity to the serotonin transporter who received antidepressant therapy [177].
Adult mice exposed to early life stress or adult fluoxetine treatment did not show changes in hippocampal neurogenesis. Antidepressants only affected neurogenesis in adolescent that were not exposed to early life stress, suggesting that antidepressant action works in a distinct background of age and prior stress exposure [179]. In male pups exposed to the early life stress model of limited bedding, authors observed a positive correlation between stress and TrkB expression in the prefrontal cortex, and a negative association in the hippocampus [180]. Prenatal stress can also have lasting effects on treatment responses later in life. For instance, in rodents, stress in utero increased methylation at Bdnf promoters, which was associated with a reduced Bdnf transcription and increased depressive-like behaviors in tail suspension, forced swim, and sucrose preference tests [181]. In addition, prenatal stress altered stress coping in male and female juvenile rats and increased Bdnf-expression [182]. Intergenerational effects of early life stress on Bdnf promoter methylation have been reported in rats as well. However, it is unclear, how this influences their response to antidepressants [183].
Conversely, pleasant life events have positive effects on depression treatment. In rats, environmental enrichment improved behaviors associated with depression but not anxiety [184]. Environmental enrichment for several weeks consistently increases BDNF levels in rodents [185]. Treatment with the SSRI sertraline was more effective in diminishing depressive-like behavior in the presence of standard or environmental enrichment housing compared to social isolation conditions. Conversely, early social enrichment increased adult hippocampal BDNF levels in mice [184].
Climatic changes in the environment, such as weather, are a well-known mood influencer. Accordingly, diminished light exposure affects BDNF levels as well [186]. A change in the monthly sunshine duration exerted a significant effect on paroxetine response time, with a later onset of treatment response during shorter sunshine duration [187].
In addition, sport or physical activities are beneficial for depression treatment [188]. In line with this, a rat model of depression showed higher mRNA levels of Bdnf, and the presence of a running wheel had a positive effect on the treatment response with escitalopram [189]. In rats with colorectal cancer and depressive-like behavior, treatment with quercetin and exercise improved anti-tumor and antidepressant effects by suppressing inflammatory markers and upregulating the BDNF/TrkB/ß-Catenin axis [190]. Exercise improved depressive-like behaviors in ovariectomized rats and BDNF levels in hippocampus [191], increased the expression of 5-HT, BDNF and TrkB, and improved the mobility in the forced swim test in socially isolated rat pups [192]. Furthermore, exercise alleviated stress-induced social isolation via 5-HT1A receptor activation in rats [193]. Release of ß-hydroxybutyrate after prolonged exercise is associated with an increase in the activity of the Bdnf promoter by HDAC2 and HDAC3 in mice [194]. In addition, differential Bdnf methylation was associated with exercise in Vietnam veterans that suffered from posttraumatic stress disorder [195]. Taken together, exercise has reproducible effects on BDNF levels, which may, at least in part, mediate its’ antidepressant and mood elevating effects.
3.3. Aging
Although aging has received much attention recently, age-dependent expression of BDNF-TrkB and the mechanisms involved in depression require further investigation. Microarray data from the prefrontal cortex of healthy subjects confirmed an age-dependent BDNF downregulation and a positive correlation between BDNF and synaptic genes [196]. In addition, an age-dependent reduction in TrkB expression in the hippocampus is associated with altered spine morphology [197], a hallmark of depression [198]. Social defeat in adolescent, but not in adult rats, leads to an upregulation of BDNF-related immediate early genes [199]. Unpredictable chronic stress induced lower BDNF expression levels in aged hippocampus compared to young rats [200]. Moreover, enriched environment, which alleviates depressive-like behavior, increased Bdnf mRNA in the frontal cortex of in BDNF deficient mice independently of age, as well as in old wildtype mice [201]. Given the association between neurotrophin signaling, including BDNF and TrkB, with aging [202], an interplay between antidepressant response and age is likely. Many researchers have tried to optimize treatments for MDD patients of all ages; however, difficulties especially arise in elderly patients. This is partially due to a high variability in the efficacy and tolerability of antidepressants in the presence of age-related diseases, which require their own medicament therapies [203]. Hence, the association between the age of patients with MDD and the treatment efficacy is still not clear. While some studies reported an improved response [204], others found a lower antidepressant response in older patients [205]. In patients with MDD treated with paroxetine in a 6-week protocol, the late responders were significantly younger than the late or non-responders [179].

Table 1.
Studies on the impact of dietary factors on antidepressant response.
Table 1.
Studies on the impact of dietary factors on antidepressant response.
| Adjunctive Nutrient | Antidepressant | Outcome | Reference |
|---|---|---|---|
| Omega-3 PUFA 1000 mg/d | Sertraline | MADRS: Omega-3 PUFA < placebo | [81] |
| Ethyl-EPA 1 g/d | Fluoxetine 20 mg/d | HDRS: Ethyl-EPA < placebo | [79] |
| EPA 1, 2 or 4 g/d | Unspecified antidepressants | HDRS EPA < placebo (best 1 g/d) | [82] |
| EPA 2 g/d | Unspecified antidepressants | HDRS EPA < placebo | [84] |
| DHA 260 mg/d or 520 mg/d | Unspecified antidepressants | HDRS < after treatment resistance | [87] |
| EPA 1.8 g/d + DHA 0.4 g/d | Citalopram 20–40 mg/d | HDRS: Fatty acids < placebo | [88] |
| EPA 0.93 g/d + DHA 0.75 g/d | Sertraline 50 mg/d | BDI-II or HDRS: Fatty acids = placebo | [80] |
| EPA 1 g/d, DHA 1 g/d | Unspecified antidepressants | HDRS: EPA < DHA or placebo | [86] |
| EPA + DHA 3 g/d (EPA 0.6 g; DHA 2.4 g) | Unspecified antidepressants | HDRS-SF: EPA + DHA = placebo | [85] |
| EPA + DHA 9.6 g/d | Unspecified antidepressants | HDRS EPA + DHA < placebo | [83] |
| Palmitoylethanolamide 1200 mg/d | Citalopram 20 mg/d | HDRS: Palmitoylethanolamide < placebo | [90] |
| Creatine 4 mg/d | Fluoxetine 20–40 mg/d | CDRS-R: Creatine < placebo | [104] |
| Creatine 5 g/d | Escitalopram 20 mg/d | HDRS: creatine < placebo | [103] |
| Tryptophan 4 g/d | Fluoxetine 20 mg/d | HDRS: Tryptophan < placebo | [110] |
| DL-Tryptophan 0.1 g/kg body weight | Chlorimipramine 150 mg/d | Cronholm-Ottosson Depression Scale: n.s. | [111] |
| DL-Tryptophan 12, 15, 18 g depending on body weight | Phenelzine 60 mg/d | HDRS: DL-Tryptophan < placebo | [112] |
| SAMe 800 mg/d | Unspecified antidepressants | HDRS: < after treatment resistance | [206,207] |
| SAMe 1600 mg/d | SSRIs | HDRS: SAMe < placebo (in treatment resistance) | [116] |
| SAMe 200 mg/d, NAC 200 mg/d and folate 200 ug/d | SSRIs | HADS-A and CGI: better with SAMe, NAC and folate | [114] |
| L-Methylfolate 15 mg/d | SSRIs | QIDS-SR and CGI: better with L-Methylfolate in SSRI resistance | [129] |
| L-Methylfolate | SSRIs/SNRIs | CGI: L-Methylfolate < placebo | [130] |
| Folic acid 0.5 mg/d | Fluoxetine 20 mg/d | HDRS: Folic acid < placebo | [126] |
| Folic acid 10 mg/d | Fluoxetine 20 mg/d | HDRS: Folic acid < placebo | [127] |
| Folic acid 5 mg/d | Unspecified antidepressants | BDI-II: Folic acid = placebo | [131] |
| Folic acid 2 mg/d + Vitamin B12 0.5 mg/d + Vitamin B6 25 mg/d | Citalopram 20–40 mg/d | MADRS: Folic acid + B12 + B6 = placebo | [135] |
| Folic acid 400 ug/d + Vitamin B12 100 ug/d | Unspecified antidepressants | PHQ-9: n.s. | [134] |
| Vitamin B12 1 mg/d | SSRIs 20–40 mg/d | HDRS: with Vitamin B12 < placebo | [132] |
| TCA 100–200 mg/d | HDRS: with Vitamin B12 < placebo | [132] | |
| Vitamin C 1 g/d | Fluoxetine 10–20 mg/d | CDRS: with Vitamin C < placebo | [136] |
| Citalopram 20 mg/d | HDRS: with Vitamin C = placebo | [138] | |
| Vitamin D3 1500IU/d | Fluoxetine 20 mg/d | HDRS: with Vitamin D3 < placebo | [137] |
| Vitamin D3 300.000 U | Unspecified antidepressants | HDRS with Vitamin D3 < placebo (after 4 weeks) | [139] |
| Zinc 25 mg/d | Fluoxetine 20–60 mg/d | BDI: Zinc < placebo | [148] |
| Citalopram 20–60 mg/d | BDI: Zinc < placebo | [148] | |
| Imipramine 100–200 mg/d | BDI, HDRS, CGI, MADRS: Zinc < placebo | [150,151] | |
| Unspecified antidepressants | HDRS, BDI: Zinc < placebo | [208] | |
| Magnesium 120 mg/d | Fluoxetine 20–40 mg/d | HDRS: Magnesium = placebo | [154] |
Abbreviations: BDI: Beck Depression Inventory; CDRS: Children’s Depression Rating Scale-Revised raw score; CGI: The Clinical Global Impression; DHA: Docosahexaenoic acid; EPA: Eicosapentaenoic acid; HDRS: Hamilton Rating Scale for Depression; MADRS: Montgomery–Åsberg Depression Rating Scale; NAC: N-acetylcysteine; SAMe: S-adenosyl methionine; n.s.: not significant; PHQ-9: Patient Health Questionnaire-9; QIDS-SR: Quick Inventory of Depressive Symptomatology. Placebo refers to treatment without corresponding adjunctive nutrient. Scale values lower in experimental cases than in placebo refer to a better outcome, while equal mean refers to not significance in effects.
4. Conclusions
The response to antidepressants is multifactorial, and the great variability in treatment response has increased the interest in developing personalized treatment strategies. However, the difficulty of managing or controlling lifestyle factors makes it challenging to design such studies, particularly in humans, and many underlying mechanisms still need to be elucidated. Nevertheless, there is mounting evidence that BDNF signaling can integrate diverse environmental and therapeutic cascades and holds promise as a pharmacological jack of all trades in the depression field.
Author Contributions
Conceptualization, O.E. and A.B.; methodology, O.E.; writing—original draft preparation, S.C. and A.B.; writing—review and editing, O.E.; visualization, A.B. and S.C.; supervision, O.E.; project administration O.E. and A.B.; funding acquisition, A.B. and O.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Carl-Zeiss-Foundation (S.C., O.E.: IMPULS #P2019-01-0006). A.B. was supported by the European Union—NextGenerationEU—funds MUR D.M. 737/2021—project BEAUTIFUL, and by the University of Palermo (FFR 2019/2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Nestler, E.J.; Barrot, M.; DiLeone, R.J.; Eisch, A.J.; Gold, S.J.; Monteggia, L.M. Neurobiology of depression. Neuron 2002, 34, 13–25. [Google Scholar] [CrossRef]
- Moncrieff, J.; Moncrieff, J.; Cooper, R.E.; Stockmann, T.; Amendola, S.; Hengartner, M.P.; Horowitz, M.A. The serotonin theory of depression: A systematic umbrella review of the evidence. Mol. Psychiatry 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Bjorkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef]
- Ilchibaeva, T.; Tsybko, A.; Zeug, A.; Müller, F.E.; Guseva, D.; Bischoff, S.; Ponimaskin, E.; Naumenko, V. Serotonin Receptor 5-HT2A Regulates TrkB Receptor Function in Heteroreceptor Complexes. Cells 2022, 11, 2384. [Google Scholar] [CrossRef]
- Castrén, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar]
- Kishimoto, T.; Chawla, J.M.; Hagi, K.; Zarate, C.A.; Kane, J.M.; Bauer, M.; Correll, C.U. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: A meta-analysis of efficacy, safety and time trajectories. Psychol. Med. 2016, 46, 1459–1472. [Google Scholar] [CrossRef]
- Jelen, L.A.; Young, A.H.; Stone, J.M. Ketamine: A tale of two enantiomers. J. Psychopharmacol. 2021, 35, 109–123. [Google Scholar] [CrossRef]
- Zanos, P.; Moaddel, R.; Morris, P.J.; Georgiou, P.; Fischell, J.; Elmer, G.I.; Alkondon, M.; Yuan, P.; Pribut, H.J.; Singh, N.S.; et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016, 533, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Soppet, D.; Escandon, E.; Maragos, J.; Middlemas, D.S.; Reid, S.W.; Blair, J.; Burton, L.E.; Stanton, B.R.; Kaplan, D.R.; Hunter, T.; et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell 1991, 65, 895–903. [Google Scholar] [CrossRef]
- Yoshii, A.; Constantine-Paton, M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010, 70, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Esvald, E.E.; Tuvikene, J.; Sirp, A.; Patil, S.; Bramham, C.R.; Timmusk, T. CREB Family Transcription Factors Are Major Mediators of BDNF Transcriptional Autoregulation in Cortical Neurons. J. Neurosci. 2020, 40, 1405–1426. [Google Scholar]
- Duman, R.S.; Monteggia, L.M. A Neurotrophic Model for Stress-Related Mood Disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Saarelainen, T.; Hendolin, P.; Lucas, G.; Koponen, E.; Sairanen, M.; MacDonald, E.; Agerman, K.; Haapasalo, A.; Nawa, H.; Aloyz, R.; et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J. Neurosci. 2003, 23, 349–357. [Google Scholar] [CrossRef]
- Rantamäki, T.; Hendolin, P.; Kankaanpää, A.; Mijatovic, J.; Piepponen, P.; Domenici, E.; Chao, M.V.; Männistö, P.T.; Castrén, E. Pharmacologically diverse antidepressants rapidly activate brain-derived neurotrophic factor receptor TrkB and induce phospholipase-Cgamma signaling pathways in mouse brain. Neuropsychopharmacology 2007, 32, 2152–2162. [Google Scholar]
- Rantamäki, T.; Vesa, L.; Antila, H.; Di Lieto, A.; Tammela, P.; Schmitt, A.; Lesch, K.P.; Rios, M.; Castrén, E. Antidepressant drugs transactivate TrkB neurotrophin receptors in the adult rodent brain independently of BDNF and monoamine transporter blockade. PLoS ONE 2011, 6, e20567. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Maya Vetencourt, J.F.; Sale, A.; Viegi, A.; Baroncelli, L.; De Pasquale, R.; O’Leary, O.F.; Castrén, E.; Maffei, L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 2008, 320, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luikart, B.W.; Birnbaum, S.; Chen, J.; Kwon, C.H.; Kernie, S.G.; Bassel-Duby, R.; Parada, L.F. TrkB Regulates Hippocampal Neurogenesis and Governs Sensitivity to Antidepressive Treatment. Neuron 2008, 59, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zang, T.; Birnbaum, S.G.; Wang, Z.; Johnson, J.E.; Zhang, C.L.; Parada, L.F. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat. Commun. 2017, 8, 1668. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, Y.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 2003, 60, 804–815. [Google Scholar] [CrossRef]
- Pandey, G.N.; Ren, X.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Dwivedi, Y. Brain-derived neurotrophic factor and tyrosine kinase B receptor signalling in post-mortem brain of teenage suicide victims. Int. J. Neuropsychopharmacol. 2008, 11, 1047–1061. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Zhang, H.; Mondal, A.C.; Roberts, R.C.; Conley, R.R.; Pandey, G.N. Neurotrophin receptor activation and expression in human postmortem brain: Effect of suicide. Biol. Psychiatry 2009, 65, 319–328. [Google Scholar] [CrossRef]
- Banerjee, R.; Ghosh, A.K.; Ghosh, B.; Bhattacharyya, S.; Mondal, A.C. Decreased mRNA and Protein Expression of BDNF, NGF, and their Receptors in the Hippocampus from Suicide: An Analysis in Human Postmortem Brain. Clin. Med. Insights Pathol. 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Jang, S.W.; Liu, X.; Chan, C.B.; Weinshenker, D.; Hall, R.A.; Xiao, G.; Ye, K. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem. Biol. 2009, 16, 644–656. [Google Scholar] [CrossRef]
- Casarotto, P.C.; Girych, M.; Fred, S.M.; Kovaleva, V.; Moliner, R.; Enkavi, G.; Biojone, C.; Cannarozzo, C.; Sahu, M.P.; Kaurinkoski, K.; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299–1313.e19. [Google Scholar] [CrossRef]
- Faron-Górecka, A.; Kuśmider, M.; Gruca, P.; Pabian, P.; Korlatowicz, A.; Solich, J.; Kolasa, M.; Dziedzicka-Wasylewska, M. Pro-cognitive effect of acute imipramine administration correlates with direct interaction of BDNF with its receptor, Trkβ. Brain Res. 2022, 1789, 147948. [Google Scholar] [CrossRef]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999, 22, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, L.A.; Altar, C.A.; Blue, M.E.; Kaplan, D.R.; Tessarollo, L.; Lyons, W.E. BDNF promotes the regenerative sprouting, but not survival, of injured serotonergic axons in the adult rat brain. J. Neurosci. 2000, 20, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Nestler, E.J.; Duman, R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996, 16, 2365–2372. [Google Scholar] [CrossRef]
- Monteggia, L.M.; Barrot, M.; Powell, C.M.; Berton, O.; Galanis, V.; Gemelli, T.; Meuth, S.; Nagy, A.; Greene, R.W.; Nestler, E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA 2004, 101, 10827–10832. [Google Scholar] [CrossRef]
- Siuciak, J.A.; Lewis, D.R.; Wiegand, S.J.; Lindsay, R.M. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF). Pharmacol. Biochem. Behav. 1997, 56, 131–137. [Google Scholar] [CrossRef]
- Shirayama, Y.; Chen, A.C.H.; Nakagawa, S.; Russell, D.S.; Duman, R.S. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002, 22, 3251–3261. [Google Scholar] [CrossRef]
- Adachi, M.; Barrot, M.; Autry, A.E.; Theobald, D.; Monteggia, L.M. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol. Psychiatry 2008, 63, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Aloyz, R.; Fawcett, J.; Kaplan, D.; Murphy, R.; Miller, F. Activity-dependent activation of TrkB neurotrophin receptors in the adult CNS. Learn. Mem. 1999, 6, 216–231. [Google Scholar] [CrossRef] [PubMed]
- Balkowiec, A.; Katz, D.M. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J. Neurosci. 2000, 20, 7417–7423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jurič, D.M.; Lončar, D.; Čarman-Kržan, M. Noradrenergic stimulation of BDNF synthesis in astrocytes: Mediation via alpha1- and beta1/beta2-adrenergic receptors. Neurochem. Int. 2008, 52, 297–306. [Google Scholar] [CrossRef]
- Galter, D.; Unsicker, K. Sequential activation of the 5-HT1(A) serotonin receptor and TrkB induces the serotonergic neuronal phenotype. Mol. Cell. Neurosci. 2000, 15, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.H.; Heng, B.C.; Lim, L.W. New insights on brain-derived neurotrophic factor epigenetics: From depression to memory extinction. Ann. N. Y. Acad. Sci. 2021, 1484, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Kaneda, K. The duration of the antidepressant-like effects of a single infusion of brain-derived neurotrophic factor into the medial prefrontal cortex in mice. Behav. Brain Res. 2020, 394, 112844. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.W.; Labonté, B.; Engmann, O.; Calipari, E.S.; Juarez, B.; Lorsch, Z.; Walsh, J.J.; Friedman, A.K.; Yorgason, J.T.; Han, M.H.; et al. Essential Role of Mesolimbic Brain-Derived Neurotrophic Factor in Chronic Social Stress-Induced Depressive Behaviors. Biol. Psychiatry 2015, 80, 469–478. [Google Scholar] [CrossRef]
- Koo, J.W.; Chaudhury, D.; Han, M.H.; Nestler, E.J. Role of Mesolimbic Brain-Derived Neurotrophic Factor in Depression. Biol. Psychiatry 2019, 86, 738–748. [Google Scholar] [CrossRef]
- Molteni, R.; Calabrese, F.; Bedogni, F.; Tongiorgi, E.; Fumagalli, F.; Racagni, G.; Riva, M.A. Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. Int. J. Neuropsychopharmacol. 2006, 9, 307–317. [Google Scholar] [CrossRef]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef]
- Eisch, A.J.; Bolaños, C.A.; de Wit, J.; Simonak, R.D.; Pudiak, C.M.; Barrot, M.; Verhaagen, J.; Nestler, E.J. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: A role in depression. Biol. Psychiatry 2003, 54, 994–1005. [Google Scholar] [CrossRef]
- Furuse, K.; Ukai, W.; Hashimoto, E.; Hashiguchi, H.; Kigawa, Y.; Ishii, T.; Tayama, M.; Deriha, K.; Shiraishi, M.; Kawanishi, C. Antidepressant activities of escitalopram and blonanserin on prenatal and adolescent combined stress-induced depression model: Possible role of neurotrophic mechanism change in serum and nucleus accumbens. J. Affect. Disord. 2019, 247, 97–104. [Google Scholar] [CrossRef]
- Brancato, A.; Castelli, V.; Cavallaro, A.; Lavanco, G.; Plescia, F.; Cannizzaro, C. Pre-conceptional and Peri-Gestational Maternal Binge Alcohol Drinking Produces Inheritance of Mood Disturbances and Alcohol Vulnerability in the Adolescent Offspring. Front. Psychiatry 2018, 9, 150. [Google Scholar] [CrossRef]
- Leßmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Barreda Tomás, F.J.; Turko, P.; Heilmann, H.; Trimbuch, T.; Yanagawa, Y.; Vida, I.; Münster-Wandowski, A. BDNF Expression in Cortical GABAergic Interneurons. Int. J. Mol. Sci. 2020, 21, 1567. [Google Scholar] [CrossRef] [PubMed]
- Rafa-Zabłocka, K.; Kreiner, G.; Baginska, M.; Nalepa, I. Selective Depletion of CREB in Serotonergic Neurons Affects the Upregulation of Brain-Derived Neurotrophic Factor Evoked by Chronic Fluoxetine Treatment. Front. Neurosci. 2018, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- Leschik, J.; Gentile, A.; Cicek, C.; Péron, S.; Tevosian, M.; Beer, A.; Radyushkin, K.; Bludau, A.; Ebner, K.; Neumann, I.; et al. Brain-derived neurotrophic factor expression in serotonergic neurons improves stress resilience and promotes adult hippocampal neurogenesis. Prog. Neurobiol. 2022, 217, 102333. [Google Scholar] [CrossRef] [PubMed]
- Hisaoka-Nakashima, K.; Kajitani, N.; Kaneko, M.; Shigetou, T.; Kasai, M.; Matsumoto, C.; Yokoe, T.; Azuma, H.; Takebayashi, M.; Morioka, N.; et al. Amitriptyline induces brain-derived neurotrophic factor (BDNF) mRNA expression through ERK-dependent modulation of multiple BDNF mRNA variants in primary cultured rat cortical astrocytes and microglia. Brain Res. 2016, 1634, 57–67. [Google Scholar] [CrossRef]
- Musazzi, L.; Rimland, J.M.; Ieraci, A.; Racagni, G.; Domenici, E.; Popoli, M. Pharmacological characterization of BDNF promoters I, II and IV reveals that serotonin and norepinephrine input is sufficient for transcription activation. Int. J. Neuropsychopharmacol. 2014, 17, 779–791. [Google Scholar] [CrossRef]
- Tsankova, N.M.; Berton, O.; Renthal, W.; Kumar, A.; Neve, R.L.; Nestler, E.J. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006, 9, 519–525. [Google Scholar] [CrossRef]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, T.; Li, G.; Xu, G.; Cheng, J.; Gao, S. The role of BDNF exon I region methylation in the treatment of depression with sertraline and its clinical diagnostic value. J. Clin. Lab. Anal. 2021, 35, e23993. [Google Scholar] [CrossRef]
- Li, L.; Wang, T.; Chen, S.; Yue, Y.; Xu, Z.; Yuan, Y. DNA methylations of brain-derived neurotrophic factor exon VI are associated with major depressive disorder and antidepressant-induced remission in females. J. Affect. Disord. 2021, 295, 101–107. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, C.; Lv, Q.; Bao, C.; Sun, H.; Ma, G.; Fang, Y.; Yi, Z.; Cai, W. Association of DNA methylation in BDNF with escitalopram treatment response in depressed Chinese Han patients. Eur. J. Clin. Pharmacol. 2018, 74, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Tadić, A.; Müller-Engling, L.; Schlicht, K.F.; Kotsiari, A.; Dreimüller, N.; Kleimann, A.; Bleich, S.; Lieb, K.; Frieling, H. Methylation of the promoter of brain-derived neurotrophic factor exon IV and antidepressant response in major depression. Mol. Psychiatry 2014, 19, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.; Wang, X.; He, Y.; Xia, Y.; Sweeney, J.A.; Kopp, R.F.; Liu, C.; Chen, C. Drug Response-Related DNA Methylation Changes in Schizophrenia, Bipolar Disorder, and Major Depressive Disorder. Front. Neurosci. 2021, 15, 674273. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Franz, H.; Vezzali, R.; Bovio, P.; Heidrich, S.; Dehghanian, F.; Lagunas, N.; Belzung, C.; Krieglstein, K.; Vogel, T. Neuronal Activity, TGFβ-Signaling and Unpredictable Chronic Stress Modulate Transcription of Gadd45 Family Members and DNA Methylation in the Hippocampus. Cereb. Cortex 2017, 27, 4166–4181. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.J.; Biojone, C.; Terceti, M.S.; Guimarães, F.S.; Gomes, M.V.; Joca, S.R. Antidepressant-like effect induced by systemic and intra-hippocampal administration of DNA methylation inhibitors. Br. J. Pharmacol. 2011, 164, 1711–1721. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Pei, L.; Li, Y.N.; Zheng, H.; Yang, S.; Wan, Y.; Mao, L.; Xia, Y.P.; He, Q.W.; Li, M.; et al. Alleviative effects of fluoxetine on depressive-like behaviors by epigenetic regulation of BDNF gene transcription in mouse model of post-stroke depression. Sci. Rep. 2017, 7, 14926. [Google Scholar] [CrossRef]
- Seo, M.K.; Ly, N.N.; Lee, C.H.; Cho, H.Y.; Choi, C.M.; Nhu, L.H.; Lee, J.G.; Lee, B.J.; Kim, G.M.; Yoon, B.J.; et al. Early life stress increases stress vulnerability through BDNF gene epigenetic changes in the rat hippocampus. Neuropharmacology 2016, 105, 388–397. [Google Scholar] [CrossRef]
- Hutchinson, A.N.; Deng, J.V.; Cohen, S.; West, A.E. Phosphorylation of MeCP2 at Ser421 contributes to chronic antidepressant action. J. Neurosci. 2012, 32, 14355–14363. [Google Scholar] [CrossRef][Green Version]
- Park, S.W.; Seo, M.K.; Lee, J.G.; Hien, L.T.; Kim, Y.H. Effects of maternal separation and antidepressant drug on epigenetic regulation of the brain-derived neurotrophic factor exon I promoter in the adult rat hippocampus. Psychiatry Clin. Neurosci. 2018, 72, 255–265. [Google Scholar] [CrossRef]
- Homan, P.; Grob, S.; Milos, G.; Schnyder, U.; Eckert, A.; Lang, U.; Hasler, G. The role of BDNF, leptin, and catecholamines in reward learning in bulimia nervosa. Int. J. Neuropsychopharmacol. 2014, 18, 1–8. [Google Scholar] [CrossRef][Green Version]
- Hoepner, C.T.; McIntyre, R.S.; Papakostas, G.I. Impact of supplementation and nutritional interventions on pathogenic processes of mood disorders: A review of the evidence. Nutrients 2021, 13, 767. [Google Scholar] [CrossRef]
- Manosso, L.M.; Moretti, M.; Rodrigues, A.L.S. Nutritional strategies for dealing with depression. Food Funct. 2013, 4, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive nutraceuticals for depression: A systematic review and meta-analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Aly, J.; Engmann, O. The Way to a Human’s Brain Goes Through Their Stomach: Dietary Factors in Major Depressive Disorder. Front. Neurosci. 2020, 14, 582–853. [Google Scholar] [CrossRef] [PubMed]
- Vines, A.; Delattre, A.M.; Lima, M.M.; Rodrigues, L.S.; Suchecki, D.; Machado, R.B.; Tufik, S.; Pereira, S.I.; Zanata, S.M.; Ferraz, A.C. The role of 5-HT₁A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology 2012, 62, 184–191. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J. Neurotrauma 2007, 24, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Moon, H.J.; Kim, S.H. N-3 polyunsaturated fatty acid consumption produces neurobiological effects associated with prevention of depression in rats after the forced swimming test. J. Nutr. Biochem. 2012, 23, 924–928. [Google Scholar] [CrossRef]
- Paduchová, Z.; Katrenčíková, B.; Vaváková, M.; Laubertová, L.; Nagyová, Z.; Garaiova, I.; Ďuračková, Z.; Trebatická, J. The Effect of Omega-3 Fatty Acids on Thromboxane, Brain-Derived Neurotrophic Factor, Homocysteine, and Vitamin D in Depressive Children and Adolescents: Randomized Controlled Trial. Nutrients 2021, 13, 1095. [Google Scholar] [CrossRef]
- Jazayeri, S.; Tehrani-Doost, M.; Keshavarz, S.A.; Hosseini, M.; Djazayery, A.; Amini, H.; Jalali, M.; Peet, M. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Aust. N. Z. J. Psychiatry 2008, 42, 192–198. [Google Scholar] [CrossRef]
- Carney, R.M.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Steinmeyer, B.C.; Harris, W.S. Omega-3 augmentation of sertraline in treatment of depression in patients with coronary heart disease: A randomized controlled trial. JAMA 2009, 302, 1651–1657. [Google Scholar] [CrossRef]
- Jahangard, L.; Sadeghi, A.; Ahmadpanah, M.; Holsboer-Trachsler, E.; Sadeghi Bahmani, D.; Haghighi, M.; Brand, S. Influence of adjuvant omega-3-polyunsaturated fatty acids on depression, sleep, and emotion regulation among outpatients with major depressive disorders—Results from a double-blind, randomized and placebo-controlled clinical trial. J. Psychiatr. Res. 2018, 107, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Peet, M.; Horrobin, D.F. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry 2002, 59, 913–919. [Google Scholar] [CrossRef]
- Su, K.P.; Huang, S.Y.; Chiu, C.C.; Shen, W.W. Omega-3 fatty acids in major depressive disorder: A preliminary double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 2003, 13, 267–271. [Google Scholar] [CrossRef]
- Nemets, B.; Stahl, Z.; Belmaker, R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry 2002, 159, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Silvers, K.M.; Woolley, C.C.; Hamilton, F.C.; Watts, P.M.; Watson, R.A. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins. Leukot. Essent. Fat. Acids 2005, 72, 211–218. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Karamati, M.; Shariati-Bafghi, S.E. Eicosapentaenoic acid versus docosahexaenoic acid in mild-to-moderate depression: A randomized, double-blind, placebo-controlled trial. Eur. Neuropsychopharmacol. 2013, 23, 636–644. [Google Scholar] [CrossRef]
- Smith, D.J.; Sarris, J.; Dowling, N.; O’Connor, M.; Ng, C.H. Adjunctive low-dose docosahexaenoic acid (DHA) for major depression: An open-label pilot trial. Nutr. Neurosci. 2018, 21, 224–228. [Google Scholar] [CrossRef]
- Gertsik, L.; Poland, R.E.; Bresee, C.; Rapaport, M.H. Omega-3 fatty acid augmentation of citalopram treatment for patients with major depressive disorder. J. Clin. Psychopharmacol. 2012, 32, 61–64. [Google Scholar] [CrossRef]
- Mocking, R.J.; Verburg, H.F.; Westerink, A.M.; Assies, J.; Vaz, F.M.; Koeter, M.W.; Ruhé, H.G.; Schene, A.H. Fatty acid metabolism and its longitudinal relationship with the hypothalamic-pituitary-adrenal axis in major depression: Associations with prospective antidepressant response. Psychoneuroendocrinology 2015, 59, 1–13. [Google Scholar] [CrossRef]
- Ghazizadeh-Hashemi, M.; Ghajar, A.; Shalbafan, M.R.; Ghazizadeh-Hashemi, F.; Afarideh, M.; Malekpour, F.; Ghaleiha, A.; Ardebili, M.E.; Akhondzadeh, S. Palmitoylethanolamide as adjunctive therapy in major depressive disorder: A double-blind, randomized and placebo-controlled trial. J. Affect. Disord. 2018, 232, 127–133. [Google Scholar] [CrossRef]
- Able, J.A.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P.; McNamara, R.K. Omega-3 fatty acid deficient male rats exhibit abnormal behavioral activation in the forced swim test following chronic fluoxetine treatment: Association with altered 5-HT1A and alpha2A adrenergic receptor expression. J. Psychiatr. Res. 2014, 50, 42–50. [Google Scholar] [CrossRef]
- McNamara, R.K.; Able, J.A.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P.; Lipton, J.W. Omega-3 fatty acid deficiency does not alter the effects of chronic fluoxetine treatment on central serotonin turnover or behavior in the forced swim test in female rats. Pharmacol. Biochem. Behav. 2013, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, G.I.; Ionescu, D.F. Towards new mechanisms: An update on therapeutics for treatment-resistant major depressive disorder. Mol. Psychiatry 2015, 20, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Scapagnini, G.; Colombrita, C.; Mazzola, C.; Alkon, D.L.; Drago, F. Behavioral effects of dietary cholesterol in rats tested in experimental models of mild stress and cognition tasks. Eur. Neuropsychopharmacol. 2008, 18, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Canale, R.E.; Marshall, K.E.; Kabir, M.M.; Bloomer, R.J. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: A summary of available findings. Nutr. J. 2011, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Elesawy, B.H.; Raafat, B.M.; Al Muqbali, A.; Abbas, A.M.; Sakr, H.F. The Impact of Intermittent Fasting on Brain-Derived Neurotrophic Factor, Neurotrophin 3, and Rat Behavior in a Rat Model of Type 2 Diabetes Mellitus. Brain Sci. 2021, 11, 242. [Google Scholar] [CrossRef]
- Stapel, B.; Fraccarollo, D.; Westhoff-Bleck, M.; Bauersachs, J.; Lichtinghagen, R.; Jahn, K.; Burkert, A.; Buchholz, V.; Bleich, S.; Frieling, H.; et al. Impact of fasting on stress systems and depressive symptoms in patients with major depressive disorder: A cross-sectional study. Sci. Rep. 2022, 12, 7642. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Lv, J.; Tang, F.; Liu, L.; Sun, Z.; Wang, L.; Siwela, S.P.; Wang, Y.; Song, Y.; et al. Additive antidepressant-like effects of fasting with imipramine via modulation of 5-HT2 receptors in the mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 48, 199–206. [Google Scholar] [CrossRef]
- Liu, L.; Li, B.; Zhou, Y.; Wang, L.; Tang, F.; Shao, D.; Jiang, X.; Zhao, H.; Cui, R.; Li, Y. Antidepressant-like effect of Fuzi total alkaloid on ovariectomized mice. J. Pharmacol. Sci. 2012, 120, 280–287. [Google Scholar] [CrossRef]
- Sial, O.K.; Gnecco, T.; Cardona-Acosta, A.M.; Vieregg, E.; Cardoso, E.A.; Parise, L.F.; Bolaños-Guzmán, C.A. Exposure to Vicarious Social Defeat Stress and Western-Style Diets During Adolescence Leads to Physiological Dysregulation, Decreases in Reward Sensitivity, and Reduced Antidepressant Efficacy in Adulthood. Front. Neurosci. 2021, 15, 701–919. [Google Scholar] [CrossRef]
- Ågren, H.; Niklasson, F. Creatinine and creatine in CSF: Indices of brain energy metabolism in depression. J. Neural Transm. 1988, 74, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.P.; Pazini, F.L.; Lieberknecht, V.; Rodrigues, A.L.S. Subchronic administration of creatine produces antidepressant-like effect by modulating hippocampal signaling pathway mediated by FNDC5/BDNF/Akt in mice. J. Psychiatr. Res. 2018, 104, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, I.K.; Yoon, S.; Kim, T.S.; Hwang, J.; Kim, J.E.; Won, W.; Bae, S.; Renshaw, P.F. A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am. J. Psychiatry 2012, 169, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.G.; Sung, Y.H.; Hellem, T.L.; Fiedler, K.K.; Shi, X.; Jeong, E.K.; Renshaw, P.F. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011, 135, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; D’Anci, K.E.; Kanarek, R.B.; Renshaw, P.F. Sex-specific antidepressant effects of dietary creatine with and without sub-acute fluoxetine in rats. Pharmacol. Biochem. Behav. 2012, 101, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Cowen, P.J. Psychopharmacology of 5-HT(1A) receptors. Nucl. Med. Biol. 2000, 27, 437–439. [Google Scholar] [CrossRef]
- Cowen, P.J.; Browning, M. What has serotonin to do with depression? World Psychiatry 2015, 14, 158–160. [Google Scholar] [CrossRef]
- Weber, L.J.; Horita, A. A study of 5-hydroxytryptamine formation from L-tryptophan in the brain and other tissues. Biochem. Pharmacol. 1965, 14, 1141–1149. [Google Scholar] [CrossRef]
- Van Der Stelt, H.M.; Broersen, L.M.; Olivier, B.; Westenberg, H.G.M. Effects of dietary tryptophan variations on extracellular serotonin in the dorsal hippocampus of rats. Psychopharmacology 2004, 172, 137–144. [Google Scholar] [CrossRef]
- Levitan, R.; Shen, J.H.; Jindal, R.; Driver, H.S.; Kennedy, S.H.; Shapiro, C.M. Preliminary randomized double-blind placebo-controlled trial of tryptophan combined with fluoxetine to treat major depressive disorder: Antidepressant and hypnotic effects. J. Psychiatry Neurosci. 2000, 25, 337–346. [Google Scholar]
- Wålinder, J.; Skott, A.; Carlsson, A.; Nagy, A.; Roos, B.E. Potentiation of the antidepressant action of clomipramine by tryptophan. Arch. Gen. Psychiatry 1976, 33, 1384–1389. [Google Scholar] [CrossRef]
- Glassman, A.H.; Platman, S.R. Potentiation of a monoamine oxidase inhibitor by tryptophan. J. Psychiatr. Res. 1969, 7, 83–88. [Google Scholar] [CrossRef]
- Arnold, O.; Saletu, B.; Anderer, P.; Assandri, A.; di Padova, C.; Corrado, M. Saletu-Zyhlarz, G.M. Double-blind, placebo-controlled pharmacodynamic studies with a nutraceutical and a pharmaceutical dose of ademetionine (SAMe) in elderly subjects, utilizing EEG mapping and psychometry. Eur. Neuropsychopharmacol. 2005, 15, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ielmini, M.; Caselli, I.; Ceccon, F.; Diurni, M.; Poloni, N.; Callegari, C. Selective Serotonin Reuptake Inhibitors and Nutraceutical Combination in Major Depression Disorder: A Case-Control Study. Psychopharmacol. Bull. 2021, 51, 31–39. [Google Scholar]
- De Berardis, D.; Orsolini, L.; Serroni, N.; Girinelli, G.; Iasevoli, F.; Tomasetti, C.; de Bartolomeis, A.; Mazza, M.; Valchera, A.; Fornaro, M.; et al. A comprehensive review on the efficacy of S-Adenosyl-L-methionine in Major Depressive Disorder. CNS Neurol. Disord. Drug Targets 2016, 15, 35–44. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Fava, M. S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: A double-blind, randomized clinical trial. Am. J. Psychiatry 2010, 167, 942–948. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Stough, C.; Mischoulon, D.; Bousman, C.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. S-Adenosylmethionine (SAMe) monotherapy for depression: An 8-week double-blind, randomised, controlled trial. Psychopharmacology 2020, 237, 209–218. [Google Scholar] [CrossRef]
- Panizzutti, B.; Bortolasci, C.; Hasebe, K.; Kidnapillai, S.; Gray, L.; Walder, K.; Berk, M.; Mohebbi, M.; Dodd, S.; Gama, C.; et al. Mediator effects of parameters of inflammation and neurogenesis from a N-acetyl cysteine clinical-trial for bipolar depression. Acta Neuropsychiatr. 2018, 30, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.C.; Chan, M.H.; Lee, M.Y.; Chen, Y.C.; Chen, H.H. N,N-dimethylglycine differentially modulates psychotomimetic and antidepressant-like effects of ketamine in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 71, 7–13. [Google Scholar] [CrossRef]
- Lin, J.C.; Lee, M.Y.; Chan, M.H.; Chen, Y.C.; Chen, H.H. Betaine enhances antidepressant-like, but blocks psychotomimetic effects of ketamine in mice. Psychopharmacology 2016, 233, 3223–3235. [Google Scholar] [CrossRef]
- Lazarou, C.; Kapsou, M. The role of folic acid in prevention and treatment of depression: An overview of existing evidence and implications for practice. Complement. Ther. Clin. Pract. 2010, 16, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Carney, S.M.; Goodwin, G.M.; Geddes, J.R. Folate for depressive disorders: Systematic review and meta-analysis of randomized controlled trials. J. Psychopharmacol. 2004, 18, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Bottiglieri, T.; Laundy, M.; Crellin, R.; Toone, B.K.; Carney, M.W.; Reynolds, E.H. Homocysteine, folate, methylation, and monoamine metabolism in depression. J. Neurol. Neurosurg. Psychiatry 2000, 69, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.W.; Trivedi, M.H.; Rush, A.J. Folate and unipolar depression. J. Altern. Complement. Med. 2008, 14, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cong, Y.; Liu, H. Folic acid ameliorates depression-like behaviour in a rat model of chronic unpredictable mild stress. BMC Neurosci. 2020, 21, 1. [Google Scholar] [CrossRef]
- Coppen, A.; Bailey, J. Enhancement of the antidepressant action of fluoxetine by folic acid: A randomised, placebo controlled trial. J. Affect. Disord. 2000, 60, 121–130. [Google Scholar] [CrossRef]
- Resler, G.; Lavie, R.; Campos, J.; Mata, S.; Urbina, M.; García, A.; Apitz, R.; Lima, L. Effect of folic acid combined with fluoxetine in patients with major depression on plasma homocysteine and vitamin B12, and serotonin levels in lymphocytes. Neuroimmunomodulation 2008, 15, 145–152. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Öngür, D.; Iosifescu, D.V.; Mischoulon, D.; Fava, M. Cholesterol in mood and anxiety disorders: Review of the literature and new hypotheses. Eur. Neuropsychopharmacol. 2004, 14, 135–142. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Shelton, R.C.; Zajecka, J.M.; Etemad, B.; Rickels, K.; Clain, A.; Baer, L.; Dalton, E.D.; Sacco, G.R.; Schoenfeld, D.; et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: Results of two randomized, double-blind, parallel-sequential trials. Am. J. Psychiatry 2012, 169, 1267–1274. [Google Scholar] [CrossRef]
- Ginsberg, L.; Oubre, A.; Daoud, Y. L-methylfolate Plus SSRI or SNRI from Treatment Initiation Compared to SSRI or SNRI Monotherapy in a Major Depressive Episode. Innov. Clin. Neurosci. 2011, 8, 19–28. [Google Scholar]
- Bedson, E.; Bell, D.; Carr, D.; Carter, B.; Hughes, D.; Jorgensen, A.; Lewis, H.; Lloyd, K.; McCaddon, A.; Moat, S.; et al. Folate Augmentation of Treatment—Evaluation for Depression (FolATED): Randomised trial and economic evaluation. Health Technol. Assess. 2014, 18, 1–159. [Google Scholar] [CrossRef] [PubMed]
- Syed, E.; Wasay, M.; Awan, S. Vitamin B12 supplementation in treating major depressive disorder: A randomized controlled trial. Open Neurol. J. 2013, 7, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Trautmann, C.; Bock, A.; Urbach, A.; Hübner, A.; Engmann, O. Acute vitamin B12 supplementation evokes antidepressant response and alters Ntrk-2. Neuropharmacology 2020, 171, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Christensen, H.; Aiken, A.; Batterham, P.J.; Walker, J.; Mackinnon, A.J.; Fenech, M.; Hickie, I.B. No clear potentiation of antidepressant medication effects by folic acid + vitamin B12 in a large community sample. J. Affect. Disord. 2011, 130, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.P.; Ford, A.H.; Hirani, V.; Singh, V.; van Bockxmeer, F.M.; McCaul, K.; Flicker, L. B vitamins to enhance treatment response to antidepressants in middle-aged and older adults: Results from the B-VITAGE randomised, double-blind, placebo-controlled trial. Br. J. Psychiatry 2014, 205, 450–457. [Google Scholar] [CrossRef]
- Amr, M.; El-Mogy, A.; Shams, T.; Vieira, K.; Lakhan, S.E. Efficacy of vitamin C as an adjunct to fluoxetine therapy in pediatric major depressive disorder: A randomized, double-blind, placebo-controlled pilot study. Nutr. J. 2013, 12, 31. [Google Scholar] [CrossRef]
- Khoraminya, N.; Tehrani-Doost, M.; Jazayeri, S.; Hosseini, A.; Djazayery, A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust. N. Z. J. Psychiatry 2013, 47, 271–275. [Google Scholar] [CrossRef]
- Sahraian, A.; Ghanizadeh, A.; Kazemeini, F. Vitamin C as an adjuvant for treating major depressive disorder and suicidal behavior, a randomized placebo-controlled clinical trial. Trials 2015, 16, 94. [Google Scholar] [CrossRef]
- Zanetidou, S.; Belvederi Murri, M.; Buffa, A.; Malavolta, N.; Anzivino, F.; Bertakis, K. Vitamin D supplements in geriatric major depression. Int. J. Geriatr. Psychiatry 2011, 26, 1209–1210. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, L. Vitamin D3/vitamin D receptor signaling mitigates symptoms of post-stroke depression in mice by upregulating hippocampal BDNF expression. Neurosci. Res. 2021, 170, 306–313. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.C.; Fierke, C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012, 26, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Mlyniec, K. Interaction between Zinc, GPR39, BDNF and Neuropeptides in Depression. Curr. Neuropharmacol. 2021, 19, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- Młyniec, K.; Nowak, G. Zinc deficiency induces behavioral alterations in the tail suspension test in mice. Effect of antidepressants. Pharmacol. Rep. 2012, 64, 249–255. [Google Scholar] [CrossRef]
- Młyniec, K.; Budziszewska, B.; Reczyński, W.; Sowa-Kućma, M.; Nowak, G. The role of the GPR39 receptor in zinc deficient-animal model of depression. Behav. Brain Res. 2013, 238, 30–35. [Google Scholar] [CrossRef]
- Młyniec, K.; Budziszewska, B.; Reczyński, W.; Doboszewska, U.; Pilc, A.; Nowak, G. Zinc deficiency alters responsiveness to antidepressant drugs in mice. Pharmacol. Rep. 2013, 65, 579–592. [Google Scholar] [CrossRef]
- Doboszewska, U.; Szewczyk, B.; Sowa-Kućma, M.; Młyniec, K.; Rafało, A.; Ostachowicz, B.; Lankosz, M.; Nowak, G. Antidepressant activity of fluoxetine in the zinc deficiency model in rats involves the NMDA receptor complex. Behav. Brain Res. 2015, 287, 323–330. [Google Scholar] [CrossRef]
- Ranjbar, E.; Shams, J.; Sabetkasaei, M.; M-Shirazi, M.; Rashidkhani, B.; Mostafavi, A.; Bornak, E.; Nasrollahzadeh, J. Effects of zinc supplementation on efficacy of antidepressant therapy, inflammatory cytokines, and brain-derived neurotrophic factor in patients with major depression. Nutr. Neurosci. 2014, 17, 65–71. [Google Scholar] [CrossRef]
- Nowak, G.; Siwek, M.; Dudek, D.; Zieba, A.; Pilc, A. Effect of zinc supplementation on antidepressant therapy in unipolar depression: A preliminary placebo-controlled study. Pol. J. Pharmacol. 2003, 55, 1143–1147. [Google Scholar]
- Siwek, M.; Dudek, D.; Schlegel-Zawadzka, M.; Morawska, A.; Piekoszewski, W.; Opoka, W.; Zieba, A.; Pilc, A.; Popik, P.; Nowak, G. Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J. Affect. Disord. 2010, 126, 447–452. [Google Scholar] [CrossRef]
- Siwek, M.; Dudek, D.; Paul, I.A.; Sowa-Kućma, M.; Zieba, A.; Popik, P.; Pilc, A.; Nowak, G. Zinc supplementation augments efficacy of imipramine in treatment resistant patients: A double blind, placebo-controlled study. J. Affect. Disord. 2009, 118, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, R. Magnesium metabolism and its disorders. Clin. Biochem. Rev. 2003, 24, 47–66. [Google Scholar] [PubMed]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional aspects of depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Ryszewska-Pokraśniewicz, B.; Mach, A.; Skalski, M.; Januszko, P.; Wawrzyniak, Z.M.; Poleszak, E.; Nowak, G.; Pilc, A.; Radziwoń-Zaleska, M. Effects of magnesium supplementation on unipolar depression: A placebo-controlled study and review of the importance of dosing and magnesium status in the therapeutic response. Nutrients 2018, 10, 1014. [Google Scholar] [CrossRef]
- Duncan, L.E.; Keller, M.C. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am. J. Psychiatry 2011, 168, 1041–1049. [Google Scholar] [CrossRef]
- Domschke, K.; Tidow, N.; Schwarte, K.; Deckert, J.; Lesch, K.P.; Arolt, V.; Zwanzger, P.; Baune, B.T. Serotonin transporter gene hypomethylation predicts impaired antidepressant treatment response. Int. J. Neuropsychopharmacol. 2014, 17, 1167–1176. [Google Scholar] [CrossRef]
- Kang, H.J.; Kim, J.M.; Stewart, R.; Kim, S.Y.; Bae, K.Y.; Kim, S.W.; Shin, I.S.; Shin, M.G.; Yoon, J.S. Association of SLC6A4 methylation with early adversity, characteristics and outcomes in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 44, 23–28. [Google Scholar] [CrossRef]
- Zimmermann, N.; Zschocke, J.; Perisic, T.; Yu, S.; Holsboer, F.; Rein, T. Antidepressants inhibit DNA methyltransferase 1 through reducing G9a levels. Biochem. J. 2012, 448, 93–102. [Google Scholar] [CrossRef]
- Detich, N.; Bovenzi, V.; Szyf, M. Valproate induces replication-independent active DNA demethylation. J. Biol. Chem. 2003, 278, 27586–27592. [Google Scholar] [CrossRef]
- Klengel, T.; Binder, E.B. Gene×environment interactions in the prediction of response to antidepressant treatment. Int. J. Neuropsychopharmacol. 2013, 16, 701–711. [Google Scholar] [CrossRef]
- Tomita, T.; Yasui-Furukori, N.; Nakagami, T.; Kaneda, A.; Kaneko, S. The association between sunshine duration and paroxetine response time in patients with major depressive disorder. J. Affect. Disord. 2012, 136, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Kessler, R.C.; Walters, E.E.; MacLean, C.; Neale, M.C.; Heath, A.C.; Eaves, L.J. Stressful life events, genetic liability, and Onset of an episode of Major depression in Women. Depress. Sci. Ment. Heal. 2013, 152, 833–842. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Neale, M.C.; Kendler, K.S. Genetic epidemiology of major depression: Review and meta-analysis. Am. J. Psychiatry 2000, 157, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Ising, M.; Lucae, S.; Binder, E.B.; Bettecken, T.; Uhr, M.; Ripke, S.; Kohli, M.A.; Hennings, J.M.; Horstmann, S.; Kloiber, S.; et al. A genomewide association study points to multiple loci that predict antidepressant drug treatment outcome in depression. Arch. Gen. Psychiatry 2009, 66, 966–975. [Google Scholar] [CrossRef]
- Garriock, H.A.; Kraft, J.B.; Shyn, S.I.; Peters, E.J.; Yokoyama, J.S.; Jenkins, G.D.; Reinalda, M.S.; Slager, S.L.; McGrath, P.J.; Hamilton, S.P. A Genomewide Association Study of Citalopram Response in Major Depressive Disorder. Biol. Psychiatry 2010, 67, 133–138. [Google Scholar] [CrossRef]
- Uher, R.; Perroud, N.; Ng, M.Y.; Hauser, J.; Henigsberg, N.; Maier, W.; Mors, O.; Placentino, A.; Rietschel, M.; Souery, D.; et al. Genome-wide pharmacogenetics of antidepressant response in the GENDEP project. Am. J. Psychiatry 2010, 167, 555–564. [Google Scholar] [CrossRef]
- Horstmann, S.; Lucae, S.; Menke, A.; Hennings, J.M.; Ising, M.; Roeske, D.; Müller-Myhsok, B.; Holsboer, F.; Binder, E.B. Polymorphisms in GRIK4, HTR2A, and FKBP5 show interactive effects in predicting remission to antidepressant treatment. Neuropsychopharmacology 2010, 35, 727–740. [Google Scholar] [CrossRef]
- Poggini, S.; Matte Bon, G.; Golia, M.T.; Ciano Albanese, N.; Viglione, A.; Poleggi, A.; Limatola, C.; Maggi, L.; Branchi, I. Selecting antidepressants according to a drug-by-environment interaction: A comparison of fluoxetine and minocycline effects in mice living either in enriched or stressful conditions. Behav. Brain Res. 2021, 408, 113256. [Google Scholar] [CrossRef]
- Alboni, S.; van Dijk, R.M.; Poggini, S.; Milior, G.; Perrotta, M.; Drenth, T.; Brunello, N.; Wolfer, D.P.; Limatola, C.; Amrein, I.; et al. Fluoxetine effects on molecular, cellular and behavioral endophenotypes of depression are driven by the living environment. Mol. Psychiatry 2017, 22, 635. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Yen, J.Y.; Watson, B.O. Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS ONE 2019, 14, e0215554. [Google Scholar] [CrossRef] [PubMed]
- Berton, O.; McClung, C.A.; Dileone, R.J.; Krishnan, V.; Renthal, W.; Russo, S.J.; Graham, D.; Tsankova, N.M.; Bolanos, C.A.; Rios, M.; et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 2006, 311, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 2000, 23, 477–501. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Reus, V.I.; Manfredi, F.; Ingbar, J.; Brizendine, L.; Weingartner, H. Ketoconazole administration in hypercortisolemic depression. Am. J. Psychiatry 1993, 150, 810–812. [Google Scholar] [PubMed]
- Chang, H.S.; Won, E.; Lee, H.Y.; Ham, B.J.; Lee, M.S. Association analysis for corticotropin releasing hormone polymorphisms with the risk of major depressive disorder and the response to antidepressants. Behav. Brain Res. 2015, 292, 116–124. [Google Scholar] [CrossRef]
- Duman, R.S.; Li, N. A neurotrophic hypothesis of depression: Role of synaptogenesis in the actions of NMDA receptor antagonists. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2475–2484. [Google Scholar] [CrossRef]
- Nanni, V.; Uher, R.; Danese, A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: A meta-analysis. Am. J. Psychiatry 2012, 169, 141–151. [Google Scholar] [CrossRef]
- Nemeroff, C.B.; Heim, C.M.; Thase, M.E.; Klein, D.N.; Rush, A.J.; Schatzberg, A.F.; Ninan, P.T.; McCullough, J.P., Jr.; Weiss, P.M.; Dunner, D.L.; et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc. Natl. Acad. Sci. USA 2003, 100, 14293–14296. [Google Scholar] [CrossRef]
- Navailles, S.; Hof, P.R.; Schmauss, C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J. Comp. Neurol. 2008, 509, 372–381. [Google Scholar] [CrossRef]
- Wearick-Silva, L.E.; Orso, R.; Martins, L.A.; Creutzberg, K.C.; Centeno-Silva, A.; Xavier, L.L.; Grassi-Oliveira, R.; Mestriner, R.G. Dual influences of early life stress induced by limited bedding on walking adaptability and Bdnf/TrkB and Drd1/Drd2 gene expression in different mouse brain regions. Behav. Brain Res. 2019, 359, 66–72. [Google Scholar] [CrossRef]
- Zheng, Y.; Fan, W.; Zhang, X.; Dong, E. Gestational stress induces depressive-like and anxiety-like phenotypes through epigenetic regulation of BDNF expression in offspring hippocampus. Epigenetics 2016, 11, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Pallarés, M.E.; Monteleone, M.C.; Pastor, V.; Grillo Balboa, J.; Alzamendi, A.; Brocco, M.A.; Antonelli, M.C. Early-Life Stress Reprograms Stress-Coping Abilities in Male and Female Juvenile Rats. Mol. Neurobiol. 2021, 58, 5837–5856. [Google Scholar] [CrossRef] [PubMed]
- Coley, E.J.L.; Demaestri, C.; Ganguly, P.; Honeycutt, J.A.; Peterzell, S.; Rose, N.; Ahmed, N.; Holschbach, M.; Trivedi, M.; Brenhouse, H.C. Cross-Generational Transmission of Early Life Stress Effects on HPA Regulators and Bdnf Are Mediated by Sex, Lineage, and Upbringing. Front. Behav. Neurosci. 2019, 13, 101. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, E.; Erol, K.; Ulupinar, E. Effects of sertraline on behavioral alterations caused by environmental enrichment and social isolation. Pharmacol. Biochem. Behav. 2012, 101, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Nasca, C.; Barnhill, O.; DeAngelis, P.; Watson, K.; Lin, J.; Beasley, J.; Young, S.P.; Myoraku, A.; Dobbin, J.; Bigio, B.; et al. Multidimensional predictors of antidepressant responses: Integrating mitochondrial, genetic, metabolic and environmental factors with clinical outcomes. Neurobiol. Stress 2021, 15, 100–407. [Google Scholar] [CrossRef]
- Takeuchi, N.; Nonen, S.; Kato, M.; Wakeno, M.; Takekita, Y.; Kinoshita, T.; Kugawa, F. Therapeutic Response to Paroxetine in Major Depressive Disorder Predicted by DNA Methylation. Neuropsychobiology 2018, 75, 81–88. [Google Scholar] [CrossRef]
- Branchi, I.; D’Andrea, I.; Sietzema, J.; Fiore, M.; Di Fausto, V.; Aloe, L.; Alleva, E. Early social enrichment augments adult hippocampal BDNF levels and survival of BRDU-positive cells while increasing anxiety- and ‘depression’-like behavior. J. Neurosci. Res. 2006, 83, 965–973. [Google Scholar] [CrossRef]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the treatment of depression and anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Karpova, N.N.; Rantamäki, T.; Di Lieto, A.; Lindemann, L.; Hoener, M.C.; Castrén, E. Darkness reduces BDNF expression in the visual cortex and induces repressive chromatin remodeling at the BDNF gene in both hippocampus and visual cortex. Cell. Mol. Neurobiol. 2010, 30, 1117–1123. [Google Scholar] [CrossRef]
- Sadighparvar, S.; Darband, S.G.; Yousefi, B.; Kaviani, M.; Ghaderi-Pakdel, F.; Mihanfar, A.; Babaei, G.; Mobaraki, K.; Majidinia, M. Combination of quercetin and exercise training attenuates depression in rats with 1,2-dimethylhydrazine-induced colorectal cancer: Possible involvement of inflammation and BDNF signalling. Exp. Physiol. 2020, 105, 1598–1609. [Google Scholar] [CrossRef]
- Lu, J.; Xu, Y.; Hu, W.; Gao, Y.; Ni, X.; Sheng, H.; Liu, Y. Exercise ameliorates depression-like behavior and increases hippocampal BDNF level in ovariectomized rats. Neurosci. Lett. 2014, 573, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.W.; Jung, S.Y.; Lee, S.W.; Lee, S.J.; Seo, T.B.; Kim, Y.P.; Kim, D.Y. Treadmill exercise ameliorates social isolation-induced depression through neuronal generation in rat pups. J. Exerc. Rehabil. 2017, 13, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Lim, B.-V.; Kim, K.; Seo, J.-H.; Kim, C.-J. Treadmill exercise alleviates stress-induced impairment of social interaction through 5-hydroxytryptamine 1A receptor activation in rats. J. Exerc. Rehabil. 2015, 11, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Voisey, J.; Lawford, B.; Bruenig, D.; Harvey, W.; Morris, C.P.; Young, R.M.; Mehta, D.; PTSD Initiative. Differential BDNF methylation in combat exposed veterans and the association with exercise. Gene 2019, 698, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Lewis, D.A.; Sibille, E. The Role of BDNF in Age-Dependent Changes of Excitatory and Inhibitory Synaptic Markers in the Human Prefrontal Cortex. Neuropsychopharmacology 2016, 41, 3080–3091. [Google Scholar] [CrossRef]
- von Bohlen und Halbach, O.; Krause, S.; Medina, D.; Sciarretta, C.; Minichiello, L.; Unsicker, K. Regional- and age-dependent reduction in trkB receptor expression in the hippocampus is associated with altered spine morphologies. Biol. Psychiatry 2006, 59, 793–800. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 8056370. [Google Scholar] [CrossRef]
- Coppens, C.M.; Siripornmongcolchai, T.; Wibrand, K.; Alme, M.N.; Buwalda, B.; de Boer, S.F.; Koolhaas, J.M.; Bramham, C.R. Social Defeat during Adolescence and Adulthood Differentially Induce BDNF-Regulated Immediate Early Genes. Front. Behav. Neurosci. 2011, 5, 72. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Y.J.; Jiang, H.; Liu, D.X.; Zhang, Q.; Fan, S.J.; Pan, F. Effects of unpredictable chronic stress on behavior and brain-derived neurotrophic factor expression in CA3 subfield and dentate gyrus of the hippocampus in different aged rats. Chin. Med. J. 2009, 122, 1564–1569. [Google Scholar]
- Dong, B.E.; Xue, Y.; Sakata, K. The effect of enriched environment across ages: A study of anhedonia and BDNF gene induction. Genes. Brain. Behav. 2018, 17, e12485. [Google Scholar] [CrossRef] [PubMed]
- Spina, E.; Scordo, M.G. Clinically significant drug interactions with antidepressants in the elderly. Drugs Aging 2002, 19, 299–320. [Google Scholar] [CrossRef] [PubMed]
- Calati, R.; Salvina Signorelli, M.; Balestri, M.; Marsano, A.; De Ronchi, D.; Aguglia, E.; Serretti, A. Antidepressants in elderly: Metaregression of double-blind, randomized clinical trials. J. Affect. Disord. 2013, 147, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bjørnebekk, A.; Mathé, A.A.; Gruber, S.H.M.; Brené, S. Housing conditions modulate escitalopram effects on antidepressive-like behaviour and brain neurochemistry. Int. J. Neuropsychopharmacol. 2008, 11, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Kok, R.M.; Van Baarsen, C.; Nolen, W.A.; Heeren, T.J. Early response as predictor of final remission in elderly depressed patients. Int. J. Geriatr. Psychiatry 2009, 24, 1299–1303. [Google Scholar] [CrossRef]
- De Berardis, D.; Marini, S.; Serroni, N.; Rapini, G.; Iasevoli, F.; Valchera, A.; Signorelli, M.; Aguglia, E.; Perna, G.; Salone, A.; et al. S-Adenosyl-L-Methionine augmentation in patients with stage II treatment-resistant major depressive disorder: An open label, fixed dose, single-blind study. Sci. World J. 2013, 2013, 204649. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: An 8-week double-blind, randomized, controlled trial. Eur. Neuropsychopharmacol. 2018, 28, 1126–1136. [Google Scholar] [CrossRef]
- Nowak, G.; Szewczyk, B.; Wieronska, J.M.; Branski, P.; Palucha, A.; Pilc, A.; Sadlik, K.; Piekoszewski, W. Antidepressant-like effects of acute and chronic treatment with zinc in forced swim test and olfactory bulbectomy model in rats. Brain Res. Bull. 2003, 61, 159–164. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).