Impaired Aversive Memory Formation in GPR37L1KO Mice
Abstract
1. Introduction
2. Results
2.1. Schematic of the Experimental Design
2.2. Effects on Locomotion, Anxiety-like and Depression-like Behavior in Adult and Middle-Aged WT and GPR37L1KO Male and Female Mice
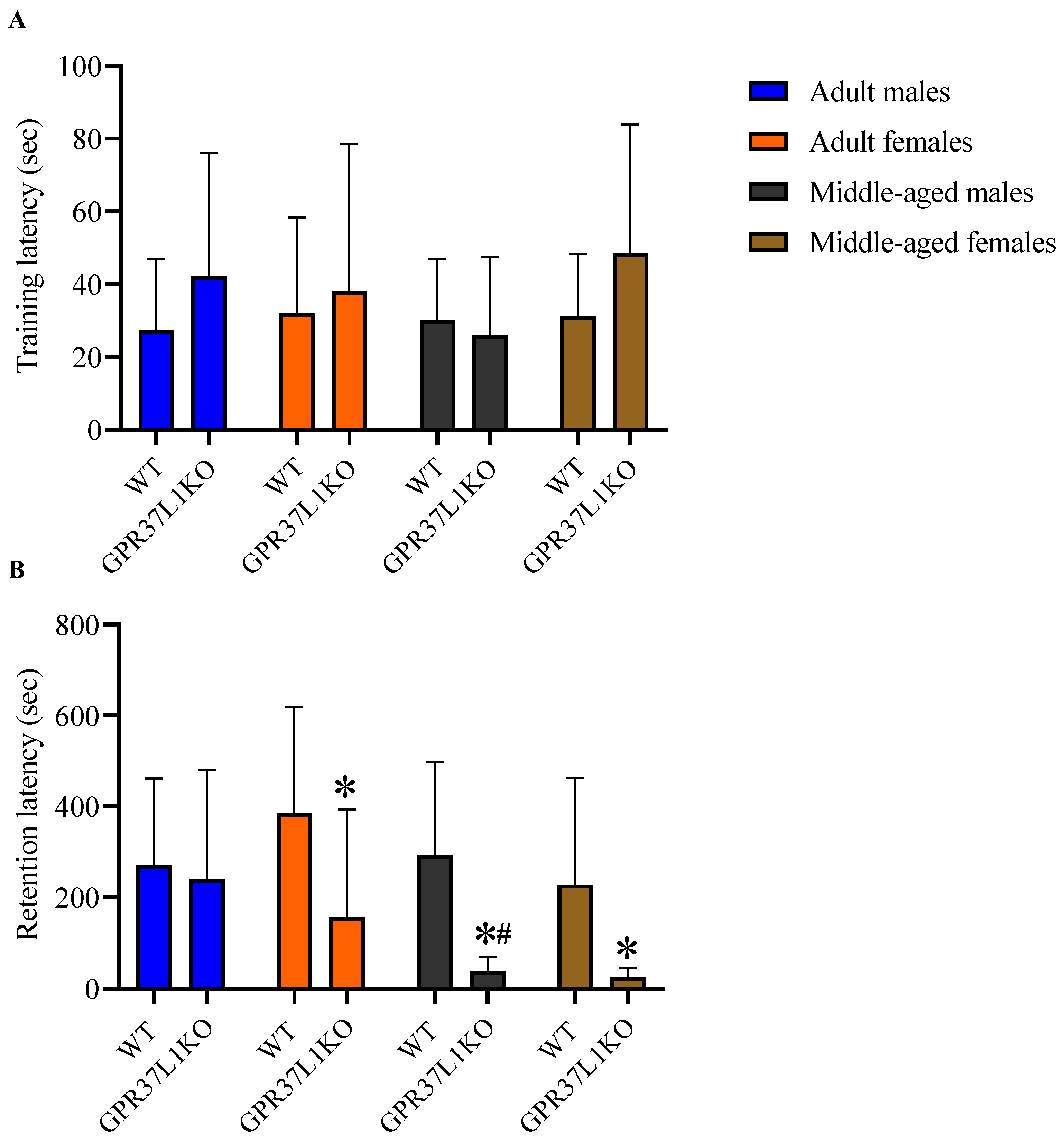
2.3. Effect of GPR37L1 Deletion on Aversive Memory Processing
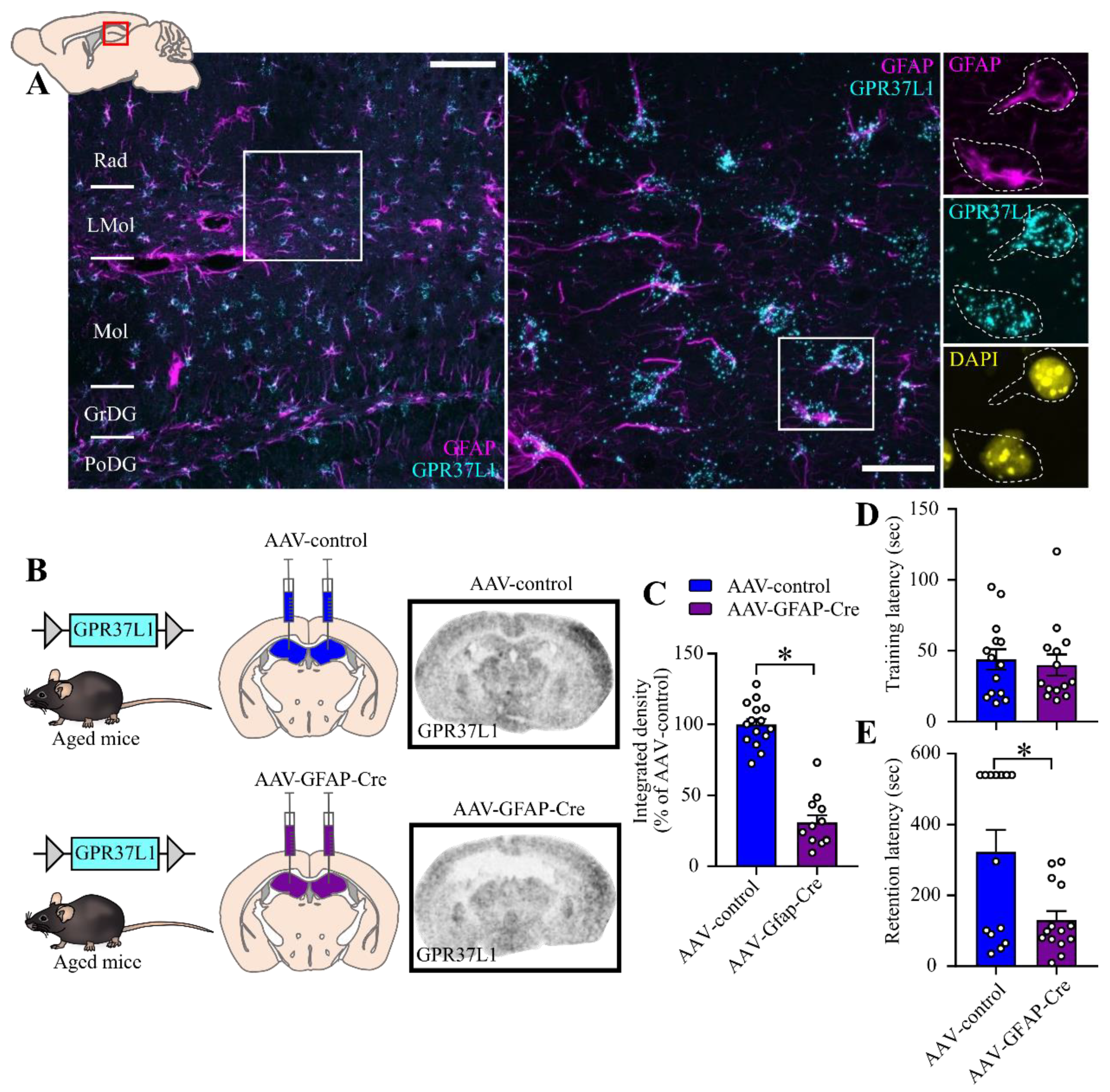
2.4. Effect of Astrocyte-Specific GPR37L1KD in the Hippocampus on Fear Related Behavior
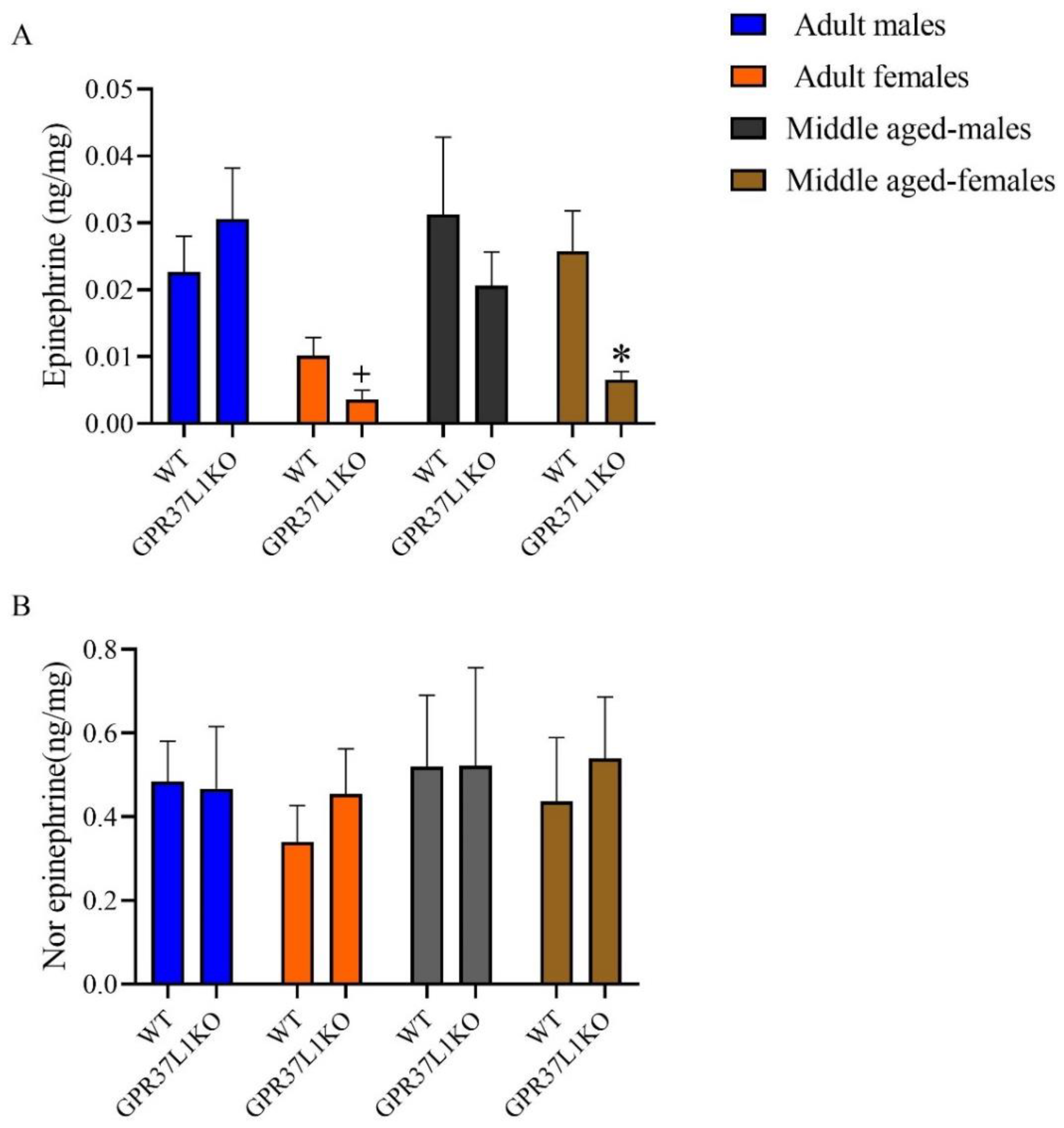
2.5. The Level of Epinephrine and Nor-Epinephrine in the Dorsal Hippocampus in Adult and Middle Aged WT and GPR37L1KO Male and Female Mice after PAT
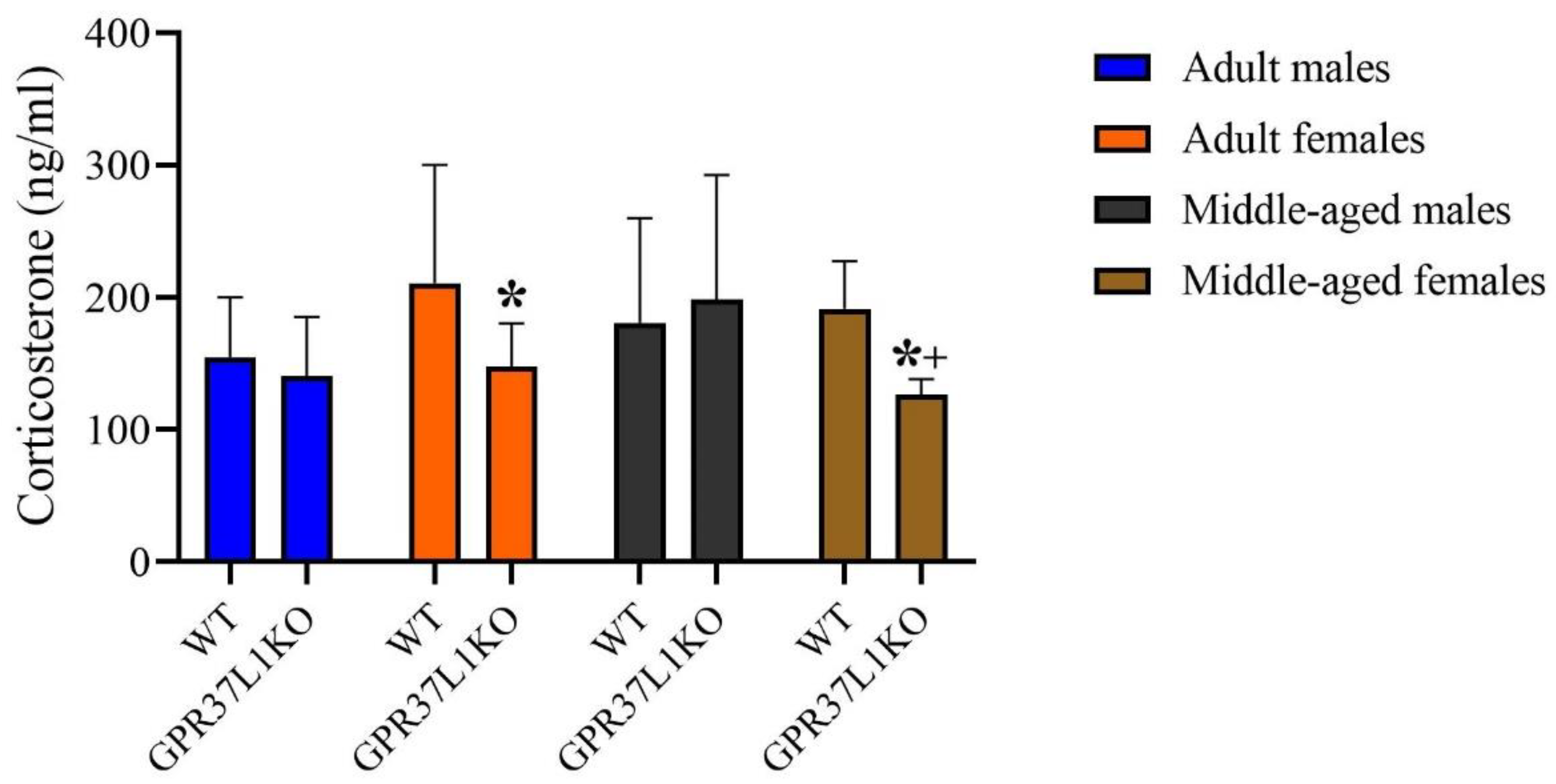
2.6. Effect of GPR37L1KO on the Corticosterone Level after PAT
2.7. Effect of GPR37L1KO on Body Weight
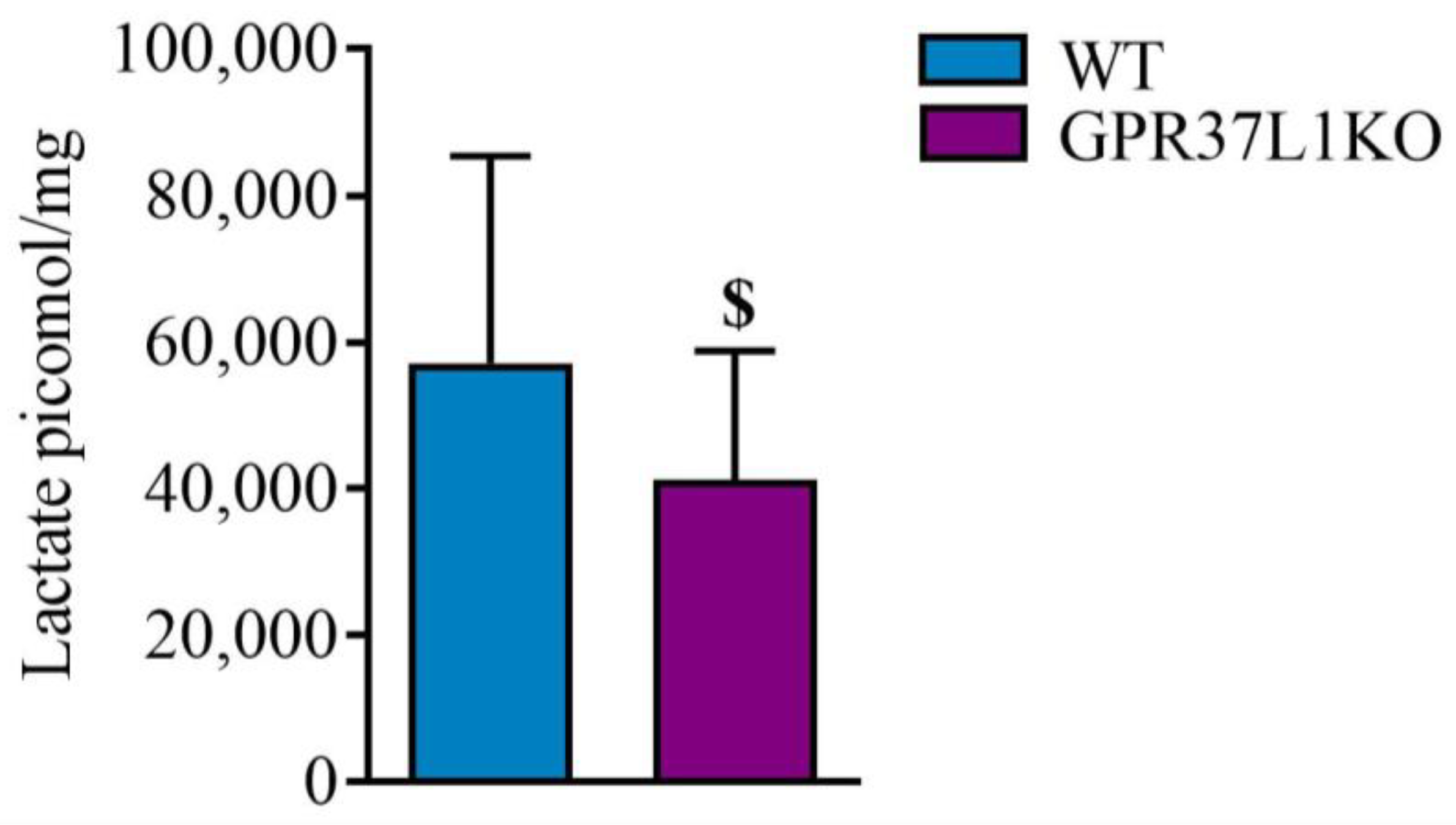
2.8. Effect of GPR37L1KO on the Level of Lactate in the Dorsal Hippocampus
3. Discussion
4. Concluding Remarks
5. Materials and Methods
5.1. Subjects and Housing
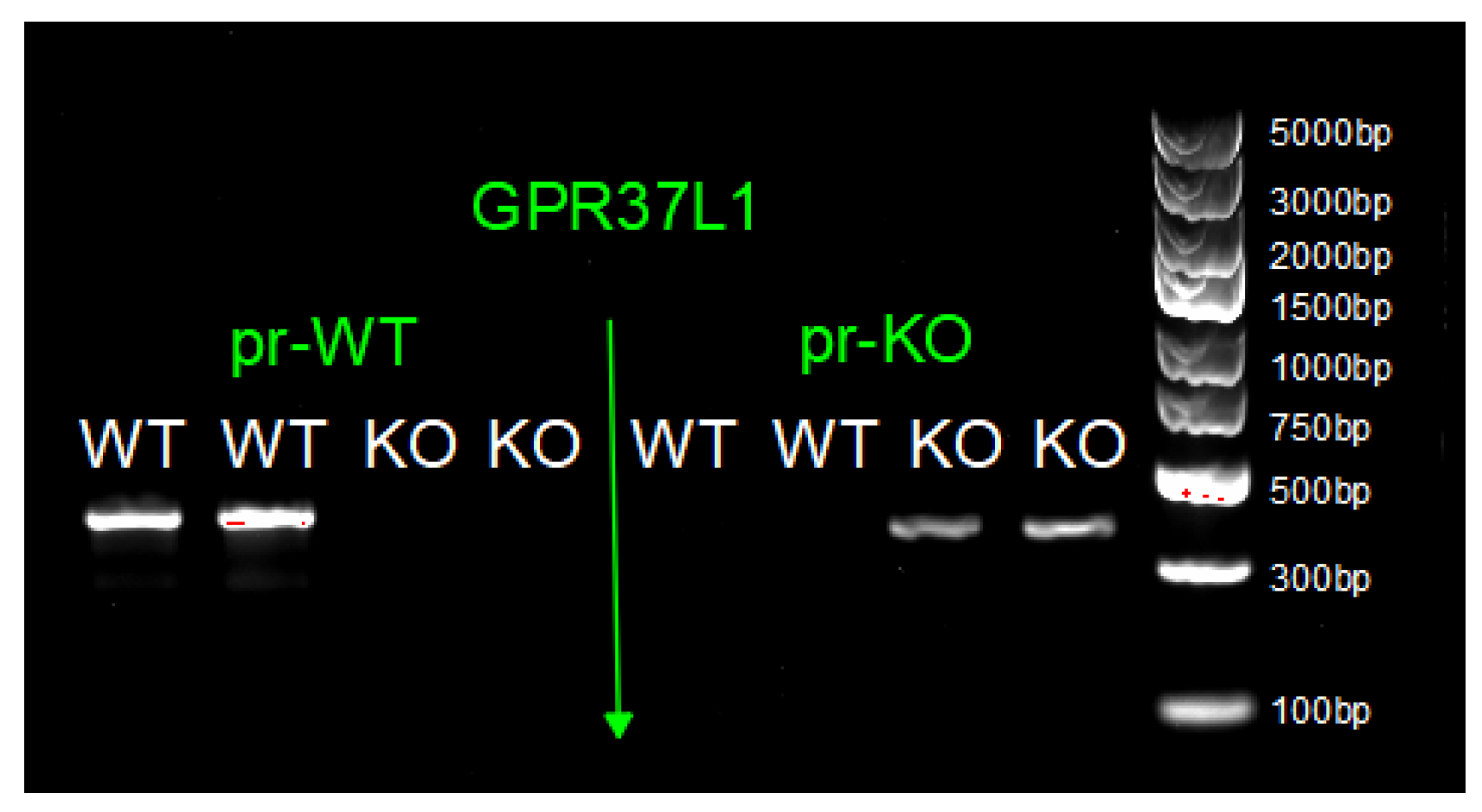
5.2. Breeding and Genotyping of Global GPR37L1 KO Mice
5.3. General Experimental Plan
5.4. Behavioral Tests
5.4.1. Open Field Test
5.4.2. Elevated plus Maze
5.4.3. Light Dark Box
5.4.4. Sucrose Preference Test
5.4.5. Passive Avoidance Task
5.5. Corticosterone Assay
5.6. High Performance Liquid Chromatography (HPLC)
5.7. GPR37L1KD in the Hippocampus
5.8. In Situ Hybridization (ISH) Experiments
5.9. Lactate Measurement in the Hippocampus
5.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jolly, S.; Bazargani, N.; Quiroga, A.C.; Pringle, N.P.; Attwell, D.; Richardson, W.D.; Li, H. G protein-coupled receptor 37-like 1 modulates astrocyte glutamate transporters and neuronal NMDA receptors and is neuroprotective in ischemia. Glia 2018, 66, 47–61. [Google Scholar] [CrossRef]
- Ngo, T.; Wilkins, B.P.; So, S.S.; Keov, P.; Chahal, K.K.; Finch, A.M.; Coleman, J.L.J.; Kufareva, I.; Smith, N.J. Orphan receptor GPR37L1 remains unliganded. Nat. Chem. Biol. 2021, 17, 383–386. [Google Scholar] [CrossRef]
- Yang, H.J.; Vainshtein, A.; Maik-Rachline, G.; Peles, E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat. Commun. 2016, 7, 10884. [Google Scholar] [CrossRef] [PubMed]
- Valdenaire, O.; Giller, T.; Breu, V.; Ardati, A.; Schweizer, A.; Richards, J.G. A new family of orphan G protein-coupled receptors predominantly expressed in the brain. FEBS Lett. 1998, 424, 193–196. [Google Scholar] [CrossRef]
- Ito, J.; Ito, M.; Nambu, H.; Fujikawa, T.; Tanaka, K.; Iwaasa, H.; Tokita, S. Anatomical and histological profiling of orphan G-protein-coupled receptor expression in gastrointestinal tract of C57BL/6J mice. Cell Tissue Res. 2009, 338, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Min, K.D.; Asakura, M.; Liao, Y.; Nakamaru, K.; Okazaki, H.; Takahashi, T.; Fujimoto, K.; Ito, S.; Takahashi, A.; Asanuma, H.; et al. Identification of genes related to heart failure using global gene expression profiling of human failing myocardium. Biochem. Biophys. Res. Commun. 2010, 393, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Homma, T.; Onouchi, S.; Shimizu, Y.; Shiina, T.; Nabeka, H.; Matsuda, S.; Saito, S. Expression of the g protein-coupled receptor (GPR) 37 and GPR37L1 in the mouse digestive system. J. Vet. Med. Sci. 2021, 83, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.C.; Giddens, M.M.; Schaefer, S.A.; Hall, R.A. GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proc. Natl. Acad. Sci. USA 2013, 110, 9529–9534. [Google Scholar] [CrossRef] [PubMed]
- Alavi, M.S.; Karimi, G.; Roohbakhsh, A. The role of orphan G protein-coupled receptors in the pathophysiology of multiple sclerosis: A review. Life Sci. 2019, 224, 33–40. [Google Scholar] [CrossRef]
- Mouat, M.A.; Jackson, K.L.; Coleman, J.L.J.; Paterson, M.R.; Graham, R.M.; Head, G.A.; Smith, N.J. Deletion of Orphan G Protein-Coupled Receptor GPR37L1 in Mice Alters Cardiovascular Homeostasis in a Sex-Specific Manner. Front. Pharmacol. 2021, 11, 600266. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mosienko, V.; Cardoso, B.V.; Prokudina, D.; Huentelman, M.; Teschemacher, A.G.; Kasparov, S. Glio- and neuro-protection by prosaposin is mediated by orphan G-protein coupled receptors GPR37L1 and GPR. Glia 2018, 66, 2414–2426. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Oasa, S.; Ciruela, F.; Terenius, L.; Vukojević, V.; Svenningsson, P. Cytosolic GPR37, but not GPR37L1, multimerization and its reversal by Parkin: A live cell imaging study. FASEB J. 2021, 35, e22055. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Shoji, M. Pael-R Is Accumulated in Lewy Bodies of Parkinson’s Disease. Ann. Neurol. 2004, 55, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Inoue, H.; Kataoka, A.; Hua-qin, W.; Masuda, M.; Ikeda, T.; Tsukita, K.; Soda, M.; Kodama, T.; Fuwa, T.; et al. Pael receptor is involved in dopamine metabolism in the nigrostriatal system. Neurosci. Res. 2007, 59, 413–425. [Google Scholar] [CrossRef]
- Zhang, X.; Mantas, I.; Fridjonsdottir, E.; Andrén, P.E.; Chergui, K.; Svenningsson, P. Deficits in Motor Performance, Neurotransmitters and Synaptic Plasticity in Elderly and Experimental Parkinsonian Mice Lacking GPR. Front. Aging Neursci. 2020, 12, 84. [Google Scholar] [CrossRef]
- Mandillo, S.; Golini, E.; Marazziti, D.; Di Pietro, C.; Matteoni, R.; Tocchini-Valentini, G.P. Mice lacking the Parkinson’s related GPR37/PAEL receptor show non-motor behavioral phenotypes: Age and gender effect. Genes Brain Behav. 2013, 12, 465–477. [Google Scholar] [CrossRef]
- Tomita, H.; Ziegler, M.E.; Kim, H.B.; Evans, S.J.; Choudary, P.V.; Li, J.Z.; Meng, F.; Dai, M.; Myers, R.M.; Neal, C.R.; et al. G protein-linked signaling pathways in bipolar and major depressive disorders. Front. Genet. 2013, 4, 297. [Google Scholar] [CrossRef]
- Aarsland, D.; Påhlhagen, S.; Ballard, C.G.; Ehrt, U.; Svenningsson, P. Depression in Parkinson disease—Epidemiology, mechanisms and management. Nat. Rev. Neurol. 2012, 8, 35–47. [Google Scholar] [CrossRef]
- Marazziti, D.; Di Pietro, C.; Golini, E.; Mandillo, S.; La Sala, G.; Matteoni, R.; Tocchini-Valentini, G.P. Precocious cerebellum development and improved motor functions in mice lacking the astrocyte cilium-, patched 1-associated Gpr37l1 receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 16486–16491. [Google Scholar] [CrossRef]
- Giddens, M.M.; Wong, J.C.; Schroeder, J.P.; Farrow, E.G.; Smith, B.M.; Owino, S.; Soden, S.E.; Meyer, R.C.; Saunders, C.; LePichon, J.B.; et al. GPR37L1 modulates seizure susceptibility: Evidence from mouse studies and analyses of a human GPR37L1 variant. Neurobiol. Dis. 2017, 106, 181–190. [Google Scholar] [CrossRef]
- Gao, V.; Suzuki, A.; Magistretti, P.J.; Lengacher, S.; Pollonini, G.; Steinman, M.Q.; Alberini, C.M. Astrocytic β2- Adrenergic receptors mediate hippocampal long- Term memory consolidation. Proc. Natl. Acad. Sci. USA 2016, 113, 8526–8531. [Google Scholar] [CrossRef] [PubMed]
- Verkerke, M.; Hol, E.M.; Middeldorp, J. Physiological and Pathological Ageing of Astrocytes in the Human Brain. Neurochem. Res. 2021, 46, 2662–2675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.P.; Guzowski, J.F.; Thomas, S.A. Mapping neuronal activation and the influence of adrenergic signaling during contextual memory retrieval. Learn Mem. 2000, 12, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Atsak, P.; Hauer, D.; Campolongo, P.; Schelling, G.; McGaugh, J.L.; Roozendaal, B. Glucocorticoids interact with the hippocampal endocannabinoid system in impairing retrieval of contextual fear memory. Proc. Natl. Acad. Sci. USA 2012, 109, 3504–3509. [Google Scholar] [CrossRef]
- Steinman, M.Q.; Gao, V.; Alberini, C.M. The Role of Lactate-Mediated Metabolic Coupling between Astrocytes and Neurons in Long-Term Memory Formation. Front. Integr. Neurosci. 2016, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Ahn, S.; Yang, E.-J.; Kim, H.; Chong, Y.H.; Kim, H.S. Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Mol. Brain 2016, 9, 72. [Google Scholar] [CrossRef]
- Sandi, C.; Rose, S.P.R. Corticosterone enhances long-term retention in one-day-old chicks trained in a weak passive avoidance learning paradigm. Brain Res. 1994, 647, 106–112. [Google Scholar] [CrossRef]
- Loscertales, M.; Rose, S.P.; Sandi, C. The corticosteroid synthesis inhibitors metyrapone and aminoglutethimide impair long-term memory for a passive avoidance task in day-old chicks. Brain Res. 1997, 769, 357–361. [Google Scholar] [CrossRef]
- Cahill, L.; Alkire, M.T. Epinephrine enhancement of human memory consolidation: Interaction with arousal at encoding. Neurobiol. Learn. Mem. 2003, 79, 194–198. [Google Scholar] [CrossRef]
- De Kloet, E.R.; Oitzl, M.S.; Joëls, M. Stress and cognition: Are corticosteroids good or bad guys? Trends Neurosci. 1999, 22, 422–426. [Google Scholar] [CrossRef]
- Buchanan, T.W.; Lovallo, W.R. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 2001, 26, 307–317. [Google Scholar] [CrossRef]
- Coleman, J.L.J.; Mouat, M.A.; Wu, J.; Jancovski, N.; Bassi, J.K.; Chan, A.Y.; Humphreys, D.T.; Mrad, N.; Yu, Z.Y.; Ngo, T.; et al. Orphan receptor GPR37L1 contributes to the sexual dimorphism of central cardiovascular control. Biol. Sex Differ. 2018, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Asico, L.D.; Ma, X.; Konkalmatt, P.R. G protein-coupled receptor 37L1 regulates renal sodium transport and blood pressure. Am. J. Physiol.-Ren. Physiol. 2019, 316, F506–F516. [Google Scholar] [CrossRef]
- Thu Nguyen, T.; Dammer, E.B.; Owino, S.A.; Giddens, M.M.; Madaras, N.S.; Duong, D.M.; Seyfried, N.T.; Hall, R.A. Quantitative Proteomics Reveal an Altered Pattern of Protein Expression in Brain Tissue from Mice Lacking GPR37 and GPR37L1. J. Proteome Res. 2020, 19, 744–755. [Google Scholar] [CrossRef]
- Walf, A.A.; Koonce, C.; Manley, K.; Frye, C.A. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav. Brain Res. 2009, 196, 254–260. [Google Scholar] [CrossRef]
- Gangitano, D.; Salas, R.; Teng, Y.; Perez, E.; De Biasi, M. Progesterone modulation of a5 nAChR subunits influences anxiety-related behavior during estrus cycle. Genes Brain Behav. 2009, 8, 398–406. [Google Scholar] [CrossRef]
- Galeeva, A.; Tuohimaa, P. Analysis of mouse plus-maze behavior modulated by ovarian steroids. Behav. Brain Res. 2001, 119, 41–47. [Google Scholar] [CrossRef]
- Veenit, V.; Zhang, X.; Ambrosini, A.; Sousa, V.; Svenningsson, P. The effect of early life stress on emotional behaviors in gpr37ko mice. Int. J. Mol. Sci. 2022, 23, 410. [Google Scholar] [CrossRef] [PubMed]
- Prut, L.; Belzung, C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur. J. Pharmacol. 2003, 463, 3–33. [Google Scholar] [CrossRef]
- Pereira, M.; Martynhak, B.J.; Andreatini, R.; Svenningsson, P. 5-HT6 receptor agonism facilitates emotional learning. Front. Pharmacol. 2015, 6, 200. [Google Scholar] [CrossRef]
- Pellow, S.; Chopin, P.; File, S.E.; Briley, M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 1985, 14, 149–167. [Google Scholar] [CrossRef]
- Crawley, J.N. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol. Biochem. Behav. 1981, 15, 695–699. [Google Scholar] [CrossRef]
- Eriksson, T.M.; Alvarsson, A.; Stan, T.L.; Zhang, X.; Hascup, K.N.; Hascup, E.R.; Kehr, J.; Gerhardt, G.A.; Warner-Schmidt, J.; Arango-Lievano, M.; et al. Bidirectional regulation of emotional memory by 5-HT 1B receptors involves hippocampal p. Mol. Psychiatry 2013, 18, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.C.; Mantas, I.; Stroth, N.; Hager, T.; Pereira, M.; Jiang, H.; Jabre, S.; Paslawski, W.; Stiedl, O.; Svenningsson, P. P11 deficiency increases stress reactivity along with HPA axis and autonomic hyperresponsiveness. Mol. Psychiatry 2021, 26, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Le Moine, C.; Aubert, I.; Burbaud, P.; Fredholm, B.B.; Bloch, B. Cellular distribution of adenosine A2A receptor mRNA in the primate striatum. J. Comp. Neurol. 1998, 399, 229–240. [Google Scholar] [CrossRef]



| Primer | Sequence, 5′–3′ |
|---|---|
| GPR37L1KO, forward | GCAGCGCATCGCCTTCTATC |
| WT, forward | CACAGCTACTACTTGAAGAG |
| Common, reverse | ACACCTGCCTGTTCATCTGG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veenit, V.; Zhang, X.; Paslawski, W.; Mantas, I.; Svenningsson, P. Impaired Aversive Memory Formation in GPR37L1KO Mice. Int. J. Mol. Sci. 2022, 23, 14290. https://doi.org/10.3390/ijms232214290
Veenit V, Zhang X, Paslawski W, Mantas I, Svenningsson P. Impaired Aversive Memory Formation in GPR37L1KO Mice. International Journal of Molecular Sciences. 2022; 23(22):14290. https://doi.org/10.3390/ijms232214290
Chicago/Turabian StyleVeenit, Vandana, Xiaoqun Zhang, Wojciech Paslawski, Ioannis Mantas, and Per Svenningsson. 2022. "Impaired Aversive Memory Formation in GPR37L1KO Mice" International Journal of Molecular Sciences 23, no. 22: 14290. https://doi.org/10.3390/ijms232214290
APA StyleVeenit, V., Zhang, X., Paslawski, W., Mantas, I., & Svenningsson, P. (2022). Impaired Aversive Memory Formation in GPR37L1KO Mice. International Journal of Molecular Sciences, 23(22), 14290. https://doi.org/10.3390/ijms232214290






