Properties of Protein Isolates from Marine Hydrobionts Obtained by Isoelectric Solubilisation/Precipitation: Influence of Temperature and Processing Time
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties of Protein Isolates
2.1.1. Chemical Composition and Protein Yield
2.1.2. Amino Acid Composition
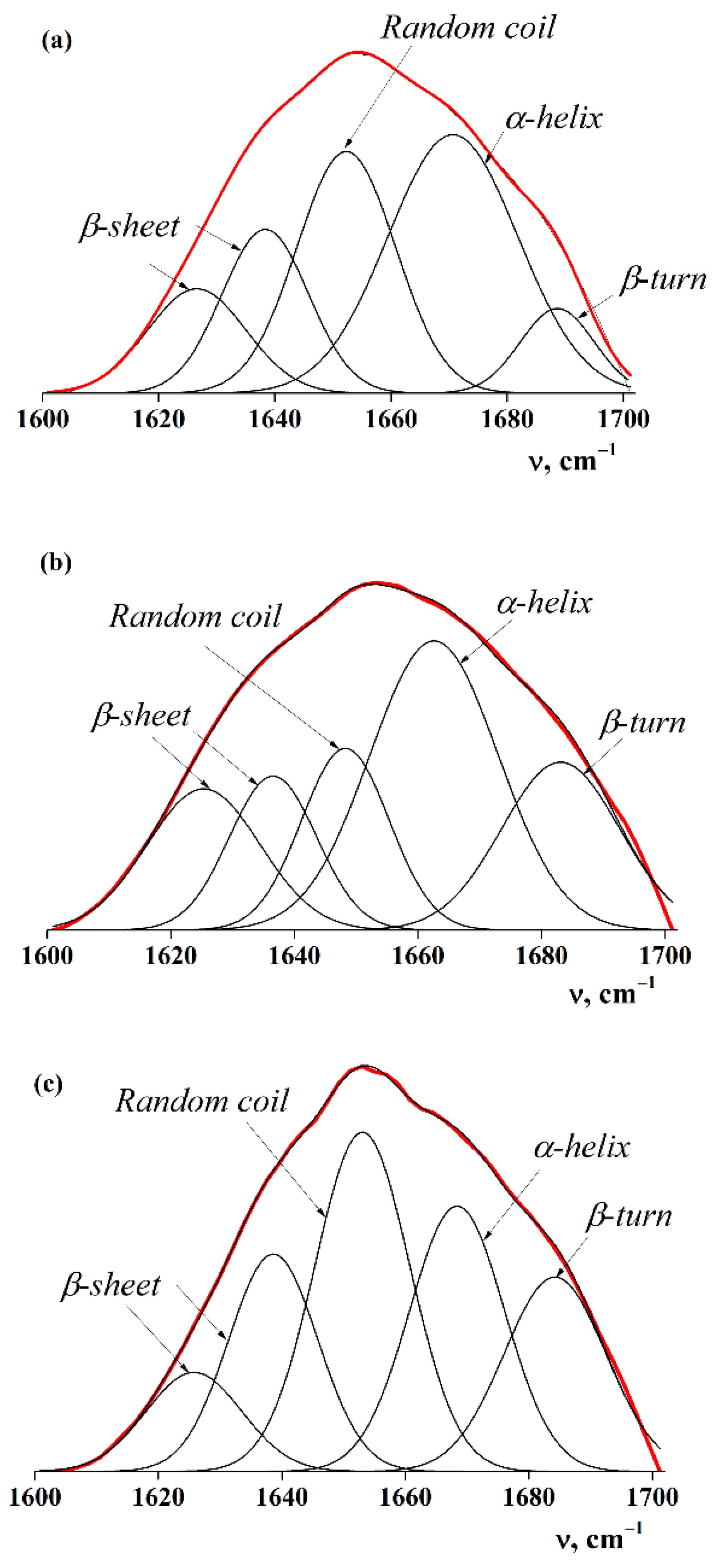
2.1.3. Secondary Structure of Proteins
2.2. Functional Properties of Protein Isolates
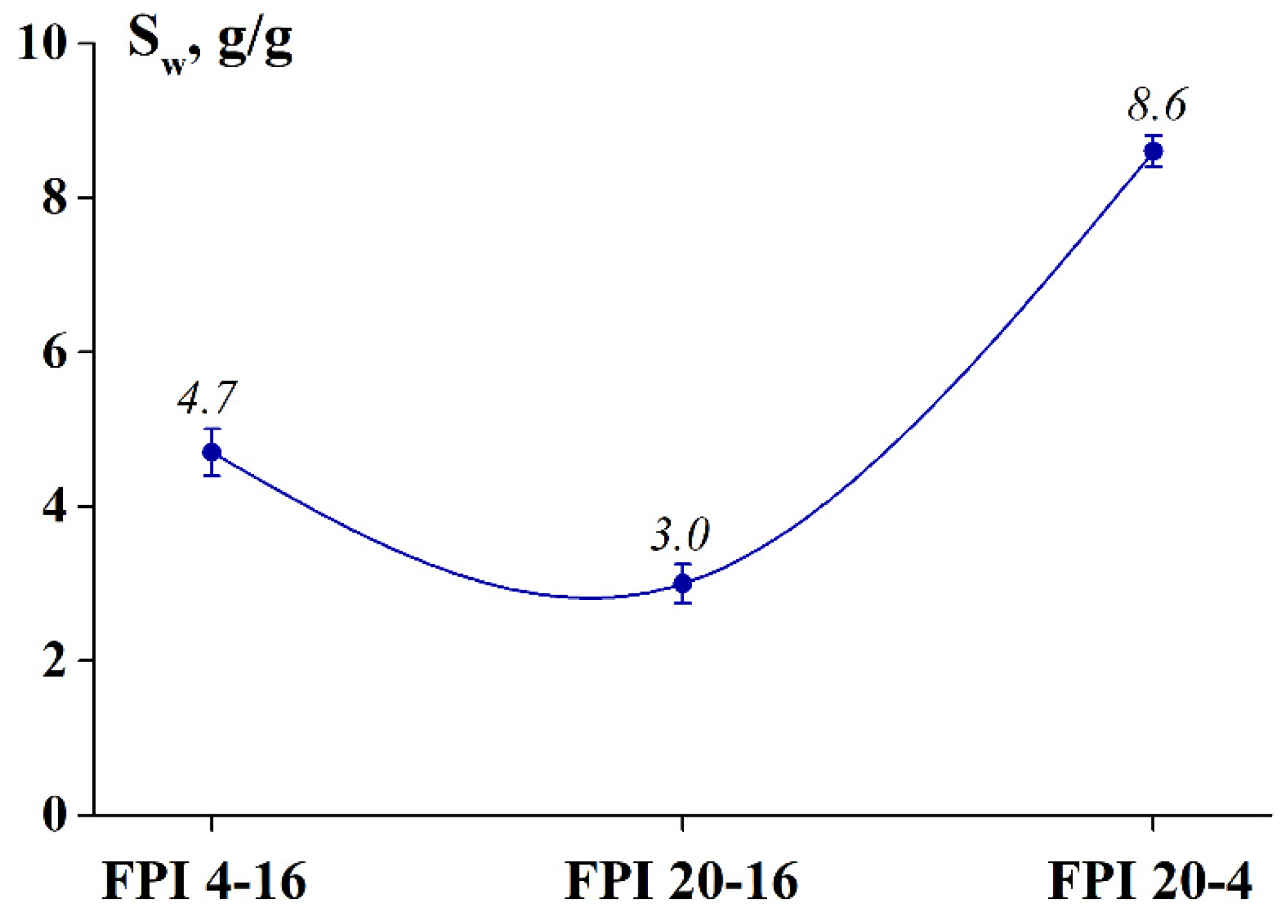
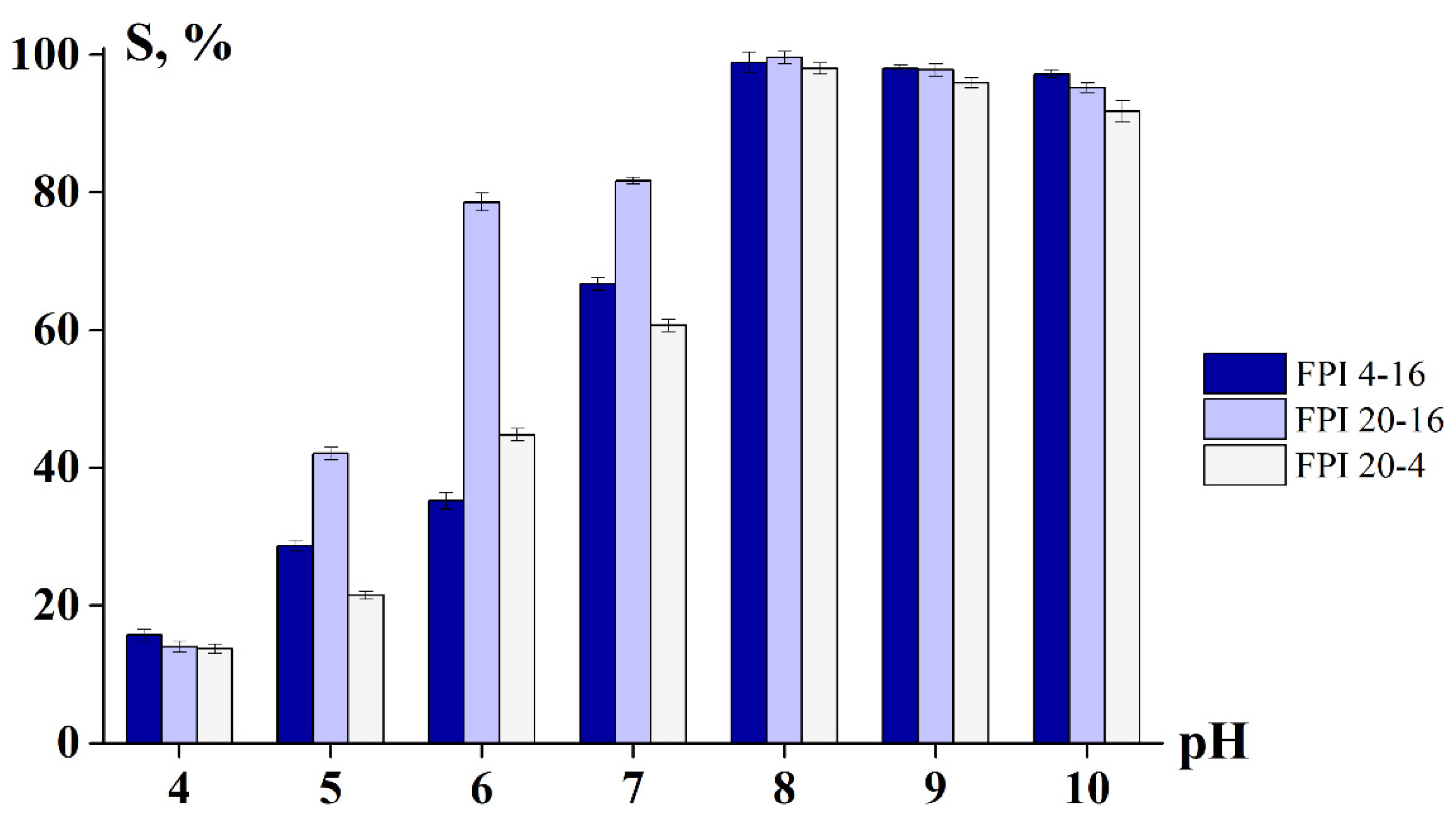
2.2.1. Degree of Swelling and Solubility
2.2.2. Emulsifying Ability and Dispersion of Concentrated Emulsions
3. Materials and Methods
3.1. Raw Materials
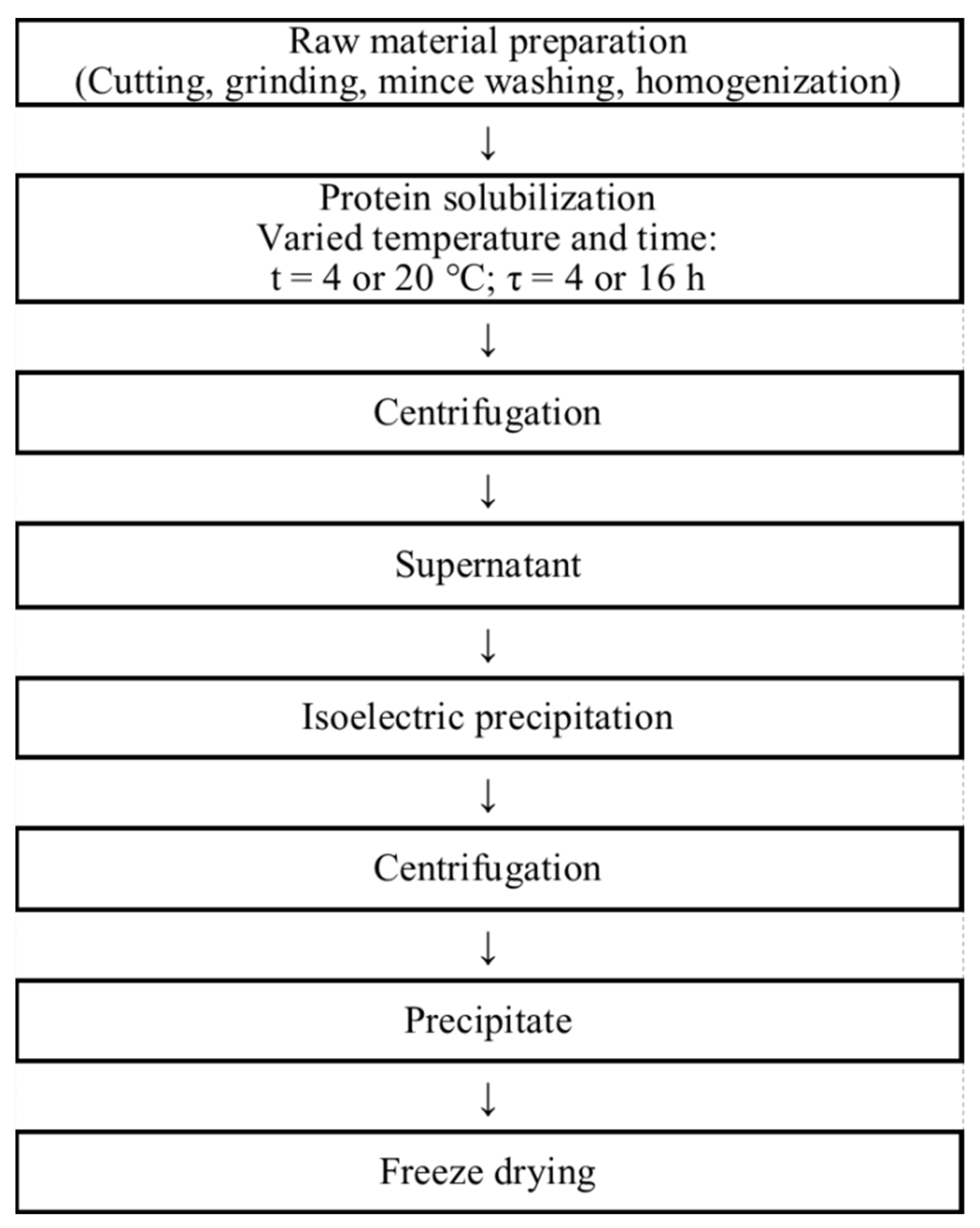
3.2. Preparation of Fish Protein Isolates
3.3. Chemical Composition of Raw Materials and Fish Protein Isolates
3.4. Amino Acid Composition
3.5. Secondary Protein Structure
3.6. Swelling Ability
3.7. Solubility
3.8. Emulsifying Ability
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacou, L.; Leonil, J.; Gagnaire, V. Functional properties of peptides: From single peptide solutions to a mixture of peptides in food products. Food Hydrocoll. 2016, 57, 187–199. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.D.A.; Kumar, S.; Bhat, H.F. Emerging processing technologies for improved digestibility of muscle proteins. Trends Food Sci. Technol. 2021, 110, 226–239. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Hosseini, S.V. Proteins, peptides, and amino acids. In Nutraceutical and Functional Food Components, 1st ed.; Galanakis, C.M., Ed.; Elsevier, Inc.: Amsterdam, The Netherlands, 2017; pp. 15–38. [Google Scholar] [CrossRef]
- Zhang, T.; Dou, W.; Zhang, X.; Zhao, Y.; Zhang, Y.; Jiang, L.; Sui, X. The development history and recent updates on soy protein-based meat alternatives. Trends Food Sci. Technol. 2021, 109, 702–710. [Google Scholar] [CrossRef]
- Otero, D.M.; da Rocha Lemos Mendes, G.; da Silva Lucas, A.J.; Christ-Ribeiro, A.; Ribeiro, C.D.F. Exploring alternative protein sources: Evidence from patents and articles focusing on food markets. Food Chem. 2022, 394, 133486. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Wardhani, D.H.; Kusworo, T.D.; Djaeni, M.; Ping, T.C.; Ma'rifat Fajar Azis, Y. Fish protein concentrate for human consumption: A review of its preparation by solvent extraction methods and potential for food applications. Ann. Agric. Sci. 2022, 67, 42–59. [Google Scholar] [CrossRef]
- Momen, S.; Alavi, F.; Aider, M. Alkali-mediated treatments for extraction and functional modification of proteins: Critical and application review. Trends Food Sci. Technol. 2021, 110, 778–797. [Google Scholar] [CrossRef]
- Nicolai, T.; Durand, D. Controlled food protein aggregation for new functionality. Curr. Opin. Colloid Interface Sci. 2013, 18, 249–256. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Kumar, S.; Bhat, H.F.; Aadil, R.M.; Bekhit, A.E.D.A. Ultrasonication as an emerging technology for processing of animal derived foods: A focus on in vitro protein digestibility. Trends Food Sci. Technol. 2022, 124, 309–322. [Google Scholar] [CrossRef]
- Yang, J.; Liu, G.; Zeng, H.; Chen, L. Effects of high pressure homogenization on faba bean protein aggregation in relation to solubility and interfacial properties. Food Hydrocoll. 2018, 83, 275–286. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Nissen, S.H.; Schmidt, J.M.; Gregersen, S.; Hammershøj, M.; Møller, A.H.; Danielsen, M.; Stødkilde, L.; Nebel, C.; Dalsgaard, T.K. Increased solubility and functional properties of precipitated Alfalfa protein concentrate subjected to pH shift processes. Food Hydrocoll. 2021, 119, 106874. [Google Scholar] [CrossRef]
- Nisov, A.; Kakko, T.; Alakomi, H.-L.; Lantto, R.; Honkapaa, K. Comparison of enzymatic and pH shift methods to extract protein from whole Baltic herring (Clupea harengus membras) and roach (Rutilus rutilus). Food Chem. 2022, 373, 131524. [Google Scholar] [CrossRef]
- Deleu, L.J.; Lambrecht, M.A.; Van de Vondel, J.; Delcour, J.A. The impact of alkaline conditions on storage proteins of cereals and pseudo-cereals. Curr. Opin. Food Sci. 2019, 25, 98–103. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Liang, Y. Effect of pH-Shift Processing and Surimi Processing on Atlantic Croaker (Micropogonias undulates) Muscle Proteins. J. Food Sci. 2006, 71, 304–312. [Google Scholar] [CrossRef]
- Abdollahi, M.; Undeland, I. Structural, functional, and sensorial properties of protein isolate produced from salmon, cod, and herring by-products. Food Bioproc. Techol. 2018, 11, 1733–1749. [Google Scholar] [CrossRef]
- Marmon, S.K.; Undeland, I. Protein isolation from gutted herring (Clupea harengus) using pH-shift processes. J. Agric. Food Chem. 2010, 58, 10480–10486. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Jaczynski, J. Protein recovery from rainbow trout (Oncorhynchus mykiss) processing byproducts via isoelectric solubilization/precipitation and Its gelation properties as affected by functional additives. J. Agric. Food Chem. 2007, 55, 9079–9088. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, S.; Cao, J.; Chen, S.; Wang, W.; Qin, X. Fish protein isolates recovered from silver carp (Hypophthalmichthys molitrix) by-products using alkaline pH solubilization and precipitation. J. Aquat. Food Prod. Technol. 2016, 25, 400–413. [Google Scholar] [CrossRef]
- Chomnawang, C.; Yongsawatdigul, J. Protein recovery of tilapia frame by-products by pH-shift method. J. Aquat. Food Prod. Technol. 2013, 22, 112–120. [Google Scholar] [CrossRef]
- Davenport, M.; Kristinsson, H. Channel catfish (Ictalurus punctatus) muscle protein isolate performance processed under different acid and alkali pH values. J. Food Sci. 2011, 76, E240–E247. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H. Effects of calcium ion on gel properties and gelation of tilapia (Oreochromis niloticus) protein isolates processed with pH shift method. Food Chem. 2019, 277, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Undeland, I. Physicochemical and gel-forming properties of protein isolated from salmon, cod and herring by-products using the pH-shift method. Lebensm. Wiss. Technol. 2019, 101, 678–684. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zhu, Z.; Zeng, Q.; Xin, M. Recovery of tilapia (Oreochromis niloticus) protein isolate by high-intensity ultrasound-aided alkaline isoelectric solubilization/precipitation process. Food Bioproc. Techol. 2015, 8, 758–769. [Google Scholar] [CrossRef]
- Surasania, V.K.R.; Khatkarb, S.K.; Singhba, S. Effect of process variables on solubility andrecovery yields of proteins from pangas (Pangasius pangasius) frames obtained by alkaline solubilization method: Characteristics of isolates. Food Bioprod. Process. 2017, 106, 137–146. [Google Scholar] [CrossRef]
- Chaijan, M.; Panpipat, W.; Benjakul, S. Physicochemical andgelling properties of short-bodied mackerel (Rastrelligerbrachysoma) protein isolate prepared using alkaline-aidedprocess. Food Bioprod. Process. 2010, 88, 174–180. [Google Scholar] [CrossRef]
- Derkach, S.R.; Grokhovsky, V.A.; Kuranova, L.K.; Volchenko, V.I. Nutrient analysis of underutilized fish species for the production of protein food. Foods Raw Mater. 2017, 5, 15–23. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Vebster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons Inc.: New York, NY, USA, 2005. [Google Scholar]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Application; John Wiley & Sons Ltd.: Chichester, UK, 2004. [Google Scholar]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures. Acta Biochim. Biophys. Sinica. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Weng, W.; Zheng, H.; Su, W. Characterization of edible films based on tilapia (Tilapia zillii) scale gelatin with different extraction pH. Food Hydrocoll. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Cortés-Ruiz, J.A.; Pacheco-Aguilar, R.; Ramírez-Suárez, J.C.; Lugo-Sánchez, M.E.; García-Orozco, K.D.; Sotelo-Mundo, R.R.; Peña-Ramos, A. Conformational changes in proteins recovered from jumbo squid (Dosidicus gigas) muscle through pH shift washing treatments. Food Chem. 2016, 196, 769–775. [Google Scholar] [CrossRef]
- Pezeshk, S.; Rezaei, M.; Hosseini, H.; Abdollahi, M. Impact of pH-shift processing combined with ultrasonication on structural and functional properties of proteins isolated from rainbow trout by-products. Food Hydrocoll. 2021, 118, 106768. [Google Scholar] [CrossRef]
- Bučko, S.; Katona, J.; Popović, L.; Vaštag, Ž.; Petrović, L.; Vučinić–Vasić, M. Investigation on solubility, interfacial and emulsifying properties of pumpkin (Cucurbita pepo) seed protein isolate. LWT—Food Sci. Technol. 2015, 64, 609–615. [Google Scholar] [CrossRef]
- Yuliana, M.; Truong, C.T.; Huynh, L.H.; Ho, Q.P.; Ju, Y.-H. Isolation and characterization of protein isolated from defatted cashew nut shell: Influence of pH and NaCl on solubility and functional properties. LWT Food Sci. Technol. 2014, 55, 621–626. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, L.; Wei, F.; Xie, B.; Huang, F.H.; Huang, W.; Shi, J.; Huang, Q.; Tian, B.; Xue, S. Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 2011, 124, 1458–1465. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C.-H. Emulsifying properties of vicilins: Dependence on the protein type and concentration. Food Hydrocoll. 2014, 36, 278–286. [Google Scholar] [CrossRef]
- Kiosseoglou, V.; Paraskevopoulou, A. Functional and Physicochemical Properties of Pulse Proteins. In Pulse Food: Processing, Quality and Nutraceutical Applications; Tiwari, B.K., Gowen, A., McKenna, B., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2011; pp. 57–90. [Google Scholar]
- Hultin, H.O.; Kristinsson, H.G.; Lanier, T.C.; Park, J.W. Process for recovery of functional proteins be pH shifts. In Surimi and Surimi Sea Food; Park, J.W., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 107–139. [Google Scholar]
- Chang, S.K.C.; Zhang, Y. Protein analysis. In Food Analysis, 5th ed.; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 315–331. ISBN 978-3-319-45776-5. [Google Scholar] [CrossRef]
- Official Methods of Analysis, 20th ed.; AOAC International: New York, NY, USA, 2016.
- Brent, L.; Frederick, W. A method for quantitative amino acid analysis using precolumn o-phthalaldehyde derivatization and high performance liquid chromatography. J. Chromatogr. Sci. 1991, 19, 259–265. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Chromatographic determination of amino acids by the use of automatic recording equipment. Methods Enzymol. 1963, 6, 819–831. [Google Scholar] [CrossRef]
- Rabek, J.F. Experimental Methods in Polymer Chemistry. Physical Principles and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 1980. [Google Scholar]
- Derkach, S.R.; Kuchina, Y.A.; Baryshnikov, A.V.; Kolotova, D.S.; Voron'ko, N.G. Tailoring cod gelatin structure and physical properties with acid and alkaline extraction. Polymers 2019, 11, 1724. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physico-chemical properties of acetylated starches from Indian black gram (Phaseolus mungo L.) cultivars. J. Food Sci. Technol. 2015, 52, 4078–4089. [Google Scholar] [CrossRef]
- Cordero-De-Los-Santos, M.Y.; Osuna-Castro, J.A.; Borodanenko, A.; Paredes-López, O. Physicochemical and functional characterisation of amaranth (Amaranthus hypochondriacus) protein isolates obtained by isoelectric precipitation and micellisation. Food Sci. Technol. Int. 2005, 11, 269–280. [Google Scholar] [CrossRef]
- Yasumatsu, K.; Sawada, K.; Moritaka, S.; Misaki, M.; Toda, J.; Wada, T.; Ishi, K. Whipping and emulsifying properties. Agric. Biol. Chem. 1972, 36, 719–727. [Google Scholar] [CrossRef]
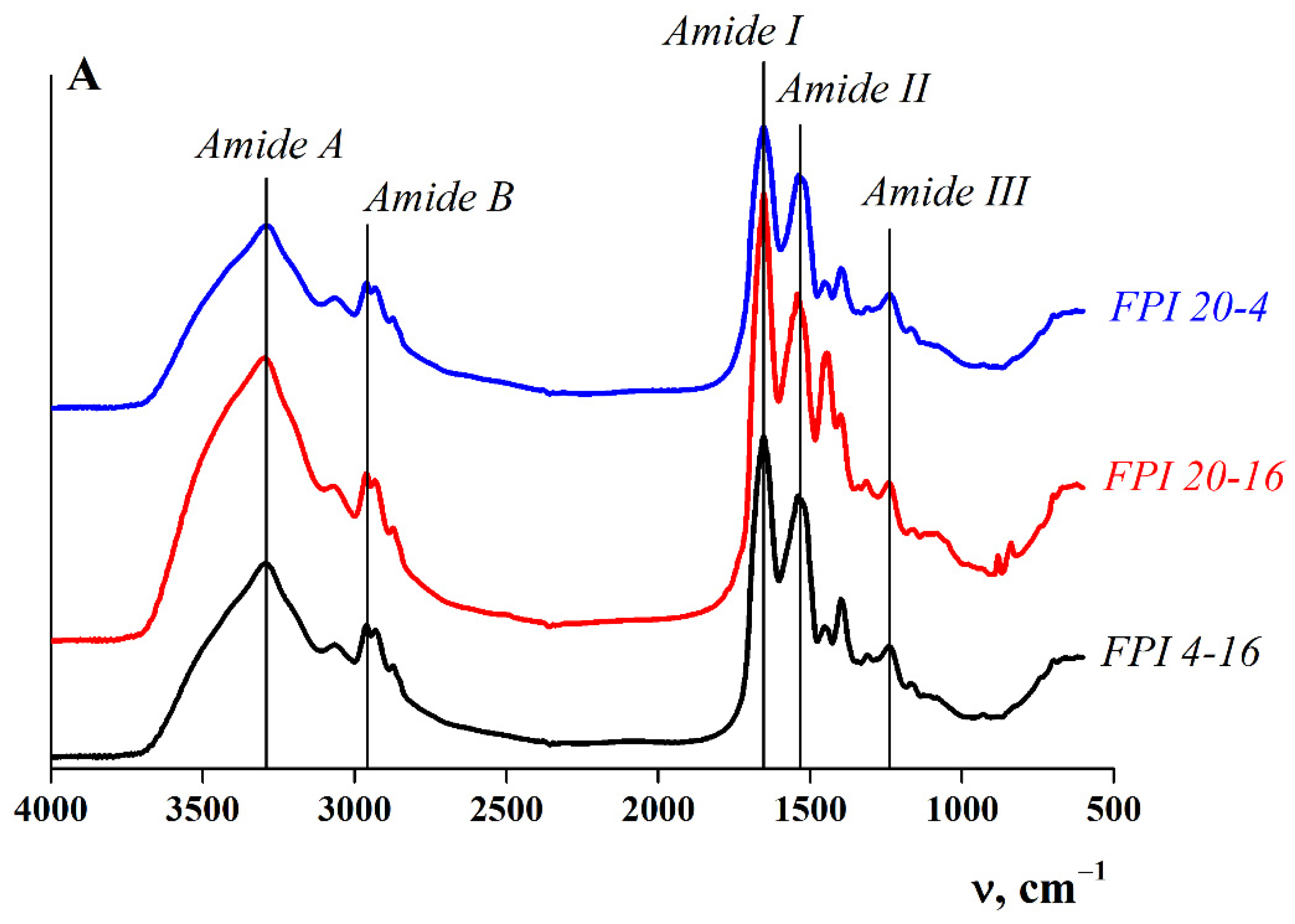
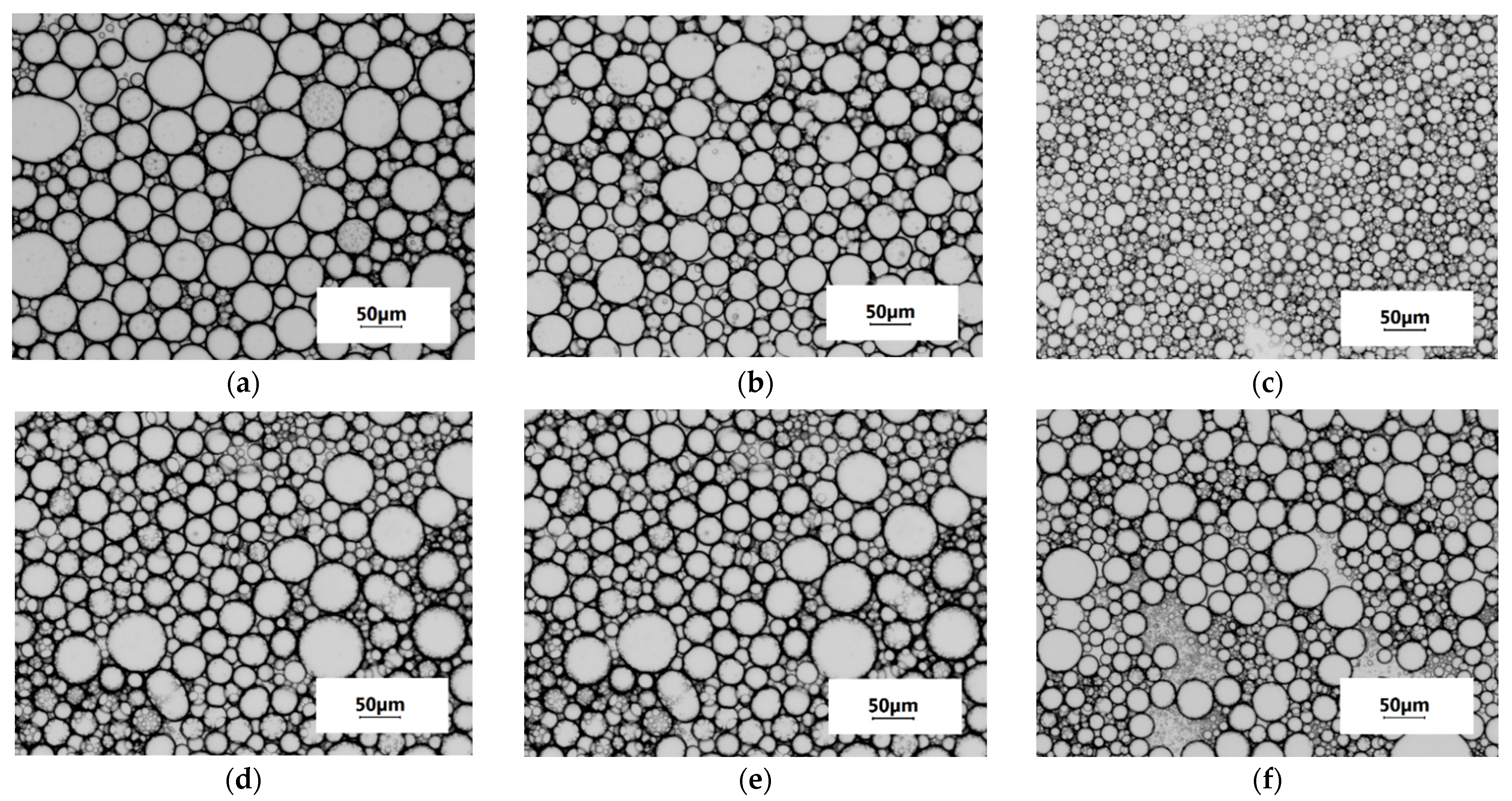
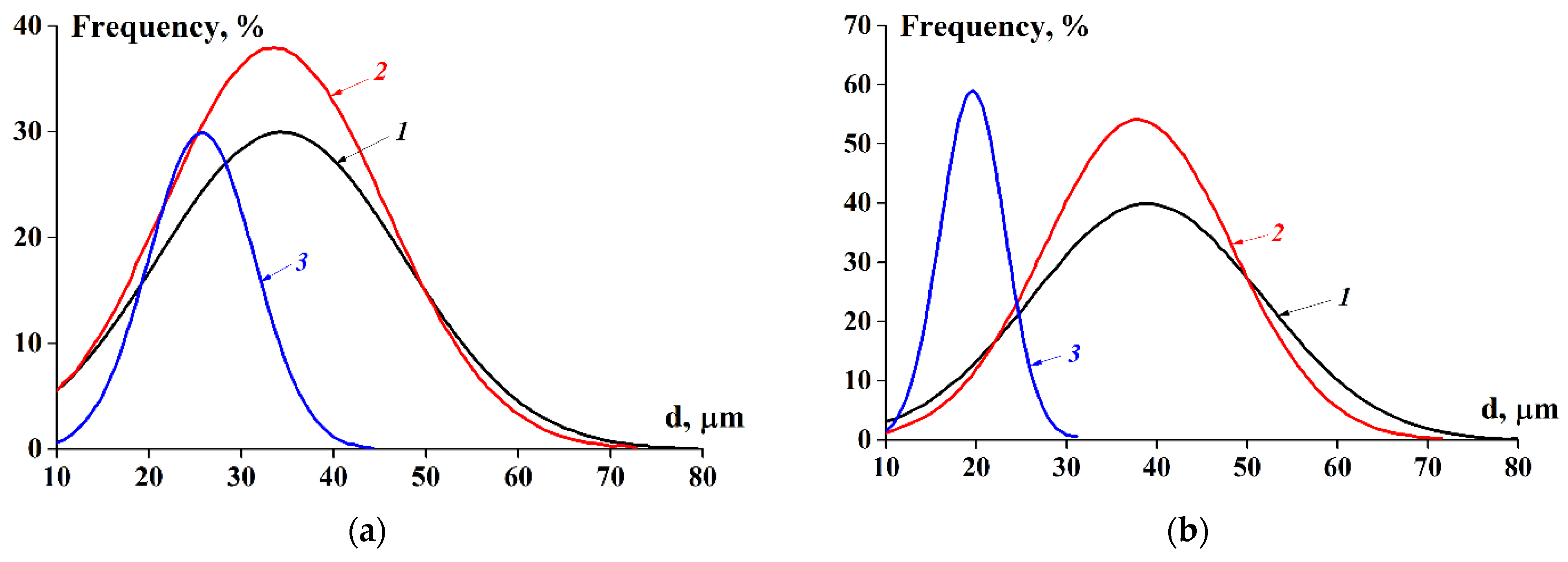
| Samples | Solubilisation Conditions | Moisture Content ωM, % | Total Nitrogen NT, % | Protein * P, % | Fat, ωF, % | Ash, ωA, % | Protein Yield, B, %, w/w | |
|---|---|---|---|---|---|---|---|---|
| t, °C | τ, h | |||||||
| Muscle tissue of northern blue whiting | − | − | 81.1 ± 2.4 | 2.6 ± 0.1 | 16.3 ± 0.6 | 0.3 ± 0.2 | 1.4 ± 0.2 | − |
| FPI 4-16 | 4 ± 1 | 16 | 3.9 ± 0.1 | 15.2 ± 0.1 | 95.1 ± 0.2 | − | 1.0 ± 0.1 | 44.5 ± 1.0 |
| FPI 20-16 | 20 ± 1 | 16 | 3.8 ± 0.1 | 15.3 ± 0.1 | 95.3 ± 0.2 | − | 0.9 ± 0.1 | 45.4 ± 1.0 |
| FPI 20-4 | 20 ± 1 | 4 | 4.9 ± 0.2 | 15.0 ± 0.1 | 93.8 ± 0.3 | − | 1.2 ± 0.1 | 44.0 ± 1.0 |
| Amino Acids | Muscle Tissue of Northern Blue Whiting [27] | FPI 4-16 | FPI 20-16 | FPI 20-4 |
|---|---|---|---|---|
| Glutamic acid (HAA) | 13.1 | 16.3 | 16.2 | 16.6 |
| Aspartic acid (HAA) | 9.0 | 9.2 | 9.3 | 9.6 |
| Leucine (EAA) | 8.0 | 9.0 | 8.9 | 8.9 |
| Lysine (EAA, HAA) | 9.0 | 8.6 | 8.1 | 8.7 |
| Arginine (HAA) | 5.8 | 5.4 | 5.4 | 5.1 |
| Alanine | 6.5 | 5.4 | 5.3 | 5.3 |
| Isoleucine (EAA) | 5.2 | 5.1 | 5.0 | 5.0 |
| Phenylalanine (EAA) | 4.1 | 4.8 | 4.6 | 4.7 |
| Valine (EAA) | 5.5 | 4.7 | 4.7 | 4.7 |
| Methionine (EAA) | 3.1 | 4.6 | 3.8 | 4.4 |
| Tyrosine | 2.4 | 4.3 | 4.1 | 4.2 |
| Serine (HAA) | 4.1 | 4.1 | 4.0 | 4.2 |
| Glycine (HAA) | 9.6 | 3.1 | 2.8 | 3.1 |
| Threonine (EAA, HAA) | 5.2 | 2.8 | 2.7 | 2.9 |
| Histidine (HAA) | 3.9 | 2.0 | 1.8 | 1.9 |
| Proline | 2.5 | 2.4 | 2.2 | 2.5 |
| EAA, total | 40.1 | 39.6 | 37.8 | 39.3 |
| HAA, total | 59.7 | 51.5 | 50.3 | 52.1 |
| Conformational State | Wave Number ν, cm−1 [31] | FPI 4-16 | FPI 20-16 | FPI 20-4 |
|---|---|---|---|---|
| β-Turn/β-sheet | 1625–1626 | 11.7 | 9.0 | 15.7 |
| 1636–1638 | 16.1 | 18.3 | 13.2 | |
| Random coil | 1649–1652 | 27.1 | 30.6 | 15.7 |
| α-Helix | 1664–1668 | 38.1 | 23.8 | 36.5 |
| β-Turn/β-sheet | 1684–1688 | 7.0 | 18.3 | 18.9 |
| Characteristic | C(FPI 4-16), % (w/v) | C(FPI 20-4), % (w/v) | ||||
|---|---|---|---|---|---|---|
| 1.0 | 2.0 | 5.0 | 1.0 | 2.0 | 5.0 | |
| υ0, cm3/h | 1.63 ± 0.09 | 0.86 ± 0.05 | 0.03 ± 0.02 | 1.55 ± 0.09 | 0.73 ± 0.05 | 0.21 ± 0.04 |
| X, % | 67.0 ± 0.2 | 68.5 ± 0.1 | 85.1 ± 0.5 | 68.7 ± 0.2 | 73.8 ± 0.6 | 75.5 ± 0.5 |
| φ (concentrated emulsion), % | 0.74 ± 0.02 | 0.71 ± 0.04 | 0.64 ± 0.04 | 0.71 ± 0.02 | 0.68 ± 0.01 | 0.68 ± 0.02 |
| d (concentrated emulsion), μm | 41 ± 3 | 35 ± 2 | 18 ± 2 | 31 ± 2 | 30 ± 2 | 25 ± 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkach, S.R.; Kuchina, Y.A.; Kolotova, D.S.; Petrova, L.A.; Volchenko, V.I.; Glukharev, A.Y.; Grokhovsky, V.A. Properties of Protein Isolates from Marine Hydrobionts Obtained by Isoelectric Solubilisation/Precipitation: Influence of Temperature and Processing Time. Int. J. Mol. Sci. 2022, 23, 14221. https://doi.org/10.3390/ijms232214221
Derkach SR, Kuchina YA, Kolotova DS, Petrova LA, Volchenko VI, Glukharev AY, Grokhovsky VA. Properties of Protein Isolates from Marine Hydrobionts Obtained by Isoelectric Solubilisation/Precipitation: Influence of Temperature and Processing Time. International Journal of Molecular Sciences. 2022; 23(22):14221. https://doi.org/10.3390/ijms232214221
Chicago/Turabian StyleDerkach, Svetlana R., Yuliya A. Kuchina, Daria S. Kolotova, Ludmila A. Petrova, Vasily I. Volchenko, Andrei Yu. Glukharev, and Vladimir A. Grokhovsky. 2022. "Properties of Protein Isolates from Marine Hydrobionts Obtained by Isoelectric Solubilisation/Precipitation: Influence of Temperature and Processing Time" International Journal of Molecular Sciences 23, no. 22: 14221. https://doi.org/10.3390/ijms232214221
APA StyleDerkach, S. R., Kuchina, Y. A., Kolotova, D. S., Petrova, L. A., Volchenko, V. I., Glukharev, A. Y., & Grokhovsky, V. A. (2022). Properties of Protein Isolates from Marine Hydrobionts Obtained by Isoelectric Solubilisation/Precipitation: Influence of Temperature and Processing Time. International Journal of Molecular Sciences, 23(22), 14221. https://doi.org/10.3390/ijms232214221







