Salbutamol in the Management of Asthma: A Review
Abstract
1. Asthma Overview
2. Short-Acting β2-Agonists
3. Salbutamol: A First Approach
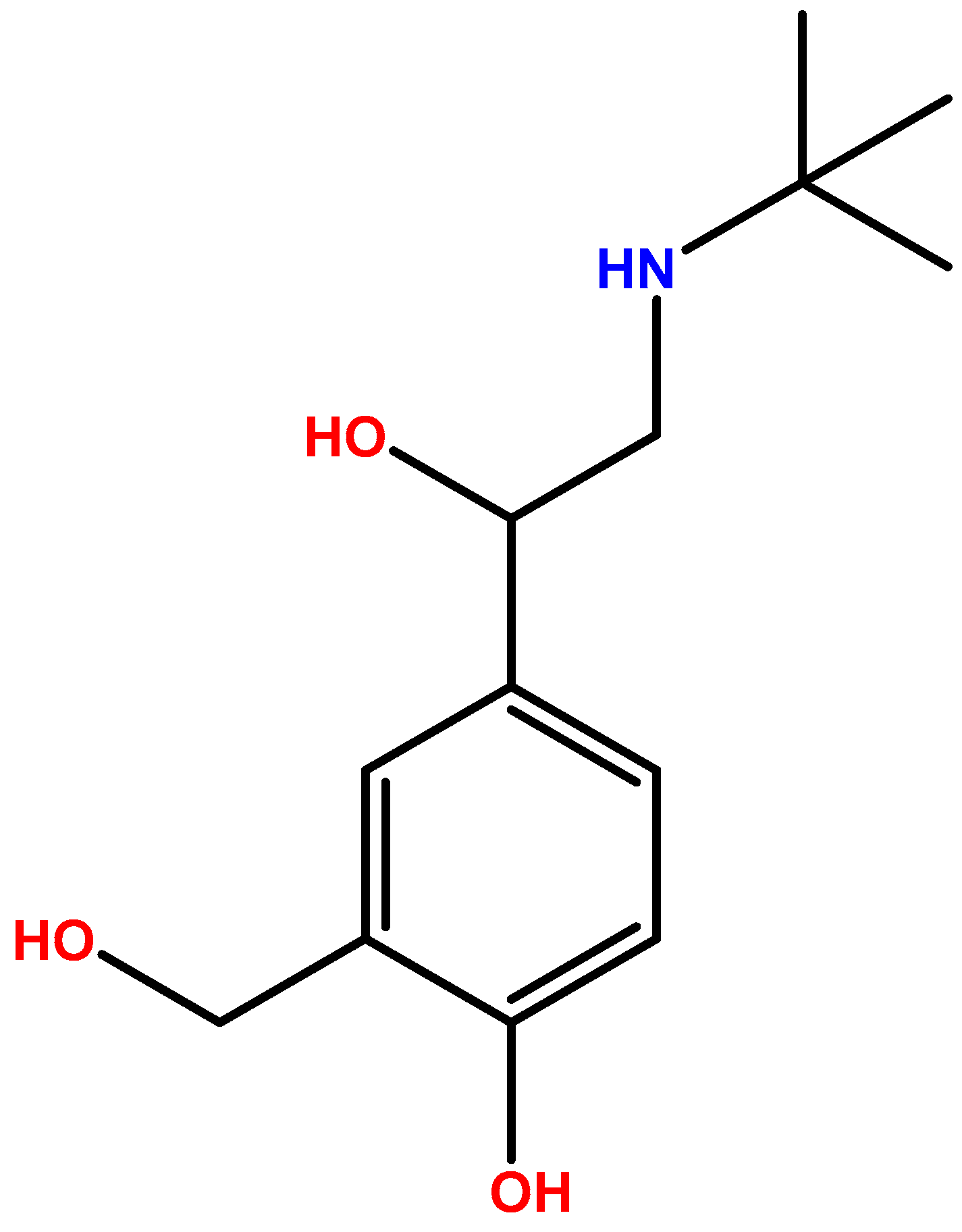
3.1. Chemistry
3.2. Pharmacokinetics and Metabolism
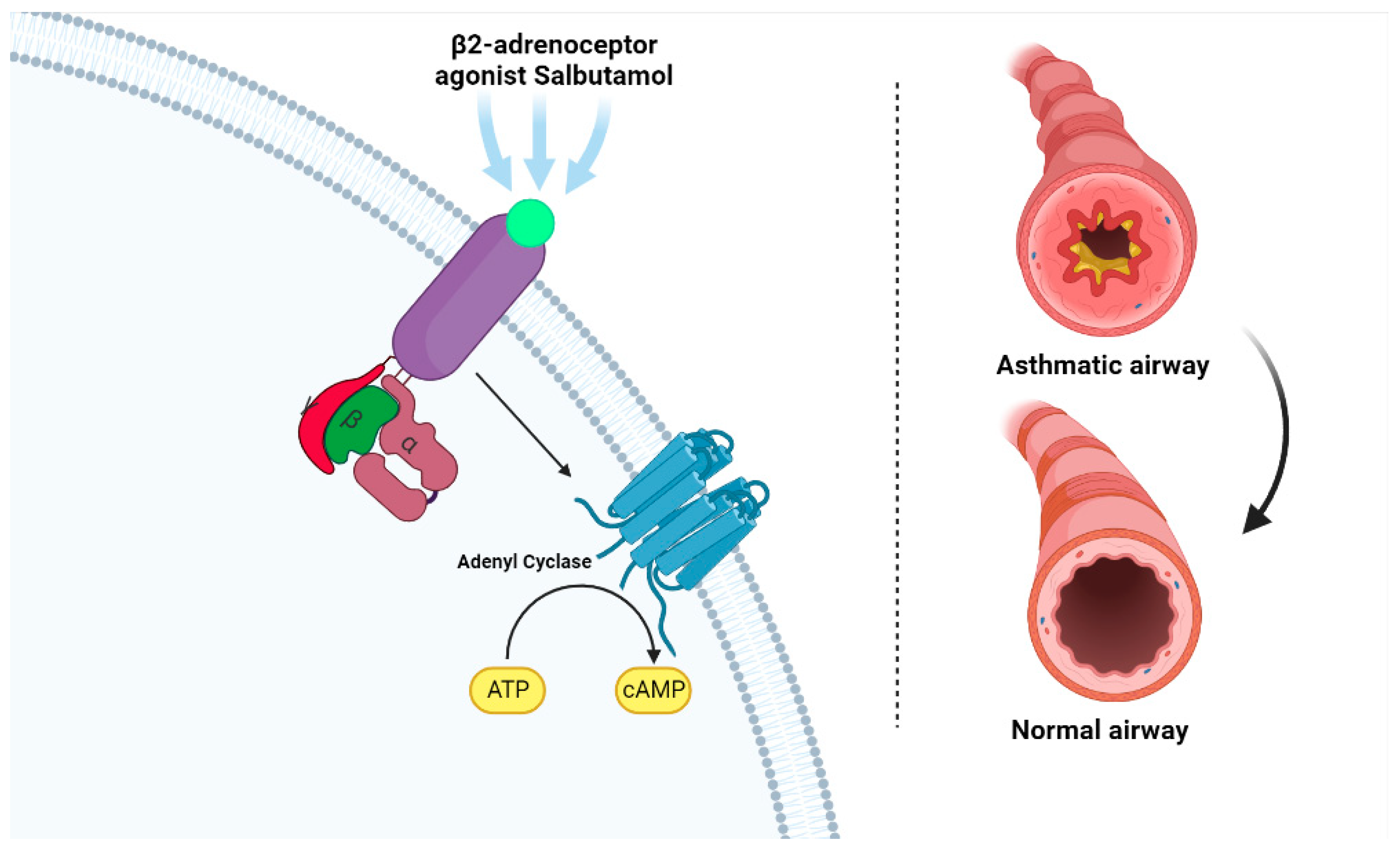
3.3. Mechanism of Action
3.4. Pharmacodynamic Properties
3.5. Adverse Effects
3.6. Clinical Efficacy
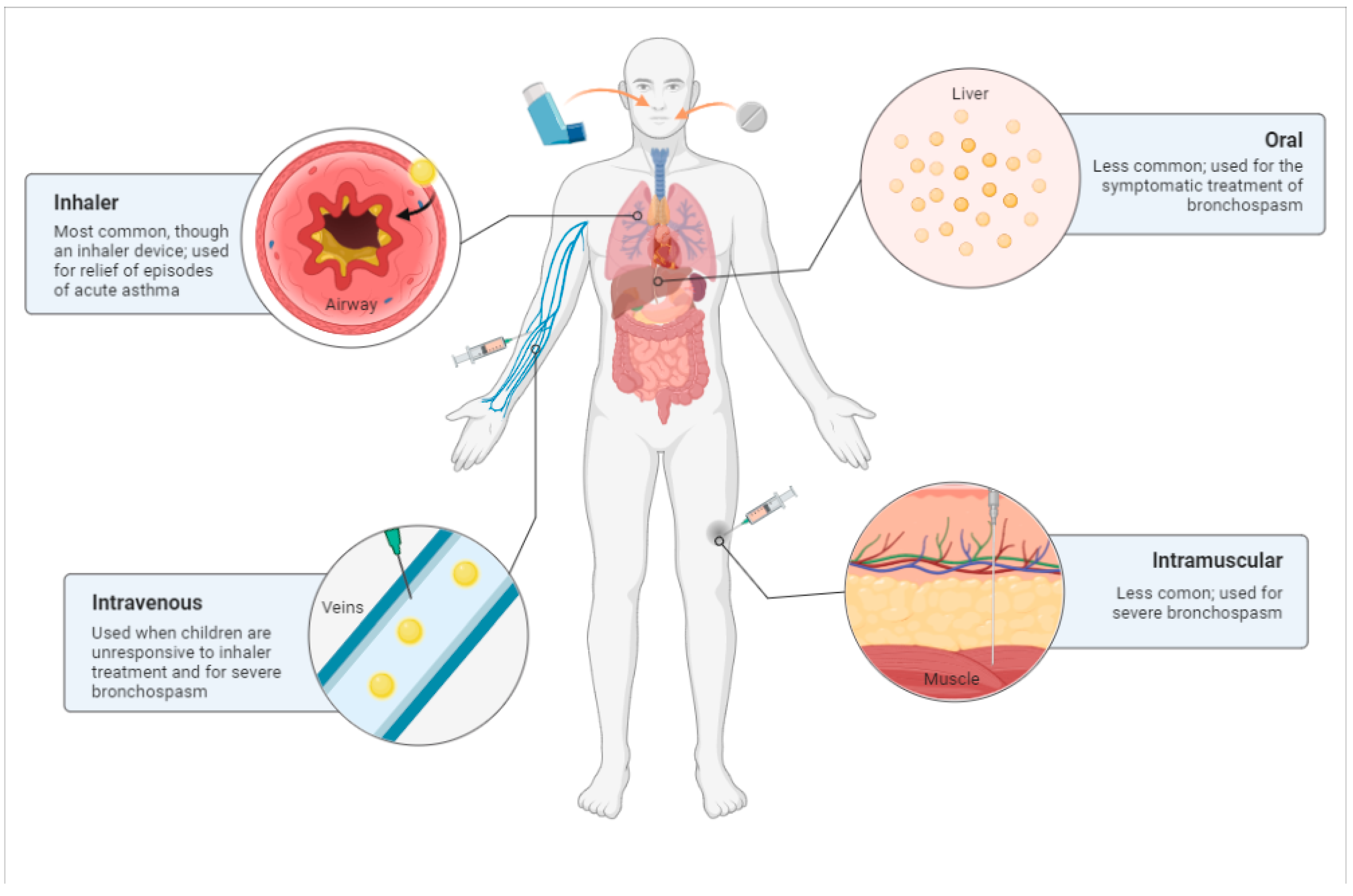
3.7. Routes of Administration
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mims, J.W. Asthma: Definitions and Pathophysiology. Int. Forum Allergy Rhinol. 2015, 5, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Guidelines for the Diagnosis and Management of Asthma 2007 (EPR-3)|NHLBI, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma (accessed on 7 October 2022).
- Cevhertas, L.; Ogulur, I.; Maurer, D.J.; Burla, D.; Ding, M.; Jansen, K.; Koch, J.; Liu, C.; Ma, S.; Mitamura, Y.; et al. Advances and Recent Developments in Asthma in 2020. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 3124–3146. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Bhat, G.; Pianosi, P. What Is New in the Management of Childhood Asthma? Indian J. Pediatr. 2018, 85, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Brightling, C.; Pedersen, S.E.; Reddel, H.K. Asthma. Lancet 2018, 391, 783–800. [Google Scholar] [CrossRef]
- Pavord, I.D.; Beasley, R.; Agusti, A.; Anderson, G.P.; Bel, E.; Brusselle, G.; Cullinan, P.; Custovic, A.; Ducharme, F.M.; Fahy, J.V.; et al. After Asthma: Redefining Airways Diseases. Lancet 2018, 391, 350–400. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Kabesch, M.; Liang, L.; Dixon, A.L.; Strachan, D.; Heath, S.; Depner, M.; Von Berg, A.; Bufe, A.; Rietschel, E.; et al. Genetic Variants Regulating ORMDL3 Expression Contribute to the Risk of Childhood Asthma. Nature 2007, 448, 470–473. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O.C.M. A Large-Scale, Consortium-Based Genomewide Association Study of Asthma. N. Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef]
- Portelli, M.A.; Hodge, E.; Sayers, I. Genetic Risk Factors for the Development of Allergic Disease Identified by Genome-Wide Association. Clin. Exp. Allergy 2015, 45, 21–31. [Google Scholar] [CrossRef]
- Rehman, A.; Amin, F.; Sadeeqa, S. Prevalence of Asthma and Its Management: A Review. J. Pak. Med. Assoc. 2018, 68, 1823–1827. [Google Scholar]
- Chronic Respiratory Diseases: Asthma. Available online: https://www.who.int/news-room/questions-and-answers/item/chronic-respiratory-diseases-asthma (accessed on 7 October 2022).
- Types of Asthma—Causes, Symptoms & Treatment|ACAAI Patient. Available online: https://acaai.org/asthma/types-of-asthma/ (accessed on 7 October 2022).
- What Are Different Types of Asthma and Are They Common? Available online: https://asthma.net/types (accessed on 7 October 2022).
- Global Initiative for Asthma GINA. Global Strategy for Asthma Management and Prevention. 2022 Global Initiative for Asthma Guidelines; Global Initiative for Asthma: Fontana, WI, USA, 2022. [Google Scholar]
- Asher, M.; Montefort, S.; Bjorksten, B.; Lai, C.; Strachan, D.; Weiland, S.; Williams, H.; Group, I.P.T. study Worldwide Time Trends in the Prevalence of Symptoms of Asthma, Allergic Rhinoconjunctivitis, and Eczema in Childhood. Lancet 2006, 368, 733–743. [Google Scholar] [CrossRef]
- Moores, G.; Boulet, L.-P.; Gershon, A.S.; Bateman, E.D.; To, T.; Stanojevic, S.; Cruz, A.A. Global Asthma Prevalence in Adults: Findings from the Cross-Sectional World Health Survey. BMC Public Health 2012, 12, 204. [Google Scholar]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Strachan, D.P. Hay Fever, Hygiene, and Household Size. J. Fam. Hist. 1989, 20, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W.; Martinelli, R.; Brunet, L.R. Innate Immune Responses to Mycobacteria and the Downregulation of Atopic Responses. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 337–342. [Google Scholar] [CrossRef]
- Leynaert, B.; Sunyer, J.; Garcia-Esteban, R.; Svanes, C.; Jarvis, D.; Cerveri, I.; Dratva, J.; Gislason, T.; Heinrich, J.; Janson, C.; et al. Gender Differences in Prevalence, Diagnosis and Incidence of Allergic and Non-Allergic Asthma: A Population-Based Cohort. Thorax 2012, 67, 625–631. [Google Scholar] [CrossRef]
- Nanda, A.; Baptist, A.P.; Divekar, R.; Parikh, N.; Seggev, J.S.; Yusin, J.S.; Nyenhuis, S.M. Asthma in the Older Adult. J. Asthma 2020, 57, 241–252. [Google Scholar] [CrossRef]
- Papi, A.; Blasi, F.; Canonica, G.W.; Morandi, L.; Richeldi, L.; Rossi, A. Treatment Strategies for Asthma: Reshaping the Concept of Asthma Management. Allergy Asthma Clin. Immunol. 2020, 16, 75. [Google Scholar] [CrossRef]
- Hills, T.; Beasley, R. The History and Future of Short-Acting Beta2-Agonist Therapy in Asthma. Respirology 2020, 25, 246–248. [Google Scholar] [CrossRef]
- Khan Mohammad Beigi, P. Expert Panel Report 3 (EPR3): Guidelines for the Diagnosis and Management. J. Allergy Clin. Immunol. 2007, 120, 13–18. [Google Scholar] [CrossRef]
- Kwah, J.H.; Peters, A.T. Asthma in Adults: Principles of Treatment. Allergy Asthma Proc. 2019, 40, 396–402. [Google Scholar] [CrossRef]
- Martin, M.J.; Harrison, T.W. Is It Time to Move Away from Short-Acting Beta-Agonists in Asthma Management? Eur. Respir. J. 2019, 53, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Blais, L.; Ernst, P. Patterns of Increasing β-Agonist Use and the Risk of Fatal or near-Fatal Asthma. Eur. Respir. J. 1994, 7, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Suissa, S.; Ernst, P.; Boivin, J.F.; Horwitz, R.I.; Habbick, B.; Cockroft, D.; Blais, L.; McNutt, M.; Buist, A.S.; Spitzer, W.O. A Cohort Analysis of Excess Mortality in Asthma and the Use of Inhaled β-Agonists. Am. J. Respir. Crit. Care Med. 1994, 149, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Sears, M.R.; Taylor, D.R.; Print, C.G.; Lake, D.C.; Li, Q.; Flannery, E.M.; Yates, D.M.; Lucas, M.K.; Herbison, G.P. Regular Inhaled Beta-Agonist Treatment in Bronchial Asthma. Lancet 1990, 336, 1391–1396. [Google Scholar] [CrossRef]
- Quint, J.K.; Arnetorp, S.; Kocks, J.W.H.; Kupczyk, M.; Nuevo, J.; Plaza, V.; Cabrera, C.; Raherison-Semjen, C.; Walker, B.; Penz, E.; et al. Short-Acting Beta-2-Agonist Exposure and Severe Asthma Exacerbations: SABINA Findings From Europe and North America. J. Allergy Clin. Immunol. Pract. 2022, 10, 2297–2309.e10. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; McParland, C.P.; Britto, S.A.; Swystun, V.A.; Rutherford, B.C. Regular Inhaled Salbutamol and Airway Responsiveness to Allergen. Lancet 1993, 342, 833–837. [Google Scholar] [CrossRef]
- Hancox, R.J.; Cowan, J.O.; Flannery, E.M.; Herbison, G.P.; Mclachlan, C.R.; Taylor, D.R. Bronchodilator Tolerance and Rebound Bronchoconstriction during Regular Inhaled β-Agonist Treatment. Respir. Med. 2000, 94, 767–771. [Google Scholar] [CrossRef]
- Reddel, H.K.; FitzGerald, J.M.; Bateman, E.D.; Bacharier, L.B.; Becker, A.; Brusselle, G.; Buhl, R.; Cruz, A.A.; Fleming, L.; Inoue, H.; et al. GINA 2019: A Fundamental Change in Asthma Management: Treatment of Asthma with Short-Acting Bronchodilators Alone Is No Longer Recommended for Adults and Adolescents. Eur. Respir. J. 2019, 53, 1901046. [Google Scholar] [CrossRef]
- Reddel, H.K.; Bacharier, L.B.; Bateman, E.D.; Brightling, C.E.; Brusselle, G.G.; Buhl, R.; Cruz, A.A.; Duijts, L.; Drazen, J.M.; FitzGerald, J.M.; et al. Global Initiative for Asthma Strategy 2021: Executive Summary and Rationale for Key Changes. Eur. Respir. J. 2022, 59, 14–35. [Google Scholar] [CrossRef]
- Maselli, D.J.; Peters, J.I. Medication Regimens for Managing Acute Asthma. Respir. Care 2018, 63, 783–796. [Google Scholar] [CrossRef]
- Looijmans-van den Akker, I.; Werkhoven, A.; Verheij, T. Over-Prescription of Short-Acting Beta Agonists in the Treatment of Asthma. Fam. Pract. 2021, 38, 612–616. [Google Scholar] [CrossRef] [PubMed]
- AstraZeneca SABINA Programme Demonstrates SABA Reliever Overuse Is a Global Issue in Asthma Management. Available online: https://www.astrazeneca.com/media-centre/medical-releases/sabina-programme-demonstrates-saba-reliever-overuse-is-a-global-issue-in-asthma-management.html (accessed on 4 November 2022).
- Makhlouf, K.; Weiner, H.L.; Khoury, S.J. Potential of Β2-Adrenoceptor Agonists as Add-on Therapy for Multiple Sclerosis Focus on Salbutamol (Albuterol). CNS Drugs 2002, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Salbutamol: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01001 (accessed on 7 October 2022).
- Vet, N.J.; de Winter, B.C.M.; Koninckx, M.; Boeschoten, S.A.; Boehmer, A.L.M.; Verhallen, J.T.; Plötz, F.B.; Vaessen-Verberne, A.A.; van der Nagel, B.C.H.; Knibbe, C.A.J.; et al. Population Pharmacokinetics of Intravenous Salbutamol in Children with Refractory Status Asthmaticus. Clin. Pharmacokinet. 2020, 59, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Boulton, D.W.; Fawcett, J.P. Enantioselective Disposition of Salbutamol in Man Following Oral and Intravenous Administration. Br. J. Clin. Pharmacol. 1996, 41, 35–40. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Model List of Essential Medicines, 22nd List; Technical Document; WHO: Geneva, Switzerland, 2021; Volume 2021. [Google Scholar]
- Ullmann, N.; Caggiano, S.; Cutrera, R. Salbutamol and Around. Ital. J. Pediatr. 2015, 41, A74. [Google Scholar] [CrossRef]
- Taylor, D.R.; Town, G.I.; Herbison, G.P.; Boothman-Burrell, D.; Flannery, E.M.; Hancox, B.; Harré, E.; Laubscher, K.; Linscott, V.; Ramsay, C.M.; et al. Asthma Control during Long Term Treatment with Regular Inhaled Salbutamol and Salmeterol. Thorax 1998, 53, 744–752. [Google Scholar] [CrossRef]
- Gauvreau, G.M.; Jordana, M.; Watson, R.M.; Cockcroft, D.W.; O’Byrne, P.M. Effect of Regular Inhaled Albuterol on Allergen-Induced Late Responses and Sputum Eosinophils in Asthmatic Subjects. Am. J. Respir. Crit. Care Med. 1997, 156, 1738–1745. [Google Scholar] [CrossRef]
- Ritchie, A.; Wiater, E.; Edwards, M.; Montminy, M.; Johnston, S. B2-Agonists Enhance Asthma-Relevant Inflammatory Mediators in Human Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2018, 58, 128–132. [Google Scholar] [CrossRef]
- Patel, M.; Thomson, N.C. (R)-Salbutamol in the Treatment of Asthma and Chronic Obstructive Airways Disease. Expert Opin. Pharmacother. 2011, 12, 1133–1141. [Google Scholar] [CrossRef]
- Asmus, M.J.; Hendeles, L.; Weinberger, M.; Ahrens, R.C.; Bisgaard, H.; Lötvall, J.; O’Byrne, P.M.; Cockcroft, D.W. Levalbuterol Has Not Been Established to Have Therapeutic Advantage over Racemic Albuterol. J. Allergy Clin. Immunol. 2002, 110, 325. [Google Scholar] [CrossRef]
- Ramsay, C.M.; Cowan, J.; Flannery, E.; McLachlan, C.; Taylor, D.R. Bronchoprotective and Bronchodilator Effects of Single Doses of (S)- Salbutamol, (R)-Salbutamol and Racemic Salbutamol in Patients with Bronchial Asthma. Eur. J. Clin. Pharmacol. 1999, 55, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, D.W.; Swystun, V.A. Effect of Single Doses of S-Salbutamol, R-Salbutamol, Racemic Salbutamol, and Placebo on the Airway Response to Methacholine. Thorax 1997, 52, 845–848. [Google Scholar] [CrossRef]
- Gumbhir-Shah, K.; Kellerman, D.J.; Degraw, S.; Koch, P.; Jusko, W.J. Pharmacokinetics and Pharmacodynamics of Cumulative Single Doses of Inhaled Salbutamol Enantiomers in Asthmatic Subjects. Pulm. Pharmacol. Ther. 1999, 12, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Skoner, D.P. Pharmacokinetics, Pharmacodynamics, and the Delivery of Pediatric Bronchodilator Therapy. J. Allergy Clin. Immunol. 2000, 106, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Elers, J.; Pedersen, L.; Henninge, J.; Lund, T.K.; Hemmersbach, P.; Dalhoff, K.; Backer, V. Blood and Urinary Concentrations of Salbutamol in Asthmatic Subjects. Med. Sci. Sports Exerc. 2010, 42, 244–249. [Google Scholar] [CrossRef]
- Lewis, L.D.; McLaren, M.; Essex, E.; Cochrane, G.M. Plasma Concentrations of Salbutamol in Acute Severe Asthmatics. Aust. N. Z. J. Med. 1990, 20, 204–207. [Google Scholar] [CrossRef]
- Morgan, D.; Paull, J.; Richmond, B.; Wilson-Evered, E.; Ziccone, S. Pharmacokinetics of Intravenous and Oral Salbutamol and Its Sulphate Conjugate. Br. J. Clin. Pharmacol. 1986, 22, 587–593. [Google Scholar] [CrossRef]
- Schmekel, B.; Rydberg, I.; Norlander, B.; Sjöswärd, K.N.; Ahlner, J.; Andersson, R.G.G. Stereoselective Pharmacokinetics of S-Salbutamol after Administration of the Racemate in Healthy Volunteers. Eur. Respir. J. 1999, 13, 1230–1235. [Google Scholar] [CrossRef][Green Version]
- Ahrens, R.C.; Smith, G.D.; Pharm, D. Albuterol: An Adrenergic Agent for Use in the Treatment of Asthma Pharmacology, Pharmacokinetics and Clinical Use. Pharmacotherapy 1984, 4, 105–121. [Google Scholar] [CrossRef]
- Sjöswärd, K.N.; Hmani, M.; Davidsson, A.; Söderkvist, P.; Schmekel, B. Single-Isomer R-Salbutamol Is Not Superior to Racemate Regarding Protection for Bronchial Hyperresponsiveness. Respir. Med. 2004, 98, 990–999. [Google Scholar] [CrossRef][Green Version]
- Ward, J.K.; Dow, J.; Dallow, N.; Eynott, P.; Milleri, S.; Ventresca, G. Pietro Enantiomeric Disposition of Inhaled, Intravenous and Oral Racemic-Salbutamol in Man—No Evidence of Enantioselective Lung Metabolism. Br. J. Clin. Pharmacol. 2000, 49, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Newman, S.; Borgström, L. Airway Deposition and Airway Effects of Antiasthma Drugs Delivered from Metered-Dose Inhalers. Eur. Respir. J. 1997, 10, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Emeryk, A.; Emeryk-Maksymiuk, J. Short-Acting Inhaled B2-Agonists: Why, Whom, What, How? Adv. Respir. Med. 2020, 88, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Story, R.E. Bronchodilators. In Allergy and Asthma: Practical Diagnosis and Management, 2nd ed.; StatPearls Publishing: Tampa, FL, USA, 2021; pp. 585–598. ISBN 9783319308357. [Google Scholar]
- Libretto, S.E. A Review of the Toxicology of Salbutamol (Albuterol). Arch. Toxicol. 1994, 68, 213–216. [Google Scholar] [CrossRef]
- Price, A.H.; Clissold, S.P. Salbutamol in the 1980s: A Reappraisal of Its Clinical Efficacy. Drugs 1989, 38, 77–122. [Google Scholar] [CrossRef]
- Macnee, W.; Douglas, N.J.; Sudlow, M.F. Effects of Inhalation of β-Sympathomimetic and Atropine-like Drugs on Airway Calibre in Normal Subjects. Clin. Sci. 1982, 63, 137–143. [Google Scholar] [CrossRef]
- Riedel, F.; von der Hart, H. Variable Response to Inhaled Salbutamol of Different Lung Function Parameters in Healthy Children. Lung 1986, 164, 333–338. [Google Scholar] [CrossRef]
- Sorbini, C.A.; Grassi, V.; Tantucci, C.; Corea, L.; Bentivoglio, M.; Verdecchia, P.; Motolese, M. Ventilatory Effects of Selective Beta 1-(Prenalterol) or Beta 2-(Salbutamol) Adrenoceptor Agonist in Man. Int. J. Clin. Pharmacol. Ther. 1984, 22, 570–575. [Google Scholar]
- Corea, L.; Bentivoglio, M.; Verdecchia, P.; Motolese, M.; Augusto Sorbini, C.; Grassi, V.; Tantucci, C. Noninvasive Assessment of Chronotropic and Inotropic Response to Preferential Beta-1 and Beta-2 Adrenoceptor Stimulation. Clin. Pharmacol. Ther. 1984, 35, 776–781. [Google Scholar] [CrossRef]
- Smith, S.R.; Ryder, C.; Kendall, M.J.; Holder, R. Cardiovascular and Biochemical Responses to Nebulised Salbutamol in Normal Subjects. Br. J. Clin. Pharmac 1984, 18, 641–644. [Google Scholar] [CrossRef][Green Version]
- Kung, M.; Croley, S.W.; Phillips, B.A. Systemic Cardiovascular and Metabolic Effects Associated with the Inhalation of an Increased Dose of Albuterol. Influence of Mouth Rinsing and Gargling. Chest 1987, 91, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Winter, R.J.D.; Langford, J.A.; Rudd, R.M. Effects of Oral and Inhaled Salbutamol and Oral Pirbuterol on Right and Left Ventricular Function in Chronic Bronchitis. Br. Med. J. 1984, 288, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, P.D.V.; Dawson, J.R.; Foale, R.A.; Timmis, A.D.; Poole-Wilson, P.A.; Sutton, G.C. Salbutamol in Treatment of Heart Failure. Br. Heart J. 1980, 43, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Whyte, K.F.; Addis, G.J.; Whitesmith, R.; Reid, J.L. The Mechanism of Salbutamol-Induced Hypokalaemia. Br. J. Clin. Pharmacol. 1987, 23, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Kendall, M.J. Metabolic Responses to Beta2 Stimulants. J. R. Coll. Physicians Lond. 1984, 18, 190–194. [Google Scholar] [PubMed]
- Wager, J.; Fredholm, B.; Lunell, N.-O.; Persson, B. Metabolic and Circulatory Effects of Intravenous and Oral Salbutamol in Late Pregnancy in Diabetic and Non-Diabetic Women. Acta Obstet. Gynecol. Scand. 1982, 61, 41–46. [Google Scholar] [CrossRef]
- Chazan, R.; Droszcz, W.; Bobilewicz, D.; Maruchin, J.E. Changes in Plasma High Density Lipoproteins (HDL) Levels after Salbutamol. Int. J. Clin. Pharmacol. Ther. Toxicol. 1985, 23, 427–429. [Google Scholar]
- Jenkins, C.R.; Marlin, G.E. The Metabolic Actions of Intravenous Salbutamol and Aminophylline Singly and in Combination. Br. J. Clin. Pharmacol. 1981, 11, 197–201. [Google Scholar] [CrossRef][Green Version]
- Lecrubier, Y.; Puech, A.J.; Jouvent, R.; Simon, P.; Widlocher, D. A Beta Adrenergic Stimulant (Salbutamol) versus Clomipramine in Depression: A Controlled Study. Br. J. Psychiatry 1980, 136, 354–358. [Google Scholar] [CrossRef]
- Gummerus, M.; Halonen, O. Prophylactic Long-term Oral Tocolysis of Multiple Pregnancies. BJOG Int. J. Obstet. Gynaecol. 1987, 94, 249–251. [Google Scholar] [CrossRef]
- de Almeida, G.M.; Scola, R.H.; Ducci, R.D.P.; Cirino, R.H.D.; Cláudia, S.K.K.; Lorenzoni, P.J.; Lima, P.H.S.; de Oliveira, L.P.; Werneck, L.C. Does Oral Salbutamol Improve Fatigue in Multiple Sclerosis? A Pilot Placebo-Controlled Study. Mult. Scler. Relat. Disord. 2020, 46, 102586. [Google Scholar] [CrossRef] [PubMed]
- Konnie, H.P. Pharmaceuticals. In Clinical Veterinary Toxicology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2004; Volume 2, pp. 282–336. [Google Scholar]
- Hellier, J.; Baudrimont, M.; Dussaule, J.; Berenbaum, F. Reversible Selective B2-Adrenoceptor Agonist-Induced Myopathy. Rheumatology 2002, 3, 90–91. [Google Scholar] [CrossRef]
- Martineau, L.; Horan, M.A.; Rothwell, N.J.; Little, R.A. Salbutamol, a Β2-Adrenoceptor Agonist, Increases Skeletal Muscle Strength in Young Men. Clin. Sci. 1992, 83, 615–621. [Google Scholar] [CrossRef]
- Courlet, P.; Buclin, T.; Biollaz, J.; Mazzoni, I.; Rabin, O.; Guidi, M. Model-Based Meta-Analysis of Salbutamol Pharmacokinetics and Practical Implications for Doping Control. CPT Pharmacometrics Syst. Pharmacol. 2022, 11, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Allen, H.; Backhouse, S.H.; Hull, J.H.; Price, O.J. Anti-Doping Policy, Therapeutic Use Exemption and Medication Use in Athletes with Asthma: A Narrative Review and Critical Appraisal of Current Regulations. Sport. Med. 2019, 49, 659–668. [Google Scholar] [CrossRef]
- WADA, World Anti-Doping Code; WADA: Montreal, QC, Canada, 2022.
- Neville, E.; Corris, P.A.; Vivian, J.; Nariman, S.; Gibson, G.J. Lesson of the Week Nebulised Salbutamol and Angina. Med. J. (Clin. Res. Ed.) 1982, 285, 796–797. [Google Scholar] [CrossRef][Green Version]
- Al-Hillawi, A.H.; Hayward, R.; Johnson, N.M. Incidence of Cardiac Arrhythmias in Patients Taking Slow Release Salbutamol and Slow Release Terbutaline for Asthma. Br. Med. J. 1984, 288, 367. [Google Scholar] [CrossRef] [PubMed]
- Neville, E.; Corris, P.; Vivian, J.; Nariman, S.; Gibson, G. Salbutamol Aerosol Causes a Tachycardia Due to the Inhaled Rather than the Swallowed Fraction. Br. J. Clin. Pharmacol. 1982, 9, 273–274. [Google Scholar]
- Barisione, G.; Baroffio, M.; Crimi, E.; Brusasco, V. Beta-Adrenergic Agonists. Pharmaceuticals 2010, 3, 1016–1044. [Google Scholar] [CrossRef]
- Malerba, M.; Politi, A.; Filippi, B.; Boni, E.; Grassi, V. Controlled-Release Oral Salbutamol and Cardiac Arrhythmias in Asthmatic Patients. Chest 1993, 104, 987–988. [Google Scholar] [CrossRef]
- Eedy, D.J.; Barton, K.; Stanford, C.F. Irritant Contact Facial Dermatitis Due to Nebulizer Therapy. Postgrad. Med. J. 1988, 64, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Hawker, F. Five Cases of Pulmonary Oedema Associated with Β2- Sympathomimetic Treatment of Premature Labour. Anaesth. Intensive Care 1984, 12, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Ayed, K.; Khalifa, I.L.H.; Mokaddem, S.; Jameleddine, S.B.K. Paradoxical Bronchoconstriction Caused by Β2-Adrenoceptor Agonists. Drug Target Insights 2020, 14, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, G.M.; Moonjelly, E.; Chan, L.; Olopade, C.O. Laryngospasm and Paradoxical Bronchoconstriction after Repeated Doses of Β2-Agonists Containing Edetate Disodium. Mayo Clin. Proc. 2000, 75, 285–287. [Google Scholar] [CrossRef]
- Melo, S.M.d.A.; de Oliveira, L.A.; Rocha, R.D.A.; Wanderley, J.L.F. Bronchodilator Test in Extreme Old Age: Adverse Effects of Short-Acting Beta-2 Adrenergic Agonists with Clinical Repercussion and Bronchodilator Response. Rev. Assoc. Med. Bras. 2019, 65, 1343–1348. [Google Scholar] [CrossRef]
- Rohr, A.S.; Spector, S.L.; Rachelefsky, G.S.; Katz, R.M.; Siegel, S.C. Efficacy of Parenteral Albuterol in the Treatment of Asthma. Comparison of Its Metabolic Side Effects with Subcutaneous Epinephrine. Chest 1986, 89, 348–351. [Google Scholar] [CrossRef]
- Torella, R.; Grandillo, F.; Giugliano, D.; Improta, L. The Effects of Salbutamol on Some Metabolic and Endocrine Patterns of Diabetic Subjects. Pharmacol. Res. Commun. 1980, 12, 909–919. [Google Scholar] [CrossRef]
- Sahan, M.; Yılmaz, M.; Gokel, Y.; Erden, E.S.; Karakus, A. Nebulized Salbutamol for Asthma: Effects on Serum Potassium and Phosphate Levels at the 60min. Rev. Port. Pneumol. 2013, 19, 200–203. [Google Scholar] [CrossRef]
- Najout, H.; Moutawakil, M.; Elkoundi, A.; Doghmi, N.; Bekkali, H. Salbutamol-Induced Severe Lactic Acidosis in Acute Asthma. SAGE Open Med. Case Rep. 2020, 8, 2050313X2096902. [Google Scholar] [CrossRef]
- Sharif, Z.; Al-Alawi, M. Beware of Beta! A Case of Salbutamol-Induced Lactic Acidosis in Severe Asthma. BMJ Case Rep. 2018, 2018, 2017–2019. [Google Scholar] [CrossRef]
- Phoophiboon, V.; Singhagowinta, P.; Boonkaya, S.; Sriprasart, T. Salbutamol-Induced Lactic Acidosis in Status Asthmaticus Survivor. BMC Pulm. Med. 2021, 21, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.H.; Chu, D.M.; Wang, C.L.; Yang, K.D. Hypokalemia and Salbutamol Therapy in Asthma. Pediatr. Pulmonol. 1999, 27, 27–31. [Google Scholar] [CrossRef]
- Chua, S.; Razvi, K.; Wong, M.T.; Tay, R.; Arulkumaran, S. Is There a Need to Treat Hypokalaemia Associated with Intravenous Salbutamol Infusion? J. Obstet. Gynaecol. Res. 1997, 23, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Udezue, E.; D’Souza, L.; Mahajan, M. Hypokalemia after Normal Doses of Nebulized Albuterol (Salbutamol). Am. J. Emerg. Med. 1995, 13, 168–171. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, E.; Olaizola Mendibil, A.; San Martín Díez, M.d.L.Á.; Burzako Sánchez, A.; Esteban-Fernández, A.; Sánchez Álvarez, E. Recomendaciones Para El Manejo de La Hiperpotasemia En Urgencias. Emerg. Rev. Soc. Esp. Med. Emerg. 2022, 34, 287–297. [Google Scholar]
- Murdoch, I.A.; Dos Anjos, R.; Haycock, G.B. Treatment of Hyperkalaemia with Intravenous Salbutamol. Arch. Dis. Child. 1991, 66, 527. [Google Scholar] [CrossRef][Green Version]
- Taylor, D.R.; Wilkins, G.T.; Herbison, G.P.; Flannery, E.M. Interaction between Corticosteroid and β-Agonist Drugs; Biochemical and Cardiovascular Effects in Normal Subjects. Chest 1992, 102, 519–524. [Google Scholar] [CrossRef]
- Lai, C.; Legge, J.; Friend, J. Air-Driven Nebulised High-Dose Salbutamol in Severe Chronic Obstructive Airways Disease: Is It Safe? Respiration 1991, 58, 249–254. [Google Scholar] [CrossRef]
- Aktar, F.; Köstü, M.; Ünal, M.; Çaksen, H. Albuterol Intoxication in a Child. J. Emerg. Med. 2013, 45, 98–99. [Google Scholar] [CrossRef]
- Khanna, P.; Davies, R. Hallucinations Associated with Administration of Salbutamol via a Nebuliser. BMJ 1986, 292, 1430. [Google Scholar]
- Littenberg, B.; Wheeler, M.; Smith, D. A Randomized Controlled Trial of Oral Albuterol in Acute Cough. J. Farmacol. Pract. 1996, 42, 49–54. [Google Scholar]
- Pratt, H.F. Abuse of Salbutamol Inhalers in Young People. Clin. Exp. Allergy 1982, 12, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Uysalol, M.; Yildiz, R.; Ozunal, Z.G. Is Seizure an Adverse Effect of Salbutamol in the Pediatric Population? Balk. Med. J. 2022, 39, 340. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Hussain, S.; Akkala, S.; Klugarová, J.; Pokorná, A.; Klugar, M.; Walters, E.H.; Hopper, I.; Campbell, J.A.; Taylor, B.; et al. Beta-Adrenergic Drugs and Risk of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2022, 80, 101670. [Google Scholar] [CrossRef]
- Finkel, M.J. Salbutamol: Lack of Evidence of Tumour Induction in Man. Br. Med. J. 1978, 1, 649. [Google Scholar] [CrossRef]
- Milner, A. Bronchodilators drugs in Childhood Asthma. Arch Dis Child. 1981, 56, 84–85. [Google Scholar] [CrossRef][Green Version]
- Boeschoten, S.A.; Buysse, C.M.P.; de Winter, B.C.M.; van Rosmalen, J.; de Jongste, J.C.; de Jonge, R.C.; Heisterkamp, S.G.J.; van Woensel, J.B.; Kneyber, M.C.J.; van Zwol, A.; et al. Efficacy of a Loading Dose of IV Salbutamol in Children with Severe Acute Asthma Admitted to a PICU: A Randomized Controlled Trial. Eur. J. Pediatr. 2022, 181, 3701–3709. [Google Scholar] [CrossRef]
- Kearns, N.; Williams, M.; Bruce, P.; Black, M.; Kearns, C.; Sparks, J.; Braithwaite, I.; Weatherall, M.; Beasley, R. Single Dose of Budesonide/Formoterol Turbuhaler Compared to Salbutamol PMDI for Speed of Bronchodilator Onset in Asthma: A Randomised Cross-over Trial. Thorax, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Jat, K.R.; Khairwa, A. Levalbuterol versus Albuterol for Acute Asthma: A Systematic Review and Meta-Analysis. Pulm. Pharmacol. Ther. 2013, 26, 239–248. [Google Scholar] [CrossRef]
- Gawchik, S.M.; Saccar, C.L.; Noonan, M.; Reasner, D.S.; DeGraw, S.S. The Safety and Efficacy of Nebulized Levalbuterol Compared with Racemic Albuterol and Placebo in the Treatment of Asthma in Pediatric Patients. J. Allergy Clin. Immunol. 1999, 103, 615–621. [Google Scholar] [CrossRef]
- Tribe, A.E.; Wong, R.M.; Robinson, J.S. A Controlled Trial of Intravenous Salbutamol and Aminophylline in Acute Asthma. Med. J. Aust. 1976, 2, 749–752. [Google Scholar] [CrossRef]
- Spring, J.; Clague, J.; Ind, P. A Comparison of the Effect of Salmeterol and Salbutamol in Normal Subjects. Br. J. Clin. Pharmacol. 1992, 33, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Lundback, B.; Rawlinson, D.W.; Palmer, J.B.D. Twelve Month Comparison of Salmeterol and Salbutamol as Dry Powder Formulations in Asthmatic Patients. Thorax 1993, 48, 148–153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sichletidis, L.; Daskalopoulou, E.; Kyriazis, G.; Kosmidou, I.; Koupidou, S.; Pechlivanidis, T.; Chloros, D. Comparative Efficacy of Salbutamol and Salmeterol in Exercise-Induced Asthma. J. Int. Med. Res. 1993, 21, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, B.J.; Aziz, I. Bronchodilator Response to Albuterol after Regular Formoterol and Effects of Acute Corticosteroid Administration. Chest 2000, 117, 156–162. [Google Scholar] [CrossRef]
- Van der Woude, H.J.; Winter, T.H.; Aalbers, R. Decreased Bronchodilating Effect of Salbutamol in Relieving Methacholine Induced Moderate to Severe Bronchoconstriction during High Dose Treatment with Long Acting Β2 Agonists. Thorax 2001, 56, 529–535. [Google Scholar] [CrossRef]
- LaForce, C.; Chipps, E.B.; Albers, F.C.; Reilly, L.; Johnsson, E.; Andrews, H.; Cappelletti, C.; Maes, A.; Papi, A. Albuterol/Budesonide for the Treatment of Exercise-Induced Bronchoconstriction in Patients with Asthma: The TYREE Study. Annu. Allergy Asthma Immunol. 2022, 128, 169–177. [Google Scholar] [CrossRef]
- Papi, A.; Chipps, B.E.; Beasley, R.; Panettieri, R.A., Jr.; Israel, E.; Cooper, M.; Dunsire, L.; Jeynes-Ellis, A.; Johnsson, E.; Rees, R.; et al. Albuterol-Budesonide Fixed-Dose Combination Rescue Inhaler for Asthma. N. Engl. J. Med. 2022, 386, 2071–2083. [Google Scholar] [CrossRef]
- Tudela, J.; Martínez, M.; Valdivia, R.; Romo, J.; Portillo, M.; Rangel, R. Effects of Budesonide Combined with Salbutamol on Pulmonary Function and Peripheral Blood Eosinophiles and IgE in Patients with Acute Attack of Bronchial Asthms. Nature 2010, 388, 539–547. [Google Scholar]
- Sarhan, H.A.; El-Garhy, O.H.; Ali, M.A.; Youssef, N.A. The Efficacy of Nebulized Magnesium Sulfate Alone and in Combination with Salbutamol in Acute Asthma. Drug Des. Devel. Ther. 2016, 10, 1927–1933. [Google Scholar] [CrossRef]
- Smyth, E.T.; Pavord, I.D.; Wong, C.S.; Wisniewski, A.F.Z.; Williams, J.; Tattersfield, A.E. Interaction and Dose Equivalence of Salbutamol and Salmeterol in Patients with Asthma. Br. Med. J. 1993, 306, 543–545. [Google Scholar] [CrossRef]
- Ruffin, R.E.; Latimer, K.M.; Crockett, A.J.; Blight, M.M. A Comparative Bronchodilator Study of Salbutamol and Salbutamol Sulphate That Were Administered by Metered-Dose Inhalers. Med. J. Aust. 1989, 150, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Haahtela, T.; Alanko, K.; Muittari, A.; Lahdensuo, A.; Sahlstrom, K.; Vilkka, V. The Superiority of Combination Beclomethasone and Salbutamol over Standard Dosing of Salbutamol in the Treatment of Chronic Asthma. Annu. Allergy 1989, 62, 63–66. [Google Scholar]
- Taitinen, L.A.; Poppius, H. Combination of Oxitropium Bromide and Salbutamol in the Treatment of Asthma with Pressurized Aerosols. Br. J. Dis. Chest 1986, 80, 179–186. [Google Scholar] [CrossRef]
- Iramain, R.; López-Herce, J.; Coronel, J.; Spitters, C.; Guggiari, J.; Bogado, N. Inhaled Salbutamol plus Ipratropium in Moderate and Severe Asthma Crises in Children. J. Asthma 2011, 48, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Madaan, A. Nebulized Salbutamol Vs Salbutamol and Ipratropium Combination in Asthma. Indian J. Pediatr. 2004, 71, 121–124. [Google Scholar] [CrossRef]
- Dawson, K.P.; Fergusson, D.M. Effects of Oral Theophylline and Oral Salbutamol in the Treatment of Asthma. Arch. Dis. Child. 1982, 57, 674–676. [Google Scholar] [CrossRef]
- Desche, P.; Cournot, A.; Duchier, J.; Prost, J.F. Airway Response to Salbutamol and to Ipratropium Bromide after Non-Selective and Cardioselective Beta-Blocker. Eur. J. Clin. Pharmacol. 1987, 32, 343–346. [Google Scholar] [CrossRef]
- Lipworth, B.J.; McDevitt, D.G.; Struthers, A.D. Prior Treatment with Diuretic Augments the Hypokalemic and Electrocardiographic Effects of Inhaled Albuterol. Am. J. Med. 1989, 86, 653–657. [Google Scholar] [CrossRef]
- Spiro, S.G.; May, C.S.; Johnson, A.J.; Paterson, J.W. Intravenous Injection of Salbutamol in the Management of Asthma. Thorax 1975, 30, 236. [Google Scholar]
- Shann, F. Dose of Intravenous Infusions of Terbatuline and Salbutamol. Crit. Care Med. 2000, 28, 2179. [Google Scholar] [CrossRef]
- Sellers, W.F.S.; Messahel, B. Rapidly Repeated Intravenous Boluses of Salbutamol for Acute Severe Asthma. Anaesthesia 2003, 58, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Starkey, E.S.; Mulla, H.; Sammons, H.M.; Pandya, H.C. Intravenous Salbutamol for Childhood Asthma: Evidence-Based Medicine? Arch. Dis. Child. 2014, 99, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Semple, P.D.; Legge, J.S.; Habeshaw, T. Intramuscular Salbutamol in Acute Asthma. Br. J. Clin. Pharmacol. 1976, 3, 935. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dawson, K.; Penna, A.; Manglick, P. Acute Asthma, Salbutamol and Hyperglycaemia. Acta Paediatr. 1995, 84, 305–307. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Milner, A.D.; Swarbrick, A. Nebulised Salbutamol Does Have a Protective Effect on Airways in Children under 1 Year Old. Arch. Dis. Child. 1988, 63, 479–483. [Google Scholar] [CrossRef]
- Welch, M.J. Pharmacokinetics, Pharmacodynamics, and Clinical Efficacy of Albuterol RespiClickTM Dry-Powder Inhaler in the Treatment of Asthma. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1109–1119. [Google Scholar] [CrossRef]
- Salbutamol: Inhaler to Relieve Asthma and Breathlessness—NHS. Available online: https://www.nhs.uk/medicines/salbutamol-inhaler/ (accessed on 10 October 2022).
- Werner, H.A. Status Asthmaticus in Children: A Review. Chest 2001, 119, 1913–1929. [Google Scholar] [CrossRef]
- Fok, T.F.; Monkman, S.; Dolovich, M.; Gray, S.; Coates, G.; Paes, B.; Rashid, F.; Newhouse, M.; Kirpalani, H. Efficiency of Aerosol Medication Delivery from a Metered Dose Inhaler versus Jet Nebulizer in Infants with Bronchopulmonary Dysplasia. Pediatr. Pulmonol. 1996, 21, 301–309. [Google Scholar] [CrossRef]
- Tukiainen, H.; Terho, E.O. Comparison of Inhaled Salbutamol Powder and Aerosol in Asthmatic Patients with Low Peak Expiratory Flow Level. Eur. J. Clin. Pharmacol. 1985, 27, 645–647. [Google Scholar] [CrossRef]
- Harrison, B.A.; Pierce, R.J. Comparison of Wet and Dry Aerosol Salbutamol. Aust. N. Z. J. Med. 1983, 13, 29–33. [Google Scholar] [CrossRef]
- Jenkins, S.C.; Heaton, R.W.; Fulton, T.J.; Moxham, J. Comparison of Domiciliary Nebulized Salbutamol and Salbutamol from a Metered-Dose Inhaler in Stable Chronic Airflow Limitation. Chest 1987, 91, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Dawson, K.P.; Unter, C.E.M.; Deo, S.; Fergusson, D.M. Inhalation Powder and Oral Salbutamol Combination. Arch. Dis. Child. 1986, 61, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Collins-Williams, C. Oral Salbutamol Therapy of Asthma in Young Children. J. Asthma Res. 1977, 15, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Legge, J.S.; Gaddie, J.; Palmer, K.N.V. Comparison of Two Oral Selective B2-Adrenergic Stimulant Drugs in Bronchial Asthma. Br. Med. J. 1971, 1, 637–639. [Google Scholar] [CrossRef][Green Version]
- Parker, S.S.; Choo-Kang, Y.F.J.; Cooper, E.J.; Cameron, S.J.; Grant, I.W.B. Bronchodilator Effect of Oral Salbutamol in Asthmatics Treated with Corticosteroids. Br. Med. J. 1971, 4, 139–142. [Google Scholar] [CrossRef]
- Grimwood, K.; Johnson-Barrett, J.J.; Taylor, B. Salbutamol: Tablets, Inhalational Powder, or Nebuliser? Br. Med. J. 1981, 282, 105–106. [Google Scholar] [CrossRef]
- Scherer, G.W. Mechanics of Syneresis I. Theory. J. Non. Cryst. Solids 1989, 108, 18–27. [Google Scholar] [CrossRef]
- Anderson, H.R. Intravenous Infusion of Salbutamol in Severe Acute Asthma. Br. Med. J. 1978, 1, 1620. [Google Scholar] [CrossRef][Green Version]
- Biddiscombe, M.F.; Usmani, O.S. Is There Room for Further Innovation in Inhaled Therapy for Airways Disease? Breathe 2018, 14, 216–224. [Google Scholar] [CrossRef]
- Charriot, J.; Vachier, I.; Halimi, L.; Gamez, A.S.; Boissin, C.; Salama, M.; Cucu-Jarjour, A.; Ahmed, E.; Bourdin, A. Future Treatment for Asthma. Eur. Respir. Rev. 2016, 25, 77–92. [Google Scholar] [CrossRef]
- Masefield, S.; Edwards, J.; Hansen, K.; Hamerlijnck, D.; Lisspers, K.; Van Der Schee, M.; Silva, L.; Matthews, J.; Gaga, M.; Adcock, I.; et al. The Future of Asthma Research and Development: A Roadmap from the European Asthma Research and Innovation Partnership (EARIP). Eur. Respir. J. 2017, 49, 1602295. [Google Scholar] [CrossRef] [PubMed]
- Ramey, O.L.; Silva Almodóvar, A.; Nahata, M.C. Medication Adherence in Medicare-Enrolled Older Adults with Asthma before and during the Coronavirus Disease 2019 Pandemic. Ann. Allergy Asthma Immunol. 2022, 128, 561–567.e1. [Google Scholar] [CrossRef] [PubMed]
| Severity Level | Clinical Characteristics |
|---|---|
| Mild asthma | Controlled using as-needed ICS-formoterol, or with low dose ICS with as-needed SABA |
| Moderate asthma | Controlled with low- or medium-dose ICS-LABA |
| Severe asthma | Requires high-dose ICS-LABA to prevent it from becoming uncontrollable, or asthma that is still uncontrolled despite this treatment |
| Clinical Use | Inhaler (100 μg) | Dry Powder Inhaler (200 μg) | Nebulizer (5 mg/mL) | Oral Syrup (2 mg/5 mL) | Oral Tablets (2 or 4 mg) | Intramuscular Subcutaneous | Intravenous |
|---|---|---|---|---|---|---|---|
| Intermittent asthma attacks or acute bronchospasm | A: 1 to 2 puffs every 4 h up to 4 times a day C: 1 puff every 4 h up to 4 times a day | 1 puff up to 4 times per day | AC: 0.5 to 1 mL | A: 5 mL to 20 mL, up to 4 times a day C: 2.5 or 5 mL, 3 or 4 times a day | A: 4 mg, 3 or 4 times a day C: 1 or 2 mg, 3 or 4 times a day | A: 500 μg every 4 h | A: 250 μg injected slowly |
| Exercise-induced bronchoconstriction | A: 2 puffs 15 min before exercise C: 1 puff 15 min before exercise | 1 puff 10 to 15 min before exercise | NA | NA | NA | NA | NA |
| Continuous treatment | NA | NA | 1 to 2 mg per hour | NA | NA | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, L.; Vale, N. Salbutamol in the Management of Asthma: A Review. Int. J. Mol. Sci. 2022, 23, 14207. https://doi.org/10.3390/ijms232214207
Marques L, Vale N. Salbutamol in the Management of Asthma: A Review. International Journal of Molecular Sciences. 2022; 23(22):14207. https://doi.org/10.3390/ijms232214207
Chicago/Turabian StyleMarques, Lara, and Nuno Vale. 2022. "Salbutamol in the Management of Asthma: A Review" International Journal of Molecular Sciences 23, no. 22: 14207. https://doi.org/10.3390/ijms232214207
APA StyleMarques, L., & Vale, N. (2022). Salbutamol in the Management of Asthma: A Review. International Journal of Molecular Sciences, 23(22), 14207. https://doi.org/10.3390/ijms232214207







