The Impact of P-Glycoprotein on Opioid Analgesics: What’s the Real Meaning in Pain Management and Palliative Care?
Abstract
1. Introduction
2. What Is P-gp?
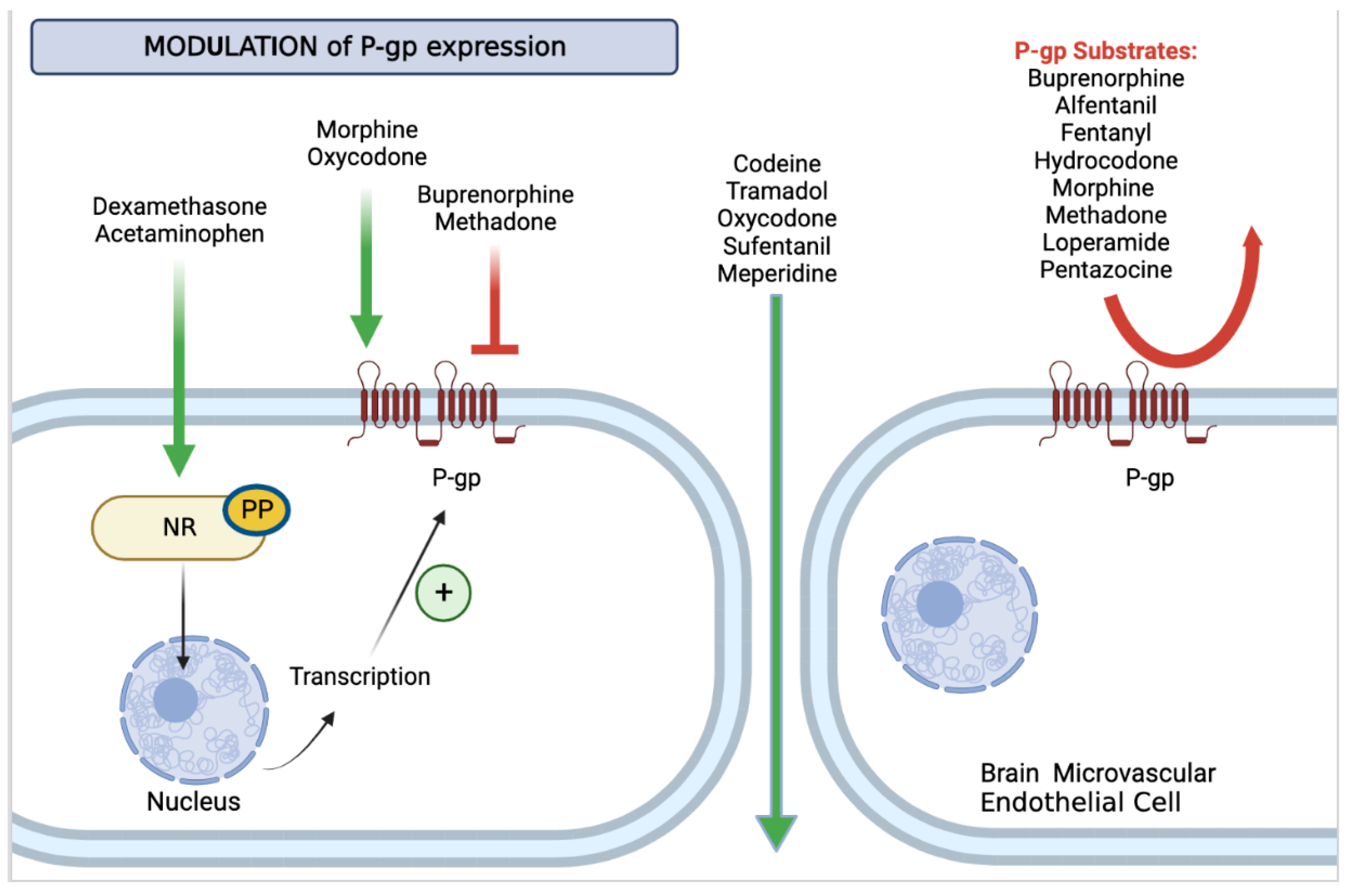
2.1. P-gp Regulation
2.2. P-gp Inhibition
2.3. P-gp and Pain/Inflammation
2.4. P-gp Polymorphisms
2.5. Opioids and P-gp
2.6. The Role of P-gp in Drug–Drug Interactions
2.7. Expression of P-gp during Aging
2.8. P-gp and Peripherally Acting Mu-Opioid Receptors Antagonists (PAMORAs)
2.9. P-gp and Neuropathic Pain Medications
2.10. P-gp and Non-Opioidergic Pain-Relievers
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mercadante, S.; Coluzzi, F. Factors Influencing Pain Expression in Patients with Cancer: An Expert Opinion. Pain Ther. 2021, 10, 765–775. [Google Scholar] [CrossRef]
- Varrassi, G.; Coluzzi, F.; Guardamagna, V.A.; Puntillo, F.; Sotgiu, G.; Vellucci, R.; Rational Use of Analgesics (RUA) Group. Personalizing Cancer Pain Therapy: Insights from the Rational Use of Analgesics (RUA) Group. Pain Ther. 2021, 10, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Remiao, F.; Cisternino, S.; Decleves, X. Opioids and the Blood-Brain Barrier: A Dynamic Interaction with Consequences on Drug Disposition in Brain. Curr. Neuropharmacol. 2017, 15, 1156–1173. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.P.; Tome, M.E.; Davis, T.P. The opioid epidemic: A central role for the blood brain barrier in opioid analgesia and abuse. Fluids Barriers CNS 2017, 14, 32. [Google Scholar] [CrossRef]
- Davis, T.P.; Sanchez-Covarubias, L.; Tome, M.E. P-glycoprotein trafficking as a therapeutic target to optimize CNS drug delivery. Adv. Pharmacol. 2014, 71, 25–44. [Google Scholar] [CrossRef]
- Suzuki, T.; Zaima, C.; Moriki, Y.; Fukami, T.; Tomono, K. P-glycoprotein mediates brain-to-blood efflux transport of buprenorphine across the blood–brain barrier. J. Drug Target. 2007, 15, 67–74. [Google Scholar] [CrossRef]
- Tournier, N.; Decleves, X.; Saubamea, B.; Scherrmann, J.M.; Cisternino, S. Opioid Transport by ATP-Binding Cassette Transporters at the Blood-Brain Barrier: Implications for Neuropsychopharmacology. Curr. Pharm. Des. 2011, 17, 2829–2842. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Koren, G. P-glycoprotein in the developing human brain: A review of the effects of ontogeny on the safety of opioids in neonates. Ther. Drug Monit. 2014, 36, 699–705. [Google Scholar] [CrossRef]
- Wilt, L.A.; Nguyen, D.; Roberts, A.G. Insights Into the Molecular Mechanism of Triptan Transport by P-glycoprotein. J. Pharm. Sci. 2017, 106, 1670–1679. [Google Scholar] [CrossRef]
- Fu, D.; Arias, I.M. Intracellular trafficking of P-glycoprotein. Int. J. Biochem. Cell Biol. 2012, 44, 461–464. [Google Scholar] [CrossRef]
- Levran, O.; O’Hara, K.; Peles, E.; Li, D.; Barral, S.; Ray, B.; Borg, L.; Ott, J.; Adelson, M.; Kreek, M.J. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum. Mol. Genet. 2008, 17, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gong, W.; Wang, Y.; Liu, Y.; Li, C. Exploring movement and energy in human P-glycoprotein conformational rearrangement. J. Biomol. Struct. Dyn. 2019, 37, 1104–1119. [Google Scholar] [CrossRef] [PubMed]
- Abel, B.; Tosh, D.K.; Durell, S.R.; Murakami, M.; Vahedi, S.; Jacobson, K.A.; Ambudkar, S.V. Evidence for the Interaction of A3 Adenosine Receptor Agonists at the Drug-Binding Site(s) of Human P-glycoprotein (ABCB1). Mol. Pharmacol. 2019, 96, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Kodan, A.; Futamata, R.; Kimura, Y.; Kioka, N.; Nakatsu, T.; Kato, H.; Ueda, K. ABCB1/MDR1/P-gp employs an ATP-dependent twist-and-squeeze mechanism to export hydrophobic drugs. FEBS Lett. 2021, 595, 707–716. [Google Scholar] [CrossRef]
- Thangapandian, S.; Kapoor, K.; Tajkhorshid, E. Probing cholesterol binding and translocation in P-glycoprotein. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183090. [Google Scholar] [CrossRef]
- Tome, M.E.; Herndon, J.M.; Schaefer, C.P.; Jacobs, L.M.; Zhang, Y.; Jarvis, C.K.; Davis, T.P. P-glycoprotein traffics from the nucleus to the plasma membrane in rat brain endothelium during inflammatory pain. J. Cereb. Blood Flow Metab. 2016, 36, 1913–1928. [Google Scholar] [CrossRef]
- Paterson, J.K.; Gottesman, M.M. P-Glycoprotein is not present in mitochondrial membranes. Exp. Cell Res. 2007, 313, 3100–3105. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, J.; Yang, W.Z.; Xian, Z.H.; Feng, Q.T.; Ruan, X.C. Mitochondrial expression and activity of P-glycoprotein under oxidative stress in outer blood-retinal barrier. Int. J. Ophthalmol. 2017, 10, 1055–1063. [Google Scholar] [CrossRef]
- Guo, W.; Dong, W.; Li, M.; Shen, Y. Mitochondria P-glycoprotein confers paclitaxel resistance on ovarian cancer cells. Onco Targets Ther. 2019, 12, 3881–3891. [Google Scholar] [CrossRef]
- Chan, G.N.Y.; Saldivia, V.; Yang, Y.; Pang, H.; Lannoy, I.; Bendayan, R. In Vivo Induction of P-Glycoprotein Expression at the Mouse Blood–Brain Barrier: An Intracerebral Microdialysis Study. J. Neurochem. 2013, 127, 342–352. [Google Scholar] [CrossRef]
- Seleman, M.; Chapy, H.; Cisternino, S.; Courtin, C.; Smirnova, M.; Schlatter, J.; Chiadmi, F.; Scherrmann, J.-M.; Noble, F.; Marie-Claire, C. Impact of P-glycoprotein at the blood-brain barrier on the uptake of heroin and its main metabolites: Behavioral effects and consequences on the transcriptional responses and reinforcing properties. Psychopharmacology 2014, 231, 3139–3149. [Google Scholar] [CrossRef]
- Amin, M.L. P-glycoprotein inhibition for optimal drug delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Noack, A.; Gericke, B.; von Köckritz-Blickwede, M.; Menze, A.; Noack, S.; Gerhauser, I.; Osten, F.; Naim, H.Y.; Löscher, W. Mechanism of drug extrusion by brain endothelial cells via lysosomal drug trapping and disposal by neutrophils. Proc. Natl. Acad. Sci. USA 2018, 115, E9590–E9599. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Hegelund-Myrbäck, T.; Öckinger, J.; Zhou, X.H.; Brännström, M.; Hagemann-Jensen, M.; Werkström, V.; Seidegård, J.; Grunewald, J.; Nord, M.; et al. Expression of MATE1, P-gp, OCTN1 and OCTN2, in epithelial and immune cells in the lung of COPD and healthy individuals. Respir. Res. 2018, 19, 68. [Google Scholar] [CrossRef]
- Cufer, T.; Pfeifer, M.; Vrhovec, I.; Frangez, R.; Kosec, M.; Mrhar, A.; Grabnar, I.; Golouh, R.; Vogric, S.; Sikic, B.I. Decreased cortisol secretion by adrenal glands perfused with the P-glycoprotein inhibitor valspodar and mitotane or doxorubicin. Anticancer Drugs 2000, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kammala, A.; Benson, M.; Ganguly, E.; Richardson, L.; Menon, R. Functional role and regulation of permeability-glycoprotein (P-gp) in the fetal membrane during drug transportation. Am. J. Reprod. Immunol. 2022, 87, e13515. [Google Scholar] [CrossRef]
- Hakeam, H.A.; Al-Sanea, N. Effect of major gastrointestinal tract surgery on the absorption and efficacy of direct acting oral anticoagulants (DOACs). J. Thromb. Thrombolysis 2017, 43, 343–351. [Google Scholar] [CrossRef]
- Tremblay, S.; Alloway, R.R. Clinical Evaluation of Modified Release and Immediate Release Tacrolimus Formulations. AAPS J. 2017, 19, 1332–1347. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Murakami, T.; Bodor, E.; Bodor, N. Factors and dosage formulations affecting the solubility and bioavailability of P-glycoprotein substrate drugs. Expert Opin. Drug Metab. Toxicol. 2021, 17, 555–580. [Google Scholar] [CrossRef]
- Savolainen, H.; Meerlo, P.; Elsinga, P.H.; Windhorst, A.D.; Dierckx, R.A.; Colabufo, N.A.; Van Waarde, A.; Luurtsema, G. P-glycoprotein Function in the Rodent Brain Displays a Daily Rhythm, a Quantitative In Vivo PET Study. AAPS J. 2016, 18, 1524–1531. [Google Scholar] [CrossRef]
- Murakami, T.; Bodor, E.; Bodor, N. Modulation of expression/function of intestinal P-glycoprotein under disease states. Expert Opin. Drug Metab. Toxicol. 2020, 16, 59–78. [Google Scholar] [CrossRef]
- Mercer, S.L.; Coop, A. Opioid analgesics and P-glycoprotein efflux transporters: A potential systems-level contribution to analgesic tolerance. Curr. Top. Med. Chem. 2011, 11, 1157–1164. [Google Scholar] [CrossRef]
- Mollazadeh, S.; Sahebkar, A.; Hadizadeh, F.; Behravan, J.; Arabzadeh, S. Structural and functional aspects of P-glycoprotein and its inhibitors. Life Sci. 2018, 214, 118–123. [Google Scholar] [CrossRef]
- Hassan, H.E.; Myers, A.L.; Lee, I.J.; Coop, A.; Eddington, N.D. Oxycodone induces overexpression of P-glycoprotein (ABCB1) and affects paclitaxel’s tissue distribution in Sprague Dawley rats. J. Pharm. Sci. 2007, 96, 2494–2506. [Google Scholar] [CrossRef] [PubMed]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: Review of Clinical Drug-Drug Interaction Studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Ashby, C.R., Jr.; Assaraf, Y.G.; Chen, Z.S.; Liu, H.M. Chemical molecular-based approach to overcome multidrug resistance in cancer by targeting P-glycoprotein (P-gp). Med. Res. Rev. 2021, 41, 525–555. [Google Scholar] [CrossRef]
- Qamar, Z.; Ashhar, M.U.; Annu; Qizilibash, F.F.; Sahoo, P.K.; Ali, A.; Ali, J.; Baboota, S. Lipid nanocarrier of selegiline augmented anti-Parkinson’s effect via P-gp modulation using quercetin. Int. J. Pharm. 2021, 609, 121131. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.B.; Leung, G.K.F.; Callaghan, R.; Gelissen, I.C. P-glycoprotein: A role in the export of amyloid-β in Alzheimer’s disease? FEBS J. 2020, 287, 612–625. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, E.A.; Iyer, A.M.; Mesarosova, L.; Çolakoglu, H.; Anink, J.J.; van Tellingen, O.; Maragakis, N.J.; Shefner, J.; Bunt, T.; Aronica, E. Expression and Cellular Distribution of P-Glycoprotein and Breast Cancer Resistance Protein in Amyotrophic Lateral Sclerosis Patients. J. Neuropathol. Exp. Neurol. 2020, 79, 266–276. [Google Scholar] [CrossRef]
- Garg, N.; Joshi, R.; Medhi, B. A novel approach of targeting refractory epilepsy: Need of an hour. Brain Res. Bull. 2020, 163, 14–20. [Google Scholar] [CrossRef]
- Lawson, J.; O’Brien, T.; Graham, M.; Renaud, E.; Jones, D.; Freeman, J.; Lawn, N.; Martin, J.H. Expert advice for prescribing cannabis medicines for patients with epilepsy-drawn from the Australian clinical experience. Br. J. Clin. Pharmacol. 2022, 88, 3101–3113. [Google Scholar] [CrossRef]
- Auzmendi, J.; Palestro, P.; Blachman, A.; Gavernet, L.; Merelli, A.; Talevi, A.; Calabrese, G.C.; Ramos, A.J.; Lazarowski, A. Cannabidiol (CBD) Inhibited Rhodamine-123 Efflux in Cultured Vascular Endothelial Cells and Astrocytes Under Hypoxic Conditions. Front. Behav. Neurosci. 2020, 14, 32. [Google Scholar] [CrossRef]
- Hoque, T.M.D.; Cattin, A.; Whyte-Allman, S.K.; Winchester, L.; Fletcher, C.V.; Routy, J.P.; Ancuta, P.; Bendayan, R. Antiretroviral Drug Transporters and Metabolic Enzymes in Circulating Monocytes and Monocyte-Derived Macrophages of ART-Treated People Living With HIV and HIV-Uninfected Individuals. J. Acquir. Immune Defic. Syndr. 2021, 87, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Kwatra, D.; Minocha, M.; Paturi, D.K.; Budda, B.; Mitra, A.K. Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 2011, 88, 959–971. [Google Scholar] [CrossRef] [PubMed]
- Kharasch, E.D.; Bedynek, P.S.; Walker, A.; Whittington, D.; Hoffer, C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: II. Ritonavir effects on CYP3A and Pglycoprotein activities. Clin. Pharmacol. Ther. 2008, 84, 506–512. [Google Scholar] [CrossRef]
- Marks, L.R.; Nolan, N.S.; Liang, S.Y.; Durkin, M.J.; Weimer, M.B. Infectious Complications of Injection Drug Use. Med. Clin. N. Am. 2022, 106, 187–200. [Google Scholar] [CrossRef]
- Schaefer, C.P.; Arkwright, N.B.; Jacobs, L.M.; Jarvis, C.K.; Hunn, K.C.; Largent-Milnes, T.M.; Tome, M.E.; Davis, T.P. Chronic morphine exposure potentiates p-glycoprotein trafficking from nuclear reservoirs in cortical rat brain microvessels. PLoS ONE. 2018, 13, e0192340. [Google Scholar] [CrossRef]
- Stage, T.B.; Mortensen, C.; Khalaf, S.; Steffensen, V.; Hammer, H.S.; Xiong, C.; Nielsen, F.; Poetz, O.; Svenningsen, Å.F.; Rodriguez-Antona, C.; et al. P-Glycoprotein Inhibition Exacerbates Paclitaxel Neurotoxicity in Neurons and Patients With Cancer. Clin. Pharmacol. Ther. 2020, 108, 671–680. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R., Jr.; Chen, Z.S.; Cheng, X.D.; Qin, J.J. Medicinal chemistry strategies to discover P-glycoprotein inhibitors: An update. Drug Resist. Updates 2020, 49, 100681. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Drewe, J.; Krähenbühl, S.; Huwyler, J.; Gutmann, H. Regulation of BCRP (ABCG2) and P-glycoprotein (ABCB1) by cytokines in a model of the human blood–brain barrier. Cell. Mol. Neurobiol. 2010, 30, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Ambudkar, S.V. ATP-dependent thermostabilization of human P-glycoprotein (ABCB1) is blocked by modulators. Biochem. J. 2019, 476, 3737–3750. [Google Scholar] [CrossRef]
- Stanković, T.; Dinić, J.; Podolski-Renić, A.; Musso, L.; Burić, S.S.; Dallavalle, S.; Pešić, M. Dual Inhibitors as a New Challenge for Cancer Multidrug Resistance Treatment. Curr. Med. Chem. 2019, 26, 6074–6106. [Google Scholar] [CrossRef]
- Halder, J.; Pradhan, D.; Kar, B.; Ghosh, G.; Rath, G. Nanotherapeutics approaches to overcome P-glycoprotein-mediated multi-drug resistance in cancer. Nanomedicine 2021, 40, 102494. [Google Scholar] [CrossRef]
- Wang, C.; Guan, W.; Peng, J.; Chen, Y.; Xu, G.; Dou, H. Gene/paclitaxel co-delivering nanocarriers prepared by framework-induced self-assembly for the inhibition of highly drug-resistant tumors. Acta Biomater. 2020, 103, 247–258. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, Y.; Wang, M.; Li, Z.; Su, L.; Xu, X.; Xing, C.; Li, J.; Lin, L.; Lu, C.; et al. siRNA-Based Carrier-Free System for Synergistic Chemo/Chemodynamic/RNAi Therapy of Drug-Resistant Tumors. ACS Appl. Mater. Interfaces 2021, 14, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Chen, S.; Zhang, S.; Cui, C. Cetuximab-Modified Human Serum Albumin Nanoparticles Co-Loaded with Doxorubicin and MDR1 siRNA for the Treatment of Drug-Resistant Breast Tumors. Int. J. Nanomed. 2021, 16, 7051–7069. [Google Scholar] [CrossRef]
- Clouser, A.F.; Alam, Y.H.; Atkins, W.M. Cholesterol Asymmetrically Modulates the Conformational Ensemble of the Nucleotide-Binding Domains of P-Glycoprotein in Lipid Nanodiscs. Biochemistry 2021, 60, 85–94. [Google Scholar] [CrossRef]
- Weinheimer, M.; Fricker, G.; Burhenne, J.; Mylius, P.; Schubert, R. The application of P-gp inhibiting phospholipids as novel oral bioavailability enhancers—An in vitro and in vivo comparison. Eur. J. Pharm. Sci. 2017, 108, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Vahedi, S.; Lusvarghi, S.; Pluchino, K.; Shafrir, Y.; Durell, S.R.; Gottesman, M.M.; Ambudkar, S.V. Mapping discontinuous epitopes for MRK-16, UIC2 and 4E3 antibodies to extracellular loops 1 and 4 of human P-glycoprotein. Sci. Rep. 2018, 8, 12716. [Google Scholar] [CrossRef]
- Li, F.; Mao, C.; Yeh, S.; Sun, Y.; Xin, J.; Shi, Q.; Ming, X. MRP1-targeted near infrared photoimmunotherapy for drug resistant small cell lung cancer. Int. J. Pharm. 2021, 604, 120760. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, F.; Zhu, Q.; Li, X.; Ren, H.; Fan, S.; Qian, B.; Zhai, B.; Yang, D. PD-L1 monoclonal antibody-decorated nanoliposomes loaded with Paclitaxel and P-gp transport inhibitor for the synergistic chemotherapy against multidrug resistant gastric cancers. Nanoscale Res. Lett. 2020, 15, 59. [Google Scholar] [CrossRef]
- Tome, M.E.; Jarvis, C.K.; Schaefer, C.P.; Jacobs, L.M.; Herndon, J.M.; Hunn, K.C.; Arkwright, N.B.; Kellohen, K.L.; Mierau, P.C.; Davis, T.P. Acute pain alters P-glycoprotein-containing protein complexes in rat cerebral microvessels: Implications for P-glycoprotein trafficking. J. Cereb. Blood Flow Metab. 2018, 38, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, G.; Staatz, W.D.; Sanchez-Covarrubias, L.; Finch, J.D.; Demarco, K.; Laracuente, M.L.; Ronaldson, P.T.; Davis, T.P. P-glycoprotein trafficking at the blood-brain barrier altered by peripheral inflammatory hyperalgesia. J. Neurochem. 2012, 122, 962–975. [Google Scholar] [CrossRef]
- Barakat, S.; Demeule, M.; Pilorget, A.; Regina, A.; Gingras, D.; Baggetto, L.G.; Beliveau, R. Modulation of p-glycoprotein function by caveolin-1 phosphorylation. J. Neurochem. 2007, 101, 1–8. [Google Scholar] [CrossRef]
- DosSantos, M.F.; Holanda-Afonso, R.C.; Lima, R.L.; DaSilva, A.F.; Moura-Neto, V. The role of the blood-brain barrier in the development and treatment of migraine and other pain disorders. Front. Cell Neurosci. 2014, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.R.; Ocheltree, S.M.; Hom, S.; Egleton, R.D.; Davis, T.P. Nociceptive inhibition prevents inflammatory pain induced changes in the blood-brain barrier. Brain Res. 2008, 1221, 6–13. [Google Scholar] [CrossRef]
- Sanchez-Covarrubias, L.; Slosky, L.M.; Thompson, B.J.; Zhang, Y.; Laracuente, M.; DeMarco, K.M.; Ronaldson, P.T.; Davis, T.P. P-Glycoprotein Modulates Morphine Uptake into the CNS: A Role for the Non-Steroidal Anti-Inflammatory Drug Diclofenac. PLoS ONE 2014, 9, e88516. [Google Scholar] [CrossRef]
- Bauer, B.; Hartz, A.M.; Miller, D.S. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol. Pharmacol. 2007, 71, 667–675. [Google Scholar] [CrossRef]
- Yousif, S.; Chaves, C.; Potin, S.; Margaill, I.; Scherrmann, J.M.; Declèves, X. Induction of P-glycoprotein and Bcrp at the rat blood-brain barrier following a subchronic morphine treatment is mediated through NMDA/COX-2 activation. J. Neurochem. 2012, 123, 491–503. [Google Scholar] [CrossRef]
- Chan, G.N.; Hoque, M.T.; Cummins, C.L.; Bendayan, R. Regulation of P-glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J. Neurochem. 2011, 118, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Slosky, L.M.; Thompson, B.J.; Sanchez-Covarrubias, L.; Zhang, Y.; Laracuente, M.L.; Vanderah, T.W.; Ronaldson, P.T.; Davis, T.P. Acetaminophen modulates P-glycoprotein functional expression at the blood-brain barrier by a constitutive androstane receptor-dependent mechanism. Mol. Pharmacol. 2013, 84, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Yang, X.; Hartz, A.M.S.; Olson, E.R.; Zhao, R.; Kalvass, J.C.; Pollack, G.M.; Miller, D.S. In vivo activation of human pregnane X receptor tightens the blood-brain barrier to methadone through P-glycoprotein up-regulation. Mol. Pharmacol. 2006, 70, 1212–1219. [Google Scholar] [CrossRef]
- Narang, V.S.; Fraga, C.; Kumar, N.; Shen, J.; Throm, S.; Stewart, C.F.; Waters, C.M. Dexamethasone increases expression and activity of multidrug resistance transporters at the rat blood-brain barrier. Am. J. Physiol.-Cell Physiol. 2008, 295, C440–C450. [Google Scholar] [CrossRef] [PubMed]
- Durk, M.R.; Chan, G.N.; Campos, C.R.; Peart, J.C.; Chow, E.C.Y.; Lee, E.; Cannon, R.E.; Bendayan, R.; Miller, D.S.; Pang, K.S. 1α,25-Dihydroxyvitamin D3-liganded vitamin D receptor increases expression and transport activity of P-glycoprotein in isolated rat brain capillaries and human and rat brain microvessel endothelial cells. J. Neurochem. 2012, 123, 944–953. [Google Scholar] [CrossRef]
- Mattia, C.; Coluzzi, F.; Celidonio, L.; Vellucci, R. Bone pain mechanism in osteoporosis: A narrative review. Clin. Cases Miner. Bone Metab. 2016, 13, 97–100. [Google Scholar] [CrossRef]
- Coluzzi, F.; Scerpa, M.S.; Centanni, M. The Effect of Opiates on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 325–335. [Google Scholar] [CrossRef]
- Coluzzi, F.; Taylor, R., Jr.; Pergolizzi, J.V., Jr.; Mattia, C.; Raffa, R.B. Good clinical practice guide for opioids in pain management: The three Ts—titration (trial), tweaking (tailoring), transition (tapering). Braz. J. Anesthesiol. 2016, 66, 310–317. [Google Scholar] [CrossRef]
- Dennis, B.B.; Bawor, M.; Thabane, L.; Sohani, Z.; Samaan, Z. Impact of ABCB1 and CYP2B6 genetic polymorphisms on methadone metabolism, dose and treatment response in patients with opioid addiction: A systematic review and meta-analysis. PLoS ONE 2014, 9, e86114. [Google Scholar] [CrossRef]
- Kus, T.; Aktas, G.; Kalender, M.; Demiryurek, A.T.; Ulasli, M.; Oztuzcu, S.; Sevinc, A.; Kul, S.; Camci, C. Polymorphism of CYP3A4 and ABCB1 genes increase the risk of neuropathy in breast cancer patients treated with paclitaxel and docetaxel. Onco Targets Ther. 2016, 9, 5073–5080. [Google Scholar] [CrossRef]
- Tanabe, Y.; Shimizu, C.; Hamada, A.; Hashimoto, K.; Ikeda, K.; Nishizawa, D.; Hasegawa, J.; Shimomura, A.; Ozaki, Y.; Tamura, N.; et al. Paclitaxel-induced sensory peripheral neuropathy is associated with an ABCB1 single nucleotide polymorphism and older age in Japanese. Cancer Chemother. Pharmacol. 2017, 79, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Campa, D.; Gioia, A.; Tomei, A.; Poli, P.; Barale, R. Association of ABCB1/MDR1 and OPRM1 gene polymorphisms with morphine pain relief. Clin. Pharmacol. Ther. 2008, 83, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Sia, A.T.; Sng, B.L.; Lim, E.C.; Law, H.; Tan, E.C. The influence of ATP-binding cassette sub-family B member-1 (ABCB1) genetic polymorphisms on acute and chronic pain after intrathecal morphine for caesarean section: A prospective cohort study. Int. J. Obstet. Anesth. 2010, 19, 254–260. [Google Scholar] [CrossRef]
- Coluzzi, F.; Caputi, F.F.; Billeci, D.; Pastore, A.L.; Candeletti, S.; Rocco, M.; Romualdi, P. Safe Use of Opioids in Chronic Kidney Disease and Hemodialysis Patients: Tips and Tricks for Non-Pain Specialists. Ther. Clin. Risk Manag. 2020, 16, 821–837. [Google Scholar] [CrossRef]
- Griffith, J.I.; Kim, M.; Bruce, D.J.; Peterson, C.D.; Kitto, K.F.; Mohammad, A.S.; Rathi, S.; Fairbanks, C.A.; Wilcox, G.L.; Elmquist, W.F. Central Nervous System Distribution of an Opioid Agonist Combination with Synergistic Activity. J. Pharmacol. Exp. Ther. 2022, 380, 34–46. [Google Scholar] [CrossRef]
- Nieri, P.; Romiti, N.; Adinolfi, B.; Chicca, A.; Massarelli, I.; Chieli, E. Modulation of P-glycoprotein activity by cannabinoid molecules in HK-2 renal cells. Br. J. Pharmacol. 2006, 148, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Chaves, C.; Gómez-Zepeda, D.; Auvity, S.; Menet, M.C.; Crété, D.; Labat, L.; Remião, F.; Cisternino, S.; Declèves, X. Effect of Subchronic Intravenous Morphine Infusion and Naloxone-Precipitated Morphine Withdrawal on P-gp and Bcrp at the Rat Blood-Brain Barrier. J. Pharm. Sci. 2016, 105, 350–358. [Google Scholar] [CrossRef]
- Wandel, C.; Kim, R.; Wood, M.; Wood, A. Interaction of morphine, fentanyl, sufentanil, alfentanil, and loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology 2002, 96, 913–920. [Google Scholar] [CrossRef]
- Hamabe, W.; Maeda, T.; Kiguchi, N.; Yamamoto, C.; Tokuyama, S.; Kishioka, S. Negative relationship between morphine analgesia and P-glycoprotein expression levels in brain. J. Pharmacol. Sci. 2007, 105, 353–360. [Google Scholar] [CrossRef]
- Cunningham, C.W.; Mercer, S.L.; Hassan, H.E.; Traynor, J.R.; Eddington, N.D.; Coop, A. Opioids and efflux transporters. Part 2: P-glycoprotein substrate activity of 3- and 6-substituted morphine analogs. J. Med. Chem. 2008, 51, 2316–2320. [Google Scholar] [CrossRef]
- Mattia, C.; Coluzzi, F. A look inside the association codeine-paracetamol: Clinical pharmacology supports analgesic efficacy. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 507–516. [Google Scholar] [PubMed]
- Yu, C.; Yuan, M.; Yang, H.; Zhuang, X.; Li, H. P-Glycoprotein on Blood-Brain Barrier Plays a Vital Role in Fentanyl Brain Exposure and Respiratory Toxicity in Rats. Toxicol. Sci. Toxicol. 2018, 164, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Kalvass, J.C.; Olson, E.R.; Pollack, G.M. Pharmacokinetics and pharmacodynamics of alfentanil in P-glycoprotein-competent and P-glycoprotein-deficient mice: P-glycoprotein efflux alters alfentanil brain disposition and antinociception. Drug Metab. Dispos. 2007, 35, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Scardino, M.; Tartarelli, A.; Coluzzi, F.; Corcione, A.; Lorini, F.L.; Torrano, V.; Martorano, P.P.; Quaini, S. Sublingual sufentanil tablet system for the management of acute postoperative pain in a hospital setting: An observational study. Minerva Anestesiol. 2021, 87, 156–164. [Google Scholar] [CrossRef]
- Vergari, A.; Cortegiani, A.; Rispoli, M.; Coluzzi, F.; Deni, F.; Leykin, Y.; Luca Lorini, F.; Martorano, P.P.; Paolicchi, A.; Polati, E.; et al. Sufentanil Sublingual Tablet System: From rationale of use to clinical practice. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11891–11899. [Google Scholar] [CrossRef]
- Sacerdote, P.; Coluzzi, F.; Fanelli, A. Sublingual sufentanil, a new opportunity for the improvement of postoperative pain management in Italy. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1411–1422. [Google Scholar]
- Kanaan, M.; Daali, Y.; Dayer, P.; Desmeules, J. Uptake/efflux transport of tramadol enantiomers and O-desmethyl-tramadol: Focus on P-glycoprotein. Basic Clin. Pharmacol. Toxicol. 2009, 105, 199–206. [Google Scholar] [CrossRef]
- Sheikholeslami, B.; Hamidi, M.; Lavasani, H.; Sharifzadeh, M.; Rouini, M.R. Lack of evidence for involvement of P-glycoprotein in brain uptake of the centrally acting analgesic, tramadol in the rat. J. Pharm. Pharm. Sci. 2012, 15, 606–615. [Google Scholar] [CrossRef]
- Alhaddad, H.; Cisternino, S.; Declèves, X.; Tournier, N.; Schlatter, J.; Chiadmi, F.; Risède, P.; Smirnova, M.; Besengez, C.; Scherrmann, J.M.; et al. Respiratory toxicity of buprenorphine results from the blockage of P-glycoprotein-mediated efflux of norbuprenorphine at the blood-brain barrier in mice. Crit Care Med. 2012, 40, 3215–3223. [Google Scholar] [CrossRef]
- Coluzzi, F.; Rullo, L.; Scerpa, M.S.; Losapio, L.M.; Rocco, M.; Billeci, D.; Candeletti, S.; Romualdi, P. Current and Future Therapeutic Options in Pain Management: Multi-mechanistic Opioids Involving Both MOR and NOP Receptor Activation. CNS Drugs 2022, 36, 617–632. [Google Scholar] [CrossRef]
- Brown, S.M.; Campbell, S.D.; Crafford, A.; Regina, K.J.; Holtzman, M.J.; Kharasch, E.D. P-glycoprotein is a major determinant of norbuprenorphine brain exposure and antinociception. J. Pharmacol. Exp. Ther. 2012, 343, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Moriki, Y.; Suzuki, T.; Fukami, T.; Hanano, M.; Tomono, K.; Watanabe, J. Involvement of P-glycoprotein in blood-brain barrier transport of pentazocine in rats using brain uptake index method. Biol. Pharm. Bull. 2004, 27, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, R.N.; Moulari, B.; Gromand, J.; Beduneau, A.; Lamprecht, A.; Pellequer, Y. Coadministration of P-glycoprotein modulators on loperamide pharmacokinetics and brain distribution. Drug Metab. Dispos. 2014, 42, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Lilius, T.O.; Jokinen, V.; Neuvonen, M.S.; Väänänen, A.J.; Niemi, M.; Rauhala, P.V.; Kalso, E.A. The mineralocorticoid receptor antagonist spironolactone enhances morphine antinociception. Eur. J. Pain 2014, 18, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Zahari, Z.; Lee, C.S.; Ibrahim, M.A.; Musa, N.; Mohd Yasin, M.A.; Lee, Y.Y.; Tan, S.C.; Mohamad, N.; Ismail, R. Relationship between ABCB1 polymorphisms and serum methadone concentration in patients undergoing methadone maintenance therapy (MMT). Am. J. Drug Alcohol. Abus. 2016, 42, 587–596. [Google Scholar] [CrossRef]
- Suzuki, T.; Miyata, M.; Zaima, C.; Furuishi, T.; Fukami, T.; Kugawa, F.; Tomono, K. Blood-brain barrier transport of naloxone does not involve P-glycoprotein-mediated efflux. J. Pharm. Sci. 2010, 99, 413–421. [Google Scholar] [CrossRef]
- Kanaan, M.; Daali, Y.; Dayer, P.; Desmeules, J. P-glycoprotein is not involved in the differential oral potency of naloxone and naltrexone. Fundam. Clin. Pharmacol. 2009, 23, 543–548. [Google Scholar] [CrossRef]
- Müller-Schwefe, G.; Morlion, B.; Ahlbeck, K.; Alon, E.; Coaccioli, S.; Coluzzi, F.; Huygen, F.; Jaksch, W.; Kalso, E.; Kocot-Kępska, M.; et al. Treatment for chronic low back pain: The focus should change to multimodal management that reflects the underlying pain mechanisms. Curr. Med. Res. Opin. 2017, 33, 1199–1210. [Google Scholar] [CrossRef]
- Morlion, B.; Coluzzi, F.; Aldington, D.; Kocot-Kepska, M.; Pergolizzi, J.; Mangas, A.C.; Ahlbeck, K.; Kalso, E. Pain chronification: What should a non-pain medicine specialist know? Curr. Med. Res. Opin. 2018, 34, 1169–1178. [Google Scholar] [CrossRef]
- Coluzzi, F.; Fornasari, D.; Pergolizzi, J.; Romualdi, P. From acute to chronic pain: Tapentadol in the progressive stages of this disease entity. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 1672–1683. [Google Scholar]
- Akamine, Y.; Yasui-Furukori, N.; Uno, T. Drug-Drug Interactions of P-gp Substrates Unrelated to CYP Metabolism. Curr. Drug Metab. 2019, 20, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Ruan, Y.; Taylor, R.M.; Donovan, J.L.; Markowitz, J.S.; DeVane, C.L. The brain entry of risperidone and 9-hydroxyrisperidone is greatly limited by P-glycoprotein. Int. J. Neuropsychopharmacol. 2004, 7, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Langer, O. Use of PET Imaging to Evaluate Transporter-Mediated Drug-Drug Interactions. J. Clin. Pharmacol. 2016, 56 (Suppl. S7), S143–S156. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.C.; Müller, M.; Lappin, G.; Langer, O. Positron emission tomography for use in microdosing studies. Curr. Opin. Drug Discov. Dev. 2008, 11, 104–110. [Google Scholar]
- Bauer, M.; Wulkersdorfer, B.; Karch, R.; Philippe, C.; Jäger, W.; Stanek, J.; Wadsak, W.; Hacker, M.; Zeitlinger, M.; Langer, O. Effect of P-glycoprotein inhibition at the blood-brain barrier on brain distribution of (R)-[11C]verapamil in elderly vs. young subjects. Br. J. Clin. Pharmacol. 2017, 83, 1991–1999. [Google Scholar] [CrossRef]
- Coluzzi, F.; Alvaro, D.; Caraceni, A.T.; Gianni, W.; Marinangeli, F.; Massazza, G.; Pinto, C.; Varrassi, G.; Lugoboni, F. Common Clinical Practice for Opioid-Induced Constipation: A Physician Survey. J. Pain Res. 2021, 14, 2255–2264. [Google Scholar] [CrossRef]
- Alvaro, D.; Coluzzi, F.; Gianni, W.; Lugoboni, F.; Marinangeli, F.; Massazza, G.; Pinto, C.; Varrassi, G. Opioid-Induced Constipation in Real-World Practice: A Physician Survey, 1 Year Later. Pain Ther. 2022, 11, 477–491. [Google Scholar] [CrossRef]
- Coluzzi, F.; Scerpa, M.S.; Pergolizzi, J. Naldemedine: A new option for OIBD. J. Pain Res. 2020, 13, 1209–1222. [Google Scholar] [CrossRef]
- Fukumura, K.; Kawaguchi, N.; Ishibashi, T.; Kubota, R.; Tada, Y.; Ogura, E. Clinical Drug-Drug Interaction Studies to Evaluate the Effects of a P-Glycoprotein Inhibitor, CYP3A Inhibitors, and a CYP3A Inducer on the Pharmacokinetics of Naldemedine in Healthy Subjects. Clin. Drug Investig. 2020, 40, 529–540. [Google Scholar] [CrossRef]
- Watari, R.; Matsuda, A.; Ohnishi, S.; Hasegawa, H. Minimal contribution of P-gp on the low brain distribution of naldemedine, a peripherally acting mu-opioid receptor antagonist. Drug Metab. Pharmacokinet. 2019, 34, 126–133. [Google Scholar] [CrossRef]
- Gudin, J.; Fudin, J. Peripheral Opioid Receptor Antagonists for Opioid-Induced Constipation: A Primer on Pharmacokinetic Variabilities with a Focus on Drug Interactions. J. Pain Res. 2020, 13, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Israel, R.J. Oral methylnaltrexone does not negatively impact analgesia in patients with opioid-induced constipation and chronic noncancer pain. J. Pain Res. 2018, 11, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W.; Woron, J. The role of naloxegol in the management of opioid-induced bowel dysfunction. Ther. Adv. Gastroenterol. 2016, 9, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Bui, K.; Sostek, M.; Al-Huniti, N. Simulation and Prediction of the Drug-Drug Interaction Potential of Naloxegol by Physiologically Based Pharmacokinetic Modeling. CPT Pharmacomet. Syst. Pharmacol. 2016, 5, 250–257. [Google Scholar] [CrossRef]
- Bak, M.S.; Park, H.; Kim, S.K. Neural Plasticity in the Brain during Neuropathic Pain. Biomedicines 2021, 9, 624. [Google Scholar] [CrossRef]
- Li, H.L.; Huang, Y.; Zhou, Y.L.; Teng, R.H.; Zhou, S.Z.; Lin, J.P.; Yang, Y.; Zhu, S.M.; Xu, H.; Yao, Y.X. C-X-C Motif Chemokine 10 Contributes to the Development of Neuropathic Pain by Increasing the Permeability of the Blood-Spinal Cord Barrier. Front. Immunol. 2020, 11, 477. [Google Scholar] [CrossRef]
- Lim, T.K.Y.; Shi, X.Q.; Martin, H.C.; Huang, H.; Luheshi, G.; Rivest, S.; Zhang, J. Blood-nerve barrier dysfunction contributes to the generation of neuropathic pain and allows targeting of injured nerves for pain relief. Pain 2014, 155, 954–967. [Google Scholar] [CrossRef]
- Westlund, K.N.; Zhang, M. Building and Testing PPARγ Therapeutic ELB00824 with an Improved Therapeutic Window for Neuropathic Pain. Molecules 2020, 25, 1120. [Google Scholar] [CrossRef]
- Takeshita, Y.; Sato, R.; Kanda, T. Blood-Nerve Barrier (BNB) Pathology in Diabetic Peripheral Neuropathy and In Vitro Human BNB Model. Int. J. Mol. Sci. 2020, 22, 62. [Google Scholar] [CrossRef]
- Fried, N.T.; Maxwell, C.R.; Elliott, M.B.; Oshinsky, M.L. Region-specific disruption of the blood-brain barrier following repeated inflammatory dural stimulation in a rat model of chronic trigeminal allodynia. Cephalalgia 2018, 38, 674–689. [Google Scholar] [CrossRef]
- Montague-Cardoso, K.; Malcangio, M. Changes in blood-spinal cord barrier permeability and neuroimmune interactions in the underlying mechanisms of chronic pain. Pain Rep. 2021, 6, e879. [Google Scholar] [CrossRef] [PubMed]
- Biondi, D.M. Is migraine a neuropathic pain syndrome? Curr. Pain Headache Rep. 2006, 10, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, C.A.; Moore, E.L.; Calamari, A.; Cook, J.J.; Michener, M.S.; O’Malley, S.; Miller, P.J.; Sur, C.; Williams, D.L., Jr.; Zeng, Z.; et al. Pharmacological properties of MK-3207, a potent and orally active calcitonin gene-related peptide receptor antagonist. J. Pharmacol. Exp. Ther. 2010, 333, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Ferrari, M.D.; Olesen, J.; Stovner, L.J.; Senard, J.M.; Jackson, N.C.; Poole, P.H. Eletriptan in acute migraine: A double-blind, placebo-controlled comparison to sumatriptan. Eletriptan Steering Committee. Neurology 2000, 54, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, G.; Färkkilä, M.; Burgess, G.; Forster, E.; Haughie, S.; Eletriptan Steering Committee. Eletriptan vs sumatriptan: A double-blind, placebo-controlled, multiple migraine attack study. Neurology 2002, 59, 1210–1217. [Google Scholar] [CrossRef]
- Färkkilä, M.; Olesen, J.; Dahlöf, C.; Stovner, L.J.; ter Bruggen, J.P.; Rasmussen, S.; Muirhead, N.; Sikes, C. Eletriptan for the treatment of migraine in patients with previous poor response or tolerance to oral sumatriptan. Cephalalgia 2003, 23, 463–471. [Google Scholar] [CrossRef]
- Diener, H.C.; Ryan, R.; Sun, W.; Hettiarachchi, J. The 40-mg dose of eletriptan: Comparative efficacy and tolerability versus sumatriptan 100 mg. Eur. J. Neurol. 2004, 11, 125–134. [Google Scholar] [CrossRef]
- Mandema, J.W.; Cox, E.; Alderman, J. Therapeutic benefit of eletriptan compared to sumatriptan for the acute relief of migraine pain--results of a model-based meta-analysis that accounts for encapsulation. Cephalalgia 2005, 25, 715–725. [Google Scholar] [CrossRef]
- Coluzzi, F.; Pergolizzi, J.V., Jr.; Giordan, E.; Locarini, P.; Boaro, A.; Billeci, D. Tapentadol prolonged release for managing moderate to severe chronic neck pain with or without a neuropathic component. Curr. Med. Res. Opin. 2020, 36, 651–659. [Google Scholar] [CrossRef]
- Coluzzi, F.; Rolke, R.; Mercadante, S. Pain Management in Patients with Multiple Myeloma: An Update. Cancers 2019, 11, 2037. [Google Scholar] [CrossRef]
- Mukae, T.; Fujita, W.; Ueda, H. P-glycoprotein inhibitors improve effective dose and time of pregabalin to inhibit intermittent cold stress-induced central pain. J. Pharmacol. Sci. 2016, 131, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V., Jr.; Magnusson, P.; Raffa, R.B.; LeQuang, J.A.; Coluzzi, F. Developments in combined analgesic regimens for improved safety in postoperative pain management. Expert Rev. Neurother. 2020, 20, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Duehmke, R.M.; Derry, S.; Wiffen, P.J.; Bell, R.F.; Aldington, D.; Moore, R.A. Tramadol for neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 6, CD003726. [Google Scholar] [CrossRef] [PubMed]
- Coluzzi, F.; Polati, E.; Freo, U.; Grilli, M. Tapentadol: An effective option for the treatment of back pain. J. Pain Res. 2019, 12, 1521–1528, Erratum in: J. Pain Res. 2019, 12, 1877. [Google Scholar] [CrossRef]
- Kress, H.G. Tapentadol and its two mechanisms of action: Is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur. J. Pain 2010, 14, 781–783. [Google Scholar] [CrossRef]
- Pergolizzi, J.V., Jr.; Varrassi, G.; Magnusson, P.; Breve, F.; Raffa, R.B.; Christo, P.J.; Chopra, M.; Paladini, A.; LeQuang, J.A.; Mitchell, K.; et al. Pharmacologic agents directed at the treatment of pain associated with maladaptive neuronal plasticity. Expert Opin. Pharmacother. 2022, 23, 105–116. [Google Scholar] [CrossRef]
- Pergolizzi, J.; Magnusson, P.; Coluzzi, F.; Breve, F.; LeQuang, J.A.K.; Varrassi, G. Multimechanistic Single-Entity Combinations for Chronic Pain Control: A Narrative Review. Cureus 2022, 14, e26000. [Google Scholar] [CrossRef]
- Kress, H.G.; Coluzzi, F. Tapentadol in the management of cancer pain: Current evidence and future perspectives. J. Pain Res. 2019, 12, 1553–1560. [Google Scholar] [CrossRef]
- Yang, J.; Reilly, B.G.; Davis, T.P.; Ronaldson, P.T. Modulation of Opioid Transport at the Blood-Brain Barrier by Altered ATP-Binding Cassette (ABC) Transporter Expression and Activity. Pharmaceutics 2018, 10, 192. [Google Scholar] [CrossRef]
- Singla, A.; Sloan, P. Pharmacokinetic evaluation of hydrocodone/acetaminophen for pain management. J. Opioid Manag. 2013, 9, 71–80. [Google Scholar] [CrossRef]
- Coluzzi, F.; Mattia, C. Oxycodone. Pharmacological profile and clinical data in chronic pain management. Minerva Anestesiol. 2005, 71, 451–460. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluzzi, F.; Scerpa, M.S.; Rocco, M.; Fornasari, D. The Impact of P-Glycoprotein on Opioid Analgesics: What’s the Real Meaning in Pain Management and Palliative Care? Int. J. Mol. Sci. 2022, 23, 14125. https://doi.org/10.3390/ijms232214125
Coluzzi F, Scerpa MS, Rocco M, Fornasari D. The Impact of P-Glycoprotein on Opioid Analgesics: What’s the Real Meaning in Pain Management and Palliative Care? International Journal of Molecular Sciences. 2022; 23(22):14125. https://doi.org/10.3390/ijms232214125
Chicago/Turabian StyleColuzzi, Flaminia, Maria Sole Scerpa, Monica Rocco, and Diego Fornasari. 2022. "The Impact of P-Glycoprotein on Opioid Analgesics: What’s the Real Meaning in Pain Management and Palliative Care?" International Journal of Molecular Sciences 23, no. 22: 14125. https://doi.org/10.3390/ijms232214125
APA StyleColuzzi, F., Scerpa, M. S., Rocco, M., & Fornasari, D. (2022). The Impact of P-Glycoprotein on Opioid Analgesics: What’s the Real Meaning in Pain Management and Palliative Care? International Journal of Molecular Sciences, 23(22), 14125. https://doi.org/10.3390/ijms232214125







