Influence of Microbial Treatment on the Preparation of Porous Biochar with Stepped-Up Performance and Its Application in Organic Pollutants Control
Abstract
1. Introduction
2. Results and Discussion
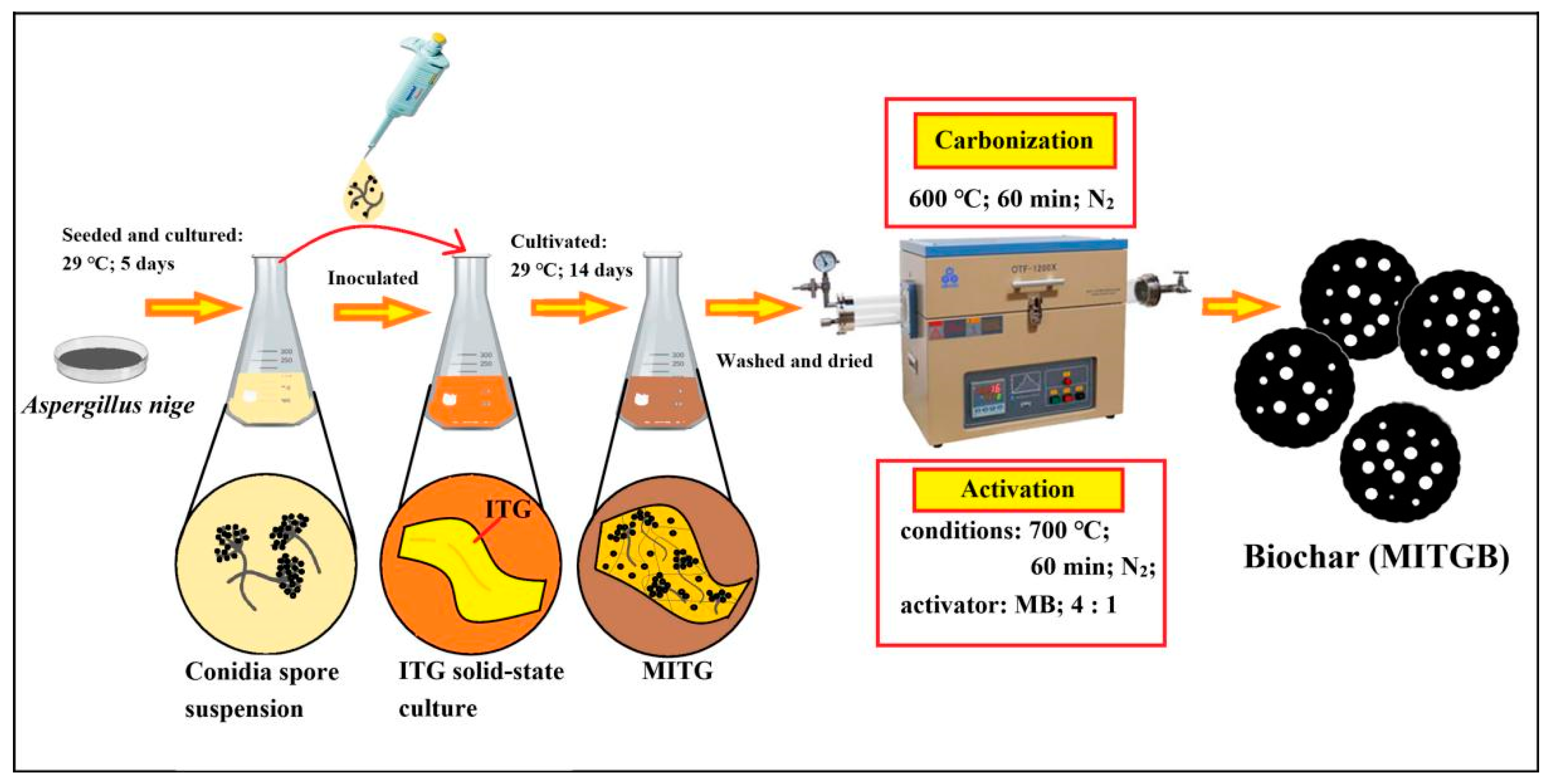
2.1. Preparation of MITGB
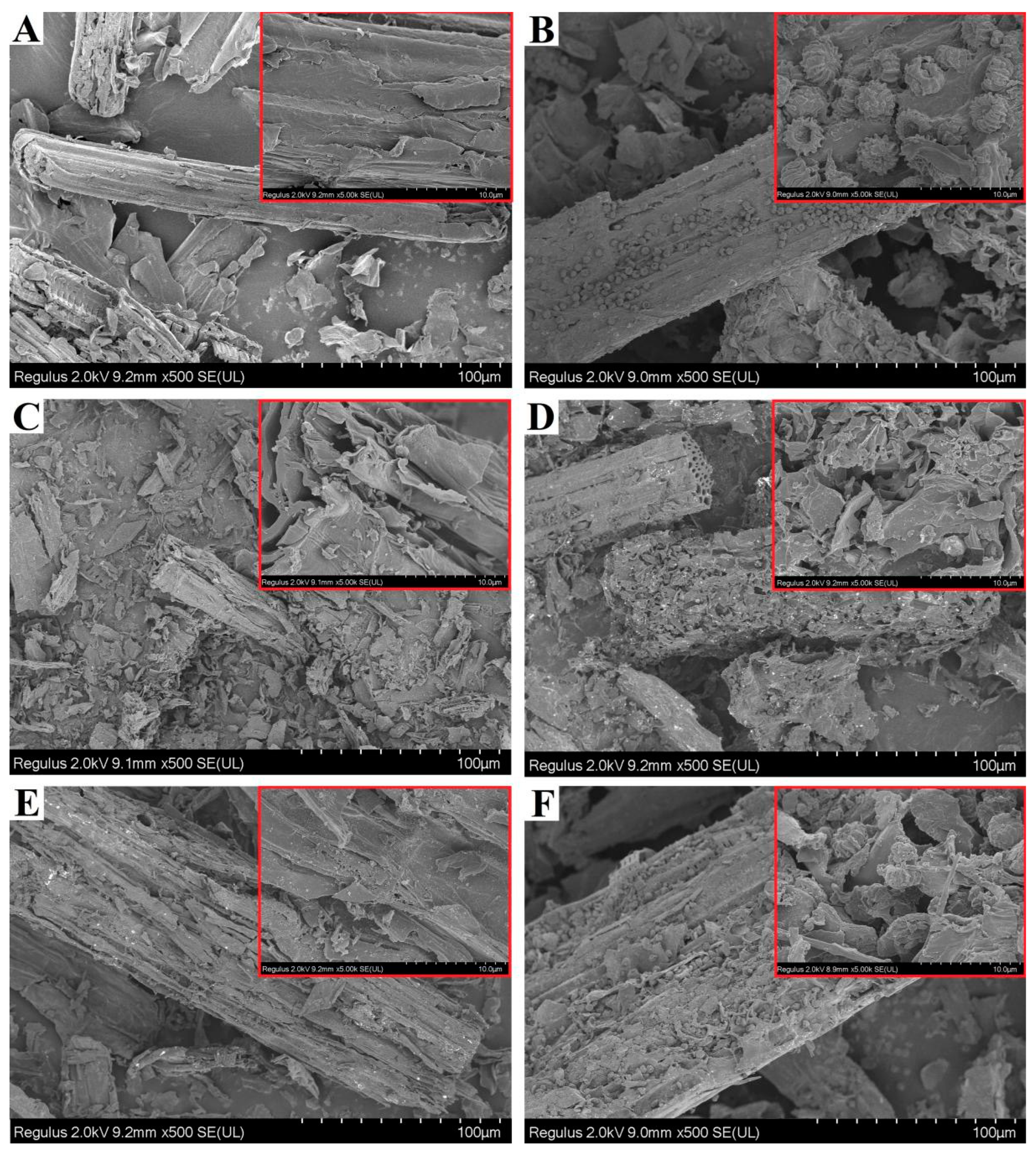
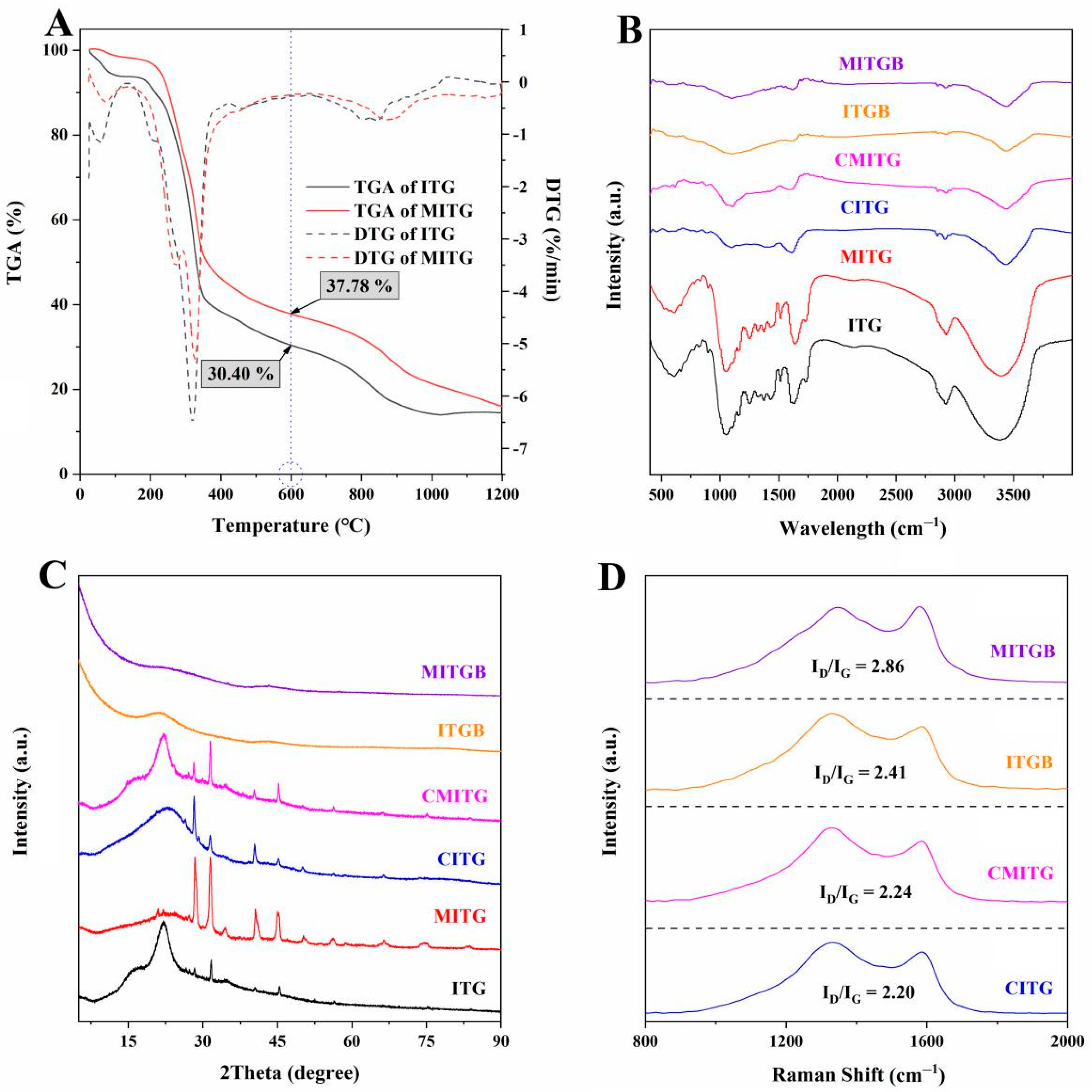
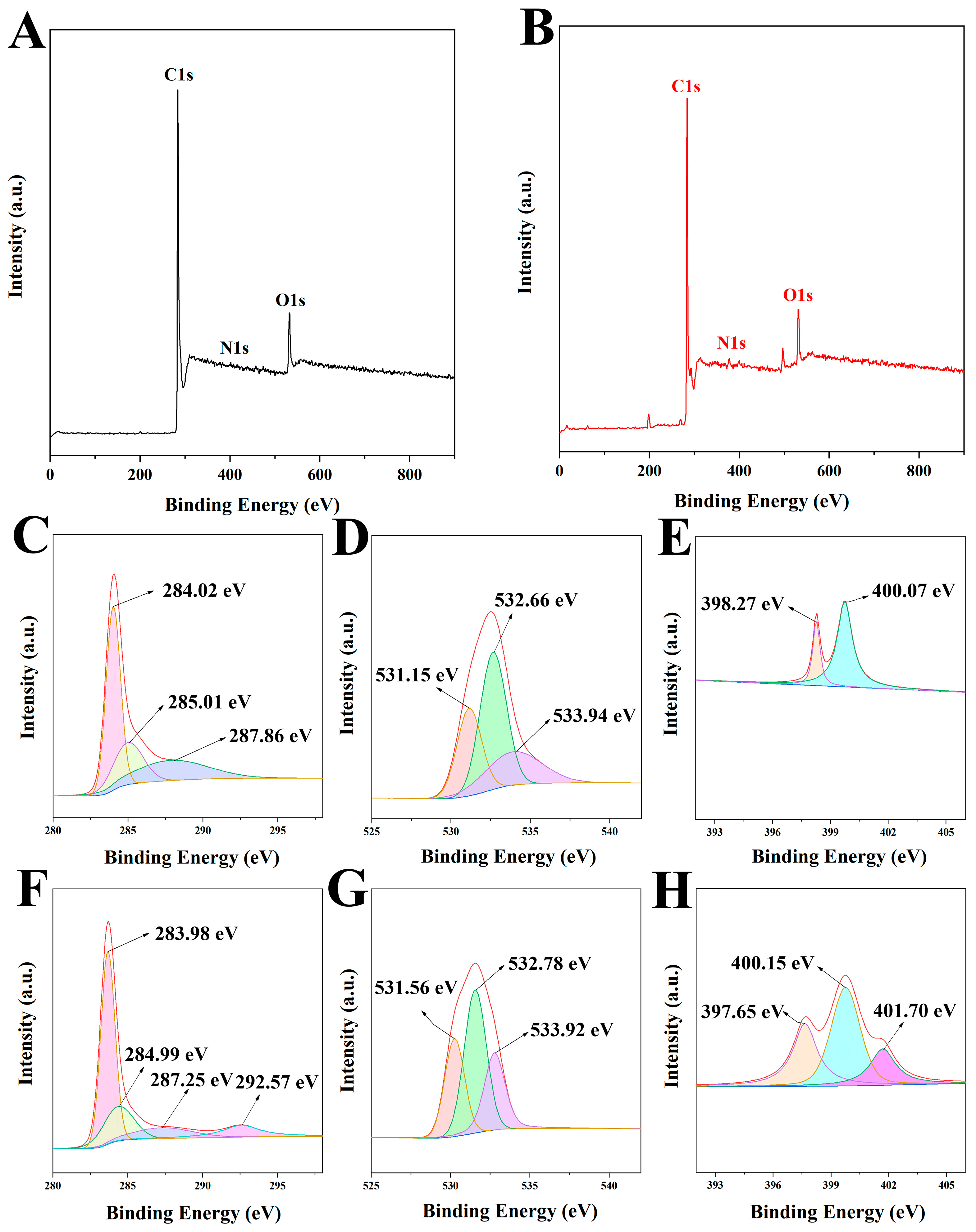
2.2. Results of Characterization
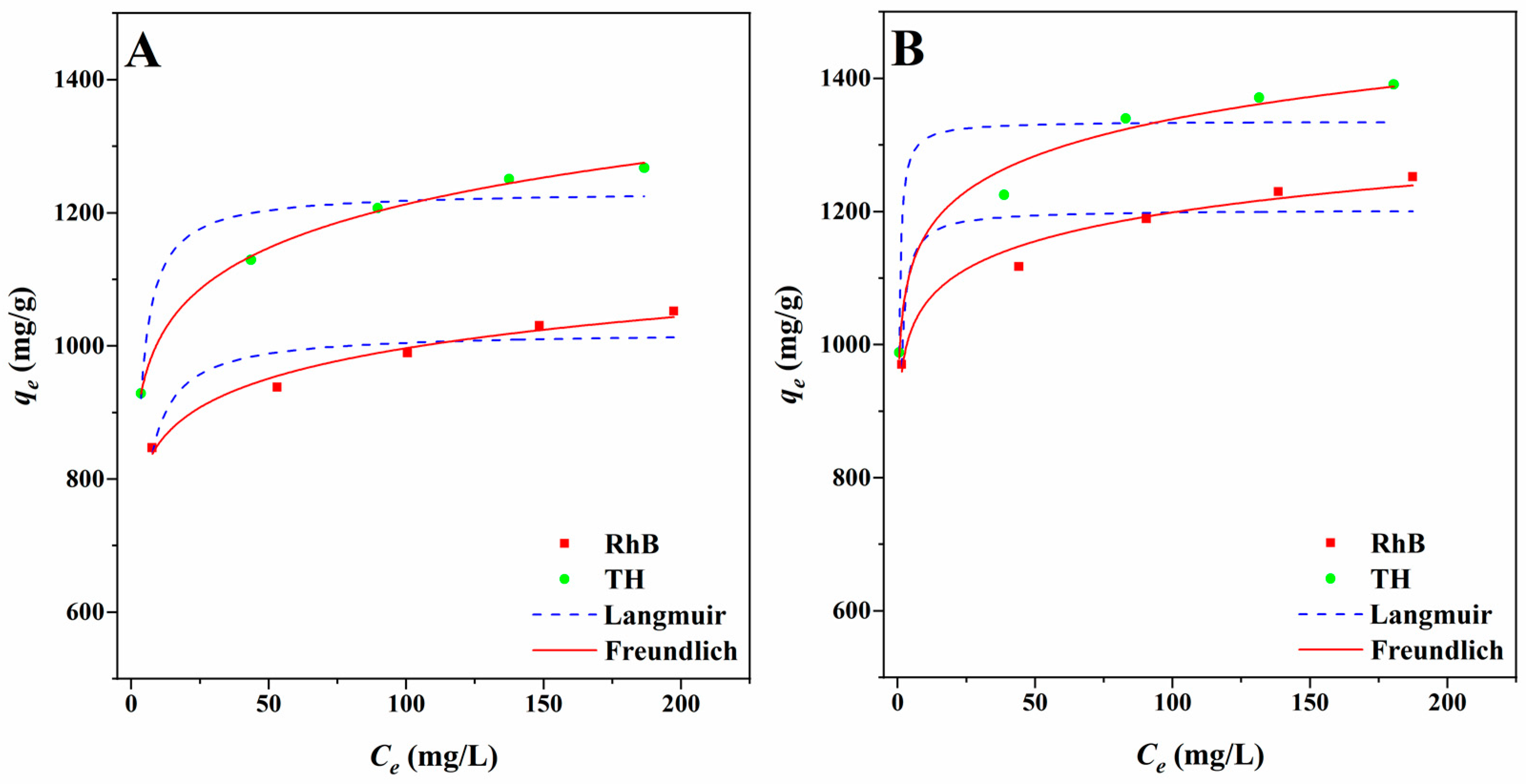
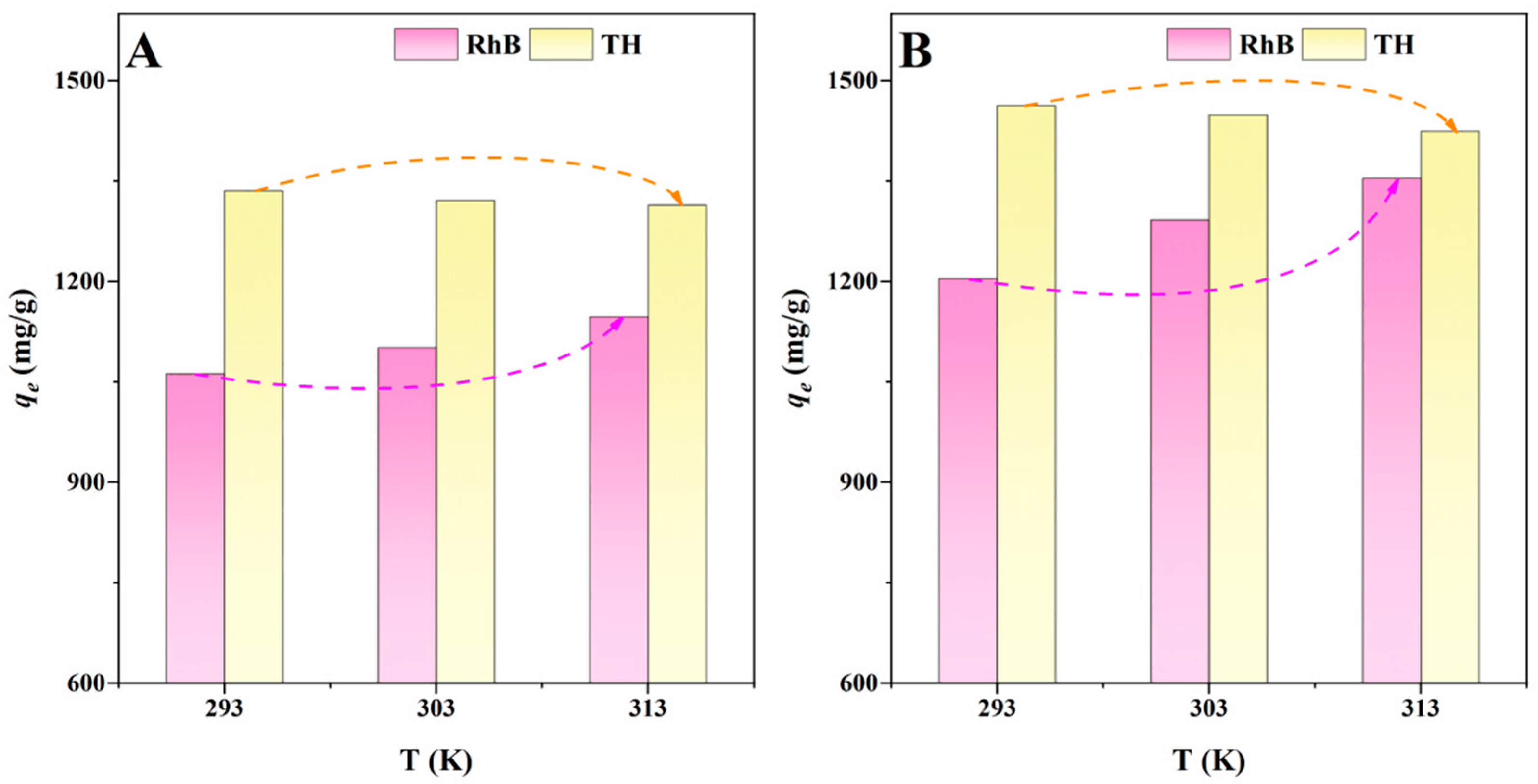
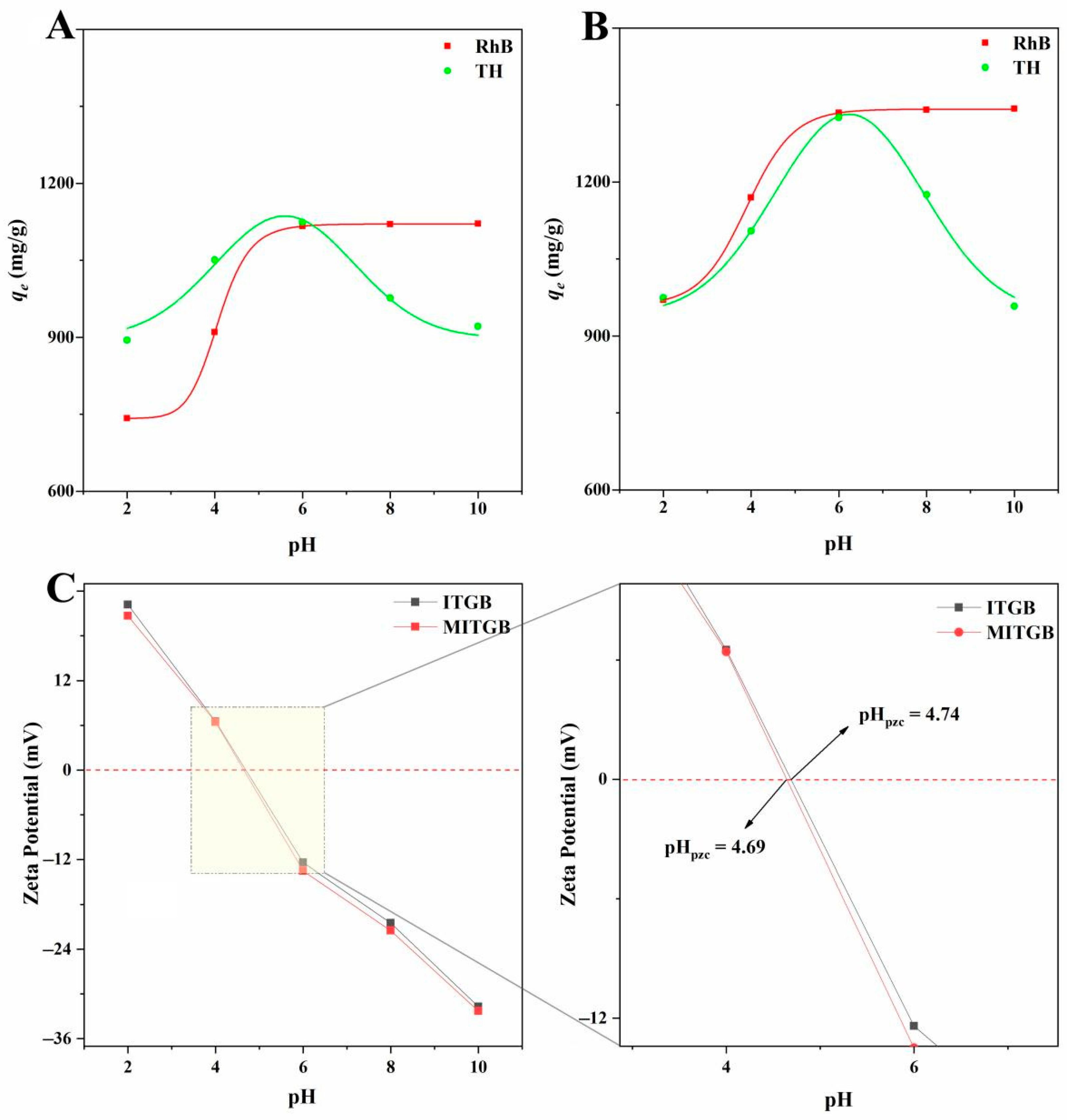
2.3. Results of Adsorption Experiments
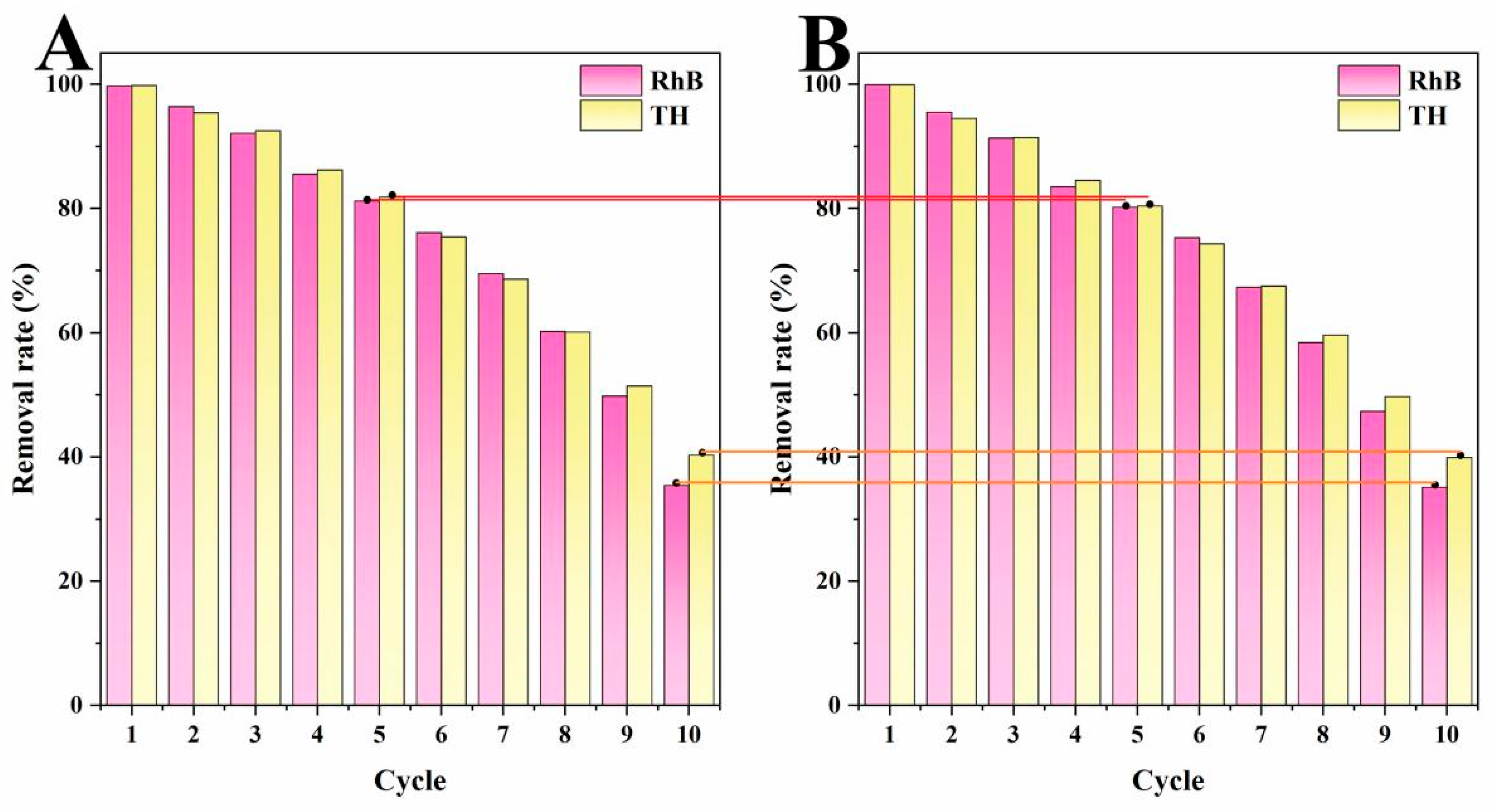
2.4. Results of Cycle Tests and Comparison with Other Biochars
| Adsorbents | SBET (m2/g) | qe for RhB (mg/g) | qe for TH (mg/g) | References |
|---|---|---|---|---|
| ITGB | 2930.0 | 1147.3 | 1335.8 | This work |
| MITGB | 3036.4 | 1354.2 | 1462.6 | This work |
| Bagasse pith biochar | 522.7 | 263.9 | − | [46] |
| Rice husk biochar | 892.0 | 518.1 | − | [47] |
| Edible fungus substrate biochar | 2767.3 | 1497.0 | − | [28] |
| Cow dung biochar | 4081.1 | 1241.0 | 1105.0 | [13] |
| Aspergillus niger/starch biochar | 3050.0 | 1188.2 | 1386.0 | [14] |
| Eucommia ulmoides lignin−based biochar | 2008.0 | − | 1163.0 | [48] |
| Fungal mycelium−agar biochar | 4343.3 | − | 1441.3 | [24] |
| Cellulose biochar | 2583.0 | − | 1854.2 | [26] |
2.5. Possible Mechanisms Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Microbial Treatment of ITG
3.3. Preparation of MITGB
3.4. Adsorption Performances
3.5. Cycling Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, L.; Lu, Q.; Zhou, H.; Shen, F.; Liu, Z.; Zhu, W.; Huang, H. Tuning oxygenated functional groups on biochar for water pollution control: A critical review. J. Hazard. Mater. 2021, 420, 126547. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Tang, J. The effects of heterogeneous environmental regulations on water pollution control: Quasi-natural experimental evidence from China. Sci. Total Environ. 2021, 751, 141550. [Google Scholar] [CrossRef] [PubMed]
- Agasti, N.; Gautam, V.; Pandey, N.; Genwa, M.; Meena, P.L.; Tandon, S.; Samantaray, R. Carbon nanotube based magnetic composites for decontamination of organic chemical pollutants in water: A review. Appl. Surf. Sci. Adv. 2022, 10, 100270. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Dong, X.; Lv, Y. Functionalized metal-organic frameworks for photocatalytic degradation of organic pollutants in environment. Chemosphere 2020, 242, 125144. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Cheng, Q.; Wang, X.; Wang, J.; Zhang, G. Sb-based photocatalysts for degradation of organic pollutants: A review. J. Clean. Prod. 2022, 367, 133060. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, H.; Dong, H.; Ma, B.; Sun, H.; Pan, T.; Jiang, R.; Zhou, R.; Shen, J.; Liu, J.; et al. Occurrence and ecological risk assessment of organic micropollutants in the lower reaches of the Yangtze River, China: A case study of water diversion. Environ. Pollut. 2018, 239, 223–232. [Google Scholar] [CrossRef]
- Daglar, H.; Altintas, C.; Erucar, I.; Heidari, G.; Zare, E.N.; Moradi, O.; Srivastava, V.; Iftekhar, S.; Keskin, S.; Sillanpaa, M. Metal-organic framework-based materials for the abatement of air pollution and decontamination of wastewater. Chemosphere 2022, 303, 135082. [Google Scholar] [CrossRef]
- Dadashi, J.; Ghasemzadeh, M.A.; Alipour, S.; Zamani, F. A review on catalytic reduction/degradation of organic pollution through silver-based hydrogels. Arab. J. Chem. 2022, 15, 104023. [Google Scholar] [CrossRef]
- Zhou, L.; Li, S.; Li, F. Damage and elimination of soil and water antibiotic and heavy metal pollution caused by livestock husbandry. Environ. Res. 2022, 215, 114188. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, Y.; Wu, J.; Zheng, X.; Chen, Y. Enhanced removal of sulfur-containing organic pollutants from actual wastewater by biofilm reactor: Insights of sulfur transformation and bacterial metabolic traits. Environ. Pollut. 2022, 313, 120187. [Google Scholar] [CrossRef]
- Feng, Y.; Tao, Y.; Meng, Q.; Qu, J.; Ma, S.; Han, S.; Zhang, Y. Microwave-combined advanced oxidation for organic pollutants in the environmental remediation: An overview of influence, mechanism, and prospective. Chem. Eng. J. 2022, 441, 135924. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, G.; Cheng, G.; Wang, X.; Liu, G.; Jin, W. Emerging membranes for separation of organic solvent mixtures by pervaporation or vapor permeation. Sep. Purif. Technol. 2022, 299, 121729. [Google Scholar] [CrossRef]
- Chen, X.; Yu, G.; Chen, Y.; Tang, S.; Su, Y. Cow dung-based biochar materials prepared via mixed base and its application in the removal of organic pollutants. Int. J. Mol. Sci. 2022, 23, 10094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, Y.; Huang, X.; Tang, S.; Chen, H.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Biological self-assembled hyphae/starch porous carbon composites for removal of organic pollutants from water. Chem. Eng. J. 2022, 450, 138264. [Google Scholar] [CrossRef]
- Shao, F.; Xu, J.; Kang, X.; Hu, Z.; Shao, Y.; Lu, C.; Zhao, C.; Ren, Y.; Zhang, J. An attempt to enhance the adsorption capacity of biochar for organic pollutants-Characteristics of CaCl2 biochar under multiple design conditions. Sci. Total Environ. 2023, 854, 158675. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, B.; Liu, Q.; Wu, C.; Li, Z. Preparation of porous biochar from heavy bio-oil for adsorption of methylene blue in wastewater. Fuel Process. Technol. 2022, 238, 107485. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Shakya, A.; Vithanage, M.; Agarwal, T. Influence of pyrolysis temperature on biochar properties and Cr(VI) adsorption from water with groundnut shell biochars: Mechanistic approach. Environ. Res. 2022, 215, 114243. [Google Scholar] [CrossRef]
- Abdulhameed, A.S.; Hum, N.N.M.F.; Rangabhashiyam, S.; Jawad, A.H.; Wilson, L.D.; Yaseen, Z.M.; Al-Kahtani, A.A.; ALOthman, Z.A. Statistical modeling and mechanistic pathway for methylene blue dye removal by high surface area and mesoporous grass-based activated carbon using K2CO3 activator. J. Environ. Chem. Eng. 2021, 9, 105530. [Google Scholar] [CrossRef]
- Kietisirirojana, N.; Tunkasiri, T.; Pengpat, K.; Khamman, O.; Intatha, U.; Eitssayeam, S. Synthesis of mesoporous carbon powder from gold beard grass pollen for use as an anode for lithium-ion batteries. Micropor. Mesopor. Mat. 2022, 331, 111565. [Google Scholar] [CrossRef]
- Rethinasabapathy, M.; Lee, J.H.; Roh, K.C.; Kang, S.; Oh, S.Y.; Park, B.; Lee, G.; Cha, Y.L.; Huh, Y.S. Silver grass-derived activated carbon with coexisting micro-, meso- and macropores as excellent bioanodes for microbial colonization and power generation in sustainable microbial fuel cells. Bioresour. Technol. 2020, 300, 122646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Porous carbon materials based on Physalis alkekengi L. husk and its application for removal of malachite green. Environ. Technol. Inno. 2021, 21, 101343. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Preparation of biomass carbon material based on Fomes fomentarius via alkali activation and its application for the removal of brilliant green in wastewater. Environ. Technol. Inno. 2021, 23, 101659. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, B.; Guo, Z.; Tang, S.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Fungal mycelium modified hierarchical porous carbon with enhanced performance and its application for removal of organic pollutants. J. Environ. Chem. Eng. 2022, 10, 108699. [Google Scholar] [CrossRef]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of lignocellulose into cellulose, hemicellulose, and lignin for preparation of porous carbon materials with enhanced performances. J. Hazard. Mater. 2021, 408, 124956. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, B.; Chen, G.; Chen, H.; Tang, S. Combining biological and chemical methods to disassemble of cellulose from corn straw for the preparation of porous carbons with enhanced adsorption performance. Int. J. Biol. Macromol. 2022, 209, 315–329. [Google Scholar] [CrossRef]
- Chen, S.; Chen, G.; Chen, H.; Sun, Y.; Yu, X.; Su, Y.; Tang, S. Preparation of porous carbon-based material from corn straw via mixed alkali and its application for removal of dye. Colloid. Surface A 2019, 568, 173–183. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, B.; Xia, Y.; Chen, H.; Chen, G.; Tang, S. Influence of mixed alkali on the preparation of edible fungus substrate porous carbon material and its application for the removal of dye. Colloid. Surface A 2021, 609, 125675. [Google Scholar] [CrossRef]
- Su, Y.; Yu, X.; Sun, Y.; Wang, G.; Chen, H.; Chen, G. An efficient strategy for enhancing enzymatic saccharification with delignified fungus Myrothecium verrucaria and solid acid. Ind. Crop. Prod. 2018, 121, 396–404. [Google Scholar] [CrossRef]
- Su, Y.; Yu, X.; Sun, Y.; Wang, G.; Chen, H.; Chen, G. Evaluation of screened lignindegrading fungi for the biological pretreatment of corn stover. Sci. Rep. 2018, 8, 5385. [Google Scholar] [CrossRef]
- Fan, C.; Yan, J.; Huang, Y.; Han, X.; Jiang, X. XRD and TG-FTIR study of the effect of mineral matrix on the pyrolysis and combustion of organic matter in shale char. Fuel 2015, 139, 502–510. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; Han, X.; Liu, J. A TG–FTIR investigation to the catalytic effect of mineral matrix in oil shaleon the pyrolysis and combustion of kerogen. Fuel 2013, 104, 307–317. [Google Scholar] [CrossRef]
- Fang, W.; Cui, L.; Zhang, Y.; Shen, H.; Xu, J.; Ren, M.; Cao, F. Investigation on a novel preparation process of B-COPNA resin from catalytic cracking diesel. Fuel 2022, 320, 123916. [Google Scholar] [CrossRef]
- Liu, B.; Chen, T.; Wang, B.; Zhou, S.; Zhang, Z.; Li, Y.; Pan, X.; Wang, N. Enhanced removal of Cd2+ from water by AHP-pretreated biochar: Adsorption performance and mechanism. J. Hazard. Mater. 2022, 438, 129467. [Google Scholar] [CrossRef]
- Ye, X.; Wu, L.; Zhu, M.; Wang, Z.; Huang, Z.; Wang, M. Lotus pollen-derived hierarchically porous carbons with exceptional adsorption performance toward Reactive Black 5: Isotherms, kinetics and thermodynamics investigations. Sep. Purif. Technol. 2022, 300, 121899. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, X.; Liu, S.; Li, X.; Wang, S.; Feng, Z.; Wu, Z.; Wang, L.; Jiang, Z.; Zhang, Y. One-step preparation of Fe/N co-doped porous biochar for chromium(VI) and bisphenol a decontamination in water: Insights to co-activation and adsorption mechanisms. Bioresour. Technol. 2022, 361, 127718. [Google Scholar] [CrossRef]
- Al-Maabreh, A.M.; Abuassaf, R.A.; Hmedat, D.A.; Alkhabbas, M.; Edris, G.; Hussein-Al-Ali, S.H.; Alawaideh, S. Adsorption characteristics of hair dyes removal from aqueous solution onto oak cupules powder coated with ZnO. Int. J. Mol. Sci. 2022, 23, 11959. [Google Scholar] [CrossRef]
- Tonk, S.; Rapo, E. Linear and nonlinear regression analysis for the adsorption of remazol dye by romanian brewery waste by-product, Saccharomyces cerevisiae. Int. J. Mol. Sci. 2022, 23, 11827. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, M.I.; Pellicer, J.A.; Gomez-Morte, T.; Aunon, D.; Gomez-Lopez, V.M.; Yanez-Gascon, M.J.; Gil-Izquierdo, A.; Ceron-Carrasco, J.P.; Crini, G.; Nunez-Delicado, E.; et al. Removal of an azo dye from wastewater through the use of two technologies: Magnetic cyclodextrin polymers and pulsed light. Int. J. Mol. Sci. 2022, 23, 8406. [Google Scholar] [CrossRef]
- Liang, D.; Tian, X.; Zhang, Y.; Zhu, G.; Gao, Q.; Liu, J.; Yu, X. A weed-derived hierarchical porous carbon with a large specific surface area for efficient dye and antibiotic removal. Int. J. Mol. Sci. 2022, 23, 6146. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, Q.; Li, H.; Wang, J.; Tai, G.; Wang, F.; Han, J.; Zhu, Y.; Wu, G. Waste-to-resource strategy to fabricate functionalized MOFs composite material based on Durian Shell biomass carbon fiber and Fe3O4 for highly efficient and recyclable dye adsorption. Int. J. Mol. Sci. 2022, 23, 5900. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.S.; Jabbar, N.M.; Alardhi, S.M.; Majdi, H.S.; Albayati, T.M. Adsorption of methyl violet dye onto a prepared bio-adsorbent from date seeds: Isotherm, kinetics, and thermodynamic studies. Heliyon 2022, 8, e10276. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Naeem, A.; Din, I.U.; Farooq, M.; Khan, I.W.; Hamayun, M.; Malik, T. Synthesis of chitosan composite of metal-organic framework for the adsorption of dyes; kinetic and thermodynamic approach. J. Hazard. Mater. 2022, 427, 127902. [Google Scholar] [CrossRef]
- Koyuncu, D.D.E.; Okur, M. Removal of AV 90 dye using ordered mesoporous carbon materials prepared via nanocasting of KIT-6: Adsorption isotherms, kinetics and thermodynamic analysis. Sep. Purif. Technol. 2021, 257, 117657. [Google Scholar] [CrossRef]
- Kumbhar, P.; Narale, D.; Bhosale, R.; Jambhale, C.; Kim, J.; Kolekar, S. Synthesis of tea waste/Fe3O4 magnetic composite (TWMC) for efficient adsorption of crystal violet dye: Isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2022, 10, 107893. [Google Scholar]
- Gad, H.M.H.; El-Sayed, A.A. Activated carbon from agricultural by-products for the removal of Rhodamine-B from aqueous solution. J. Hazard. Mater. 2009, 168, 1070–1081. [Google Scholar] [CrossRef]
- Ding, L.; Zou, B.; Gao, W.; Liu, Q.; Wang, Z.; Guo, Y.; Wang, X.; Liu, Y. Adsorption of Rhodamine-B from aqueous solution using treated rice husk-based activated carbon. Colloid. Surface A 2014, 446, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Zeng, Q.; Liang, Z.; Ye, X.; Lv, Y.; Liu, M. Preparation of Eucommia ulmoides lignin-based high-performance biochar containing sulfonic group: Synergistic pyrolysis mechanism and tetracycline hydrochloride adsorption. Bioresour. Technol. 2021, 329, 124856. [Google Scholar] [CrossRef]



| Samples | SBET (m2 g−1) | Vmicro (cm3 g−1) | Pm (nm) | Vtotal (cm3 g−1) |
|---|---|---|---|---|
| CITG | 129.9 | 0.0575 | 2.24 | 0.0729 |
| CMITG | 213.1 | 0.0924 | 1.97 | 0.1048 |
| ITGB | 2930.0 | 1.4777 | 2.08 | 1.5062 |
| MITGB | 3036.4 | 1.4607 | 1.98 | 1.5252 |
| Adsorbates | Adsorbents | Models | Parameters | C0 (mg L−1) | ||
|---|---|---|---|---|---|---|
| 50 | 100 | 200 | ||||
| RhB | ITGB | qe (mg/g) | 841.0 | 936.6 | 1030.3 | |
| PFK | k1 (min−1) | 0.0108 | 0.0110 | 0.0104 | ||
| qe.cat (mg/g) | 801.3 | 905.4 | 1003.0 | |||
| R2 | 0.9784 | 0.9889 | 0.9962 | |||
| PSK | k2 (g mg−1 min−1) | 0.0001 | 0.0001 | 0.0001 | ||
| qe.cat (mg/g) | 844.5 | 937.2 | 1035.8 | |||
| R2 | 0.9954 | 0.9991 | 0.9996 | |||
| IPD | k3 (mg g−1 min−0.5) | 14.94 | 12.23 | 12.21 | ||
| C | 684.1 | 807.5 | 900.2 | |||
| R2 | 0.9083 | 0.8114 | 0.6291 | |||
| MITGB | qe (mg/g) | 962.8 | 1111.8 | 1227.3 | ||
| PFK | k1 (min−1) | 0.0064 | 0.0059 | 0.0075 | ||
| qe.cat (mg/g) | 919.0 | 1025.5 | 1203.9 | |||
| R2 | 0.9783 | 0.9350 | 0.9957 | |||
| PSK | k2 (g mg−1 min−1) | 0.0005 | 0.0005 | 0.0005 | ||
| qe.cat (mg/g) | 967.5 | 1114.6 | 1250.7 | |||
| R2 | 0.9984 | 0.9986 | 0.9982 | |||
| IPD | k3 (mg g−1 min−0.5) | 21.86 | 22.57 | 19.44 | ||
| C | 734.9 | 876.1 | 1032.7 | |||
| R2 | 0.8099 | 0.8103 | 0.5491 | |||
| TH | ITGB | qe (mg/g) | 928.0 | 1137.9 | 1246.2 | |
| PFK | k1 (min−1) | 0.0046 | 0.0098 | 0.0089 | ||
| qe.cat (mg/g) | 893.3 | 1105.8 | 1212.8 | |||
| R2 | 0.9852 | 0.9905 | 0.9907 | |||
| PSK | k2 (g mg−1 min−1) | 0.0005 | 0.0007 | 0.0006 | ||
| qe.cat (mg/g) | 938.8 | 1145.7 | 1259.2 | |||
| R2 | 0.9998 | 0.9997 | 0.9999 | |||
| IPD | k3 (mg g−1 min−0.5) | 20.26 | 16.49 | 19.31 | ||
| C | 719.3 | 969.8 | 1051.9 | |||
| R2 | 0.7368 | 0.7208 | 0.7101 | |||
| MITGB | qe (mg/g) | 982.7 | 1218.2 | 1365.1 | ||
| PFK | k1 (min−1) | 0.0089 | 0.0090 | 0.0076 | ||
| qe.cat (mg/g) | 952.4 | 1172.6 | 1324.3 | |||
| R2 | 0.9885 | 0.9865 | 0.9911 | |||
| PSK | k2 (g mg−1 min−1) | 0.0007 | 0.0006 | 0.0006 | ||
| qe.cat (mg/g) | 989.7 | 1220.2 | 1372.2 | |||
| R2 | 0.9994 | 0.9990 | 0.9998 | |||
| IPD | k3 (mg g−1 min−0.5) | 15.64 | 20.43 | 20.06 | ||
| C | 822.9 | 1005.8 | 1158.9 | |||
| R2 | 0.7352 | 0.8005 | 0.7364 | |||
| Adsorbates | Adsorbents | Types | Parameters | |
|---|---|---|---|---|
| RhB | ITGB | Langmuir | qm (mg/g) | 1021.5 |
| KL (L/mg) | 0.5985 | |||
| R2 | 0.81 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 730.5 | ||
| nF | 14.28 | |||
| R2 | 0.98 | |||
| MITGB | Langmuir | qm (mg/g) | 1202.8 | |
| KL (L/mg) | 2.7126 | |||
| R2 | 0.82 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 938.7 | ||
| nF | 18.87 | |||
| R2 | 0.97 | |||
| TH | ITGB | Langmuir | qm (mg/g) | 1231.4 |
| KL (L/mg) | 0.8304 | |||
| R2 | 0.80 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 837.5 | ||
| nF | 12.50 | |||
| R2 | 0.99 | |||
| MITGB | Langmuir | qm (mg/g) | 1335.7 | |
| KL (L/mg) | 4.8893 | |||
| R2 | 0.86 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 1012.0 | ||
| nF | 16.46 | |||
| R2 | 0.98 | |||
| Adsorbents | Adsorbates | T (K) | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J mol−1 K−1) |
|---|---|---|---|---|---|
| ITGB | RhB | 293 | −2.73 | 3.41 | 20.94 |
| 303 | −2.92 | ||||
| 313 | −3.14 | ||||
| TH | 293 | −3.38 | −0.75 | 9.00 | |
| 303 | −3.46 | ||||
| 313 | −3.56 | ||||
| MITGB | RhB | 293 | −3.08 | 5.32 | 28.69 |
| 303 | −3.40 | ||||
| 313 | −3.66 | ||||
| TH | 293 | −3.65 | −1.23 | 8.24 | |
| 303 | −3.75 | ||||
| 313 | −3.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Xie, K.; Xiao, J.; Chen, S. Influence of Microbial Treatment on the Preparation of Porous Biochar with Stepped-Up Performance and Its Application in Organic Pollutants Control. Int. J. Mol. Sci. 2022, 23, 14082. https://doi.org/10.3390/ijms232214082
Su Y, Xie K, Xiao J, Chen S. Influence of Microbial Treatment on the Preparation of Porous Biochar with Stepped-Up Performance and Its Application in Organic Pollutants Control. International Journal of Molecular Sciences. 2022; 23(22):14082. https://doi.org/10.3390/ijms232214082
Chicago/Turabian StyleSu, Yingjie, Keyu Xie, Jiaohui Xiao, and Siji Chen. 2022. "Influence of Microbial Treatment on the Preparation of Porous Biochar with Stepped-Up Performance and Its Application in Organic Pollutants Control" International Journal of Molecular Sciences 23, no. 22: 14082. https://doi.org/10.3390/ijms232214082
APA StyleSu, Y., Xie, K., Xiao, J., & Chen, S. (2022). Influence of Microbial Treatment on the Preparation of Porous Biochar with Stepped-Up Performance and Its Application in Organic Pollutants Control. International Journal of Molecular Sciences, 23(22), 14082. https://doi.org/10.3390/ijms232214082





