Hepatocyte DAX1 Deletion Exacerbates Inflammatory Liver Injury by Inducing the Recruitment of CD4+ and CD8+ T Cells through NF-κB p65 Signaling Pathway in Mice
Abstract
1. Introduction
2. Results
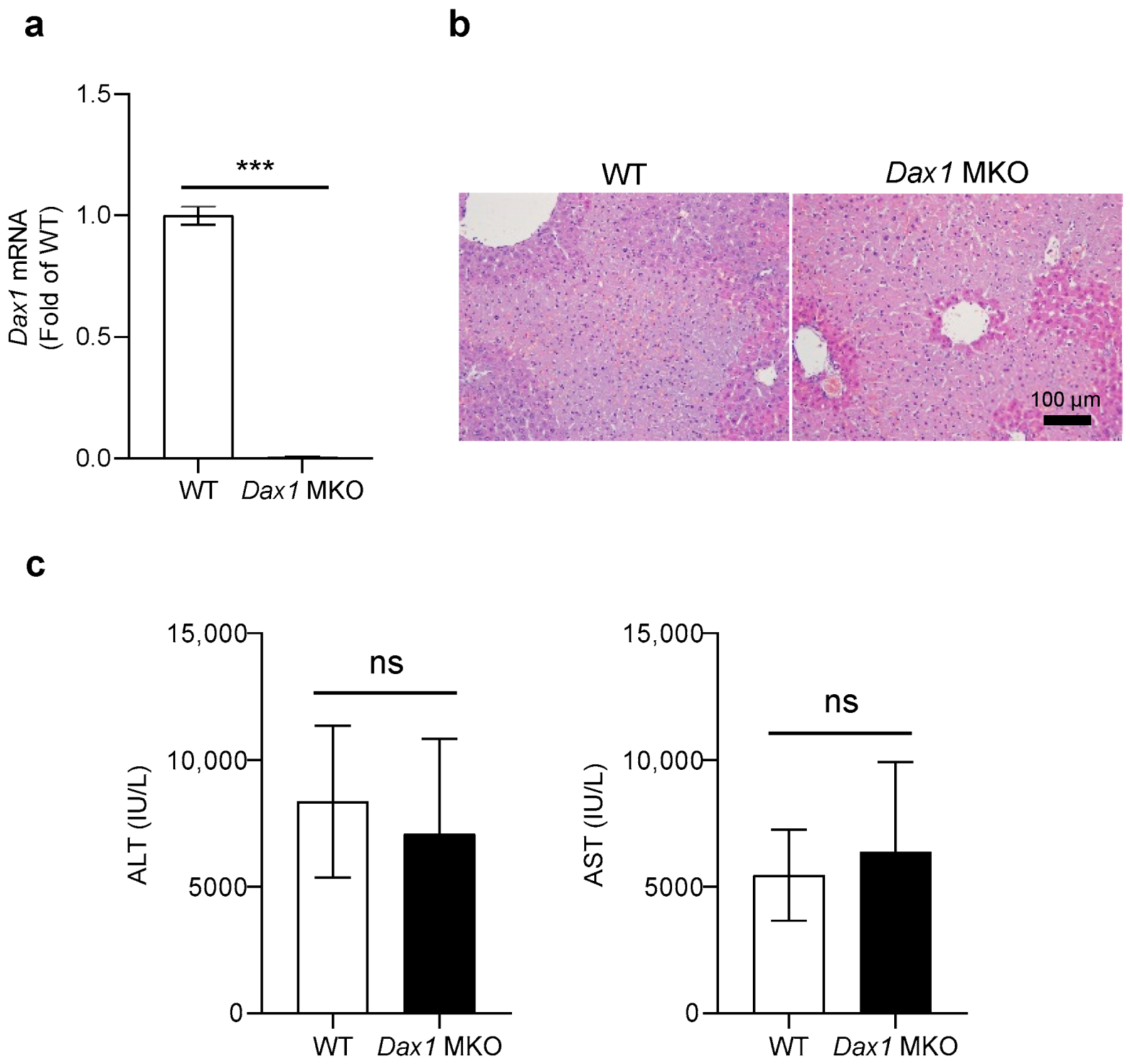
2.1. Loss of DAX1 in Myeloid Cells Does Not Affect ConA-Induced Liver Injury
2.2. DAX1 Deficiency in Hepatocytes Elevates the Susceptibility to ConA-Induced Liver Injury
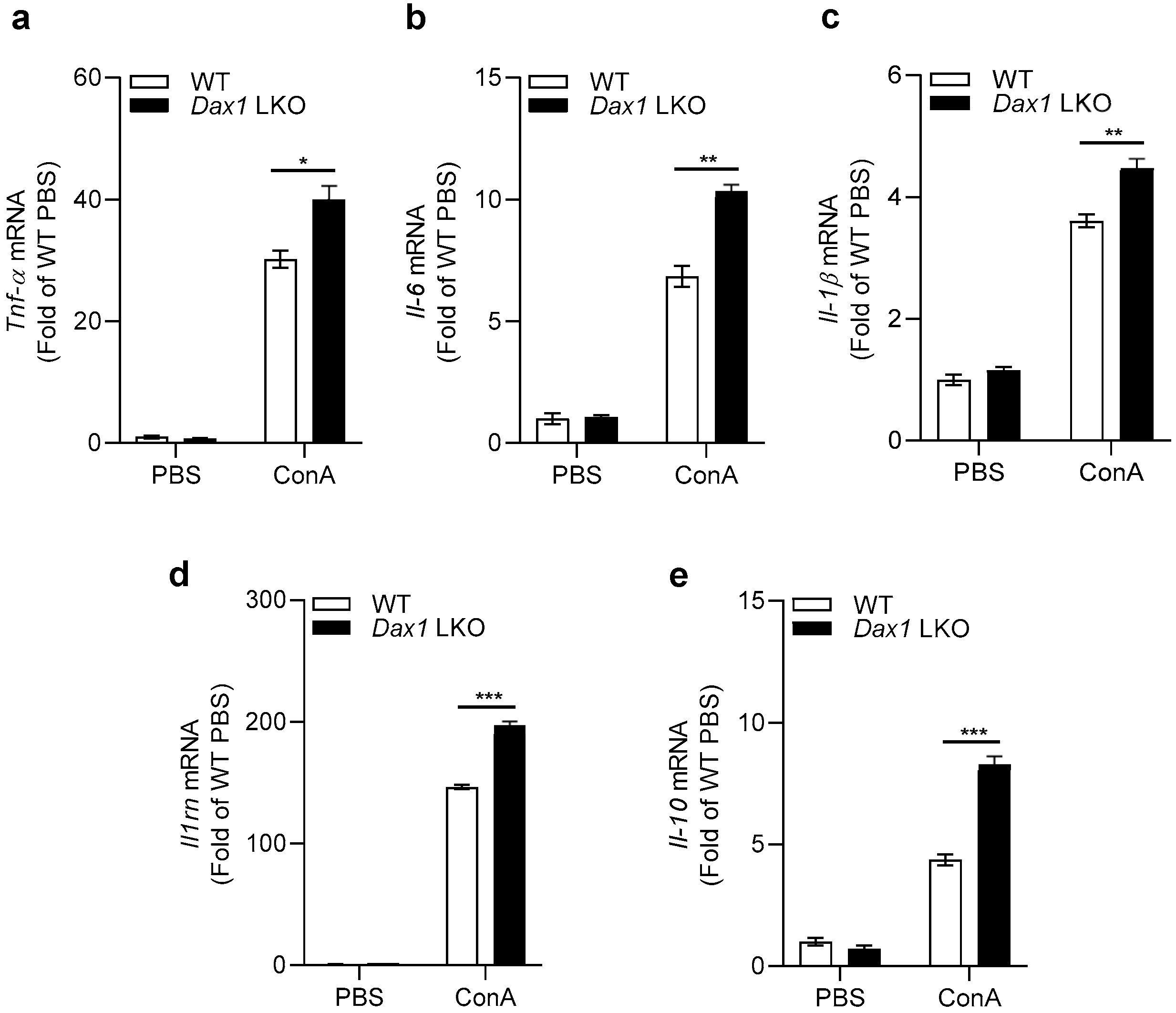
2.3. Hepatic Expression Levels of Inflammatory Cytokines Are Increased in Dax1 LKO Mice following ConA Treatment
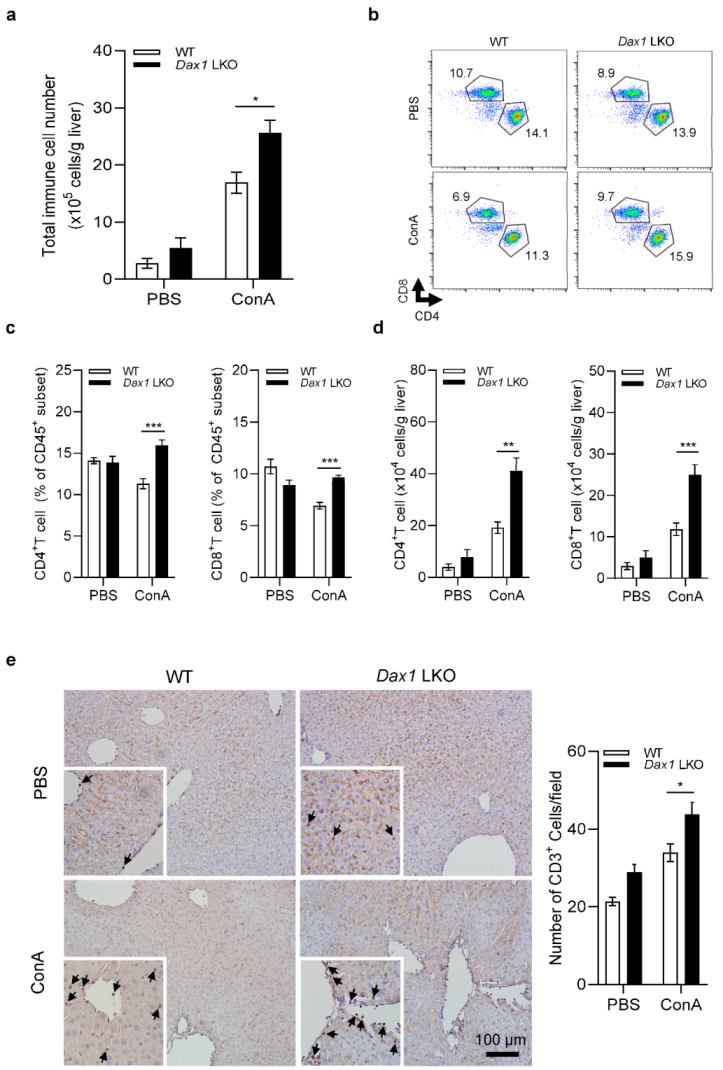
2.4. Absence of DAX1 in Hepatocytes Enhances CD4+ and CD8+ T-Cell Infiltration against ConA-Induced Liver Injury
2.5. DAX1 Modulates the Expression of Both Chemokines and Adhesion Molecules in Hepatocytes to Promote CD4+ and CD8+ T-Cell Migration
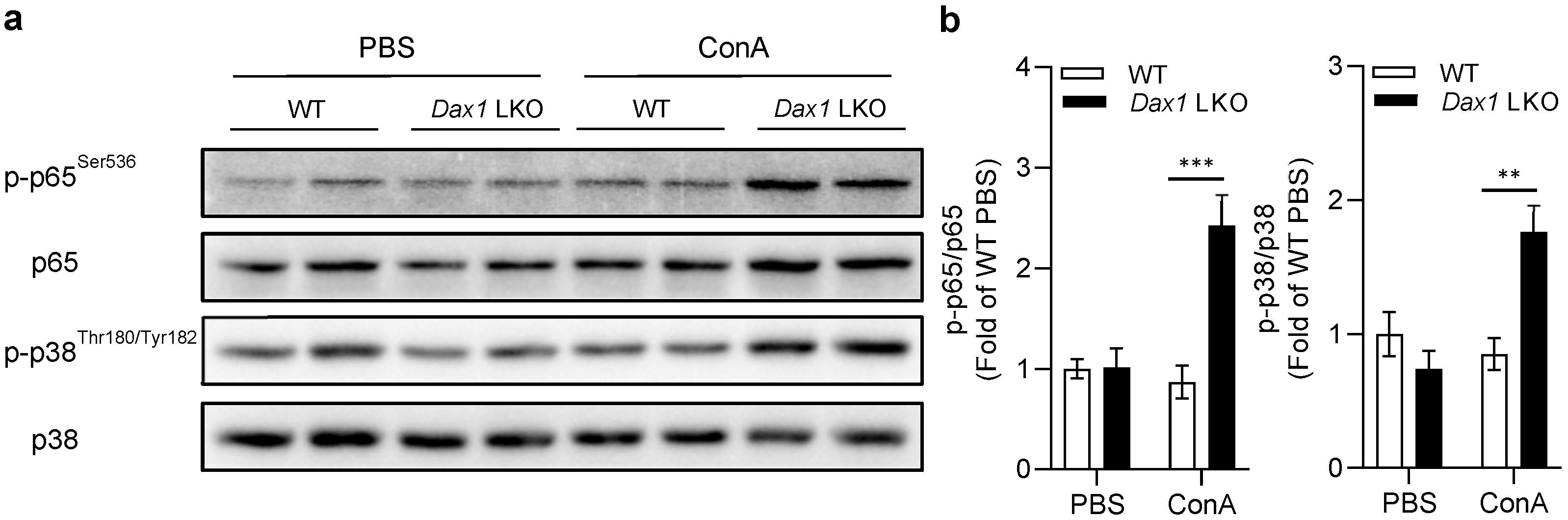
2.6. Hepatocyte DAX1 Deletion Negatively Regulates ConA-Induced Hepatic Injury via Activation of NF-κB p65
3. Discussion
4. Materials and Methods
4.1. Animals Studies
4.2. Blood Analysis
4.3. Histopathology and Immunohistochemistry
4.4. Quantitative Real-Time PCR (RT-qPCR)
4.5. Isolation of Intrahepatic Immune Cells
4.6. Flow-Cytometric Analysis of Intrahepatic Immune Cells
4.7. Bone Marrow-Derived Neutrophils
4.8. Isolation of Primary Mouse Hepatocytes and In Vitro TNFα Treatment
4.9. Western Blot Analysis
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gotthardt, D.; Riediger, C.; Weiss, K.H.; Encke, J.; Schemmer, P.; Schmidt, J.; Sauer, P. Fulminant hepatic failure: Etiology and indications for liver transplantation. Nephrol. Dial. Transplant. 2007, 22 (Suppl. 8), viii5–viii8. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, C.; Koch, A. Mechanisms of Acute Liver Failure. In Liver Immunology: Principles and Practice; Springer: Berlin/Heidelberg, Germany, 2013; pp. 373–388. [Google Scholar] [CrossRef]
- Wu, Z.; Han, M.; Chen, T.; Yan, W.; Ning, Q. Acute liver failure: Mechanisms of immune-mediated liver injury. Liver Int. Off. J. Int. Assoc. Study Liver 2010, 30, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.W.; Harmon, C.; O’Farrelly, C. Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 2016, 13, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Mendez, A.; Vargas, R.E.; Michelangeli, C. Effects of Concanavalin A, fed as a constituent of Jack bean (Canavalia ensiformis L.) seeds, on the humoral immune response and performance of broiler chickens. Poult. Sci. 1998, 77, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ballegeer, M.; Libert, C. Different Cell Types Involved in Mediating Concanavalin A Induced Liver Injury: A Comprehensive Overview. J. Gastroenterol. Hepatol. Res. 2016, 1, 1. [Google Scholar]
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 2015, 49, 12–20. [Google Scholar] [CrossRef]
- Tiegs, G.; Hentschel, J.; Wendel, A. A T cell-dependent experimental liver injury in mice inducible by concanavalin A. J. Clin. Investig. 1992, 90, 196–203. [Google Scholar] [CrossRef]
- Lalli, E.; Sassone-Corsi, P. DAX-1, an unusual orphan receptor at the crossroads of steroidogenic function and sexual differentiation. Mol. Endocrinol. 2003, 17, 1445–1453. [Google Scholar] [CrossRef]
- Niakan, K.K.; McCabe, E.R. DAX1 origin, function, and novel role. Mol. Genet. Metab. 2005, 86, 70–83. [Google Scholar] [CrossRef]
- Noh, J.R.; Kim, Y.H.; Kim, D.K.; Hwang, J.H.; Kim, K.S.; Choi, D.H.; Lee, S.J.; Lee, H.G.; Lee, T.G.; Weng, H.L.; et al. Small Heterodimer Partner Deficiency Increases Inflammatory Liver Injury Through C-X-C motif chemokine ligand 2-Driven Neutrophil Recruitment in Mice. Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 163, 254–264. [Google Scholar] [CrossRef]
- Noh, J.R.; Kim, Y.H.; Kim, D.K.; Hwang, J.H.; Kim, K.S.; Choi, D.H.; Lee, S.J.; Lee, H.G.; Lee, T.G.; Weng, H.L.; et al. Small heterodimer partner negatively regulates C-X-C motif chemokine ligand 2 in hepatocytes during liver inflammation. Sci. Rep. 2018, 8, 15222. [Google Scholar] [CrossRef]
- Bonder, C.S.; Ajuebor, M.N.; Zbytnuik, L.D.; Kubes, P.; Swain, M.G. Essential role for neutrophil recruitment to the liver in concanavalin A-induced hepatitis. J. Immunol. 2004, 172, 45–53. [Google Scholar] [CrossRef]
- Malchow, S.; Thaiss, W.; Jänner, N.; Waetzig, G.H.; Gewiese-Rabsch, J.; Garbers, C.; Yamamoto, K.; Rose-John, S.; Scheller, J. Essential role of neutrophil mobilization in concanavalin A-induced hepatitis is based on classic IL-6 signaling but not on IL-6 trans-signaling. Biochim. Biophys. Acta 2011, 1812, 290–301. [Google Scholar] [CrossRef]
- Vekemans, K.; Braet, F. Structural and functional aspects of the liver and liver sinusoidal cells in relation to colon carcinoma metastasis. World J. Gastroenterol. 2005, 11, 5095–5102. [Google Scholar] [CrossRef]
- Pang, Q.; Jin, H.; Wang, Y.; Dai, M.; Liu, S.; Tan, Y.; Liu, H.; Lu, Z. Depletion of serotonin relieves concanavalin A-induced liver fibrosis in mice by inhibiting inflammation, oxidative stress, and TGF-β1/Smads signaling pathway. Toxicol. Lett. 2021, 340, 123–132. [Google Scholar] [CrossRef]
- Oo, Y.H.; Shetty, S.; Adams, D.H. The role of chemokines in the recruitment of lymphocytes to the liver. Dig. Dis. 2010, 28, 31–44. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Solt, L.A.; May, M.J. The IkappaB kinase complex: Master regulator of NF-kappaB signaling. Immunol. Res. 2008, 42, 3–18. [Google Scholar] [CrossRef]
- Carter, A.B.; Knudtson, K.L.; Monick, M.M.; Hunninghake, G.W. The p38 mitogen-activated protein kinase is required for NF-kappaB-dependent gene expression. The role of TATA-binding protein (TBP). J. Biol. Chem. 1999, 274, 30858–30863. [Google Scholar] [CrossRef]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-kappa B activation by MAP kinase cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Guo, W.; Burris, T.P.; McCabe, E.R. Expression of DAX-1, the gene responsible for X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism, in the hypothalamic-pituitary-adrenal/gonadal axis. Biochem. Mol. Med. 1995, 56, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Nedumaran, B.; Hong, S.; Xie, Y.B.; Kim, Y.H.; Seo, W.Y.; Lee, M.W.; Lee, C.H.; Koo, S.H.; Choi, H.S. DAX-1 acts as a novel corepressor of orphan nuclear receptor HNF4alpha and negatively regulates gluconeogenic enzyme gene expression. J. Biol. Chem. 2009, 284, 27511–27523. [Google Scholar] [CrossRef] [PubMed]
- Nedumaran, B.; Kim, G.S.; Hong, S.; Yoon, Y.S.; Kim, Y.H.; Lee, C.H.; Lee, Y.C.; Koo, S.H.; Choi, H.S. Orphan nuclear receptor DAX-1 acts as a novel corepressor of liver X receptor alpha and inhibits hepatic lipogenesis. J. Biol. Chem. 2010, 285, 9221–9232. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.J.; Zimmermann, H.W.; Adams, D.H. The Role of Myeloid-Derived Cells in the Progression of Liver Disease. Front. Immunol. 2019, 10, 893. [Google Scholar] [CrossRef]
- Metzemaekers, M.; Gouwy, M.; Proost, P. Neutrophil chemoattractant receptors in health and disease: Double-edged swords. Cell. Mol. Immunol. 2020, 17, 433–450. [Google Scholar] [CrossRef]
- Karin, N. CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond. Front. Immunol. 2020, 11, 976. [Google Scholar] [CrossRef]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2022, 9, 12–27. [Google Scholar] [CrossRef]
- Lalor, P.F.; Shields, P.; Grant, A.; Adams, D.H. Recruitment of lymphocytes to the human liver. Immunol. Cell Biol. 2002, 80, 52–64. [Google Scholar] [CrossRef]
- Bunting, K.; Rao, S.; Hardy, K.; Woltring, D.; Denyer, G.S.; Wang, J.; Gerondakis, S.; Shannon, M.F. Genome-wide analysis of gene expression in T cells to identify targets of the NF-kappa B transcription factor c-Rel. J. Immunol. 2007, 178, 7097–7109. [Google Scholar] [CrossRef]
- Iademarco, M.F.; McQuillan, J.J.; Rosen, G.D.; Dean, D.C. Characterization of the promoter for vascular cell adhesion molecule-1 (VCAM-1). J. Biol. Chem. 1992, 267, 16323–16329. [Google Scholar] [CrossRef]
- Moriuchi, H.; Moriuchi, M.; Fauci, A.S. Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 1997, 158, 3483–3491. [Google Scholar]
- Ohmori, Y.; Hamilton, T.A. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J. Biol. Chem. 1993, 268, 6677–6688. [Google Scholar] [CrossRef]
- Tensen, C.P.; Flier, J.; Rampersad, S.S.; Sampat-Sardjoepersad, S.; Scheper, R.J.; Boorsma, D.M.; Willemze, R. Genomic organization, sequence and transcriptional regulation of the human CXCL 11(1) gene. Biochim. Biophys. Acta 1999, 1446, 167–172. [Google Scholar] [CrossRef]
- Van de Stolpe, A.; Caldenhoven, E.; Stade, B.G.; Koenderman, L.; Raaijmakers, J.A.; Johnson, J.P.; van der Saag, P.T. 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J. Biol. Chem. 1994, 269, 6185–6192. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Luedde, T.; Schwabe, R.F. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat. Reviews. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Jijon, H.; Allard, B.; Jobin, C. NF-kappaB inducing kinase activates NF-kappaB transcriptional activity independently of IkappaB kinase gamma through a p38 MAPK-dependent RelA phosphorylation pathway. Cell. Signal. 2004, 16, 1023–1032. [Google Scholar] [CrossRef]
- Olson, C.M.; Hedrick, M.N.; Izadi, H.; Bates, T.C.; Olivera, E.R.; Anguita, J. p38 mitogen-activated protein kinase controls NF-kappaB transcriptional activation and tumor necrosis factor alpha production through RelA phosphorylation mediated by mitogen- and stress-activated protein kinase 1 in response to Borrelia burgdorferi antigens. Infect. Immun. 2007, 75, 270–277. [Google Scholar] [CrossRef]
- Saha, R.N.; Jana, M.; Pahan, K. MAPK p38 regulates transcriptional activity of NF-kappaB in primary human astrocytes via acetylation of p65. J. Immunol. 2007, 179, 7101–7109. [Google Scholar] [CrossRef]
- Kim, Y.H.; Hwang, J.H.; Kim, K.S.; Noh, J.R.; Choi, D.H.; Kim, D.K.; Tadi, S.; Yim, Y.H.; Choi, H.S.; Lee, C.H. Metformin ameliorates acetaminophen hepatotoxicity via Gadd45β-dependent regulation of JNK signaling in mice. J. Hepatol. 2015, 63, 75–82. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, H.-J.; Suh, Y.-J.; Kim, Y.-B.; Kang, E.-J.; Choi, J.H.; Choi, Y.-K.; Lee, I.-B.; Choi, D.-H.; Seo, Y.J.; Noh, J.-R.; et al. Hepatocyte DAX1 Deletion Exacerbates Inflammatory Liver Injury by Inducing the Recruitment of CD4+ and CD8+ T Cells through NF-κB p65 Signaling Pathway in Mice. Int. J. Mol. Sci. 2022, 23, 14009. https://doi.org/10.3390/ijms232214009
Yun H-J, Suh Y-J, Kim Y-B, Kang E-J, Choi JH, Choi Y-K, Lee I-B, Choi D-H, Seo YJ, Noh J-R, et al. Hepatocyte DAX1 Deletion Exacerbates Inflammatory Liver Injury by Inducing the Recruitment of CD4+ and CD8+ T Cells through NF-κB p65 Signaling Pathway in Mice. International Journal of Molecular Sciences. 2022; 23(22):14009. https://doi.org/10.3390/ijms232214009
Chicago/Turabian StyleYun, Hyo-Jeong, Young-Joo Suh, Yu-Bin Kim, Eun-Jung Kang, Jung Hyeon Choi, Young-Keun Choi, In-Bok Lee, Dong-Hee Choi, Yun Jeong Seo, Jung-Ran Noh, and et al. 2022. "Hepatocyte DAX1 Deletion Exacerbates Inflammatory Liver Injury by Inducing the Recruitment of CD4+ and CD8+ T Cells through NF-κB p65 Signaling Pathway in Mice" International Journal of Molecular Sciences 23, no. 22: 14009. https://doi.org/10.3390/ijms232214009
APA StyleYun, H.-J., Suh, Y.-J., Kim, Y.-B., Kang, E.-J., Choi, J. H., Choi, Y.-K., Lee, I.-B., Choi, D.-H., Seo, Y. J., Noh, J.-R., Choi, H.-S., Kim, Y.-H., & Lee, C.-H. (2022). Hepatocyte DAX1 Deletion Exacerbates Inflammatory Liver Injury by Inducing the Recruitment of CD4+ and CD8+ T Cells through NF-κB p65 Signaling Pathway in Mice. International Journal of Molecular Sciences, 23(22), 14009. https://doi.org/10.3390/ijms232214009





