Nitro Dihydrocapsaicin, a Non-Pungent Capsaicin Analogue, Inhibits Cellular Senescence of Lens Epithelial Cells via Upregulation of SIRT1
Abstract
1. Introduction
2. Results and Discussion
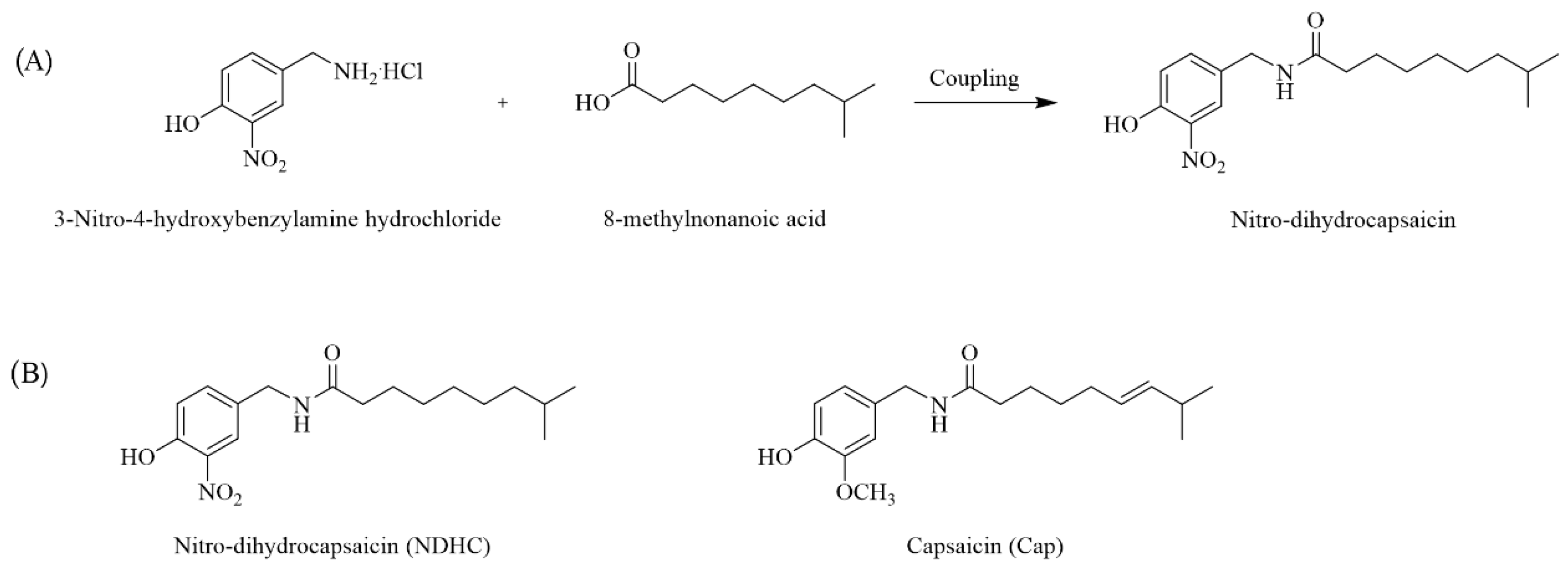
2.1. Nitro Dihydrocapsaicin (NDHC) Synthesis
2.2. Cell Viability Assay
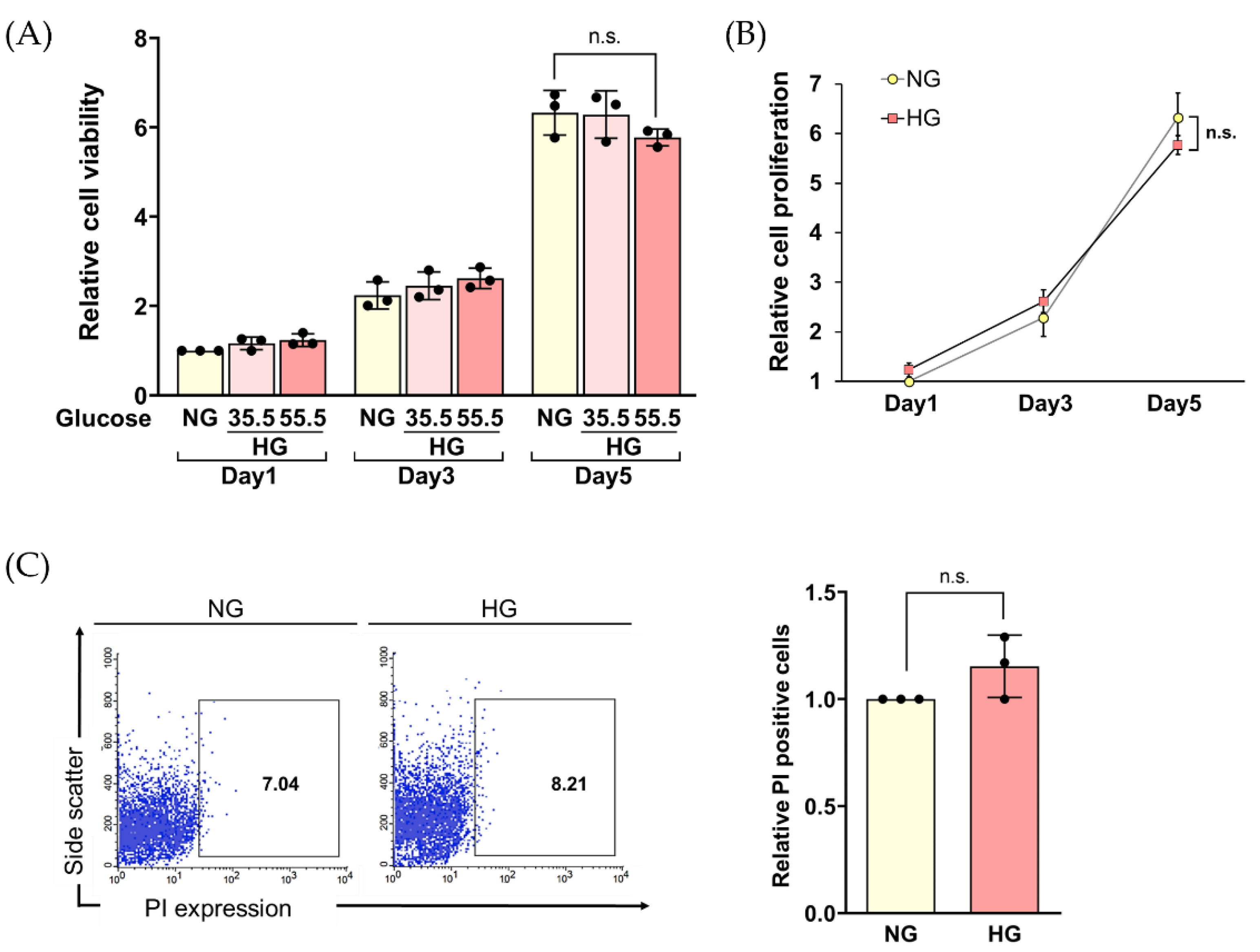
2.3. Long-Term HG Exposure Did Not Impair the Viability of HLE Cells
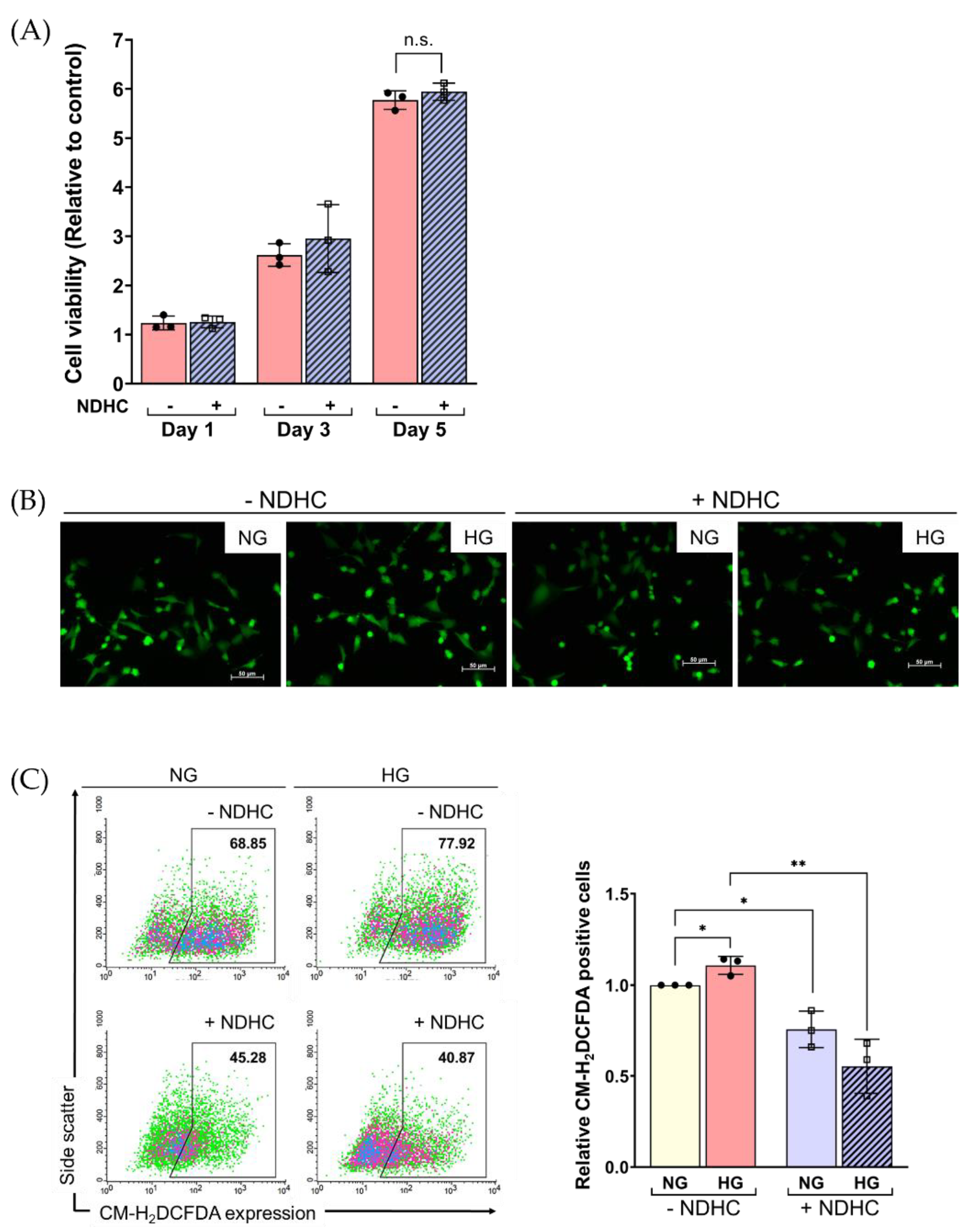
2.4. NDHC Sustained the Viability of HLE by Alleviating the Oxidative Stress Response under High-Glucose Conditions
2.5. Enhancement of Cellular ROS Production and Senescence under HG Condition
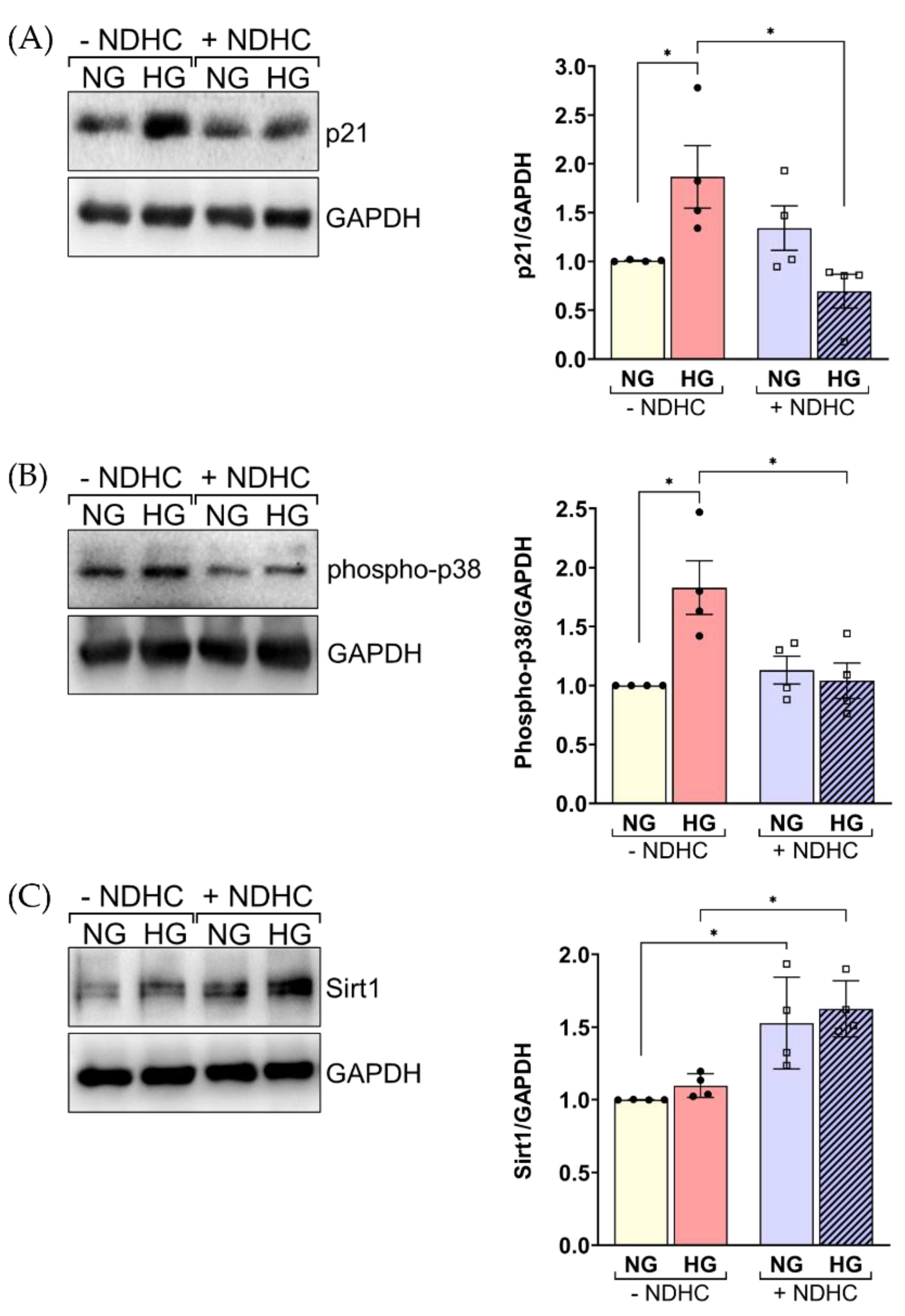
2.6. NDHC Reverses HLE Senescence by Upregulating SIRT1 and Inhibiting p21 and Phospho-p38 Expression
3. Materials and Methods
3.1. Reagents and Antibodies
3.2. A Nitro Dihydrocapsaicin Preparation
3.3. Cell Culture
3.4. Cell Viability Assay
3.5. ROS Production
3.6. SA-β gal Activity Assay
3.7. Western Blot
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haddad, N.M.N.; Sun, J.K.; Abujaber, S.; Schlossman, D.K.; Silva, P.S. Cataract Surgery and its Complications in Diabetic Patients. Semin. Ophthalmol. 2014, 29, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Wu, T.-T.; Ho, C.-Y.; Yeh, T.-C.; Sun, G.-C.; Tseng, C.-J.; Cheng, P.-W. Blocking of SGLT2 to Eliminate NADPH-Induced Oxidative Stress in Lenses of Animals with Fructose-Induced Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 7142. [Google Scholar] [CrossRef] [PubMed]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in diabetes mellitus. World J. Diabetes 2019, 10, 140–153. [Google Scholar] [CrossRef]
- Swarup, A.; Bell, B.A.; Du, J.; Han, J.Y.S.; Soto, J.; Abel, E.D.; Bravo-Nuevo, A.; FitzGerald, P.G.; Peachey, N.S.; Philp, N.J. Deletion of GLUT1 in mouse lens epithelium leads to cataract formation. Exp. Eye Res. 2018, 172, 45–53. [Google Scholar] [CrossRef]
- Jain, A.K.; Lim, G.; Langford, M.; Jain, S.K. Effect of high-glucose levels on protein oxidation in cultured lens cells, and in crystalline and albumin solution and its inhibition by vitamin B6 and N-acetylcysteine: Its possible relevance to cataract formation in diabetes. Free Radic. Biol. Med. 2002, 33, 1615–1621. [Google Scholar] [CrossRef]
- Albert-Garay, J.S.; Riesgo-Escovar, J.R.; Salceda, R. High glucose concentrations induce oxidative stress by inhibiting Nrf2 expression in rat Müller retinal cells in vitro. Sci. Rep. 2022, 12, 1261. [Google Scholar] [CrossRef]
- Chen, P.; Yao, Z.; He, Z. Resveratrol protects against high glucose-induced oxidative damage in human lens epithelial cells by activating autophagy. Exp. Med. 2021, 21, 440. [Google Scholar] [CrossRef]
- Truscott, R.J.W.; Friedrich, M.G. Molecular Processes Implicated in Human Age-Related Nuclear Cataract. Investig. Ophthalmol. Vis. Sci. 2019, 60, 5007–5021. [Google Scholar] [CrossRef]
- Hashim, Z.; Zarina, S. Advanced glycation end products in diabetic and non-diabetic human subjects suffering from cataract. Age 2011, 33, 377–384. [Google Scholar] [CrossRef]
- Bron, A.J.; Sparrow, J.; Brown, N.A.P.; Harding, J.J.; Blakytny, R. The lens in diabetes. Eye 1993, 7, 260–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Fu, Y.; Wang, X.; Wu, R.; Su, D.; Zhou, N.; Qi, Y. Metformin protects lens epithelial cells against senescence in a naturally aged mouse model. Cell Death Discov. 2022, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef]
- Bertelli, P.M.; Pedrini, E.; Hughes, D.; McDonnell, S.; Pathak, V.; Peixoto, E.; Guduric-Fuchs, J.; Stitt, A.W.; Medina, R.J. Long term high glucose exposure induces premature senescence in retinal endothelial cells. Front. Physiol. 2022, 13, 1661. [Google Scholar] [CrossRef]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Jankowski, M.; Angielski, S. High glucose concentration affects the oxidant-antioxidant balance in cultured mouse podocytes. J. Cell. Biochem. 2011, 112, 1661–1672. [Google Scholar] [CrossRef]
- Yao, Q.; Zhou, Y.; Yang, Y.; Cai, L.; Xu, L.; Han, X.; Guo, Y.; Li, P.A. Activation of Sirtuin1 by lyceum barbarum polysaccharides in protection against diabetic cataract. J. Ethnopharmacol. 2020, 261, 113165. [Google Scholar] [CrossRef] [PubMed]
- Mimura, T.; Kaji, Y.; Noma, H.; Funatsu, H.; Okamoto, S. The role of SIRT1 in ocular aging. Exp. Eye Res. 2013, 116, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, T.L.; Olawale, F.; Olisah, C.; Adetunji, A.E.; Aremu, A.O. Capsaicin: A Two-Decade Systematic Review of Global Research Output and Recent Advances Against Human Cancer. Front. Oncol. 2022, 12, 908487. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Escogido Mde, L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and pharmacological aspects of capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef] [PubMed]
- Delamere, N.A.; Shahidullah, M. Ion Transport Regulation by TRPV4 and TRPV1 in Lens and Ciliary Epithelium. Front. Physiol. 2021, 12, 834916. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Donaldson, P.J.; Petrova, R.S. Verification and spatial mapping of TRPV1 and TRPV4 expression in the embryonic and adult mouse lens. Exp. Eye Res. 2019, 186, 107707. [Google Scholar] [CrossRef] [PubMed]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the therapeutic potential of capsaicin and its analogues in pain and other diseases. Molecules. 2016, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Chapa-Oliver, A.M.; Mejía-Teniente, L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules 2016, 21, 931. [Google Scholar] [CrossRef] [PubMed]
- Krishnatreyya, H.; Hazarika, H.; Saha, A.; Chattopadhyay, P. Fundamental pharmacological expressions on ocular exposure to capsaicin, the principal constituent in pepper sprays. Sci. Rep. 2018, 8, 12153. [Google Scholar] [CrossRef]
- Baron, R. Capsaicin and nociception: From basic mechanisms to novel drugs. Lancet 2000, 356, 785–787. [Google Scholar] [CrossRef]
- Laorob, T.; Bunyapraphatsara, N.; Waranuch, N.; Pongcharoen, S.; Punyain, W.; Chancharunee, S.; Sakchaisri, K.; Pratuangdejkul, J.; Chongruchiroj, S.; Kielar, F.; et al. Enhancement of Lipolysis in 3T3-L1 Adipocytes by Nitroarene Capsaicinoid Analogs. Nat. Prod. Commun. 2021, 16, 1934578X2098794. [Google Scholar] [CrossRef]
- Malewicz, N.M.; Rattray, Z.; Oeck, S.; Jung, S.; Escamilla-Rivera, V.; Chen, Z.; Tang, X.; Zhou, J.; LaMotte, R.H. Topical Capsaicin in Poly(lactic-co-glycolic)acid (PLGA) Nanoparticles Decreases Acute Itch and Heat Pain. Int. J. Mol. Sci. 2022, 23, 5275. [Google Scholar] [CrossRef]
- Moya, C.; Marquez-Aguirre, A. The Prospective Antiobesity Effect of Capsaicin Synthetic Analogs: A Matter of Weight. Med. Chem. 2016, 6, 365–371. [Google Scholar] [CrossRef]
- Wang, J.-L. A Study of Pungency of Capsaicinoid as Affected by Their Molecular Structure Alteration. Pharmacol. Pharm. 2011, 2, 109–115. [Google Scholar] [CrossRef]
- Tsukura, Y.; Mori, M.; Hirotani, Y.; Ikeda, K.; Amano, F.; Kato, R.; Ijiri, Y.; Tanaka, K. Effects of Capsaicin on Cellular Damage and Monolayer Permeability in Human Intestinal Caco-2 Cells. Biol. Pharm. Bull. 2007, 30, 1982–1986. [Google Scholar] [CrossRef]
- Du, S.; Shao, J.; Xie, D.; Zhang, F. Decorin inhibits glucose-induced lens epithelial cell apoptosis via suppressing p22phox-p38 MAPK signaling pathway. PLoS ONE 2020, 15, e0224251. [Google Scholar] [CrossRef]
- Fu, J.; Hu, X. Simvastatin alleviates epithelial-mesenchymal transition and oxidative stress of high glucose-induced lens epithelial cells in vitro by inhibiting RhoA/ROCK signaling. Exp. Med. 2022, 23, 420. [Google Scholar] [CrossRef]
- Weil, B.R.; Abarbanell, A.M.; Herrmann, J.L.; Wang, Y.; Meldrum, D.R. High glucose concentration in cell culture medium does not acutely affect human mesenchymal stem cell growth factor production or proliferation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1735–R1743. [Google Scholar] [CrossRef] [PubMed]
- Aswamenakul, K.; Klabklai, P.; Pannengpetch, S.; Tawonsawatruk, T.; Isarankura-Na-Ayudhya, C.; Roytrakul, S.; Nantasenamat, C.; Supokawej, A. Proteomic study of in vitro osteogenic differentiation of mesenchymal stem cells in high glucose condition. Mol. Biol. Rep. 2020, 47, 7505–7516. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, H.; Wei, T.; Wang, D.; Xia, H.; Zhao, L.; Ji, J.; Gao, H. High Glucose-Induced PC12 Cell Death by Increasing Glutamate Production and Decreasing Methyl Group Metabolism. BioMed Res. Int. 2016, 2016, 4125731. [Google Scholar] [CrossRef]
- Thongin, S.; Den-udom, T.; Uppakara, K.; Sriwantana, T.; Sibmooh, N.; Laorob, T.; Boonthip, C.; Wichai, U.; Muta, K.; Ketsawatsomkron, P. Beneficial effects of capsaicin and dihydrocapsaicin on endothelial inflammation, nitric oxide production and antioxidant activity. Biomed. Pharmacother. 2022, 154, 113521. [Google Scholar] [CrossRef] [PubMed]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- de Mera-Rodríguez, J.A.; Álvarez-Hernán, G.; Gañán, Y.; Martín-Partido, G.; Rodríguez-León, J.; Francisco-Morcillo, J. Is Senescence-Associated β-Galactosidase a Reliable in vivo Marker of Cellular Senescence during Embryonic Development? Front. Cell Dev. Biol. 2021, 9, 623175. [Google Scholar] [CrossRef]
- Harney, A.; Meade, T. Molecular imaging of in vivo gene expression. Future Med. Chem. 2010, 2, 503–519. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Souroullas, G.P.; Diekman, B.O.; Krishnamurthy, J.; Hall, B.M.; Sorrentino, J.A.; Parker, J.S.; Sessions, G.A.; Gudkov, A.V.; Sharpless, N.E. Cells exhibiting strong p16INK4a promoter activation in vivo display features of senescence. Proc. Natl. Acad. Sci. USA 2019, 116, 2603–2611. [Google Scholar] [CrossRef]
- Bras, I.D.; Colitz, C.M.; Kusewitt, D.F.; Chandler, H.; Lu, P.; Gemensky-Metzler, A.J.; Wilkie, D.A. Evaluation of advanced glycation end-products in diabetic and inherited canine cataracts. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 245, 249–257. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paensuwan, P.; Laorob, T.; Ngoenkam, J.; Wichai, U.; Pongcharoen, S. Nitro Dihydrocapsaicin, a Non-Pungent Capsaicin Analogue, Inhibits Cellular Senescence of Lens Epithelial Cells via Upregulation of SIRT1. Int. J. Mol. Sci. 2022, 23, 13960. https://doi.org/10.3390/ijms232213960
Paensuwan P, Laorob T, Ngoenkam J, Wichai U, Pongcharoen S. Nitro Dihydrocapsaicin, a Non-Pungent Capsaicin Analogue, Inhibits Cellular Senescence of Lens Epithelial Cells via Upregulation of SIRT1. International Journal of Molecular Sciences. 2022; 23(22):13960. https://doi.org/10.3390/ijms232213960
Chicago/Turabian StylePaensuwan, Pussadee, Thanet Laorob, Jatuporn Ngoenkam, Uthai Wichai, and Sutatip Pongcharoen. 2022. "Nitro Dihydrocapsaicin, a Non-Pungent Capsaicin Analogue, Inhibits Cellular Senescence of Lens Epithelial Cells via Upregulation of SIRT1" International Journal of Molecular Sciences 23, no. 22: 13960. https://doi.org/10.3390/ijms232213960
APA StylePaensuwan, P., Laorob, T., Ngoenkam, J., Wichai, U., & Pongcharoen, S. (2022). Nitro Dihydrocapsaicin, a Non-Pungent Capsaicin Analogue, Inhibits Cellular Senescence of Lens Epithelial Cells via Upregulation of SIRT1. International Journal of Molecular Sciences, 23(22), 13960. https://doi.org/10.3390/ijms232213960





