Non-Ionic Surfactant Effects on Innate Pluronic 188 Behavior: Interactions, and Physicochemical and Biocompatibility Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Poloxamer 188/Surfactant Nanosystems
2.2. Fluorescence Spectroscopy Results
2.3. Thermal Characterization by MicroDSC
2.4. In Vitro Toxicity Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Poloxamer 188 and Poloxamer 188/Surfactant Colloidal Dispersions
3.2.2. Light Scattering Methods
3.2.3. MicroDSC Analysis and High-Resolution Acoustic Spectroscopy
3.2.4. Fluorescence Spectroscopy
3.2.5. In Vitro Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, H.; Lu, X.; Wang, W.; Kang, N.G.; Mays, J.W. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Karayianni, M.; Pispas, S. Block copolymer solution self-assembly: Recent advances, emerging trends, and applications. J. Polym. Sci. 2021, 59, 1874–1898. [Google Scholar] [CrossRef]
- Jiao, W.; Yang, H.; Wu, Z.; Liu, J.; Zhang, W. Self-assembled block polymer aggregates in selective solution: Controllable morphology transitions and their applications in drug delivery. Expert Opin. Drug Deliv. 2020, 17, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-Assembled Block Copolymer Aggregates: From Micelles to Vesicles and Their Biological Applications. Macromol. Rapid Commun. 2009, 30, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Alexandridis, P. Amphiphilic Block Copolymers: Self-Assembly and Applications, 1st ed.; Lindman, B., Ed.; Elsevier Science & Technology: Oxford, UK, 2014. [Google Scholar]
- Rodríguez-Hernández, J. Micro-/nanostructured polymer blends containing block copolymers. In Recent Developments in Polymer Macro, Micro and Nano Blends; Elsevier: New York, NY, USA, 2017; pp. 131–161. [Google Scholar]
- Kretzmann, J.A.; Evans, C.W.; Norret, M.; Swaminathan Iyer, K. Supramolecular Assemblies of Dendrimers and Dendritic Polymers in Nanomedicine. In Comprehensive Supramolecular Chemistry II; Elsevier: New York, NY, USA, 2017; pp. 237–256. [Google Scholar]
- Chountoulesi, M.; Perinelli, D.R.; Forys, A.; Katifelis, H.; Selianitis, D.; Chrysostomou, V.; Lagopati, N.; Bonacucina, G.; Trzebicka, B.; Gazouli, M.; et al. Studying the properties of polymer-lipid nanostructures: The role of the host lipid. J. Drug Deliv. Sci. Technol. 2022, 77, 103830. [Google Scholar] [CrossRef]
- Javan, R. Biological Structures; Elsevier Ltd.: Richmond, VA, USA, 2005. [Google Scholar]
- Kaur, P.; Garg, T.; Rath, G.; Murthy, R.S.R.; Goyal, A.K. Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. 2016, 23, 727–738. [Google Scholar] [CrossRef]
- Ai, X.; Zhong, L.; Niu, H.; He, Z. Thin-film hydration preparation method and stability test of DOX-loaded disulfide-linked polyethylene glycol 5000-lysine-di-tocopherol succinate nanomicelles. Asian J. Pharm. Sci. 2014, 9, 244–250. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, L.; Kent, B.; Wong, S.; Garvey, C.J.; Stenzel, M.H. The protein corona leads to deformation of spherical micelles. Angew. Chem. Int. Ed. Engl. 2021, 60, 10342–10349. [Google Scholar] [CrossRef]
- Heinz, H.; Pramanik, C.; Heinz, O.; Ding, Y.; Mishra, R.K.; Marchon, D.; Flatt, R.J.; Estrela-Lopis, I.; Llop, J.; Moya, S.; et al. Nanoparticle decoration with surfactants: Molecular interactions, assembly, and applications. Surf. Sci. Rep. 2017, 72, 1–58. [Google Scholar] [CrossRef]
- Kang, J.-H.; Chon, J.; Kim, Y.-I.; Lee, H.-J.; Oh, D.-W.; Lee, H.-G.; Han, C.-S.; Kim, D.-W.; Park, C.-W. Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Int. J. Nanomed. 2019, 14, 5381–5396. [Google Scholar] [CrossRef] [PubMed]
- Yeo, L.K.; Olusanya, T.O.B.; Chaw, C.S.; Elkordy, A.A. Brief effect of a small hydrophobic drug (cinnarizine) on the physicochemical characterisation of niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics 2018, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pitto-Barry, A.; Barry, N.P.E. Pluronic® block-copolymers in medicine: From chemical and biological versatility to rationalisation and clinical advances. Polym. Chem. 2014, 5, 3291–3297. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Perinelli, D.R.; Forys, A.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Liquid crystalline nanoparticles for drug delivery: The role of gradient and block copolymers on the morphology, internal organisation and release profile. Eur. J. Pharm. Biopharm. 2021, 158, 21–34. [Google Scholar] [CrossRef]
- Nambam, J.S.; Philip, J. Effects of interaction of ionic and nonionic surfactants on self-assembly of PEO-PPO-PEO triblock copolymer in aqueous solution. J. Phys. Chem. B 2012, 116, 1499–1507. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Khougaz, K.; Astafieva, I.; Eisenberg, A. Micellization in block polyelectrolyte solutions. 3. Static light scattering characterization. Macromolecules 1995, 28, 7135–7147. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Pispas, S.; Chrysina, E.D.; Forys, A.; Trzebicka, B.; Demetzos, C. Cubic lyotropic liquid crystals as drug delivery carriers: Physicochemical and morphological studies. Int. J. Pharm. 2018, 550, 57–70. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Pippa, N.; Chrysostomou, V.; Pispas, S.; Chrysina, E.D.; Forys, A.; Otulakowski, L.; Trzebicka, B.; Demetzos, C. Stimuli-responsive lyotropic liquid crystalline nanosystems with incorporated poly(2-dimethylamino ethyl methacrylate)-b-poly(Lauryl methacrylate) amphiphilic block copolymer. Polymers 2019, 11, 1400. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Naziris, N.; Stellas, D.; Massala, C.; Zouliati, K.; Pispas, S.; Demetzos, C.; Forys, A.; Marcinkowski, A.; Trzebicka, B. PEO-b-PCL grafted niosomes: The cooperativilty of amphiphilic components and their properties in vitro and in vivo. Colloids Surf. B Biointerfaces 2019, 177, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Lakowicz, J.R., Ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Abbasi Moud, A. Rheology and microscopy analysis of polymer–surfactant complexes. Colloid Polym. Sci. 2022, 300, 733–762. [Google Scholar] [CrossRef]
- Sheng, J.J. Surfactant Flooding. In Modern Chemical Enhanced Oil Recovery; Elsevier: New York, NY, USA, 2011; pp. 239–335. [Google Scholar]
- Bollenbach, L.; Buske, J.; Mäder, K.; Garidel, P. Poloxamer 188 as surfactant in biological formulations—An alternative for polysorbate 20/80? Int. J. Pharm. 2022, 620, 121706. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; Di Sarno, A.; D’Apuzzo, M.; Avallone, P.R.; Raccone, E.; Bellissimo, A.; Auriemma, F.; Grizzuti, N.; Pasquino, R. Rheology and morphology of Pluronic F68 in water. Phys. Fluids 2021, 33, 043113. [Google Scholar] [CrossRef]
- Fathy, M.; El-Badry, M. Preparation and evaluation of piroxicam—Pluronic solid dispersions. Bull. Pharm. Sci. 2003, 26, 97–108. [Google Scholar] [CrossRef]
- Sickler, B. Interpreting DSC Data. Available online: https://www.mrl.ucsb.edu (accessed on 8 October 2022).
- Manzoor, U. Differential Scanning Calorimetry. University of the Punjab. 2014. Available online: https://www.slideshare.net/umairmanzoor372/dsc-presentation-31613937 (accessed on 10 October 2022).
- Glass Transition. Available online: https://polymerdatabase.com/polymer%20physics/GlassTransition.html (accessed on 11 October 2022).
- Zhang, M.; Djabourov, M.; Bourgaux, C.; Bouchemal, K. Nanostructured fluids from pluronic® mixtures. Int. J. Pharm. 2013, 454, 599–610. [Google Scholar] [CrossRef]
- Cespi, M.; Bonacucina, G.; Mencarelli, G.; Pucciarelli, S.; Giorgioni, G.; Palmieri, G.F. Monitoring the aggregation behaviour of self-assembling polymers through high-resolution ultrasonic spectroscopy. Int. J. Pharm. 2010, 388, 274–279. [Google Scholar] [CrossRef]
- Isothermal/Adiabatic Compressibility. Available online: https://paradigms.oregonstate.edu/problem/41/ (accessed on 12 October 2022).
- Sezgin-Bayindir, Z.; Yuksel, N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech. 2012, 13, 826–835. [Google Scholar] [CrossRef]
- Abdelkader, H.; Ismail, S.; Kamal, A.; Alany, R.G. Preparation of niosomes as an ocular delivery system for naltrexone hydrochloride: Physicochemical characterization. Pharmazie 2010, 65, 811–817. [Google Scholar]
- Pramod, K.; Suneesh, C.V.; Shanavas, S.; Ansari, S.H.; Ali, J. Unveiling the compatibility of eugenol with formulation excipients by systematic drug-excipient compatibility studies. J. Anal. Sci. Technol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Rabha, B.; Bharadwaj, K.K.; Baishya, D.; Sarkar, T.; Edinur, H.A.; Pati, S. Synthesis and characterization of diosgenin encapsulated poly-ε-caprolactone-Pluronic nanoparticles and its effect on brain cancer cells. Polymers 2021, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Pepić, I.; Lovrić, J.; Filipović-Grčić, J. How do polymeric micelles cross epithelial barriers? Eur. J. Pharm. Sci. 2013, 50, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT assay: Utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef]
- Portugal, J. Re: 79% Cell Viability in MTT Assay is Indicating Induction of Apoptosis or Necrosis/Cell Toxic Effect of a Drug. Can Anyone Explain This? 2014. Available online: https://www.researchgate.net/post/79-Cell-viability-in-MTT-assay-is-indicating-induction-of-apoptosis-or-Necrosis-Cell-Toxic-effect-of-a-drug-Can-anyone-explain-this/540432b0d11b8b455d8b4619/citation/download (accessed on 6 October 2022).
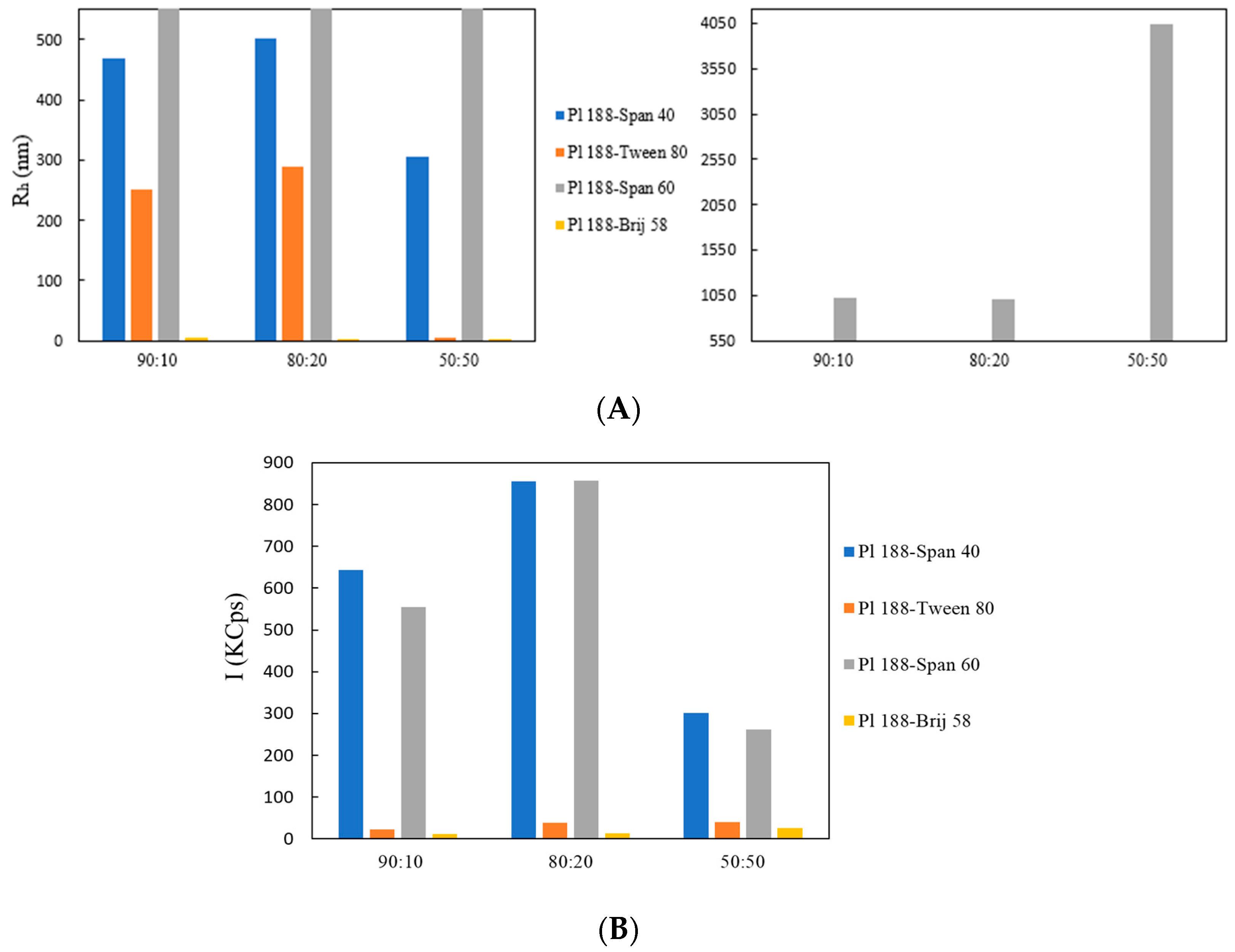
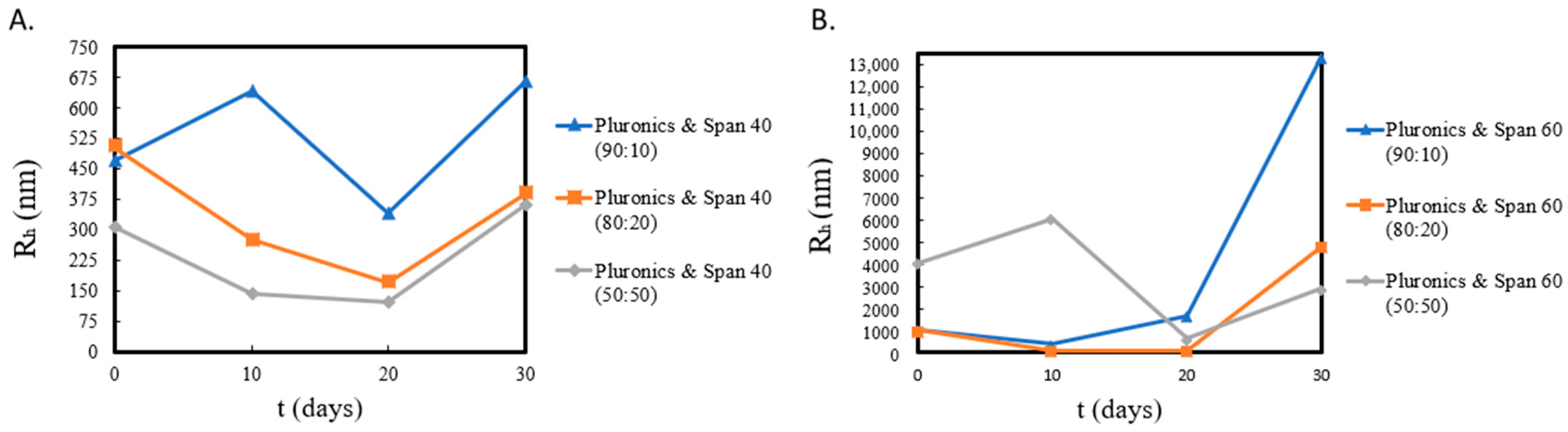



| System | Weight Ratio | Rh (nm) a | I (KCps) b | PDI c |
|---|---|---|---|---|
| Poloxamer 188 | 100:0 | 425.0 | 763 | 0.480 |
| Poloxamer 188-Tween 80 | 90:10 | 252.0 | 23 | 0.530 |
| Poloxamer 188-Tween 80 | 80:20 | 290.0 | 39 | 0.603 |
| Poloxamer 188-Tween 80 | 50:50 | 6.0 | 23 | 0.435 |
| Poloxamer 188-Span 40 | 90:10 | 470.0 | 644 | 0.520 |
| Poloxamer 188-Span 40 | 80:20 | 503.0 | 855 | 0.560 |
| Poloxamer 188-Span 40 | 50:50 | 305.0 | 302 | 0.530 |
| Poloxamer 188-Span 60 | 90:10 | 1018.0 | 555 | 0.509 |
| Poloxamer 188-Span 60 | 80:20 | 1002.0 | 857 | 0.489 |
| Poloxamer 188-Span 60 | 50:50 | 4047.0 | 261 | 0.542 |
| Poloxamer 188-Brij 58 | 90:10 | 5.0 | 11 | 0.582 |
| Poloxamer 188-Brij 58 | 80:20 | 346.0 | 13 | 0.520 |
| Poloxamer 188-Brij 58 | 50:50 | 4.0 | 26 | 0.431 |
| System | Weight Ratio | Pyrene | Dipyrene |
|---|---|---|---|
| I1/I3 | Iex/I1 | ||
| Poloxamer 188 | 100:0 | 1.17 | 0.42 |
| Poloxamer 188-Tween 80 | 90:10 | 1.18 | 0.37 |
| Poloxamer 188-Tween 80 | 80:20 | 1.16 | 0.57 |
| Poloxamer 188-Tween 80 | 50:50 | 1.08 | 0.27 |
| Poloxamer 188-Span 40 | 90:10 | 1.01 | 0.15 |
| Poloxamer 188-Span 40 | 80:20 | 0.95 | 0.12 |
| Poloxamer 188-Span 40 | 50:50 | 0.96 | 0.09 |
| Poloxamer 188-Span 60 | 90:10 | 1.21 | 0.03 |
| Poloxamer 188-Span 60 | 80:20 | 0.96 | 0.14 |
| Poloxamer 188-Span 60 | 50:50 | 0.85 | 0.12 |
| Poloxamer 188-Brij 58 | 90:10 | 0.96 | 0.99 |
| Poloxamer 188-Brij 58 | 80:20 | 1.33 | 0.70 |
| Poloxamer 188-Brij 58 | 50:50 | 1.13 | 0.65 |
| System | Weight Ratio | mDSC | HR-US (Sound Speed) | |
|---|---|---|---|---|
| Transition Temperature (°C) | Enthalpy (J/g of Solution) | Transition Temperature (°C) | ||
| Poloxamer 188 | 100:0 | 55.66 ± 0.75 | 0.041 ± 0.011 | 56.82 ± 0.96 |
| Poloxamer 188–Tween 80 | 90:10 | 53.69 ± 0.06 | 0.030 ± 0.002 | 54.86 ± 0.74 |
| Poloxamer 188–Tween 80 | 80:20 | 53.80 ± 0.12 | 0.029 ± 0.011 | 53.36 ± 0.66 |
| Poloxamer 188–Tween 80 | 50:50 | 53.18 ± 0.22 | 0.016 ± 0.013 | 52.47 ± 0.33 |
| Poloxamer 188–Span 40 | 90:10 | 53.00 ± 0.64 | 0.029 ± 0.012 | 52.27 ± 0.97 |
| Poloxamer 188–Span 40 | 80:20 | 52.30 ± 0.36 | 0.037 ± 0.009 | 50.35 ± 0.95 |
| Poloxamer 188–Span 40 | 50:50 | 45.67 ± 0.46 | 0.124 ± 0.013 | 46.61 ± 0.37 |
| Poloxamer 188–Span 60 | 90:10 | 53.20 ± 0.19 | 0.135 ± 0.012 | 54.13 ± 0.46 |
| Poloxamer 188–Span 60 | 80:20 | 55.56 ± 0.30 | 0.182 ± 0.012 | 56.12 ± 0.53 |
| Poloxamer 188–Span 60 | 50:50 | 54.22 ± 0.14 | 0.354 ± 0.010 | 55.17 ± 0.55 |
| Poloxamer 188–Brij 58 | 90:10 | 53.89 ± 0.23 | 0.070 ± 0.009 | 56.86 ± 0.45 |
| Poloxamer 188–Brij 58 | 80:20 | 53.14 ± 0.13 | 0.022 ± 0.007 | 55.66 ± 0.66 |
| Poloxamer 188–Brij 58 | 50:50 | 52.57 ± 0.16 | 0.011 ± 0.005 | 56.77 ± 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kontogiannis, O.; Selianitis, D.; Perinelli, D.R.; Bonacucina, G.; Pippa, N.; Gazouli, M.; Pispas, S. Non-Ionic Surfactant Effects on Innate Pluronic 188 Behavior: Interactions, and Physicochemical and Biocompatibility Studies. Int. J. Mol. Sci. 2022, 23, 13814. https://doi.org/10.3390/ijms232213814
Kontogiannis O, Selianitis D, Perinelli DR, Bonacucina G, Pippa N, Gazouli M, Pispas S. Non-Ionic Surfactant Effects on Innate Pluronic 188 Behavior: Interactions, and Physicochemical and Biocompatibility Studies. International Journal of Molecular Sciences. 2022; 23(22):13814. https://doi.org/10.3390/ijms232213814
Chicago/Turabian StyleKontogiannis, Orestis, Dimitrios Selianitis, Diego Romano Perinelli, Giulia Bonacucina, Natassa Pippa, Maria Gazouli, and Stergios Pispas. 2022. "Non-Ionic Surfactant Effects on Innate Pluronic 188 Behavior: Interactions, and Physicochemical and Biocompatibility Studies" International Journal of Molecular Sciences 23, no. 22: 13814. https://doi.org/10.3390/ijms232213814
APA StyleKontogiannis, O., Selianitis, D., Perinelli, D. R., Bonacucina, G., Pippa, N., Gazouli, M., & Pispas, S. (2022). Non-Ionic Surfactant Effects on Innate Pluronic 188 Behavior: Interactions, and Physicochemical and Biocompatibility Studies. International Journal of Molecular Sciences, 23(22), 13814. https://doi.org/10.3390/ijms232213814










