Antibiofilm Activity of Sundew Species against Multidrug-Resistant Escherichia coli Strains
Abstract
1. Introduction
2. Results and Discussion
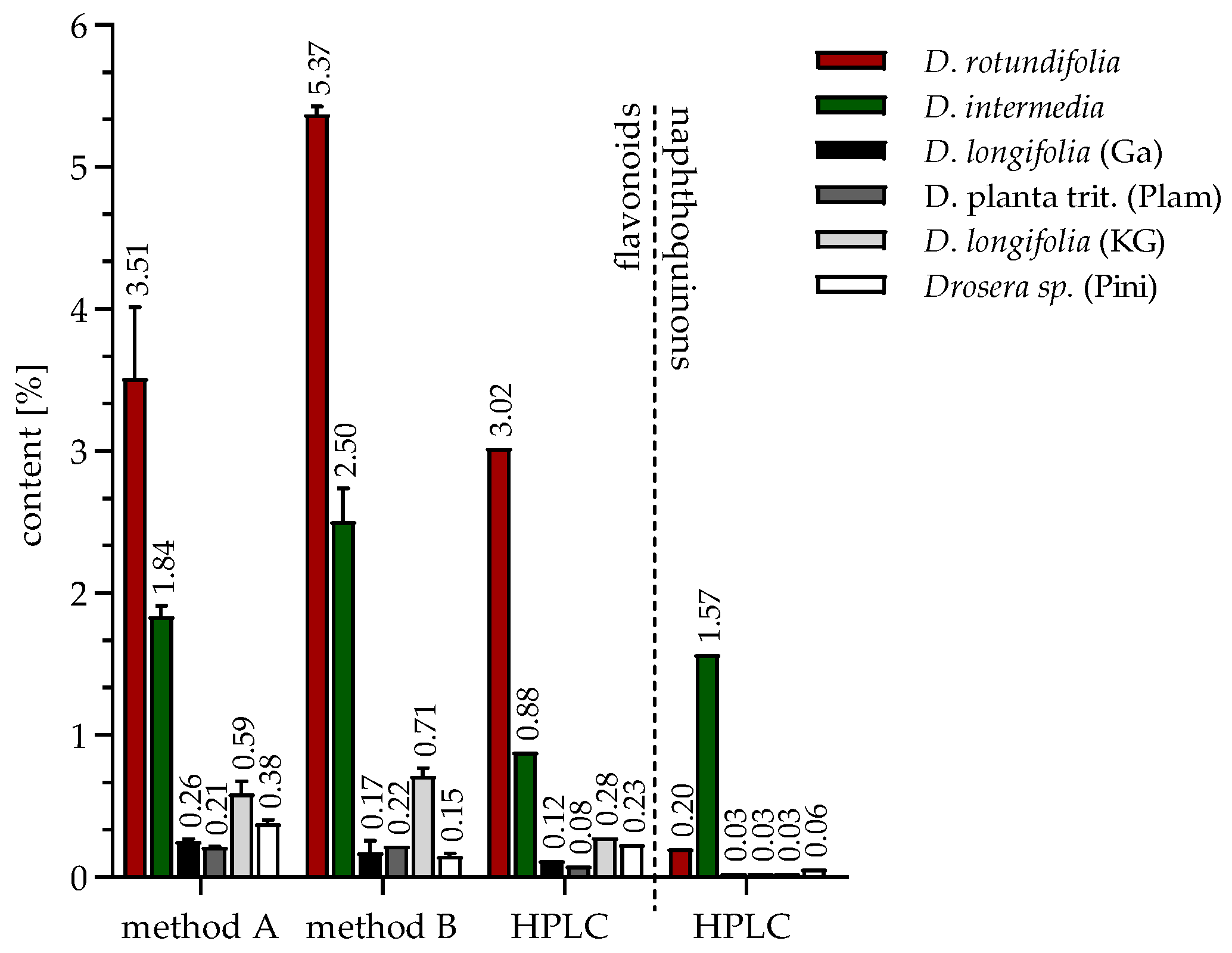
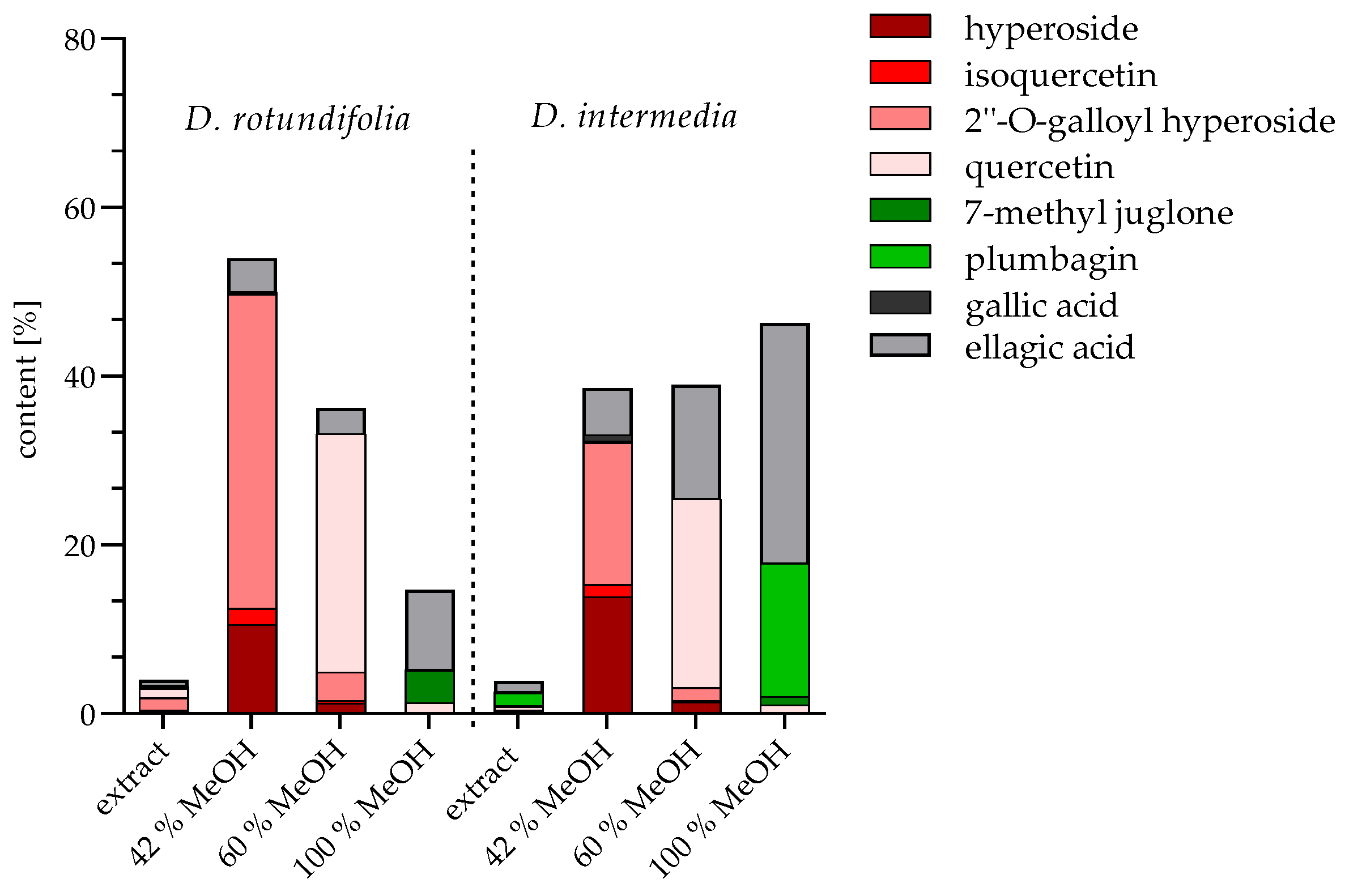
2.1. Identification and Quantification of Flavonoids and Naphthoquinones
2.1.1. Identification of 7-Methyl Juglone
2.1.2. Identification of 2″-O-Galloyl Hyperoside
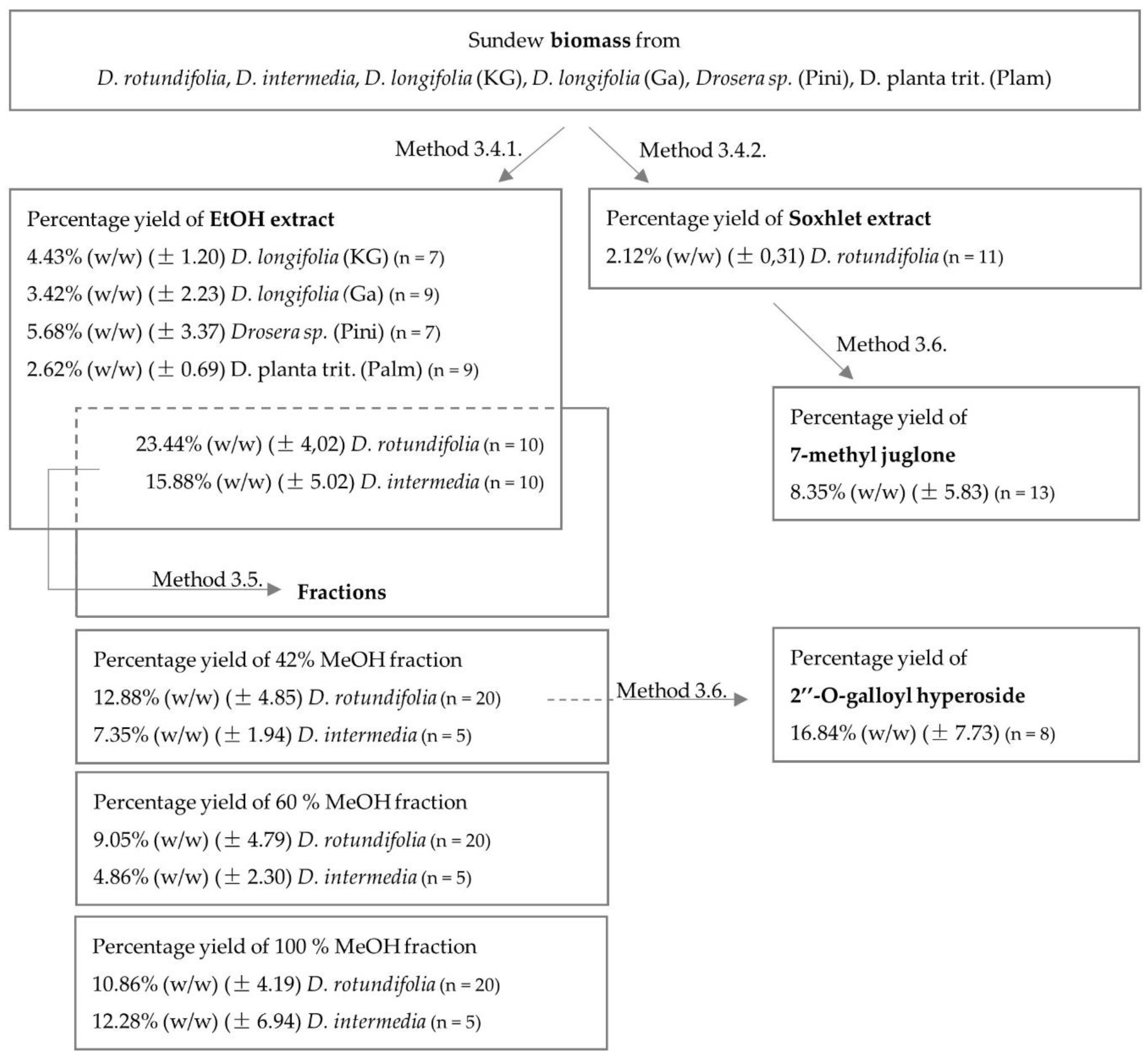
2.1.3. Quantification of Extracts and Fractions
2.2. Microbiological Testing
2.2.1. Minimum Inhibitory Concentration (MIC)
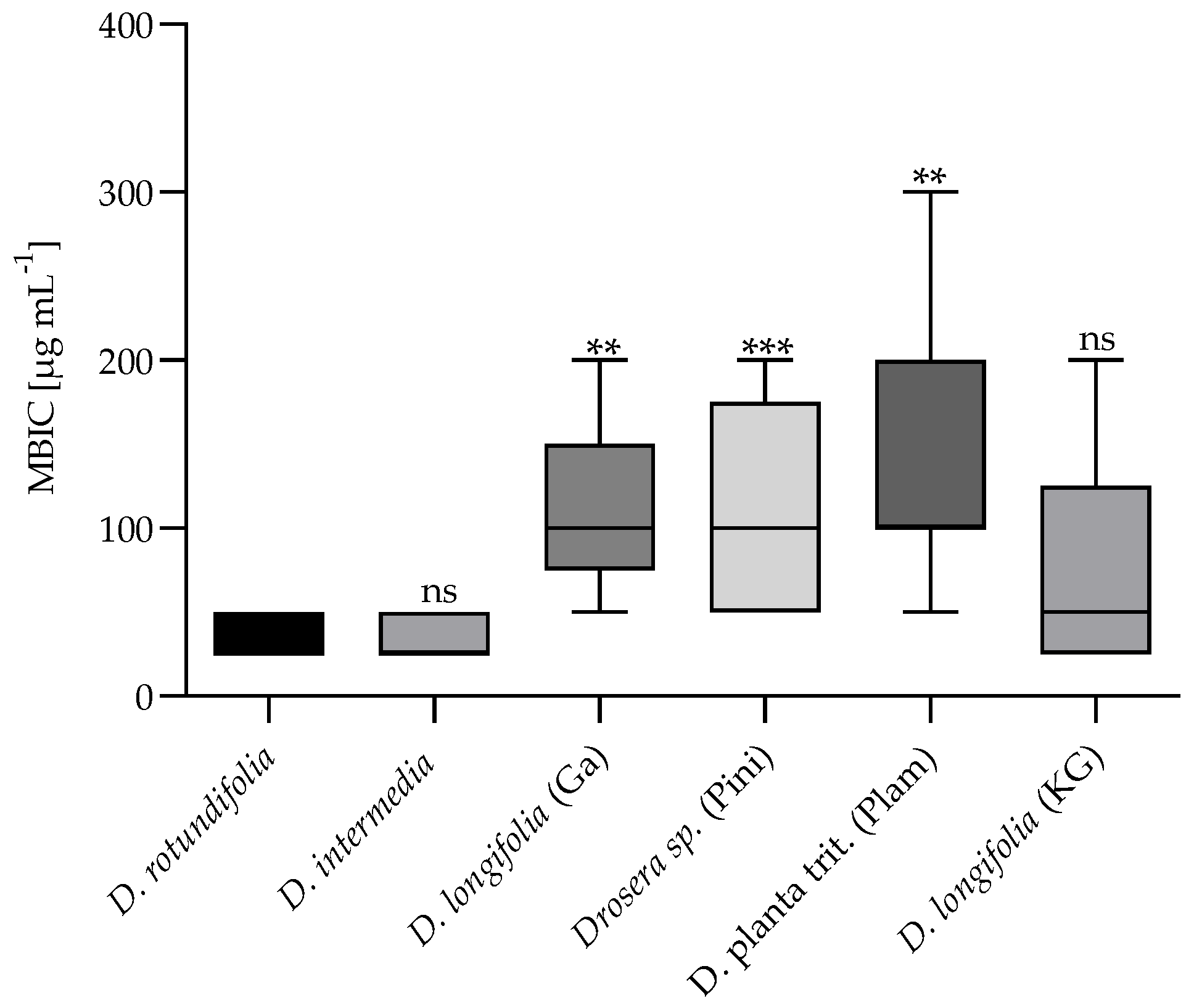
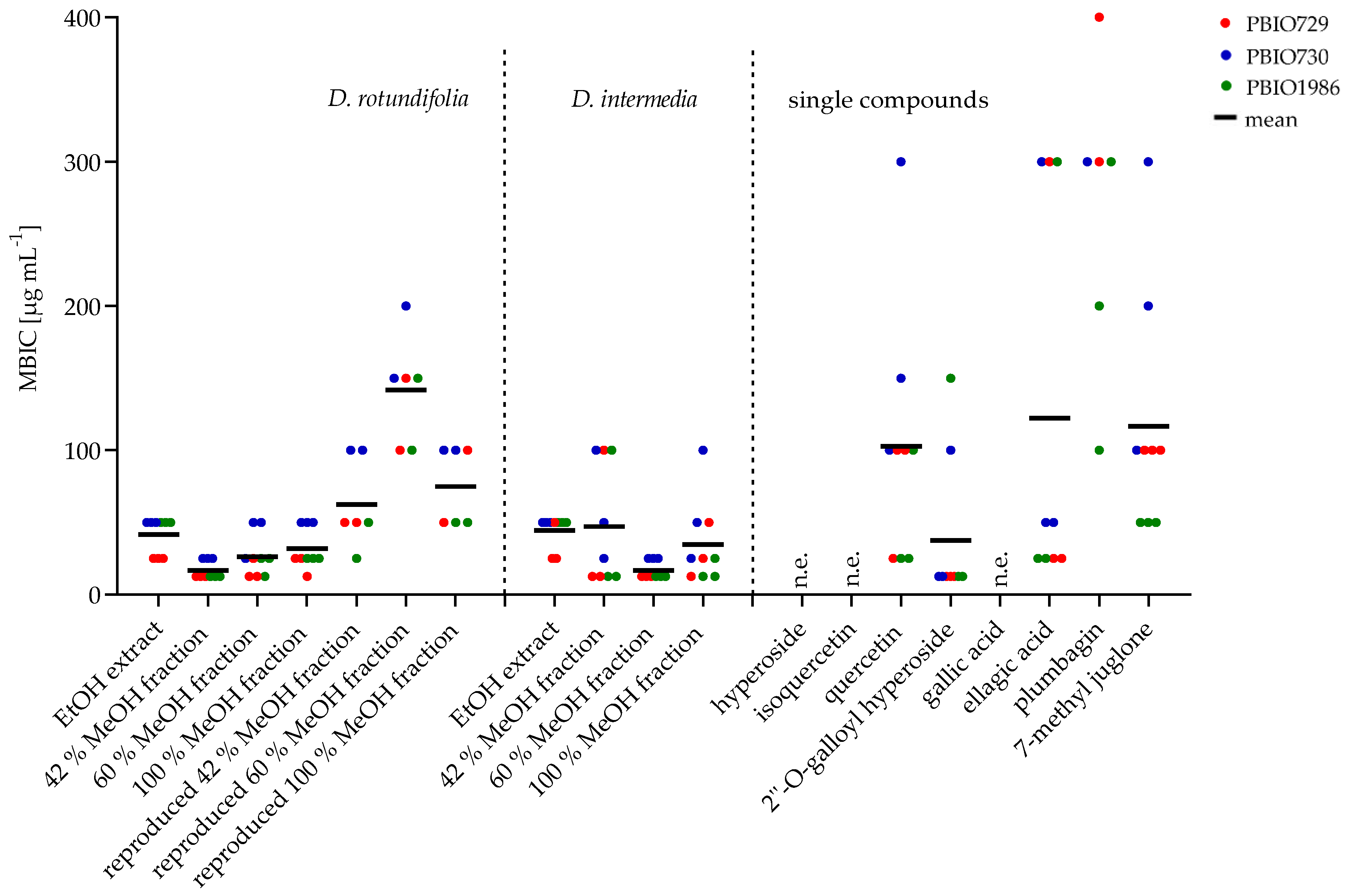
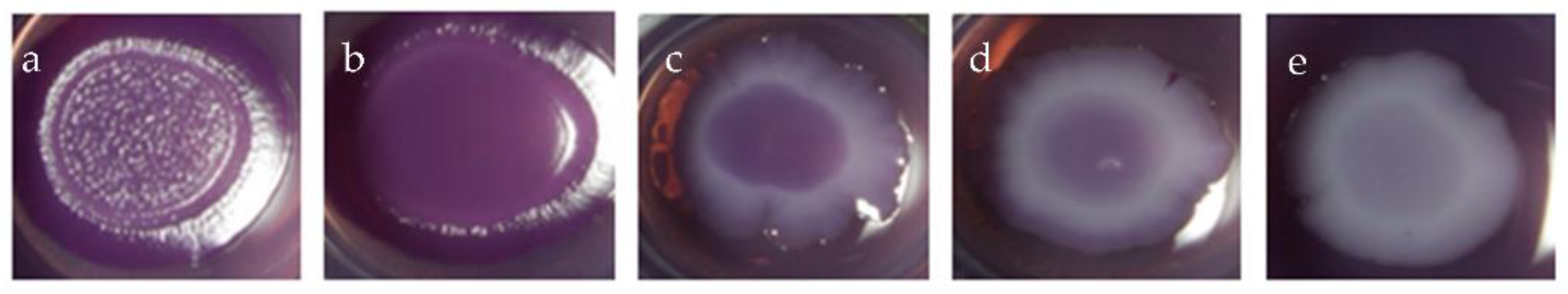
2.2.2. Minimum Biofilm Inhibitory Concentration (MBIC)
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Bacteria
3.4. Extraction
3.4.1. Ultrasonic Extraction
3.4.2. Soxhlet Extraction
3.5. Solid Phase Extraction
3.6. Size Exclusion Chromatography
3.7. Determination of Flavonoids
3.8. High Performance Liquid Chromatography
3.9. LC-MS Analysis
3.10. Minimum Inhibitory Concentration
3.11. Biofilm Assay
3.12. Software
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rivadavia, F.; Kondo, K.; Kato, M.; Hasebe, M. Phylogeny of the sundews, Drosera (Droseraceae), based on chloroplast rbcL and nuclear 18S ribosomal DNA Sequences. Am. J. Bot. 2003, 90, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.A.; van der Kooy, F. Phytochemistry of the carnivorous sundew genus Drosera (Droseraceae)—Future perspectives and ethnopharmacological relevance. Chem. Biodivers. 2013, 10, 1774–1790. [Google Scholar] [CrossRef]
- Metzing, D.; Hofbauer, N.; Ludwig, G.; Matzke-Hajek, G. Rote Liste gefährdeter Tiere, Pflanzen und Pilze Deutschlands; Naturschutz und biologische Vielfalt 70.1; Bundesamt für Naturschutz: Bonn-Bad Godesberg, Germany, 2009. [Google Scholar]
- Krenn, L.; Beyer, G.; Pertz, H.H.; Karall, E.; Kremser, M.; Galambosi, B.; Melzig, M.F. In vitro antispasmodic and anti-inflammatory effects of Drosera rotundifolia. Arzneimittelforschung 2004, 54, 402–405. [Google Scholar] [PubMed]
- Paper, D.H.; Karall, E.; Kremser, M.; Krenn, L. Comparison of the antiinflammatory effects of Drosera rotundifolia and Drosera madagascariensis in the HET-CAM assay. Phytother. Res. PTR 2005, 19, 323–326. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, B.-E.; Wink, C.; Wink, M. Handbuch der Arzneipflanzen. Ein Illustrierter Leitfaden; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2004. [Google Scholar]
- Zehl, M.; Braunberger, C.; Conrad, J.; Crnogorac, M.; Krasteva, S.; Vogler, B.; Beifuss, U.; Krenn, L. Identification and quantification of flavonoids and ellagic acid derivatives in therapeutically important Drosera species by LC-DAD, LC-NMR, NMR, and LC-MS. Anal. Bioanal. Chem. 2011, 400, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, B.; Joosten, H. Biology, ecology, use, conservation and cultivation of round-leaved sundew (Drosera rotundifolia L.): A review. Mires Peat 2016, 18, 1–28. [Google Scholar]
- Kreyling, J.; Tanneberger, F.; Jansen, F.; van der Linden, S.; Aggenbach, C.; Blüml, V.; Couwenberg, J.; Emsens, W.-J.; Joosten, H.; Klimkowska, A.; et al. Rewetting does not return drained fen peatlands to their old selves. Nat. Commun. 2021, 12, 5693. [Google Scholar] [CrossRef]
- Kämäräinen, T.; Uusitalo, J.; Jalonen, J.; Laine, K.; Hohtola, A. Regional and habitat differences in 7-methyljuglone content of Finnish Drosera rotundifolia. Phytochemistry 2003, 63, 309–314. [Google Scholar] [CrossRef]
- Kolodziej, H.; Pertz, H.H.; Humke, A. Main constituents of a commercial Drosera fluid extract and their antagonist activity at muscarinic M3 receptors in guinea-pig ileum. Die Pharm. 2002, 57, 201–203. [Google Scholar] [CrossRef]
- Melzig, M.F.; Pertz, H.H.; Krenn, L. Anti-inflammatory and spasmolytic activity of extracts from Droserae herba. Phytomedicine 2001, 8, 225–229. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm Activity of Plant Polyphenols. Molecules 2016, 21, 12. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.; Khanal, S.; Poudel, P.; Khadayat, K.; Ghaju, S.; Bhandari, A.; Lekhak, S.; Pant, N.D.; Sharma, M.; Marasini, B.P. Extended spectrum β-lactamase producing uropathogenic Escherichia coli and the correlation of biofilm with antibiotics resistance in Nepal. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Epidemiology of urinary tract infections: Incidence, morbidity, and economic costs. Am. J. Med. 2002, 113, 5–13. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norrby, S.R. Urinary tract infections: Disease panorama and challenges. J. Infect. Dis. 2001, 183 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, K.; Semmler, T.; Wieler, L.H.; Trott, D.J.; Pitout, J.; Peirano, G.; Bonnedahl, J.; Dolejska, M.; Literak, I.; Fuchs, S.; et al. Genomic and Functional Analysis of Emerging Virulent and Multidrug-Resistant Escherichia coli Lineage Sequence Type 648. Antimicrob. Agents Chemother. 2019, 63, 6. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Sharma, S.; Sharma, P.; Chandola, D.; Dang, S.; Gupta, S.; Gabrani, R. Escherichia coli biofilm: Development and therapeutic strategies. J. Appl. Microbiol. 2016, 121, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Coumans, M.; Hofinger, M.; Ramaut, J.L.; Gaspar, T. High-performance gas chromatography of 1,4-naphthoquinones from Droseraceae. Chromatographia 1984, 18, 621–622. [Google Scholar] [CrossRef]
- Medic, A.; Zamljen, T.; Hudina, M.; Veberic, R. Identification and Quantification of Naphthoquinones and Other Phenolic Compounds in Leaves, Petioles, Bark, Roots, and Buds of Juglans regia L., Using HPLC-MS/MS. Horticulturae 2021, 7, 326. [Google Scholar] [CrossRef]
- Zhou, D.; Jin, Y.; Yao, F.; Duan, Z.; Wang, Q.; Liu, J. Validated LC-MS/MS method for the simultaneous determination of hyperoside and 2″-O-galloylhyperin in rat plasma: Application to a pharmacokinetic study in rats. Biomed. Chromatogr. BMC 2014, 28, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Braunberger, C.; Zehl, M.; Conrad, J.; Wawrosch, C.; Strohbach, J.; Beifuss, U.; Krenn, L. Flavonoids as chemotaxonomic markers in the genus Drosera. Phytochemistry 2015, 118, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L.; Blaeser, U.; Hausknost-Chenicek, N. Determination of Naphthoquinones in Droserae herba by Reversed-Phase High Performance Liquid Chromatography. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 3149–3160. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Pruteanu, M.; Hernández Lobato, J.I.; Stach, T.; Hengge, R. Common plant flavonoids prevent the assembly of amyloid curli fibres and can interfere with bacterial biofilm formation. Environ. Microbiol. 2020, 22, 5280–5299. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Shady, N.H.; Abdeljawad, K.M.; Elzamkan, M.B.; Helmy, H.H.; Tarshan, E.A.; Adly, A.N.; Hussien, Y.H.; Sayed, N.G.; Zayed, A.; et al. Antimicrobial potentials of natural products against multidrug resistance pathogens: A comprehensive review. RSC Adv. 2022, 12, 29078–29102. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, S.R.; Figueiredo, M.R.; Aragão, T.V.; Kaplan MA, C. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Mem. Inst. Oswaldo Cruz 2003, 98, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Curreli, N.; Sollai, F.; Massa, L.; Comandini, O.; Rufo, A.; Sanjust, E.; Rinaldi, A.; Rinaldi, A.C. Effects of plant-derived naphthoquinones on the growth of Pleurotus sajor-caju and degradation of the compounds by fungal cultures. J. Basic Microbiol. 2001, 41, 253. [Google Scholar] [CrossRef]
- Krishnaswamy, M.; Purushothaman, K.K. Plumbagin: A study of its anticancer, antibacterial & antifungal properties. Indian J. Exp. Biol. 1980, 18, 876–877. [Google Scholar] [PubMed]
- Edenharder, R.; Tang, X. Inhibition of the mutagenicity of 2-nitrofluorene, 3-nitrofluoranthene and 1-nitropyrene by flavonoids, coumarins, quinones and other phenolic compounds. Food Chem. Toxicol. 1997, 35, 357–372. [Google Scholar] [CrossRef]
- Cohen, G.M.; d’Arcy Doherty, M. Free radical mediated cell toxicity by redox cycling chemicals. Br. J. Cancer. Suppl. 1987, 8, 46–52. [Google Scholar]
- Hassan, H.; Fridovich, I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch. Biochem. Biophys. 1979, 196, 385–395. [Google Scholar] [CrossRef]
- Neuhaus, J.M.; Wright, J.K. Chemical modification of the lactose carrier of Escherichia coli by plumbagin, phenylarsinoxide or diethylpyrocarbonate affects the binding of galactoside. Eur. J. Biochem. 1983, 137, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.; Fridovich, I. Exogenous quinones directly inhibit the respiratory NADH dehydrogenase in Escherichia coli. Arch. Biochem. Biophys. 1992, 296, 337–346. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic antimicrobial activities of epigallocatechin gallate, myricetin, daidzein, gallic acid, epicatechin, 3-hydroxy-6-methoxyflavone and genistein combined with antibiotics against ESKAPE pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef] [PubMed]
- El-Gammal, A.A.; Mansour, R. Antimicrobial Activities of Some Flavonoid Compounds. Zent. Mikrobiol. 1986, 141, 561–565. [Google Scholar] [CrossRef]
- Rauha, J.-P.; Remes, S.; Heinonen, M.; Hopia, A.; Kähkönen, M.; Kujala, T.; Pihlaja, K.; Vuorela, H.; Vuorela, P. Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Food Microbiol. 2000, 56, 3–12. [Google Scholar] [CrossRef]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σ(E) -dependent sRNA RybB. Mol. Microbiol. 2016, 101, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Schaufler, K.; Semmler, T.; Pickard, D.J.; Toro M de La Cruz F de Wieler, L.H.; Ewers, C.; Guenther, S. Carriage of Extended-Spectrum Beta-Lactamase-Plasmids Does Not Reduce Fitness but Enhances Virulence in Some Strains of Pandemic E. coli Lineages. Front. Microbiol. 2016, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Neumann, N.; Honke, M.; Povydysh, M.; Guenther, S.; Schulze, C. Evaluating Tannins and Flavonoids from Traditionally Used Medicinal Plants with Biofilm Inhibitory Effects against MRGN E. coli. Molecules 2022, 27, 7. [Google Scholar] [CrossRef] [PubMed]
- Stepanov, D.; Buchmann, D.; Schultze, N.; Wolber, G.; Schaufler, K.; Guenther, S.; Belik, V. A Combined Bayesian and Similarity-Based Approach for Predicting E. coli Biofilm Inhibition by Phenolic Natural Compounds. J. Nat. Prod. 2022, 85, 2255–2265. [Google Scholar] [CrossRef] [PubMed]
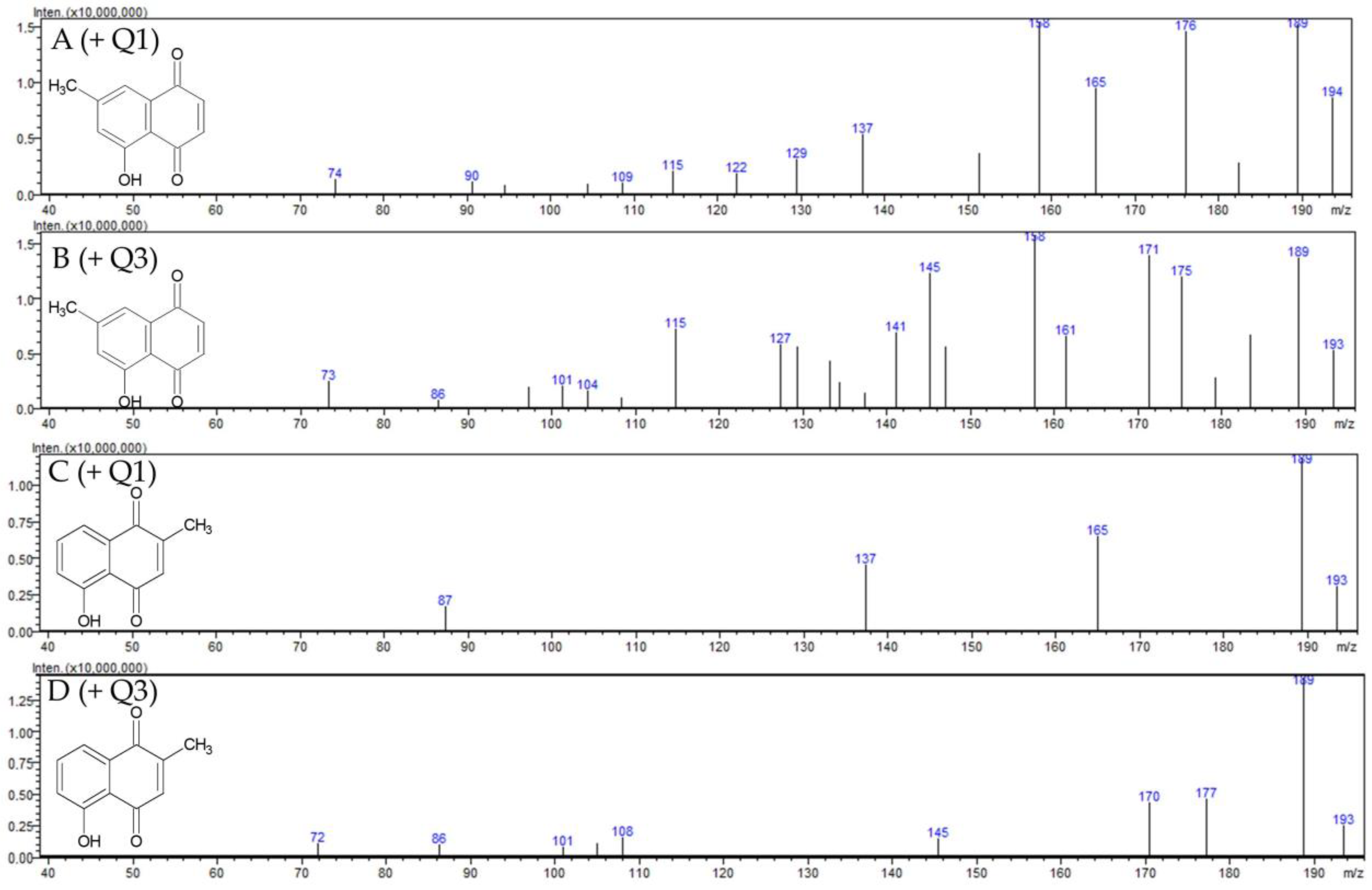
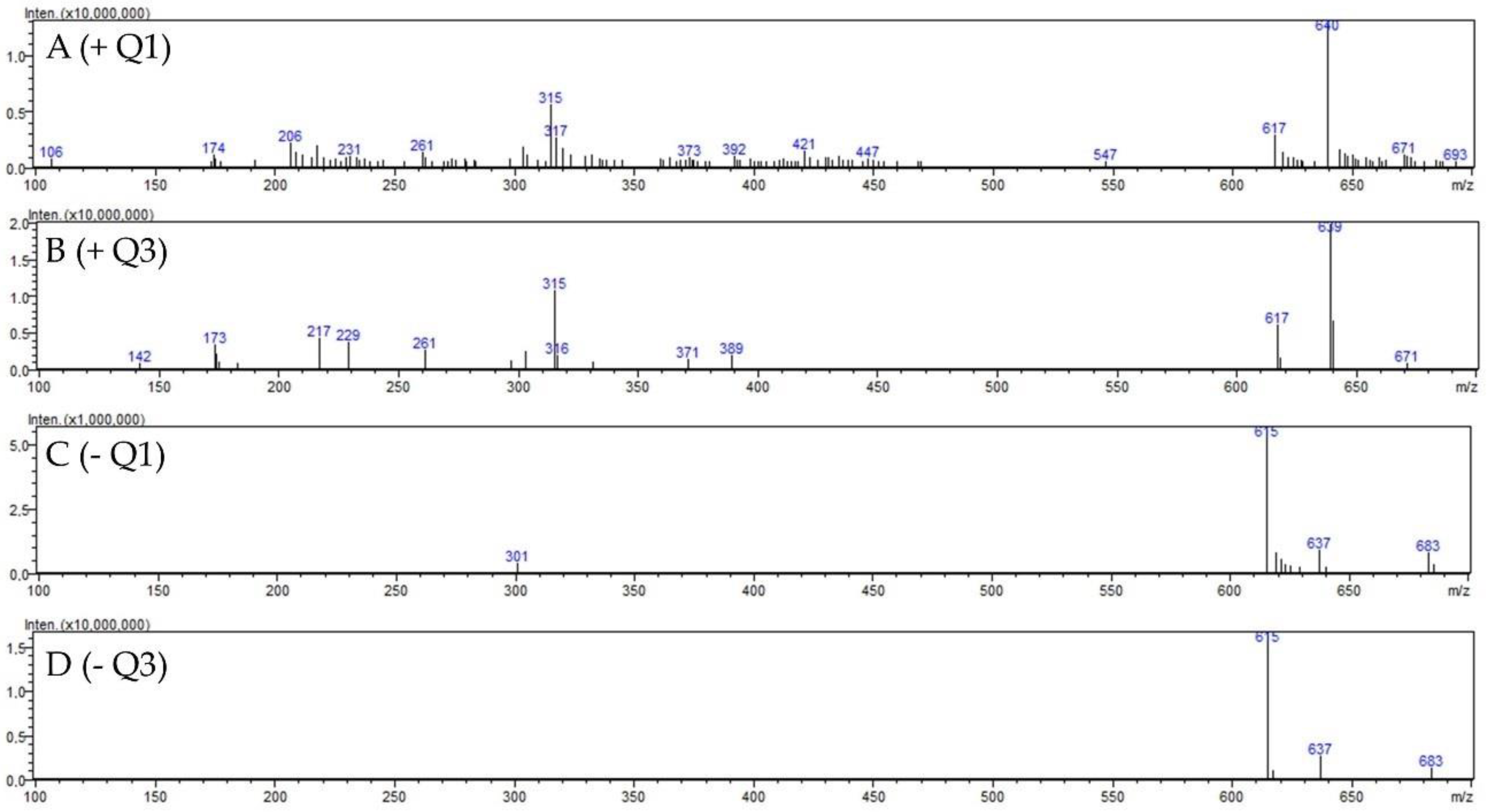
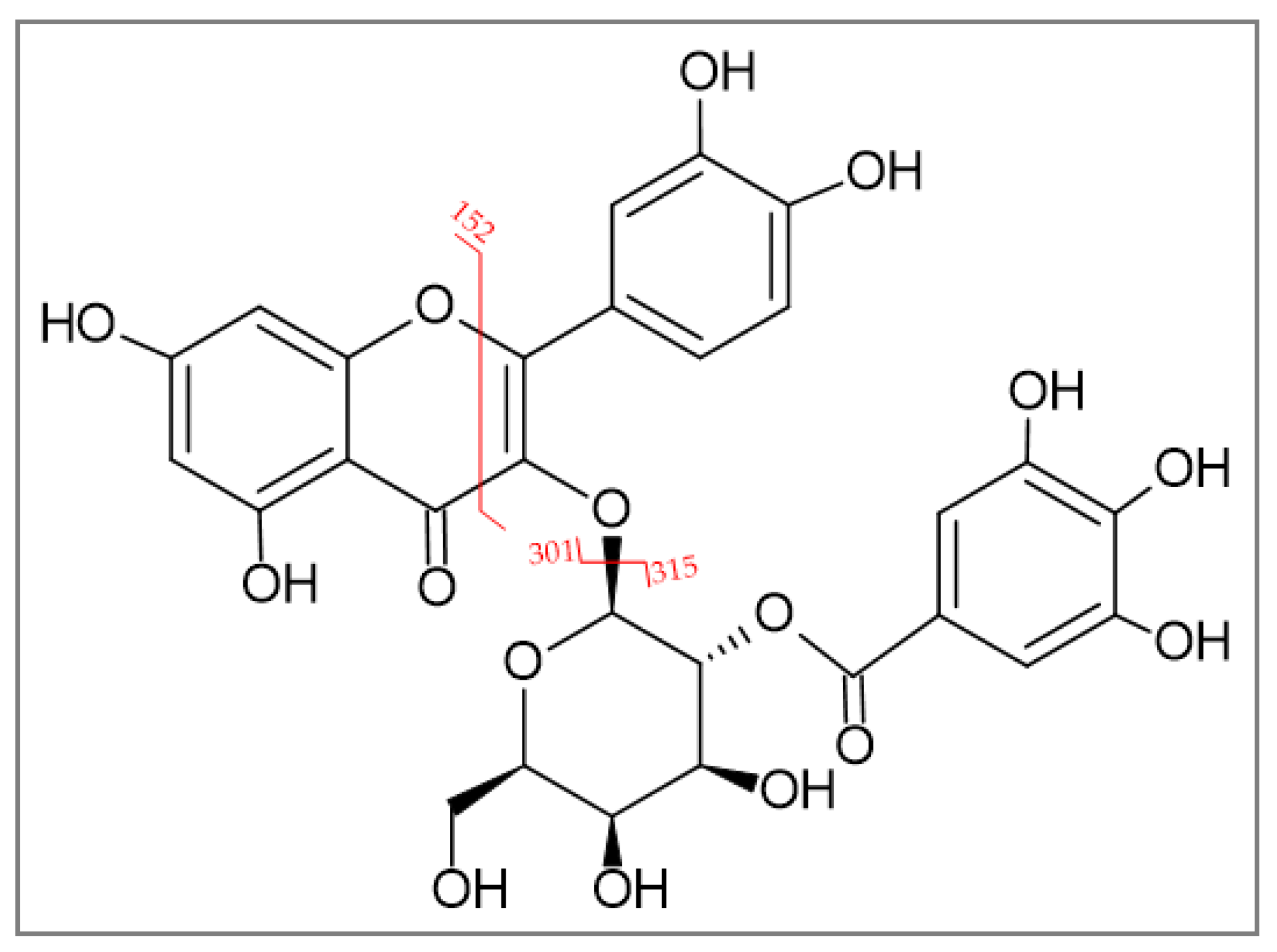
| Compound | MRM (ESI +) | Q1 pre Bias (eV) | Collisions Energy (eV) | Q3 pre Bias (eV) | Compound |
|---|---|---|---|---|---|
| 7-methyl juglone | 188.9 → 161.15 | −12 | −18 | −11 | 7-methyl juglone |
| 188.9 → 114.95 | −12 | −28 | −12 | ||
| 188.9 → 105.10 | −12 | −23 | −18 | ||
| 188.9 → 77.05 | −24 | −45 | −15 | ||
| 188.9 → 157.05 | −25 | −9 | −15 | ||
| plumbagin | 188.9 → 121.05 | −12 | −22 | −12 | plumbagin |
| 188.9 → 144.95 | −12 | −31 | −19 | ||
| 188.9 → 161.15 | −12 | −18 | −11 | ||
| 188.9 → 65.20 | −12 | −43 | −11 | ||
| 188.9 → 105.15 | −12 | −23 | −18 |
| Compound | MRM (ESI +) | MRM (ESI −) | Q1 pre Bias (V) | Collisions Energy (V) | Q3 pre Bias (V) |
|---|---|---|---|---|---|
| 2″-O-galloyl hyperoside | 617.10 → 315.05 | −30 | −14 | −22 | |
| 617.10 → 153.00 | −30 | −33 | −15 | ||
| 617.10 → 302.95 | −28 | −18 | −14 | ||
| 617.10 → 297.00 | −20 | −18 | −20 | ||
| 617.10 → 233.10 | −22 | −27 | −15 | ||
| 615.30 → 300.90 | 28 | 24 | 24 | ||
| 615.30 → 150.95 | 28 | 53 | 10 | ||
| 615.30 → 179.00 | 28 | 45 | 10 | ||
| 615.30 → 312.90 | 28 | 23 | 10 | ||
| 615.30 → 121.10 | 28 | 49 | 12 |
| Sample Name | Product Description | Company |
|---|---|---|
| D. rotundifolia | harvested fresh biomass, whole plant | Paludimed GmbH (Greifswald, Germany) |
| D. intermedia | harvested fresh biomass, whole plant | Paludimed GmbH (Greifswald, Germany) |
| D. longifolia (KG) | declared as Drosera longifolia, cut biomass | Kräutergarten (Munich, Germany) |
| D. longifolia (Ga) | declared as Drosera longifolia, cut biomass | Alfred Galke GmbH (Bad Grund, Germany) |
| Drosera sp. (Pini) | declared as Drosera sp., cut biomass | Pinisan Laboratorios (Madrid, Spain) |
| D. planta trit. (Plam) | declared as Drosera planta trit., cut biomass | Plameca (Barcelona, Spain) |
| Strain/ Data Number | Host | Origin | Toxin-Antitoxin System | Other Epigenomic Resistance Genes | Genome Accession Numbers/ | CTX-M Type, Sequence Type |
|---|---|---|---|---|---|---|
| E. coli/ IMT17433 PBIO729 | dog (C. lupus familiaris) | urinary tract infection | pemI/K, vagC/D, hok/sok | blaTEM-1, blaOXA-1, tet(A), tet(R), aadA, aac(6′)-ib-cr | ERR163891 | CTX-M-15 ST131 |
| E. coli/ IMT16316 PBIO730 | blackbird (T. merula) | feces | pemI/K, vagC/D, srnB/C, | tet(A), tet(R), sul1, sul2, strA, strB, aadA, aac(3)-II mph(A), mrx, mphR, dhfrVII | ERR163879 | CTX-M-15 ST648 |
| E. coli/ IMT17887 PBIO1986 | horse (E. ferus caballus) | soft tissue/wound infection | pemI/K, vagC/D, srnB/C | tet(A), tet(R), sul1, sul2, strA, strB, aadA, mph(A), mphR, dhfrVII | ERR163883 | CTX-M-15 ST648 |
| Compound | RT | Linear Range (µg mL−1) | R2 | Calibration Equation * | LOD ** (ng mL−1) | LOQ ** (ng mL−1) |
|---|---|---|---|---|---|---|
| hyperoside | 17.35 | 10–100 | 0.9998 | y = 1,999,316x − 28,941 | 0.62 | 1.73 |
| isoquercetin | 18.7 | 10–100 | 0.9999 | y = 1,890,097x − 15,975 | 0.65 | 1.77 |
| quercetin | 24.96 | 10–100 | 0.9999 | y = 2,740,278x − 11,469 | 0.30 | 0.82 |
| 2″-O-galloyl hyperoside | 19.43 | 10–100 | 0.9999 | y = 2,148,206x − 10,079 | 0.46 | 1.33 |
| gallic acid | 3.11 | 10–100 | 0.9999 | y = 1,327,487x + 15,200 | 3.90 | 8.15 |
| ellagic acid | 15.7 | 10–100 | 0.9905 | y = 4,978,062x − 570,036 | 1.51 | 4.51 |
| plumbagin | 28.4 | 10–200 | 0.9984 | y = 2,893,059x + 27,732 | 0.14 | 0.30 |
| 7-methyl juglone | 28.1 | 10–100 | 0.9996 | y = 2,042,891x + 7477 | 0.27 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerschler, S.; Guenther, S.; Schulze, C. Antibiofilm Activity of Sundew Species against Multidrug-Resistant Escherichia coli Strains. Int. J. Mol. Sci. 2022, 23, 13720. https://doi.org/10.3390/ijms232213720
Gerschler S, Guenther S, Schulze C. Antibiofilm Activity of Sundew Species against Multidrug-Resistant Escherichia coli Strains. International Journal of Molecular Sciences. 2022; 23(22):13720. https://doi.org/10.3390/ijms232213720
Chicago/Turabian StyleGerschler, Sandy, Sebastian Guenther, and Christian Schulze. 2022. "Antibiofilm Activity of Sundew Species against Multidrug-Resistant Escherichia coli Strains" International Journal of Molecular Sciences 23, no. 22: 13720. https://doi.org/10.3390/ijms232213720
APA StyleGerschler, S., Guenther, S., & Schulze, C. (2022). Antibiofilm Activity of Sundew Species against Multidrug-Resistant Escherichia coli Strains. International Journal of Molecular Sciences, 23(22), 13720. https://doi.org/10.3390/ijms232213720





