Chitosan–Hydroxycinnamic Acids Conjugates: Emerging Biomaterials with Rising Applications in Biomedicine
Abstract
1. Introduction
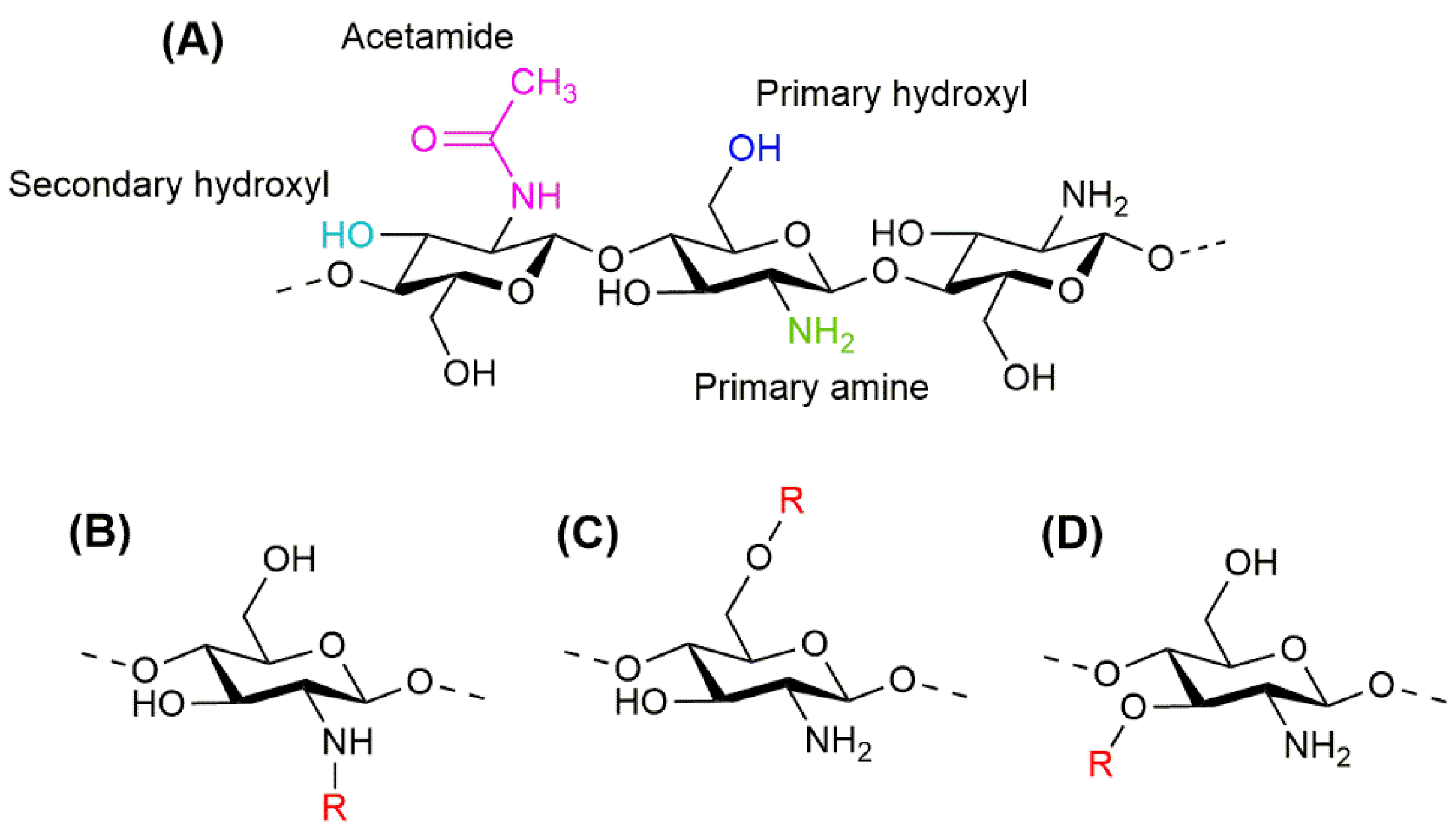
2. Chitosan
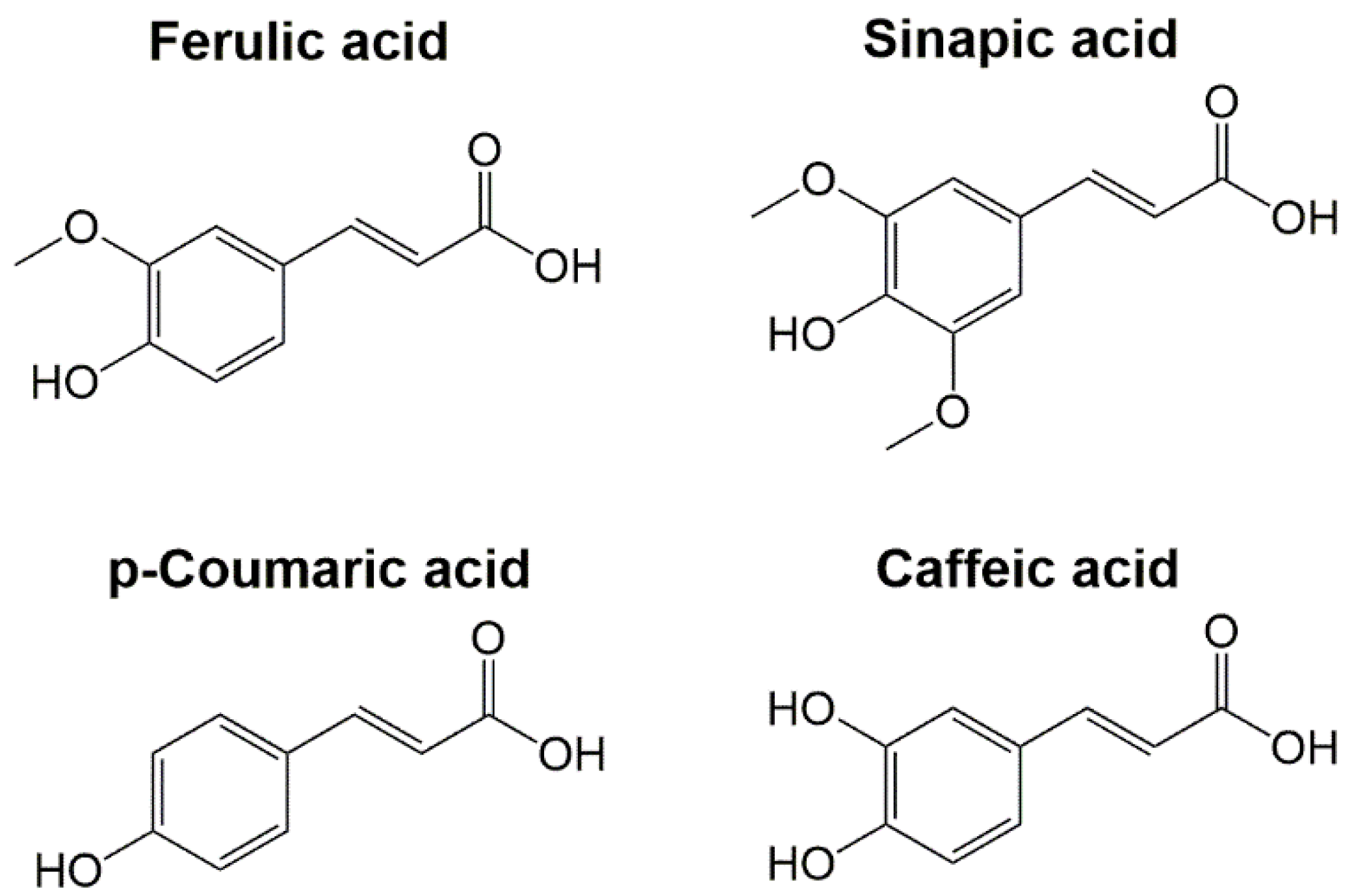
3. Hydroxycinnamic Acids
3.1. Ferulic Acid
3.2. Sinapic Acid
3.3. p-Coumaric Acid
3.4. Caffeic Acid
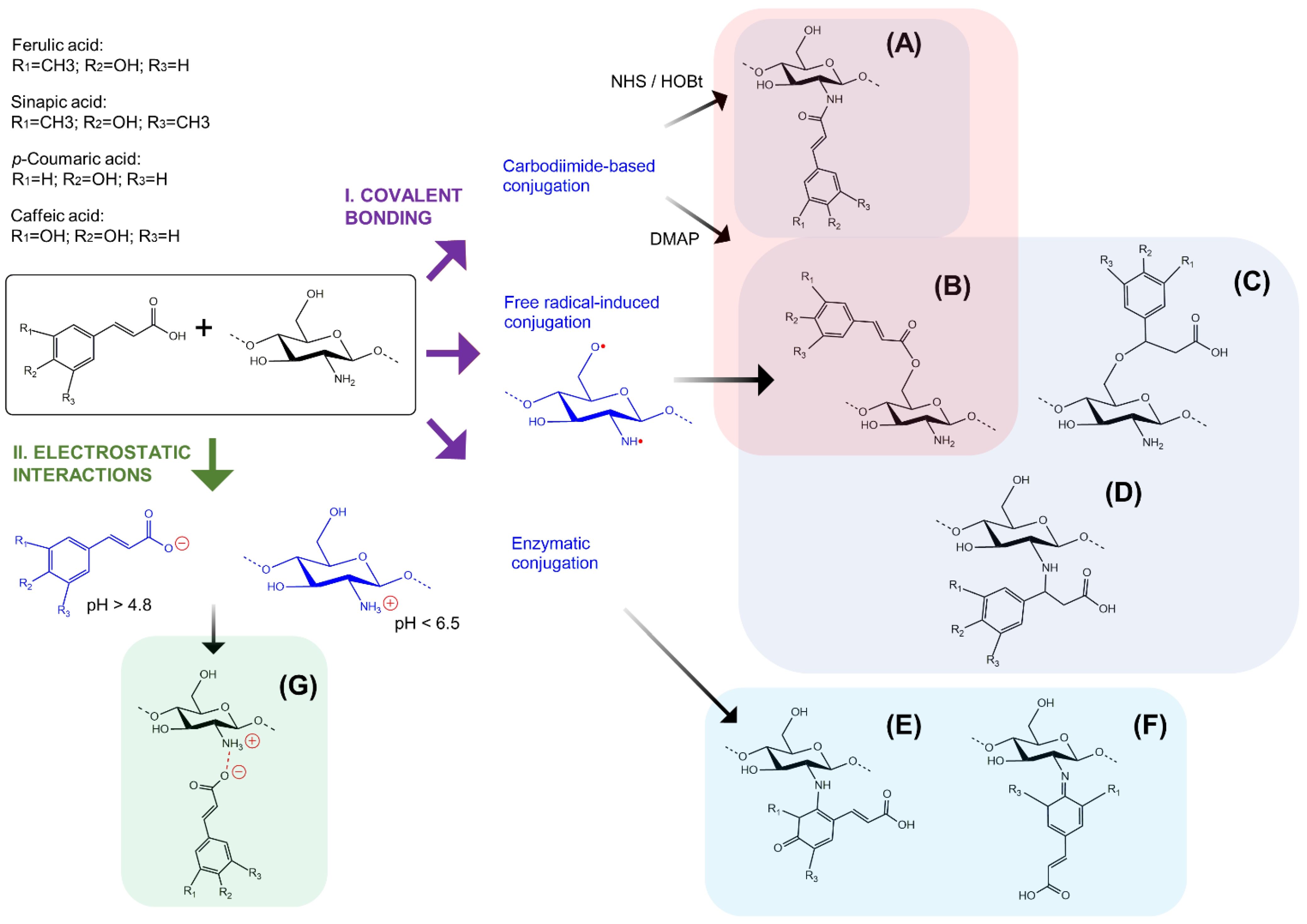
4. Conjugation of Chitosan and Hydroxycinnamic Acids
4.1. Covalent Bonding
4.1.1. Chemical Methods
4.1.2. Enzymatic Methods
4.2. Non-Covalent Interactions
5. Biomedical Applications of CS–HCAs Conjugates
5.1. Delivery of Therapeutic Molecules
5.2. Tissue Engineering and Regenerative Medicine
| Conjugate | Synthesis Method | Application | Study Type | Properties | Reference (Year) |
|---|---|---|---|---|---|
| CS-FA combined with bacterial cellulose | Ascorbic acid/hydrogen peroxide redox pair | Films for wound healing | In vitro | Enhanced water-absorbing capacity and antimicrobial activity over S. aureus and E. coli | Shen et al. (2021) [153] |
| CS-pCA combined with polyvinyl alcohol/starch films | Non-covalent interactions | Nanoparticles or films for wound healing | In vitro | CS-pCA film with higher antioxidant activity and less cytotoxicity over L929 cells CS-pCA nanoparticles with better thermal stability and enhanced antimicrobial activity over Gram-positive and Gram-negative bacteria | Lee et al. (2021) [154] |
| CS-FA/ Succinic acid | Carbodiimide-mediated | Hydrogels for brain injury repair | In vitro/in vivo | Enhanced in vitro biocompatibility with MSC and L929 cells, and cell-adhesion capacity of eASC In vivo integration to neural tissue and biocompatibility | Ojeda-Hernández et al. (2021) [115] |
| O-carboxymethyl-CS-CA combined with polyacrylamide | Non-covalent interactions | Doxycycline carrying hydrogel for wound dressing | In vitro | Controlled delivery of doxycycline, non-toxicity over HDF cells, and growth inhibition of S. aureus and E. coli | Hafezi et al. (2020) [156] |
| Glycol-CS-FA crosslinked with feruloyl-modified peptides | Carbodiimide-mediated conjugation and enzymatic (laccase) crosslinking | Wound healing hydrogels | In vitro/in vivo | Higher storage and compressive modulus Enhanced in vitro antioxidant activity and biocompatibility with NIH-3T3 cells In vivo acceleration of wound closure process and promotion of mature skin formation | Wei et al. (2019) [157] |
| CS-FA combined with PɛC | Carbodiimide-mediated | Nanofibrous material for wound dressing and for local treatment of cervical tumours | In vitro | Higher viscosity and swelling capacity, enhanced antibacterial activity against S. aureus, and higher antitumor activity over HeLa cells | Yakub et al. (2018) [152] |
| CS-FA combined with gelatin | Non-covalent interactions | Films/scaffolds for biomedical applications | In vitro | Formation of a polymer coacervation system, higher water uptake, and porosity when formic acid was used as the solvent | Nady et al. (2018) [155] |
| CS-FA | Enzymatic (laccase) | Biomaterials for tissue engineering and other biomedical applications | In vitro | Higher hydrophobic and antioxidant properties, and enhanced viability and cell-adhesion of MSC | Aljawish et al. (2016) [158] |
| CS-CA combined with PɛC | Ascorbic acid/hydrogen peroxide redox pair | Microfibrous material for wound dressing | In vitro | Increased tensile properties, higher cell attachment and proliferation of NHDF-neo cells, enhanced antimicrobial effect against S. aureus | Oh et al. (2016) [151] |
| Glycol CS-FA | Carbodiimide-mediated | Nanoparticles for functional restoration of traumatically injured spinal cord | In vitro/in vivo | In vitro protection of primary neurons from glutamate-induced excitotoxicity In vivo preservation of axons and myelin, and cavity volume, astrogliosis, and inflammatory response reduction at the spinal cord contusion injury site | Wu et al. (2014) [159] |
5.3. QS-HCAs as Active Ingredients
5.3.1. Antimicrobial
5.3.2. Antitumoral
5.3.3. Antioxidant
| Conjugate | Synthesis Method | Application | Study Type | Properties | Reference (Year) |
|---|---|---|---|---|---|
| CS-FA | Ascorbic acid/hydrogen peroxide redox pair | Biomaterial with antibacterial activity | In vitro | Bactericidal action against L. monocytogenes and S. aureus, and bacteriostatic action against P. aeruginosa | Dasagrandhi et al. (2018) [162] |
| CS-CA, CS-FA, CS-SA | Ascorbic acid/hydrogen peroxide redox pair | Antibacterial agents to control antibiotic- resistant acne-related bacteria | In vitro | Enhanced antimicrobial activity, especially from CS-CA Reduction of MIC values of antibiotics against antibiotic-resistant P. acnes and P. aeruginosa | Kim et al. (2017) [163] |
| CS-FA | Ascorbic acid/hydrogen peroxide redox pair | Antibacterial agent to use in combination with antibiotics against methicillin-resistant S. aureus | In vitro | Higher antioxidant activity and restoration of susceptibility of methicillin-resistant S. aureus to β-lactams | Eom et al. (2016) [164] |
| CS-FA, CS-CA, CS-SA | Ascorbic acid/hydrogen peroxide redox pair | Antibacterial agent against methicillin-resistant/susceptible S. aureus and foodborne pathogens | In vitro | Good biocompatibility over hCLC and mouse macrophage RAW26.7 cells Highest antibacterial activity from CS-FA Highest antioxidant activity from CS-CA Highest lipid peroxidation from CS-SA | Lee et al. (2014) [161] |
| CS quaternary ammonium derivatives-FA, -pCA, -SA | Functional groups conversion | Antioxidant and antitumor agent | In vitro | Enhanced antioxidant properties Antitumor activity over A549 cells Good biocompatibility with L929 cells | Li et al. (2020) [124] |
| CS-CA | Carbodiimide- mediated | Anticancer agent | In vitro | Enhanced antitumour and anti-invasive effects over CT26 colorectal carcinoma cells | Lee et al. (2013) [165] |
| CS-CA | Ascorbic acid/hydrogen peroxide redox pair | Hepatoprotective agent | In vitro/in vivo | In vitro lipid peroxidation activity In vivo enhancement of antioxidant enzymes Reduction of pro-inflammatory molecules | Park et al. (2017) [122] |
| CS-FA, CS-CA | Ascorbic acid/hydrogen peroxide redox pair | Antioxidant agent | In vitro/in vivo | Decreased thermal stability and crystallinity In vivo increase of antioxidant enzymes and decrease of malondialdehyde levels in D-galactose-induced ageing mice CS-CA with the highest in vitro and in vivo antioxidant activity | Liu et al. (2014) [166] |
6. Perspectives and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Joyce, K.; Fabra, G.T.; Bozkurt, Y.; Pandit, A. Bioactive potential of natural biomaterials: Identification, retention and assessment of biological properties. Signal Transduct. Target. Ther. 2021, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Ramya, R.; Venkatesan, J.; Kim, S.K.; Sudha, P.N. Biomedical Applications of Chitosan: An Overview. J. Biomater. Tissue Eng. 2012, 2, 100–111. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Taghizadeh, A.; Yazdi, M.K.; Zarrintaj, P.; Stadler, F.J.; Ramsey, J.D.; Habibzadeh, S.; Hosseini Rad, S.; Naderi, G.; Saeb, M.R.; et al. Chitosan-based inks for 3D printing and bioprinting. Green Chem. 2022, 24, 62–101. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Arguelles-Monal, W.M.; Lizardi-Mendoza, J.; Fernandez-Quiroz, D.; Recillas-Mota, M.T.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A Natural Biopolymer with a Wide and Varied Range of Applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Pires, C.T.G.V.M.T.; Vilela, J.A.P.; Airoldi, C. The Effect of Chitin Alkaline Deacetylation at Different Condition on Particle Properties. Procedia Chem. 2014, 9, 220–225. [Google Scholar] [CrossRef]
- Svensson, S.E.; Oliveira, A.O.; Adolfsson, K.H.; Heinmaa, I.; Root, A.; Kondori, N.; Ferreira, J.A.; Hakkarainen, M.; Zamani, A. Turning food waste to antibacterial and biocompatible fungal chitin/chitosan monofilaments. Int. J. Biol. Macromol. 2022, 209, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Żukiewicz-Sobczak, W.; Sobczak, P.; Zawiślak, K.; Zagórski, J.; Wojtyła-Buciora, P.; Wojtyła, A. Physical and chemical properties comparison of fungal and crustaceous chitosan. J. Health Inequalities 2015, 1, 7–14. [Google Scholar] [CrossRef]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef] [PubMed]
- Gortari, M.C.; Hours, R.A. Biotechnological processes for chitin recovery out of crustacean waste: A mini-review. Electron. J. Biotechnol. 2013, 16, 14. [Google Scholar]
- Tan, Y.N.; Lee, P.P.; Chen, W.N. Microbial extraction of chitin from seafood waste using sugars derived from fruit waste-stream. AMB Express 2020, 10, 17. [Google Scholar] [CrossRef]
- Zhang, H.; Yun, S.; Song, L.; Zhang, Y.; Zhao, Y. The preparation and characterization of chitin and chitosan under large-scale submerged fermentation level using shrimp by-products as substrate. Int. J. Biol. Macromol. 2017, 96, 334–339. [Google Scholar] [CrossRef]
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic Modifications of Chitin, Chitosan, and Chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef]
- Poshina, D.N.; Raik, S.V.; Poshin, A.N.; Skorik, Y.A. Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polym. Degrad. Stab. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Farhadihosseinabadi, B.; Zarebkohan, A.; Eftekhary, M.; Heiat, M.; Moosazadeh Moghaddam, M.; Gholipourmalekabadi, M. Crosstalk between chitosan and cell signaling pathways. Cell. Mol. Life Sci. 2019, 76, 2697–2718. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemeş, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef] [PubMed]
- Foster, L.J.R.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a Biomaterial: Influence of Degree of Deacetylation on Its Physiochemical, Material and Biological Properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Springer: Singapore, 2020; pp. 457–489. [Google Scholar] [CrossRef]
- He, J.; Wu, F.; Wang, D.; Yao, R.; Wu, Y. Modulation of cationicity of chitosan for tuning mesenchymal stem cell adhesion, proliferation, and differentiation. Biointerphases 2015, 10, 04A304. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for Biomedical Applications: Cellulose, Chitosan, and Protein/Peptide Derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef]
- Deepthi, S.; Venkatesan, J.; Kim, S.-K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef]
- Kumar, D.; Gihar, S.; Shrivash, M.K.; Kumar, P.; Kundu, P.P. A review on the synthesis of graft copolymers of chitosan and their potential applications. Int. J. Biol. Macromol. 2020, 163, 2097–2112. [Google Scholar] [CrossRef]
- Bhavsar, C.; Momin, M.; Gharat, S.; Omri, A. Functionalized and graft copolymers of chitosan and its pharmaceutical applications. Expert Opin. Drug Deliv. 2017, 14, 1189–1204. [Google Scholar] [CrossRef]
- Godos, J.; Caraci, F.; Micek, A.; Castellano, S.; D’Amico, E.; Paladino, N.; Ferri, R.; Galvano, F.; Grosso, G. Dietary Phenolic Acids and Their Major Food Sources Are Associated with Cognitive Status in Older Italian Adults. Antioxidants 2021, 10, 700. [Google Scholar] [CrossRef]
- Coman, V.; Vodnar, D.C. Hydroxycinnamic acids and human health: Recent advances. J. Sci. Food Agric. 2020, 100, 483–499. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds: Current industrial applications, limitations and future challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Al Jitan, S.; Alkhoori, S.A.; Yousef, L.F. Chapter 13—Phenolic Acids From Plants: Extraction and Application to Human Health. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 389–417. [Google Scholar]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Aarabi, A.; Mizani, M.; Honarvar, M.; Faghihian, H.; Gerami, A. Extraction of ferulic acid from sugar beet pulp by alkaline hydrolysis and organic solvent methods. J. Food Meas. Charact. 2015, 10, 42–47. [Google Scholar] [CrossRef]
- Salleh, N.H.M.; Daud, M.Z.M.; Arbain, D.; Ahmad, M.S.; Ismail, K.S.K. Optimization of alkaline hydrolysis of paddy straw for ferulic acid extraction. Ind. Crop. Prod. 2011, 34, 1635–1640. [Google Scholar] [CrossRef]
- Grajales-Hernández, D.A.; Armendáriz Ruiz, M.A.; Contreras-Jácquez, V.; Mateos-Díaz, J.C. Biotransformation of phenolic acids from by-products using heterogeneous biocatalysts: One more step toward a circular economy. Curr. Opin. Green Sustain. Chem. 2021, 32, 100550. [Google Scholar] [CrossRef]
- Candy, L.; Bassil, S.; Rigal, L.; Simon, V.; Raynaud, C. Thermo-mechano-chemical extraction of hydroxycinnamic acids from industrial hemp by-products using a twin-screw extruder. Ind. Crop. Prod. 2017, 109, 335–345. [Google Scholar] [CrossRef]
- Contreras-Jácquez, V.; Valenzuela-Vázquez, U.; Grajales-Hernández, D.A.; Mateos-Díaz, J.C.; Arrellano-Plaza, M.; Jara-Marini, M.E.; Asaff-Torres, A. Pilot-Scale Integrated Membrane System for the Separation and Concentration of Compounds of Industrial Interest from Tortilla Industry Wastewater (Nejayote). Waste Biomass Valorization 2021, 13, 345–360. [Google Scholar] [CrossRef]
- Torres-Mancera, M.; Cordova, J.; Rodríguez-Serrano, G.; Roussos, S.; Ramirez-Coronel, A.; Favela-Torres, E.; Saucedo-Castañeda, G. Enzymatic Extraction of Hydroxycinnamic Acids from Coffee Pulp. Food Technol. Biotechnol. 2011, 49, 369–373. [Google Scholar]
- Ferri, M.; Happel, A.; Zanaroli, G.; Bertolini, M.; Chiesa, S.; Commisso, M.; Guzzo, F.; Tassoni, A. Advances in combined enzymatic extraction of ferulic acid from wheat bran. New Biotechnol. 2020, 56, 38–45. [Google Scholar] [CrossRef]
- Uraji, M.; Arima, J.; Inoue, Y.; Harazono, K.; Hatanaka, T. Application of Two Newly Identified and Characterized Feruloyl Esterases from Streptomyces sp. in the Enzymatic Production of Ferulic Acid from Agricultural Biomass. PLoS ONE 2014, 9, e104584. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amst.) 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol Rep. (Amst.) 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Pazo-Cepeda, M.V.; Aspromonte, S.G.; Alonso, E. Extraction of ferulic acid and feruloylated arabinoxylo-oligosaccharides from wheat bran using pressurized hot water. Food Biosci. 2021, 44, 101374. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, S.; Ou, S.; Lin, J.; Wang, Y.; Peng, X.; Li, A.; Yu, B. Preparation of ferulic acid from corn bran: Its improved extraction and purification by membrane separation. Food Bioprod. Process. 2014, 92, 309–313. [Google Scholar] [CrossRef]
- Gadalkar, S.M.; Rathod, V.K. Pre-treatment of ferulic acid esterases immobilized on MNPs to enhance the extraction of ferulic acid from defatted rice bran in presence of ultrasound. Biocatal. Agric. Biotechnol. 2017, 10, 342–351. [Google Scholar] [CrossRef]
- Abramovič, H. Chapter 93—Antioxidant Properties of Hydroxycinnamic Acid Derivatives: A Focus on Biochemistry, Physicochemical Parameters, Reactive Species, and Biomolecular Interactions. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 843–852. [Google Scholar]
- Gerin, F.; Erman, H.; Erboga, M.; Sener, U.; Yilmaz, A.; Seyhan, H.; Gurel, A. The Effects of Ferulic Acid Against Oxidative Stress and Inflammation in Formaldehyde-Induced Hepatotoxicity. Inflammation 2016, 39, 1377–1386. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.-x.; Guo, S.-d.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Peres, D.D.A.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with UV filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- De Oliveira Silva, E.; Batista, R. Ferulic Acid and Naturally Occurring Compounds Bearing a Feruloyl Moiety: A Review on Their Structures, Occurrence, and Potential Health Benefits. Compr. Rev. Food Sci. Food Saf. 2017, 16, 580–616. [Google Scholar] [CrossRef] [PubMed]
- Salau, V.F.; Erukainure, O.L.; Ibeji, C.U.; Olasehinde, T.A.; Koorbanally, N.A.; Islam, M.S. Ferulic Acid Modulates Dysfunctional Metabolic Pathways and Purinergic Activities, While Stalling Redox Imbalance and Cholinergic Activities in Oxidative Brain Injury. Neurotox. Res. 2020, 37, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.-V.; Tan, J.; Town, T. Ferulic Acid Is a Nutraceutical β-Secretase Modulator That Improves Behavioral Impairment and Alzheimer-like Pathology in Transgenic Mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef]
- Chen, Y.; Xue, F.; Han, C.; Yang, H.; Han, L.; Li, K.; Li, J.; Xu, Q.; Li, Z.; Yuan, B.; et al. Ferulic acid ameliorated placental inflammation and apoptosis in rat with preeclampsia. Clin. Exp. Hypertens. 2019, 41, 524–530. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Hsiao, C.-Y.; Tsai, K.-L.; Cheng, Y.-H. Injectable thermosensitive chitosan-based hydrogel containing ferulic acid for treating peripheral arterial disease. J. Tissue Eng. Regen. Med. 2020, 14, 1438–1448. [Google Scholar] [CrossRef]
- Koh, E.-J.; Kim, K.-J.; Seo, Y.-J.; Choi, J.; Lee, B.-Y. Modulation of HO-1 by Ferulic Acid Attenuates Adipocyte Differentiation in 3T3-L1 Cells. Molecules 2017, 22, 745. [Google Scholar] [CrossRef]
- Rezaei, A.; Varshosaz, J.; Fesharaki, M.; Farhang, A.; Jafari, S.M. Improving the solubility and in vitro cytotoxicity (anticancer activity) of ferulic acid by loading it into cyclodextrin nanosponges. Int. J. Nanomed. 2019, 14, 4589–4599. [Google Scholar] [CrossRef]
- Grasso, R.; Dell’Albani, P.; Carbone, C.; Spatuzza, M.; Bonfanti, R.; Sposito, G.; Puglisi, G.; Musumeci, F.; Scordino, A.; Campisi, A. Synergic pro-apoptotic effects of Ferulic Acid and nanostructured lipid carrier in glioblastoma cells assessed through molecular and Delayed Luminescence studies. Sci. Rep. 2020, 10, 4680. [Google Scholar] [CrossRef]
- Bocco, B.M.; Fernandes, G.W.; Lorena, F.B.; Cysneiros, R.M.; Christoffolete, M.A.; Grecco, S.S.; Lancellotti, C.L.; Romoff, P.; Lago, J.H.G.; Bianco, A.C.; et al. Combined treatment with caffeic and ferulic acid from Baccharis uncinella C. DC. (Asteraceae) protects against metabolic syndrome in mice. Braz. J. Med. Biol. Res. 2016, 49, e5003. [Google Scholar] [CrossRef] [PubMed]
- Nićiforović, N.; Abramovič, H. Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Reungoat, V.; Mouterde, L.M.M.; Chadni, M.; Couvreur, J.; Isidore, E.; Allais, F.; Ducatel, H.; Ioannou, I. Simultaneous extraction and enzymatic hydrolysis of mustard bran for the recovery of sinapic acid. Food Bioprod. Process. 2021, 130, 68–78. [Google Scholar] [CrossRef]
- Moreno-González, M.; Girish, V.; Keulen, D.; Wijngaard, H.; Lauteslager, X.; Ferreira, G.; Ottens, M. Recovery of sinapic acid from canola/rapeseed meal extracts by adsorption. Food Bioprod. Process. 2020, 120, 69–79. [Google Scholar] [CrossRef]
- Teixeira, J.; Gaspar, A.; Garrido, E.M.; Garrido, J.; Borges, F. Hydroxycinnamic Acid Antioxidants: An Electrochemical Overview. BioMed Res. Int. 2013, 2013, 251754. [Google Scholar] [CrossRef]
- Chen, C. Sinapic Acid and Its Derivatives as Medicine in Oxidative Stress-Induced Diseases and Aging. Oxid. Med. Cell. Longev. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Zou, Y.; Kim, A.R.; Kim, J.E.; Choi, J.S.; Chung, H.Y. Peroxynitrite Scavenging Activity of Sinapic Acid (3,5-Dimethoxy-4-hydroxycinnamic Acid) Isolated from Brassica juncea. J. Agric. Food Chem. 2002, 50, 5884–5890. [Google Scholar] [CrossRef]
- Martinović, N.; Abramovič, H.; Poklar Ulrih, N. Inhibition of copper-induced lipid peroxidation by sinapic acid and its derivatives in correlation to their effect on the membrane structural properties. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1–8. [Google Scholar] [CrossRef]
- Silambarasan, T.; Manivannan, J.; Raja, B.; Chatterjee, S. Prevention of cardiac dysfunction, kidney fibrosis and lipid metabolic alterations in l-NAME hypertensive rats by sinapic acid—Role of HMG-CoA reductase. Eur. J. Pharmacol. 2016, 777, 113–123. [Google Scholar] [CrossRef]
- Eroğlu, C.; Avcı, E.; Vural, H.; Kurar, E. Anticancer mechanism of Sinapic acid in PC-3 and LNCaP human prostate cancer cell lines. Gene 2018, 671, 127–134. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Trivedi, R.; Vairamani, M.; Selvamurugan, N. Sinapic acid-loaded chitosan nanoparticles in polycaprolactone electrospun fibers for bone regeneration in vitro and in vivo. Carbohydr. Polym. 2019, 216, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Singh, D.; Kh, R. Sinapic Acid Alleviates Oxidative Stress and Neuro-Inflammatory Changes in Sporadic Model of Alzheimer’s Disease in Rats. Brain Sci. 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Shin, J.H.; Kim, S.S.; Seo, S.R. Sinapic Acid Controls Inflammation by Suppressing NLRP3 Inflammasome Activation. Cells 2021, 10, 2327. [Google Scholar] [CrossRef] [PubMed]
- Pandi, A.; Kalappan, V.M. Pharmacological and therapeutic applications of Sinapic acid—An updated review. Mol. Biol. Rep. 2021, 48, 3733–3745. [Google Scholar] [CrossRef]
- Cha, H.; Lee, S.; Lee, J.H.; Park, J.-W. Protective effects of p-coumaric acid against acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2018, 121, 131–139. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Arruda, C.; Pena Ribeiro, V.; Oliveira Almeida, M.; Aldana Mejía, J.A.; Casoti, R.; Kenupp Bastos, J. Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green propolis. J. Pharm. Biomed. Anal. 2020, 178, 112922. [Google Scholar] [CrossRef]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Thangaradjou, T.; Anantharaman, P. Phytochemical constituents, antioxidant properties and p-coumaric acid analysis in some seagrasses. Food Res. Int. 2013, 54, 1229–1236. [Google Scholar] [CrossRef]
- Boz, H. p-Coumaric acid in cereals: Presence, antioxidant and antimicrobial effects. Int. J. Food Sci. Technol. 2015, 50, 2323–2328. [Google Scholar] [CrossRef]
- Kong, C.-S.; Jeong, C.-H.; Choi, J.-S.; Kim, K.-J.; Jeong, J.-W. Antiangiogenic Effects of P-Coumaric Acid in Human Endothelial Cells. Phytother. Res. 2013, 27, 317–323. [Google Scholar] [CrossRef]
- Peng, J.; Zheng, T.-T.; Liang, Y.; Duan, L.-F.; Zhang, Y.-D.; Wang, L.-J.; He, G.-M.; Xiao, H.-T. p-Coumaric Acid Protects Human Lens Epithelial Cells against Oxidative Stress-Induced Apoptosis by MAPK Signaling. Oxid. Med. Cell. Longev. 2018, 2018, 8549052. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Ramakrishnan, S.; Gunasekaran, V.P.; Parkash, K.; Ramakrishnan, A.; Vijayakumar, N.; Ganeshan, M. Protective effects of p-coumaric acid on ethanol induced male reproductive toxicity. Life Sci. 2018, 209, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, G.; Min, C.-K.; Jeon, Y.-M.; Bashyal, R.; Poudel, S.B.; Kook, S.-H.; Lee, J.-C. Oral supplementation with p-coumaric acid protects mice against diabetes-associated spontaneous destruction of periodontal tissue. J. Periodontal Res. 2019, 54, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Neog, M.K.; Joshua Pragasam, S.; Krishnan, M.; Rasool, M. p-Coumaric acid, a dietary polyphenol ameliorates inflammation and curtails cartilage and bone erosion in the rheumatoid arthritis rat model. BioFactors 2017, 43, 698–717. [Google Scholar] [CrossRef]
- Li, Y.-H.; He, Q.; Chen, Y.-Z.; Du, Y.-F.; Guo, Y.-X.; Xu, J.-Y.; Qin, L.-Q. p-Coumaric acid ameliorates ionizing radiation-induced intestinal injury through modulation of oxidative stress and pyroptosis. Life Sci. 2021, 278, 119546. [Google Scholar] [CrossRef]
- Souza, T.N.; Santos, F.M.; Alves, P.R.; Ferro, J.N.; Correia, A.C.C.; Melo, T.S.; Soares, W.R.; Andrade, B.S.; Lagente, V.; Barreto, E. Local administration of p-coumaric acid decreases lipopolysaccharide-induced acute lung injury in mice: In vitro and in silico studies. Eur. J. Pharmacol. 2021, 897, 173929. [Google Scholar] [CrossRef]
- Yoon, D.S.; Cho, S.Y.; Yoon, H.J.; Kim, S.R.; Jung, U.J. Protective effects of p-coumaric acid against high-fat diet-induced metabolic dysregulation in mice. Biomed. Pharmacother. 2021, 142, 111969. [Google Scholar] [CrossRef]
- Oh, D.-R.; Kim, M.-J.; Choi, E.-J.; Kim, Y.; Lee, H.-S.; Bae, D.; Choi, C. Protective Effects of p-Coumaric Acid Isolated from Vaccinium bracteatum Thunb. Leaf Extract on Corticosterone-Induced Neurotoxicity in SH-SY5Y Cells and Primary Rat Cortical Neurons. Processes 2021, 9, 869. [Google Scholar] [CrossRef]
- Meinhart, A.D.; Damin, F.M.; Caldeirão, L.; de Jesus Filho, M.; da Silva, L.C.; da Silva Constant, L.; Filho, J.T.; Wagner, R.; Godoy, H.T. Chlorogenic and caffeic acids in 64 fruits consumed in Brazil. Food Chem. 2019, 286, 51–63. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of Antioxidant Activity of some Medicinal Plants Having High Content of Caffeic Acid Derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef]
- Moreira, G.C.; de Souza Dias, F. Mixture design and Doehlert matrix for optimization of the ultrasonic assisted extraction of caffeic acid, rutin, catechin and trans-cinnamic acid in Physalis angulata L. and determination by HPLC DAD. Microchem. J. 2018, 141, 247–252. [Google Scholar] [CrossRef]
- Konar, N.; Dalabasmaz, S.; Poyrazoglu, E.S.; Artik, N.; Colak, A. The determination of the caffeic acid derivatives of Echinacea purpurea aerial parts under various extraction conditions by supercritical fluid extraction (SFE). J. Supercrit. Fluids 2014, 89, 128–136. [Google Scholar] [CrossRef]
- Miura, C.; Matsunaga, H.; Haginaka, J. Molecularly imprinted polymer for caffeic acid by precipitation polymerization and its application to extraction of caffeic acid and chlorogenic acid from Eucommia ulmodies leaves. J. Pharm. Biomed. Anal. 2016, 127, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Li, H.; Shi, S.; Chen, X. Hollow molecular imprinted polymers towards rapid, effective and selective extraction of caffeic acid from fruits. J. Chromatogr. A 2016, 1470, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Paciello, F.; Di Pino, A.; Rolesi, R.; Troiani, D.; Paludetti, G.; Grassi, C.; Fetoni, A.R. Anti-oxidant and anti-inflammatory effects of caffeic acid: In vivo evidences in a model of noise-induced hearing loss. Food Chem. Toxicol. 2020, 143, 111555. [Google Scholar] [CrossRef]
- Owumi, S.E.; Irozuru, C.E.; Arunsi, U.O.; Oyelere, A.K. Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B1. Toxicon 2022, 207, 1–12. [Google Scholar] [CrossRef]
- Zielińska, D.; Zieliński, H.; Laparra-Llopis, J.M.; Szawara-Nowak, D.; Honke, J.; Giménez-Bastida, J.A. Caffeic Acid Modulates Processes Associated with Intestinal Inflammation. Nutrients 2021, 13, 554. [Google Scholar] [CrossRef]
- Huang, X.; Xi, Y.; Pan, Q.; Mao, Z.; Zhang, R.; Ma, X.; You, H. Caffeic acid protects against IL-1β-induced inflammatory responses and cartilage degradation in articular chondrocytes. Biomed. Pharmacother. 2018, 107, 433–439. [Google Scholar] [CrossRef]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef]
- Sorgi, C.A.; de Campos Chaves Lamarque, G.; Verri, M.P.; Nelson-Filho, P.; Faccioli, L.H.; Paula-Silva, F.W.G. Multifaceted effect of caffeic acid against Streptococcus mutans infection: Microbicidal and immunomodulatory agent in macrophages. Arch. Microbiol. 2021, 203, 2979–2987. [Google Scholar] [CrossRef]
- Jamali, N.; Mostafavi-Pour, Z.; Zal, F.; Kasraeian, M.; Poordast, T.; Nejabat, N. Antioxidant ameliorative effect of caffeic acid on the ectopic endometrial cells separated from patients with endometriosis. Taiwan. J. Obstet. Gynecol. 2021, 60, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Koga, M.; Nakagawa, S.; Kato, A.; Kusumi, I. Caffeic acid reduces oxidative stress and microglial activation in the mouse hippocampus. Tissue Cell 2019, 60, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zaitone, S.A.; Ahmed, E.; Elsherbiny, N.M.; Mehanna, E.T.; El-Kherbetawy, M.K.; ElSayed, M.H.; Alshareef, D.M.; Moustafa, Y.M. Caffeic acid improves locomotor activity and lessens inflammatory burden in a mouse model of rotenone-induced nigral neurodegeneration: Relevance to Parkinson’s disease therapy. Pharmacol. Rep. 2019, 71, 32–41. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Zabolian, A.; Saleki, H.; Farahani, M.V.; Hamzehlou, S.; Far, F.B.; Sharifzadeh, S.O.; Samarghandian, S.; Khan, H.; et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021, 171, 105759. [Google Scholar] [CrossRef] [PubMed]
- Kanimozhi, G.; Prasad, N.R. Chapter 73—Anticancer Effect of Caffeic Acid on Human Cervical Cancer Cells. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 655–661. [Google Scholar]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial Chitosan Conjugates: Current Synthetic Strategies and Potential Applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pu, H.; Liu, S.; Kan, J.; Jin, C. Synthesis, characterization, bioactivity and potential application of phenolic acid grafted chitosan: A review. Carbohydr. Polym. 2017, 174, 999–1017. [Google Scholar] [CrossRef]
- Nicolle, L.; Journot, C.M.A.; Gerber-Lemaire, S. Chitosan Functionalization: Covalent and Non-Covalent Interactions and Their Characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, M.; Ma, G.; Fang, Y.; Yang, W.; Ma, N.; Fang, D.; Hu, Q.; Pei, F. The antioxidant and antimicrobial activities of different phenolic acids grafted onto chitosan. Carbohydr. Polym. 2019, 225, 115238. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Gomez-Pinedo, U.; Hernández-Sapiéns, M.A.; Canales-Aguirre, A.A.; Espinosa-Andrews, H.; Matias-Guiu, J.; González-García, Y.; Mateos-Díaz, J.C. Biocompatibility of ferulic/succinic acid-grafted chitosan hydrogels for implantation after brain injury: A preliminary study. Mater. Sci. Eng. C 2021, 121, 111806. [Google Scholar] [CrossRef] [PubMed]
- Leiro, V.; Parreira, P.; Freitas, S.C.; Martins, M.C.L.; Pêgo, A.P. Chapter 2—Conjugation Chemistry Principles and Surface Functionalization of Nanomaterials. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 35–66. [Google Scholar]
- Madison, S.A.; Carnali, J.O. pH Optimization of Amidation via Carbodiimides. Ind. Eng. Chem. Res. 2013, 52, 13547–13555. [Google Scholar] [CrossRef]
- Christ, H.-A.; Bourgat, Y.; Menzel, H. Optimization of Critical Parameters for Carbodiimide Mediated Production of Highly Modified Chitosan. Polymers 2021, 13, 2702. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Yang, J.-M.; Chang, Y.-H.; Su, W.-W. Modification of different molecular weights of chitosan by p-Coumaric acid: Preparation, characterization and effect of molecular weight on its water solubility and antioxidant property. Int. J. Biol. Macromol. 2019, 136, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Mao, L.; Dai, L.; Yuan, F.; Gao, Y. Characterization of chitosan-ferulic acid conjugates and their application in the design of β-carotene bilayer emulsions with propylene glycol alginate. Food Hydrocoll. 2018, 80, 281–291. [Google Scholar] [CrossRef]
- Anwar, M.; Nisa, K.; Indirayati, N. Acid-base evaluation of chitosan-ferulic acid conjugate by a free radical grafting method. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Park, S.Y.; Ahn, G.; Um, J.H.; Han, E.J.; Ahn, C.-B.; Yoon, N.Y.; Je, J.-Y. Hepatoprotective effect of chitosan-caffeic acid conjugate against ethanol-treated mice. Exp. Toxicol. Pathol. 2017, 69, 618–624. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Nunes, A.; Silva, J.A.L.d.; Coimbra, M.A. Chitosan–caffeic acid–genipin films presenting enhanced antioxidant activity and stability in acidic media. Carbohydr. Polym. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Tan, W.; Zhang, J.; Guo, Z. Phenolic-containing chitosan quaternary ammonium derivatives and their significantly enhanced antioxidant and antitumor properties. Carbohydr. Res. 2020, 498, 108169. [Google Scholar] [CrossRef]
- Nagy, V.; Sahariah, P.; Hjálmarsdóttir, M.Á.; Másson, M. Chitosan-hydroxycinnamic acid conjugates: Optimization of the synthesis and investigation of the structure activity relationship. Carbohydr. Polym. 2022, 277, 118896. [Google Scholar] [CrossRef]
- Mohit, E.; Tabarzad, M.; Faramarzi, A.M. Biomedical and Pharmaceutical-Related Applications of Laccases. Curr. Protein Pept. Sci. 2020, 21, 78–98. [Google Scholar] [CrossRef]
- Shokri, Z.; Seidi, F.; Saeb, M.R.; Jin, Y.; Li, C.; Xiao, H. Elucidating the impact of enzymatic modifications on the structure, properties, and applications of cellulose, chitosan, starch and their derivatives: A review. Mater. Today Chem. 2022, 24, 100780. [Google Scholar] [CrossRef]
- Yang, C.; Han, B.; Zheng, Y.; Liu, L.; Li, C.; Sheng, S.; Zhang, J.; Wang, J.; Wu, F. The Quality Changes of Postharvest Mulberry Fruit Treated by Chitosan-g-Caffeic Acid during Cold Storage. J. Food Sci. 2016, 81, C881–C888. [Google Scholar] [CrossRef] [PubMed]
- Robert, B.; Chenthamara, D.; Subramaniam, S. Fabrication and biomedical applications of Arabinoxylan, Pectin, Chitosan, soy protein, and silk fibroin hydrogels via laccase—Ferulic acid redox chemistry. Int. J. Biol. Macromol. 2022, 201, 539–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, B.; Javvaji, V.; Kim, E.; Lee, M.E.; Raghavan, S.R.; Wang, Q.; Payne, G.F. Tyrosinase-mediated grafting and crosslinking of natural phenols confers functional properties to chitosan. Biochem. Eng. J. 2014, 89, 21–27. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, H.; Zhang, L.; Sun, Q. Horseradish peroxidase-mediated synthesis of an antioxidant gallic acid-g-chitosan derivative and its preservation application in cherry tomatoes. RSC Adv. 2018, 8, 20363–20371. [Google Scholar] [CrossRef]
- Stefani, R. Computational Study of Natural Phenolic Acid Solubility and Their Interactions with Chitosan. 2016. Available online: https://sciforum.net/manuscripts/3862/slides.pdf (accessed on 23 June 2022).
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Riccucci, G.; Ferraris, S.; Reggio, C.; Bosso, A.; Örlygsson, G.; Ng, C.H.; Spriano, S. Polyphenols from Grape Pomace: Functionalization of Chitosan-Coated Hydroxyapatite for Modulated Swelling and Release of Polyphenols. Langmuir 2021, 37, 14793–14804. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-Based Functional Materials for Skin Wound Repair: Mechanisms and Applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Cicciù, M.; Fiorillo, L.; Cervino, G. Chitosan Use in Dentistry: A Systematic Review of Recent Clinical Studies. Mar. Drugs 2019, 17, 417. [Google Scholar] [CrossRef]
- Li, J.; Cai, C.; Li, J.; Li, J.; Li, J.; Sun, T.; Wang, L.; Wu, H.; Yu, G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules 2018, 23, 2661. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Nurus Sakib, M.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Li, C.; Li, J.-B. Preparation of chitosan-ferulic acid conjugate: Structure characterization and in the application of pharmaceuticals. Int. J. Biol. Macromol. 2017, 105, 1539–1543. [Google Scholar] [CrossRef]
- Li, C.; Fang, K.; He, W.; Li, K.; Jiang, Y.; Li, J. Evaluation of chitosan-ferulic acid microcapsules for sustained drug delivery: Synthesis, characterizations, and release kinetics in vitro. J. Mol. Struct. 2021, 1227, 129353. [Google Scholar] [CrossRef]
- Chatterjee, N.S.; Anandan, R.; Navitha, M.; Asha, K.K.; Kumar, K.A.; Mathew, S.; Ravishankar, C.N. Development of thiamine and pyridoxine loaded ferulic acid-grafted chitosan microspheres for dietary supplementation. J. Food Sci. Technol. 2016, 53, 551–560. [Google Scholar] [CrossRef]
- Kamal, I.; Khedr, A.I.M.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Elshaarawy, R.F.M.; Saad, A.S. Chemotherapeutic and chemopreventive potentials of ρ-coumaric acid—Squid chitosan nanogel loaded with Syzygium aromaticum essential oil. Int. J. Biol. Macromol. 2021, 188, 523–533. [Google Scholar] [CrossRef]
- Pengpong, T.; Sangvanich, P.; Sirilertmukul, K.; Muangsin, N. Design, synthesis and in vitro evaluation of mucoadhesive p-coumarate-thiolated-chitosan as a hydrophobic drug carriers. Eur. J. Pharm. Biopharm. 2014, 86, 487–497. [Google Scholar] [CrossRef]
- Lee, S.J.; Choi, K.-C.; Kang, M.-S.; Oh, J.-S.; Jeong, Y.-I.; Lee, H.C. Self-Organized Nanoparticles of Caffeic Acid Conjugated Polysaccharide and Its Anticancer Activity. J. Nanosci. Nanotechnol. 2015, 15, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Tegl, G.; Baumann, M.; Sommer, E.; Gorji, E.G.; Borth, N.; Schleining, G.; Nyanhongo, G.S.; Guebitz, G.M. Chitosan hydrogel formation using laccase activated phenolics as cross-linkers. Carbohydr. Polym. 2017, 157, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, E.; Iaquinta, M.R.; Lanzillotti, C.; Mazziotta, C.; Maritati, M.; Montesi, M.; Sprio, S.; Tampieri, A.; Tognon, M.; Martini, F. Bioactive Materials for Soft Tissue Repair. Front. Bioeng. Biotechnol. 2021, 9, 613787. [Google Scholar] [CrossRef] [PubMed]
- Oh, G.-W.; Ko, S.-C.; Je, J.-Y.; Kim, Y.-M.; Oh, J.; Jung, W.-K. Fabrication, characterization and determination of biological activities of poly(ε-caprolactone)/chitosan-caffeic acid composite fibrous mat for wound dressing application. Int. J. Biol. Macromol. 2016, 93, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Yakub, G.; Ignatova, M.; Manolova, N.; Rashkov, I.; Toshkova, R.; Georgieva, A.; Markova, N. Chitosan/ferulic acid-coated poly(ε-caprolactone) electrospun materials with antioxidant, antibacterial and antitumor properties. Int. J. Biol. Macromol. 2018, 107, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Wang, H.; Wu, K.; Gao, J.; Li, J. Characterization and antimicrobial properties of ferulic acid grafted self-assembled bacterial cellulose-chitosan membranes. J. Appl. Polym. Sci. 2021, 138, 50824. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, M.; Wang, G.; Meng, W.; Zhang, X.; Wang, D.; Zhou, Y.; Wang, Z. Characterization of polyvinyl alcohol/starch composite films incorporated with p-coumaric acid modified chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2021, 262, 117930. [Google Scholar] [CrossRef]
- Nady, N.; Kandil, S.H. Novel Blend for Producing Porous Chitosan-Based Films Suitable for Biomedical Applications. Membranes 2018, 8, 2. [Google Scholar] [CrossRef]
- Hafezi Moghaddam, R.; Dadfarnia, S.; Shabani, A.M.H.; Amraei, R.; Hafezi Moghaddam, Z. Doxycycline drug delivery using hydrogels of O-carboxymethyl chitosan conjugated with caffeic acid and its composite with polyacrylamide synthesized by electron beam irradiation. Int. J. Biol. Macromol. 2020, 154, 962–973. [Google Scholar] [CrossRef]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic crosslinking to fabricate antioxidant peptide-based supramolecular hydrogel for improving cutaneous wound healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef]
- Aljawish, A.; Muniglia, L.; Chevalot, I. Growth of human mesenchymal stem cells (MSCs) on films of enzymatically modified chitosan. Biotechnol. Prog. 2016, 32, 491–500. [Google Scholar] [CrossRef]
- Wu, W.; Lee, S.-Y.; Wu, X.; Tyler, J.Y.; Wang, H.; Ouyang, Z.; Park, K.; Xu, X.-M.; Cheng, J.-X. Neuroprotective ferulic acid (FA)–glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials 2014, 35, 2355–2364. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Woo, J.-Y.; Ahn, C.-B.; Je, J.-Y. Chitosan–hydroxycinnamic acid conjugates: Preparation, antioxidant and antimicrobial activity. Food Chem. 2014, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Dasagrandhi, C.; Park, S.; Jung, W.-K.; Kim, Y.-M. Antibacterial and Biofilm Modulating Potential of Ferulic Acid-Grafted Chitosan against Human Pathogenic Bacteria. Int. J. Mol. Sci. 2018, 19, 2157. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yu, D.; Eom, S.-H.; Kim, S.-H.; Oh, J.; Jung, W.K.; Kim, Y.-M. Synergistic Antibacterial Effects of Chitosan-Caffeic Acid Conjugate against Antibiotic-Resistant Acne-Related Bacteria. Mar. Drugs 2017, 15, 167. [Google Scholar] [CrossRef]
- Eom, S.-H.; Kang, S.-K.; Lee, D.-S.; Myeong, J.-I.; Lee, J.; Kim, H.-W.; Kim, K.-H.; Je, J.-Y.; Jung, W.-K.; Kim, Y.-M.; et al. Synergistic Antibacterial Effect and Antibacterial Action Mode of Chitosan-Ferulic Acid Conjugate against Methicillin-Resistant Staphylococcus aureus. J. Microbiol. Biotechnol. 2016, 26, 784–789. [Google Scholar] [CrossRef]
- Lee, S.J.; Kang, M.-S.; Oh, J.-S.; Na, H.S.; Lim, Y.J.; Jeong, Y.-I.; Lee, H.C. Caffeic acid-conjugated chitosan derivatives and their anti-tumor activity. Arch. Pharmacal Res. 2013, 36, 1437–1446. [Google Scholar] [CrossRef]
- Liu, J.; Wen, X.-y.; Lu, J.-f.; Kan, J.; Jin, C.-h. Free radical mediated grafting of chitosan with caffeic and ferulic acids: Structures and antioxidant activity. Int. J. Biol. Macromol. 2014, 65, 97–106. [Google Scholar] [CrossRef]

| Conjugate | Synthesis Method | Application | Study Type | Properties | Reference (Year) |
|---|---|---|---|---|---|
| CS-pCA | Carbodiimide-mediated | Encapsulation of essential oil | In vitro | Higher thermostability, antioxidant activity, and antitumoral effect | Kamal et al. (2021) [146] |
| CS-FA | Ascorbic acid/hydrogen peroxide redox pair | BSA loading | In vitro | Higher thermal stability, swelling ratio, and superior sustained release | Li et al. (2021) [144] |
| CS-transFA, CS-CA, or CS-SA | Enzymatic (laccase) | Methylene blue loading | In vitro | CS-AC with higher swelling capacity CS-transFA and CS-CA biocompatible with HEK293 cells Sustained release independent of the grafting substrate | Huber et al. (2017) [149] |
| CS-FA | Ascorbic acid/hydrogen peroxide redox pair | BSA microencapsulation | In vitro | Higher thermal stability, lower crystallinity, and enhanced sustained release | Li et al. (2017) [143] |
| CS-FA | Ascorbic acid/hydrogen peroxide redox pair | Thiamine and pyridoxine microencapsulation | In vitro/in vivo | Higher thermal stability of thiamine/pyridoxine, lower crystallinity on modified CS, and good encapsulation rate In vivo controlled release and reduced effective dose of thiamine/pyridoxine | Chatterjee et al. (2016) [145] |
| CS-CA co- polymerized with D-b-PEG | Carbodiimide- mediated | Doxorubicin nanoencapsulation | In vitro | Enhanced water solubility Cell internalization of nanoparticles and growth inhibition of doxorubicin-resistant CT26 cells | Lee et al. (2015) [148] |
| Thiolated CS-pCA | Carbodiimide-mediated | Piperine encapsulation | In vitro | Increased swelling capacity, mucoadhesion, and controlled release of hydrophobic piperine | Pengpong et al. (2014) [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.A.; Matias-Guiu, J.; Gómez-Pinedo, U.; Mateos-Díaz, J.C. Chitosan–Hydroxycinnamic Acids Conjugates: Emerging Biomaterials with Rising Applications in Biomedicine. Int. J. Mol. Sci. 2022, 23, 12473. https://doi.org/10.3390/ijms232012473
Ojeda-Hernández DD, Canales-Aguirre AA, Matias-Guiu JA, Matias-Guiu J, Gómez-Pinedo U, Mateos-Díaz JC. Chitosan–Hydroxycinnamic Acids Conjugates: Emerging Biomaterials with Rising Applications in Biomedicine. International Journal of Molecular Sciences. 2022; 23(20):12473. https://doi.org/10.3390/ijms232012473
Chicago/Turabian StyleOjeda-Hernández, Doddy Denise, Alejandro A. Canales-Aguirre, Jordi A. Matias-Guiu, Jorge Matias-Guiu, Ulises Gómez-Pinedo, and Juan Carlos Mateos-Díaz. 2022. "Chitosan–Hydroxycinnamic Acids Conjugates: Emerging Biomaterials with Rising Applications in Biomedicine" International Journal of Molecular Sciences 23, no. 20: 12473. https://doi.org/10.3390/ijms232012473
APA StyleOjeda-Hernández, D. D., Canales-Aguirre, A. A., Matias-Guiu, J. A., Matias-Guiu, J., Gómez-Pinedo, U., & Mateos-Díaz, J. C. (2022). Chitosan–Hydroxycinnamic Acids Conjugates: Emerging Biomaterials with Rising Applications in Biomedicine. International Journal of Molecular Sciences, 23(20), 12473. https://doi.org/10.3390/ijms232012473










