MicroRNA-19b Plays a Key Role in 5-Fluorouracil Resistance and Predicts Tumor Progression in Locally Advanced Rectal Cancer Patients
Abstract
1. Introduction
2. Results
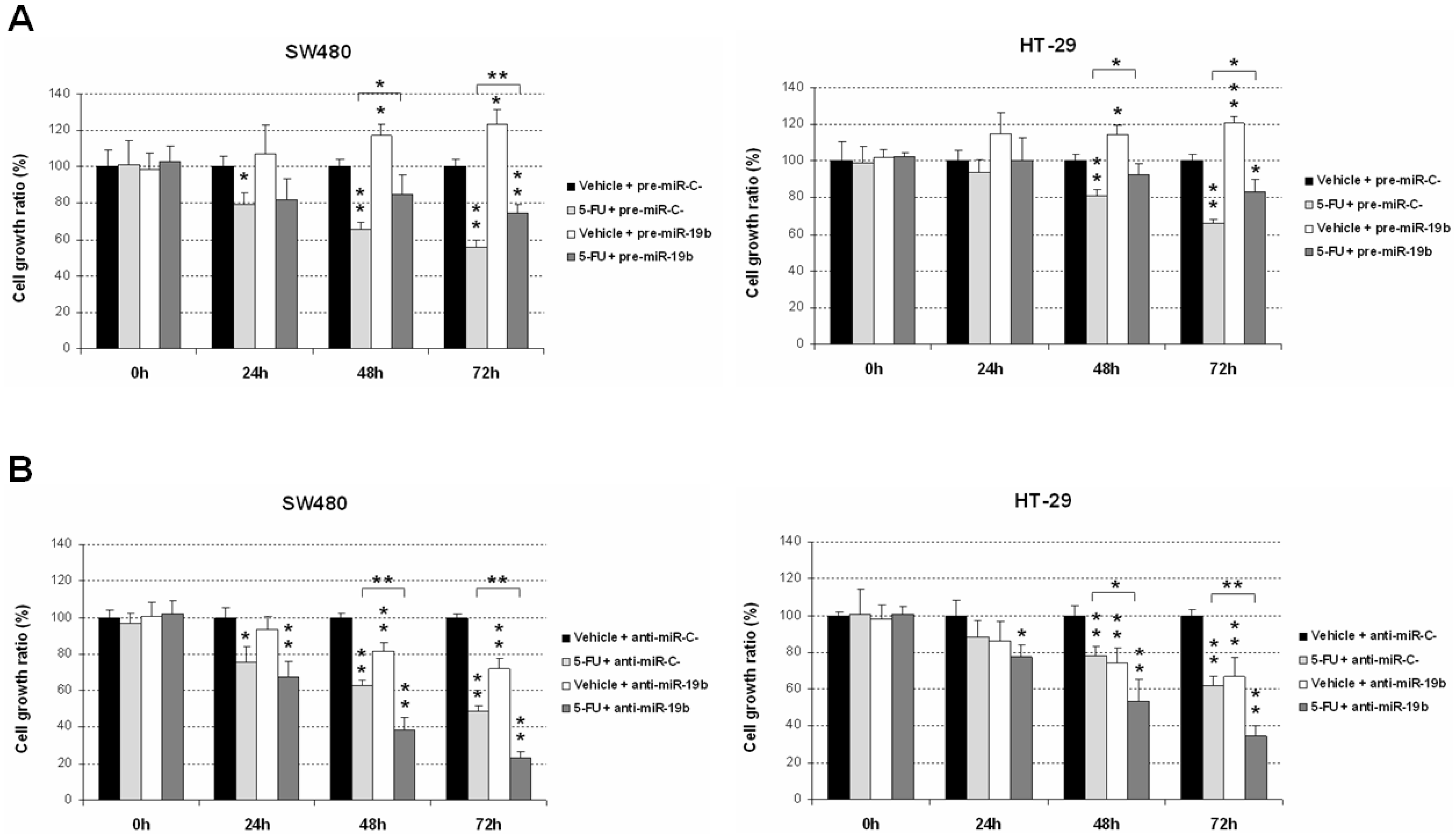
2.1. miR-19b Modulates Antitumor Effects of 5-FU Treatment
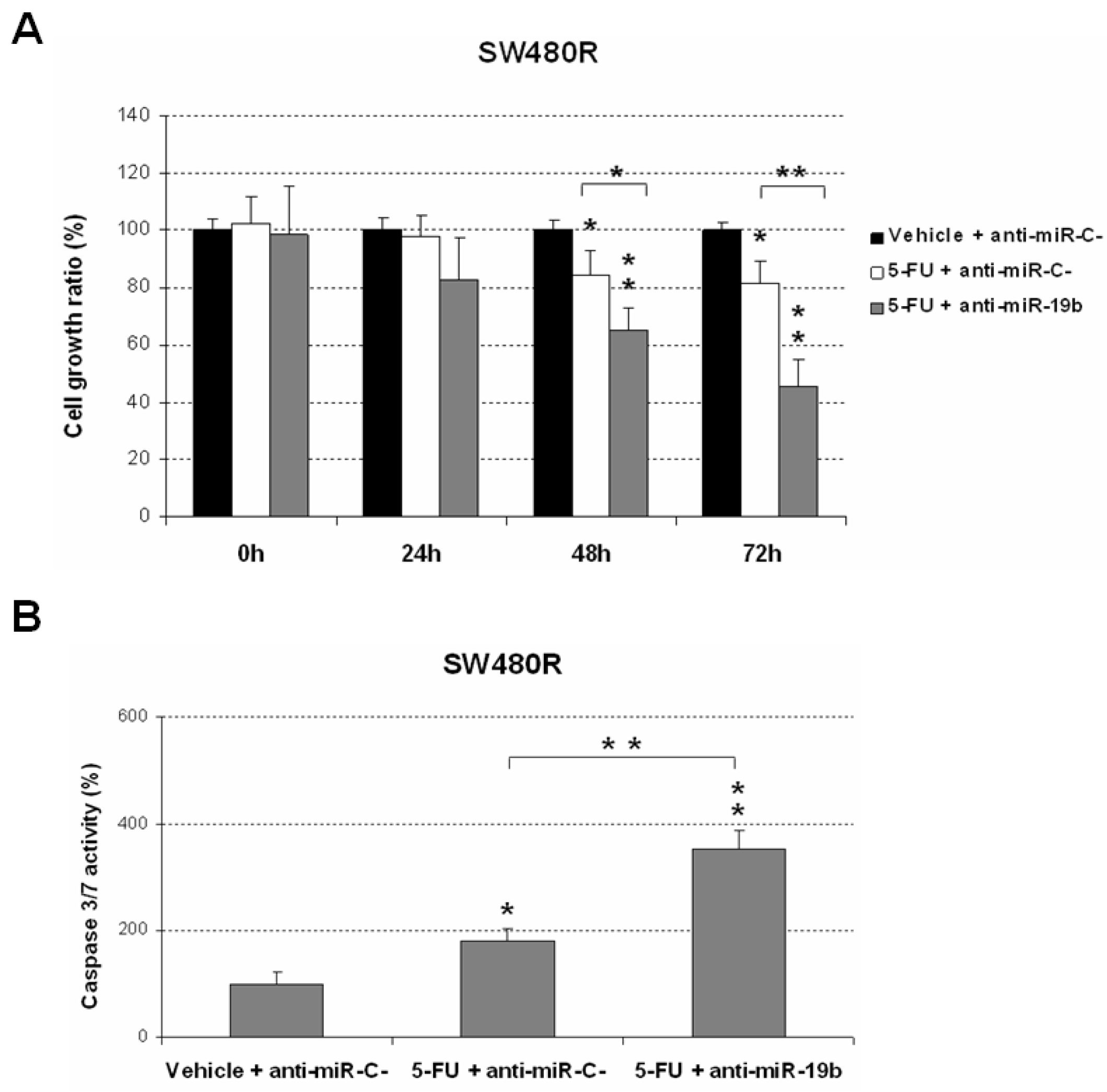
2.2. Downregulation of miR-19b Represents a Novel Molecular Strategy to Overcome 5-FU Resistance
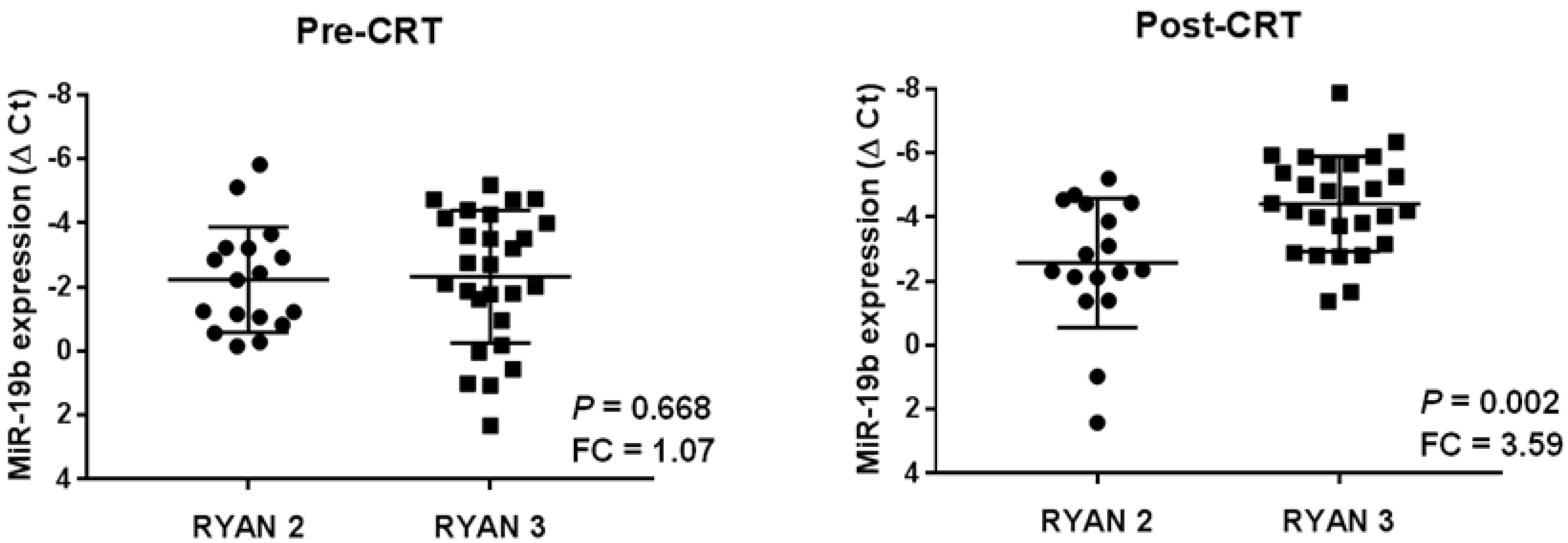
2.3. High miR-19b Levels Are Observed in Post-Treatment Samples from Those LARC Patients with Lack of Response to Preoperative CRT
2.4. miR-19b Overexpression in Post-Neoadjuvant CRT Samples Anticipates Patient Recurrence
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Transfection
4.2. Patient Samples
4.3. Nucleic Acid Isolation
4.4. Quantification of MiRNA Expression Levels
4.5. Cell Viability Assay
4.6. Analysis of Caspase Activation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory. Colorectal Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 17 January 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Feeney, G.; Sehgal, R.; Sheehan, M.; Hogan, A.; Regan, M.; Joyce, M.; Kerin, M. Neoadjuvant radiotherapy for rectal cancer management. World J. Gastroenterol. 2019, 25, 4850–4869. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, S.; Yang, T.W.W.; Johnson, N.; Bell, S.; Chin, M.; Simpson, P.; Carne, P.; Farmer, C.; Skinner, S.; Warrier, S.K.; et al. Latest evidence on the management of early-stage and locally advanced rectal cancer: A narrative review. ANZ J. Surg. 2022, 92, 365–372. [Google Scholar] [CrossRef]
- De Mattia, E.; Canzonieri, V.; Polesel, J.; Mezzalira, S.; Dalle Fratte, C.; Dreussi, E.; Roncato, R.; Bignucolo, A.; Innocente, R.; Belluco, C.; et al. SMAD3 Host and Tumor Profiling to Identify Locally Advanced Rectal Cancer Patients at High Risk of Poor Response to Neoadjuvant Chemoradiotherapy. Front. Pharmacol. 2021, 12, 778781. [Google Scholar] [CrossRef]
- Yoo, R.N.; Kim, H.J. Total neoadjuvant therapy in locally advanced rectal cancer: Role of systemic chemotherapy. Ann. Gastroenterol. Surg. 2019, 3, 356–367. [Google Scholar] [CrossRef]
- Izzotti, A.; Ceccaroli, C.; Geretto, M.; Ruggieri, F.G.; Schenone, S.; Di Maria, E. Predicting Response to Neoadjuvant Therapy in Colorectal Cancer Patients the Role of Messenger-and Micro-RNA Profiling. Cancers 2020, 12, 1652. [Google Scholar] [CrossRef]
- Sauer, R.; Fietkau, R.; Wittekind, C.; Rödel, C.; Martus, P.; Hohenberger, W.; Tschmelitsch, J.; Sabitzer, H.; Karstens, J.-H.; Becker, H.; et al. Adjuvant vs. neoadjuvant radiochemotherapy for locally advanced rectal cancer: The German trial CAO/ARO/AIO-94. Color. Dis. 2003, 5, 406–415. [Google Scholar] [CrossRef]
- van Gijn, W.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.M.; Putter, H.; Wiggers, T.; Rutten, H.J.T.; Påhlman, L.; Glimelius, B.; van de Velde, C.J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011, 12, 575–582. [Google Scholar] [CrossRef]
- Riihimäki, M.; Hemminki, A.; Sundquist, J.; Hemminki, K. Patterns of metastasis in colon and rectal cancer. Sci. Rep. 2016, 6, 29765. [Google Scholar] [CrossRef]
- Meltzer, S.; Bjørnetrø, T.; Lyckander, L.G.; Flatmark, K.; Dueland, S.; Samiappan, R.; Johansen, C.; Kalanxhi, E.; Ree, A.H.; Redalen, K.R. Circulating Exosomal miR-141-3p and miR-375 in Metastatic Progression of Rectal Cancer. Transl. Oncol. 2020, 13, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; van Stiphout, R.G.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.-F.; Bujko, K.; Cionini, L.; et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J. Clin. Oncol. 2011, 29, 3163–3172. [Google Scholar] [CrossRef] [PubMed]
- Peeters, K.C.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.K.; Putter, H.; Wiggers, T.; Rutten, H.; Pahlman, L.; Glimelius, B.; Leer, J.W.; et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann. Surg. 2007, 246, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Sebag-Montefiore, D.; Stephens, R.J.; Steele, R.; Monson, J.; Grieve, R.; Khanna, S.; Quirke, P.; Couture, J.; de Metz, C.; Myint, A.S.; et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): A multicentre, randomised trial. Lancet 2009, 373, 811–820. [Google Scholar] [CrossRef]
- Riesco-Martinez, M.C.; Fernandez-Martos, C.; Gravalos-Castro, C.; Espinosa-Olarte, P.; La Salvia, A.; Robles-Diaz, L.; Modrego-Sanchez, A.; Garcia-Carbonero, R. Impact of Total Neoadjuvant Therapy vs. Standard Chemoradiotherapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-Analysis of Randomized Trials. Cancers 2020, 12, 3655. [Google Scholar] [CrossRef] [PubMed]
- Papaccio, F.; Roselló, S.; Huerta, M.; Gambardella, V.; Tarazona, N.; Fleitas, T.; Roda, D.; Cervantes, A. Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer. Cancers 2020, 12, 3611. [Google Scholar] [CrossRef]
- Weiser, M.R. Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: PRODIGE 23 Trial. Ann. Surg. Oncol. 2022, 29, 1493–1495. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- Jin, J.; Tang, Y.; Hu, C.; Jiang, L.M.; Jiang, J.; Li, N.; Liu, W.-Y.; Chen, S.-L.; Li, S.; Lu, N.-N.; et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J. Clin. Oncol. 2022, 40, 1681–1692. [Google Scholar] [CrossRef]
- Strubberg, A.M.; Madison, B.B. MicroRNAs in the etiology of colorectal cancer: Pathways and clinical implications. Dis. Model. Mech. 2017, 10, 197–214. [Google Scholar] [CrossRef]
- Huang, T.C.; Peng, K.C.; Kuo, T.T.; Lin, L.C.; Liu, B.C.; Ye, S.P.; Chu, C.-C.; Hsia, S.-M.; Chang, H.-Y. Predicting Agents That Can Overcome 5-FU Resistance in Colorectal Cancers via Pharmacogenomic Analysis. Biomedicines 2021, 9, 882. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cai, J.L.; Huang, P.Z.; Kang, L.; Huang, M.J.; Wang, L.; Wang, J.P. miR19b-3p promotes the growth and metastasis of colorectal cancer via directly targeting ITGB8. Am. J. Cancer Res. 2017, 7, 1996–2008. [Google Scholar] [PubMed]
- Calin, G.A.; Sevignani, C.; Dumitru, C.D.; Hyslop, T.; Noch, E.; Yendamuri, S.; Shimizu, M.; Rattan, S.; Bullrich, F.; Negrini, M.; et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 2999–3004. [Google Scholar] [CrossRef]
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, U.; Berger, F.; Hashemi Gheinani, A.; Burgener, S.S.; Monastyrskaya, K.; Vassella, E. miR-19b enhances proliferation and apoptosis resistance via the EGFR signaling pathway by targeting PP2A and BIM in non-small cell lung cancer. Mol. Cancer 2018, 17, 44. [Google Scholar] [CrossRef]
- Olive, V.; Bennett, M.J.; Walker, J.C.; Ma, C.; Jiang, I.; Cordon-Cardo, C.; Li, Q.-J.; Lowe, S.W.; Hannon, G.J.; He, L. miR-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009, 23, 2839–2849. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Z.; Han, X.; Jiang, L.; Ge, R.; Wang, X.; Li, J. Up-regulation of microRNA-19b is associated with metastasis and predicts poor prognosis in patients with colorectal cancer. Int. J. Clin. Exp. Pathol. 2018, 11, 3952–3960. [Google Scholar] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef]
- Kong, Y.W.; Ferland-McCollough, D.; Jackson, T.J.; Bushell, M. microRNAs in cancer management. Lancet Oncol. 2012, 13, 249–258. [Google Scholar] [CrossRef]
- De Palma, F.D.E.; Luglio, G.; Tropeano, F.P.; Pagano, G.; D’Armiento, M.; Kroemer, G.; Maiuri, M.C.; De Palma, G.D. The Role of Micro-RNAs and Circulating Tumor Markers as Predictors of Response to Neoadjuvant Therapy in Locally Advanced Rectal Cancer. Int. J. Mol. Sci. 2020, 21, 7040. [Google Scholar] [CrossRef]
- Imedio, L.; Cristóbal, I.; Rubio, J.; Santos, A.; Rojo, F.; García-Foncillas, J. MicroRNAs in Rectal Cancer: Functional Significance and Promising Therapeutic Value. Cancers 2020, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Farazi, T.A.; Hoell, J.I.; Morozov, P.; Tuschl, T. MicroRNAs in human cancer. Adv. Exp. Med. Biol. 2013, 774, 1–20. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Thomson, J.M.; Hemann, M.T.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.W.; Hannon, G.J.; et al. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, H.; Seto, M. A microRNA cluster as a target of genomic amplification in malignant lymphoma. Leukemia 2005, 19, 2013–2016. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, S.; Zhang, X.; Chen, G.; Liang, H.; Yu, M.; Liao, Z.; Zhou, Y.; Zhang, C.-Y.; Wang, T.; et al. Oncogenic miR-19a and miR-19b co-regulate tumor suppressor MTUS1 to promote cell proliferation and migration in lung cancer. Protein Cell 2017, 8, 455–466. [Google Scholar] [CrossRef]
- Navarro, A.; Beà, S.; Fernández, V.; Prieto, M.; Salaverria, I.; Jares, P.; Hartmann, E.; Mozos, A.; López-Guillermo, A.; Villamor, N.; et al. MicroRNA expression, chromosomal alterations, and immunoglobulin variable heavy chain hypermutations in Mantle cell lymphomas. Cancer Res. 2009, 69, 7071–7078. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, K.; Zhi, Y.; Wu, Y.; Chen, B.; Bai, J.; Wang, X. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin. Transl. Med. 2021, 11, 478. [Google Scholar] [CrossRef]
- Jiang, T.; Ye, L.; Han, Z.; Liu, Y.; Yang, Y.; Peng, Z.; Fan, J. miR-19b-3p promotes colon cancer proliferation and oxaliplatin-based chemoresistance by targeting SMAD4: Validation by bioinformatics and experimental analyses. J. Exp. Clin. Cancer Res. 2017, 36, 131. [Google Scholar] [CrossRef]
- Sun, T.; Yin, Y.F.; Jin, H.G.; Liu, H.R.; Tian, W.C. Exosomal microRNA-19b targets FBXW7 to promote colorectal cancer stem cell stemness and induce resistance to radiotherapy. Kaohsiung J. Med. Sci. 2022, 38, 108–119. [Google Scholar] [CrossRef]
- Kurokawa, K.; Tanahashi, T.; Iima, T.; Yamamoto, Y.; Akaike, Y.; Nishida, K.; Masuda, K.; Kuwano, Y.; Murakami, Y.; Fukushima, M.; et al. Role of miR-19b and its target mRNAs in 5-fluorouracil resistance in colon cancer cells. J. Gastroenterol. 2012, 47, 883–895. [Google Scholar] [CrossRef]
- Cristóbal, I.; Manso, R.; Rincón, R.; Caramés, C.; Senin, C.; Borrero, A.; Martínez-Useros, J.; Rodríguez, M.; Zazo, S.; Aguilera, O.; et al. PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential. Mol. Cancer Ther. 2014, 13, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Salvi, S.; Foca, F.; Teodorani, N.; Saragoni, L.; Puccetti, M.; Passardi, A.; Tamberi, S.; Avanzolini, A.; Lucci, E.; et al. miR-17-92a-1 cluster host gene (MIR17HG) evaluation and response to neoadjuvant chemoradiotherapy in rectal cancer. OncoTargets Ther. 2016, 9, 2735–2742. [Google Scholar] [CrossRef][Green Version]
- Rubio, J.; Cristóbal, I.; Santos, A.; Caramés, C.; Luque, M.; Sanz-Alvarez, M.; Zazo, S.; Madoz-Gúrpide, J.; Rojo, F.; García-Foncillas, J. Low MicroRNA-19b Expression Shows a Promising Clinical Impact in Locally Advanced Rectal Cancer. Cancers 2021, 13, 1456. [Google Scholar] [CrossRef]
- Machackova, T.; Prochazka, V.; Kala, Z.; Slaby, O. Translational Potential of MicroRNAs for Preoperative Staging and Prediction of Chemoradiotherapy Response in Rectal Cancer. Cancers 2019, 11, 1545. [Google Scholar] [CrossRef] [PubMed]
- Casado, E.; Garcia, V.M.; Sanchez, J.J.; Blanco, M.; Maurel, J.; Feliu, J.; Fernández-Martos, C.; de Castro, J.; Castelo, B.; Belda-Iniesta, C.; et al. A combined strategy of SAGE and quantitative PCR provides a 13-gene signature that predicts preoperative chemoradiotherapy response and outcome in rectal cancer. Clin. Cancer Res. 2011, 17, 4145–4154. [Google Scholar] [CrossRef] [PubMed]
- Cristóbal, I.; Rincón, R.; Manso, R.; Caramés, C.; Zazo, S.; Madoz-Gúrpide, J.; Rojo, F.; García-Foncillas, J. Deregulation of the PP2A inhibitor SET shows promising therapeutic implications and determines poor clinical outcome in patients with metastatic colorectal cancer. Clin. Cancer Res. 2015, 21, 347–356. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, C.; Wang, Q.; Li, L.; Wang, M.; Li, X.; Yang, C. MicroRNA-19b Downregulates NR3C1 and Enhances Oxaliplatin Chemoresistance in Colon Cancer via the PI3K/AKT/mTOR Pathway. Clin. Med. Insights Oncol. 2021, 15, 11795549211012666. [Google Scholar] [CrossRef]
- Gu, Y.Y.; Yu, J.; Zhang, J.F.; Wang, C. Suppressing the secretion of exosomal miR-19b by gw4869 could regulate oxaliplatin sensitivity in colorectal cancer. Neoplasma 2019, 66, 39–45. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Netz, U.; Hallion, J.; Bishop, C.; Stephen, V.; Burton, J.; Paas, M.; Feagins, K.; Pan, J.; Rai, S.N.; et al. Circulating plasma microRNAs in colorectal neoplasia: A pilot study in assessing response to therapy. Transl. Oncol. 2021, 14, 100962. [Google Scholar] [CrossRef]
- Greenson, J.K.; Huang, S.-C.; Herron, C.; Moreno, V.; Bonner, J.D.; Tomsho, L.P.; Ben-Izhak, O.; Cohen, H.I.; Trougouboff, P.; Bejhar, J.; et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am. J. Surg. Pathol. 2009, 33, 126–133. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real- time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, W.; Taube, S.E.; McShane, L.M.; Cavenagh, M.M.; Altman, D.G. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): An Abridged Explanation and Elaboration. J. Natl. Cancer Inst. 2018, 110, 803–811. [Google Scholar] [CrossRef] [PubMed]

| Recurrence | No. Cases | No (%) | Yes (%) | p |
|---|---|---|---|---|
| miR-19b expression | 44 | 32 | 12 | 0.002 |
| No | 30 | 26 (86.7) | 4 (13.3) | |
| Yes | 14 | 6 (42.9) | 8 (57.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.; Cristóbal, I.; Rubio, J.; Caramés, C.; Luque, M.; Sanz-Álvarez, M.; Zazo, S.; Madoz-Gúrpide, J.; Rojo, F.; García-Foncillas, J. MicroRNA-19b Plays a Key Role in 5-Fluorouracil Resistance and Predicts Tumor Progression in Locally Advanced Rectal Cancer Patients. Int. J. Mol. Sci. 2022, 23, 12447. https://doi.org/10.3390/ijms232012447
Santos A, Cristóbal I, Rubio J, Caramés C, Luque M, Sanz-Álvarez M, Zazo S, Madoz-Gúrpide J, Rojo F, García-Foncillas J. MicroRNA-19b Plays a Key Role in 5-Fluorouracil Resistance and Predicts Tumor Progression in Locally Advanced Rectal Cancer Patients. International Journal of Molecular Sciences. 2022; 23(20):12447. https://doi.org/10.3390/ijms232012447
Chicago/Turabian StyleSantos, Andrea, Ion Cristóbal, Jaime Rubio, Cristina Caramés, Melani Luque, Marta Sanz-Álvarez, Sandra Zazo, Juan Madoz-Gúrpide, Federico Rojo, and Jesus García-Foncillas. 2022. "MicroRNA-19b Plays a Key Role in 5-Fluorouracil Resistance and Predicts Tumor Progression in Locally Advanced Rectal Cancer Patients" International Journal of Molecular Sciences 23, no. 20: 12447. https://doi.org/10.3390/ijms232012447
APA StyleSantos, A., Cristóbal, I., Rubio, J., Caramés, C., Luque, M., Sanz-Álvarez, M., Zazo, S., Madoz-Gúrpide, J., Rojo, F., & García-Foncillas, J. (2022). MicroRNA-19b Plays a Key Role in 5-Fluorouracil Resistance and Predicts Tumor Progression in Locally Advanced Rectal Cancer Patients. International Journal of Molecular Sciences, 23(20), 12447. https://doi.org/10.3390/ijms232012447







