Cathepsin S Inhibition Suppresses Experimental Systemic Lupus Erythematosus-Associated Pulmonary Arterial Remodeling
Abstract
1. Introduction
2. Results
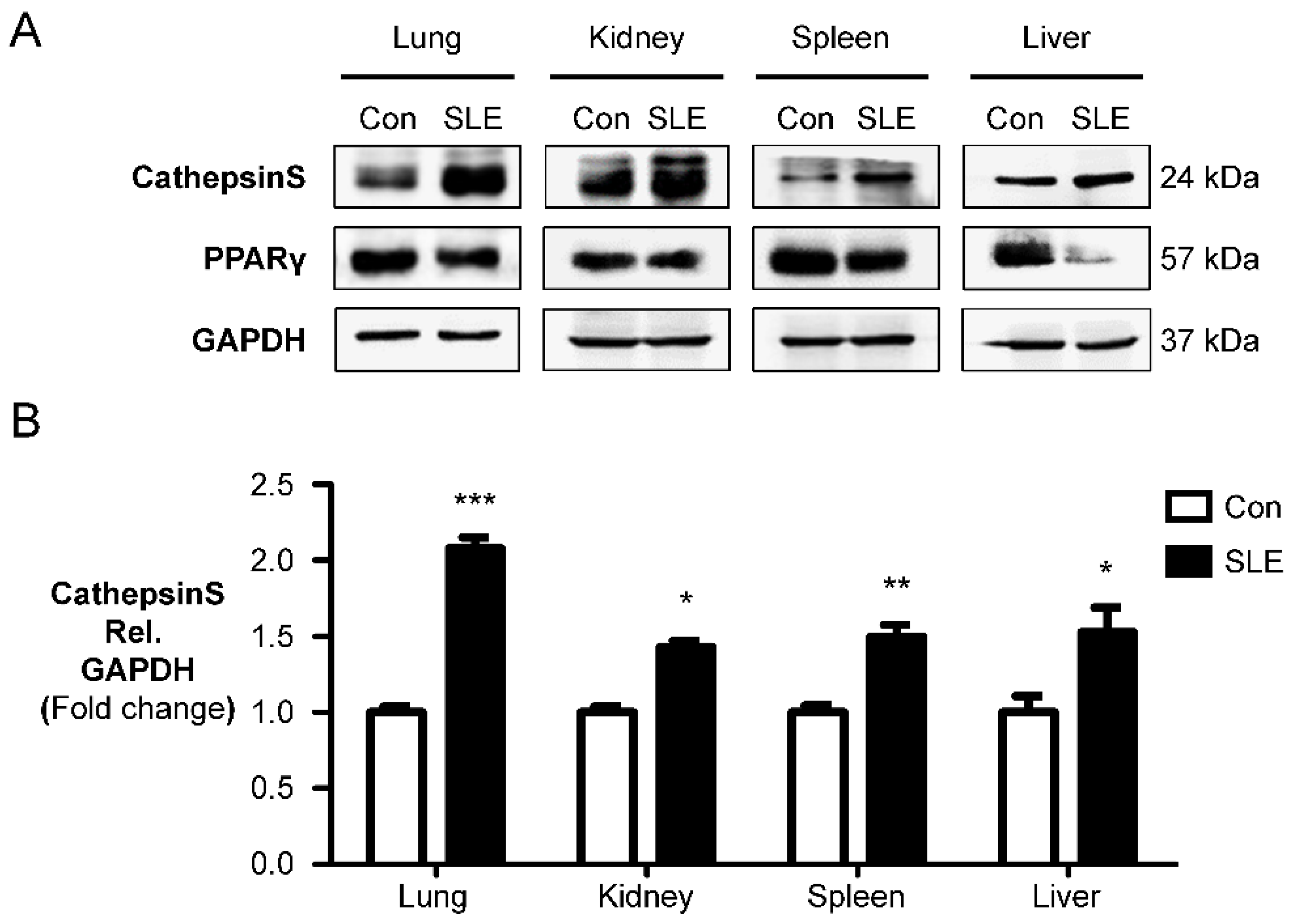
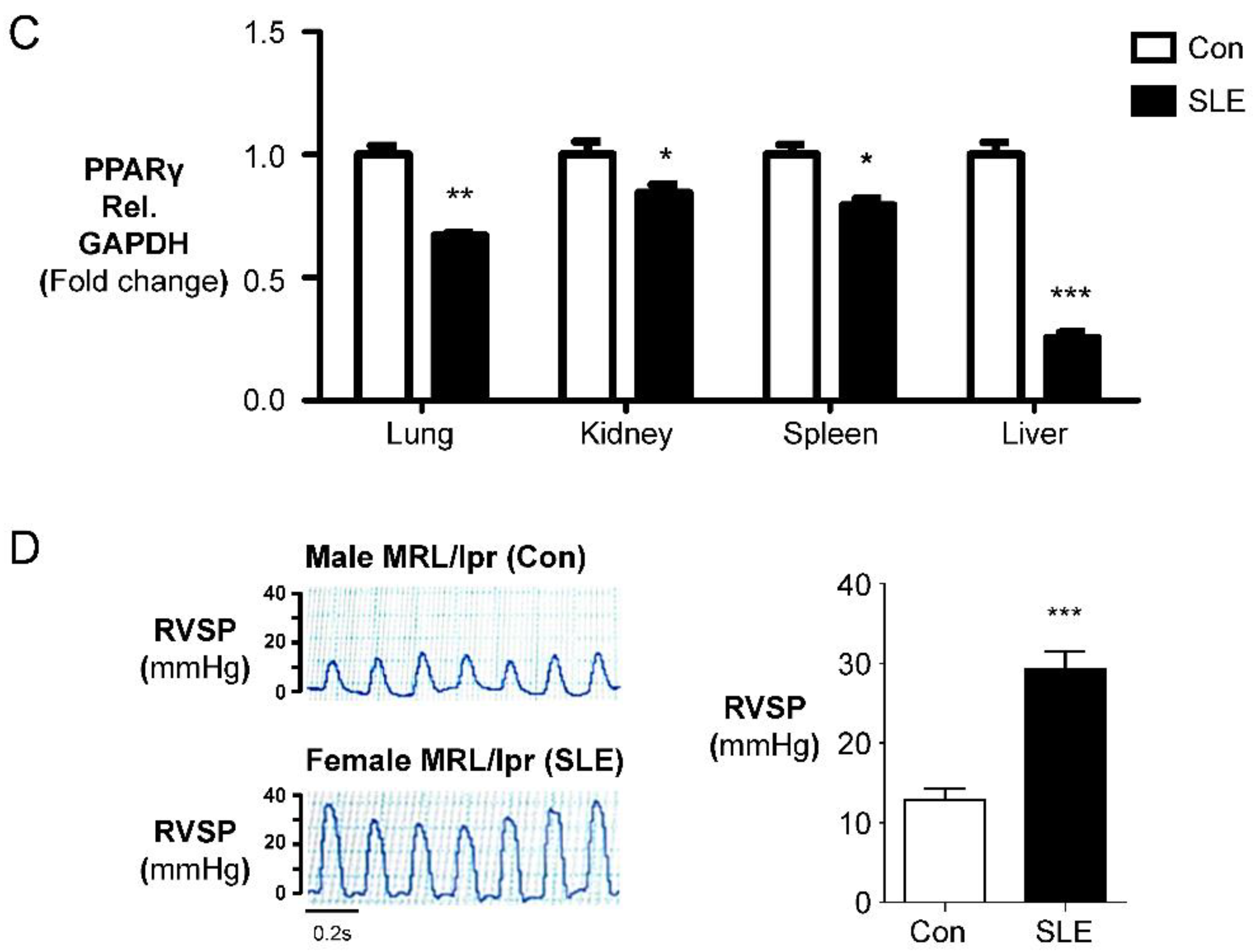
2.1. Cathepsin S Protein Level Is a Marker of Experimental SLE
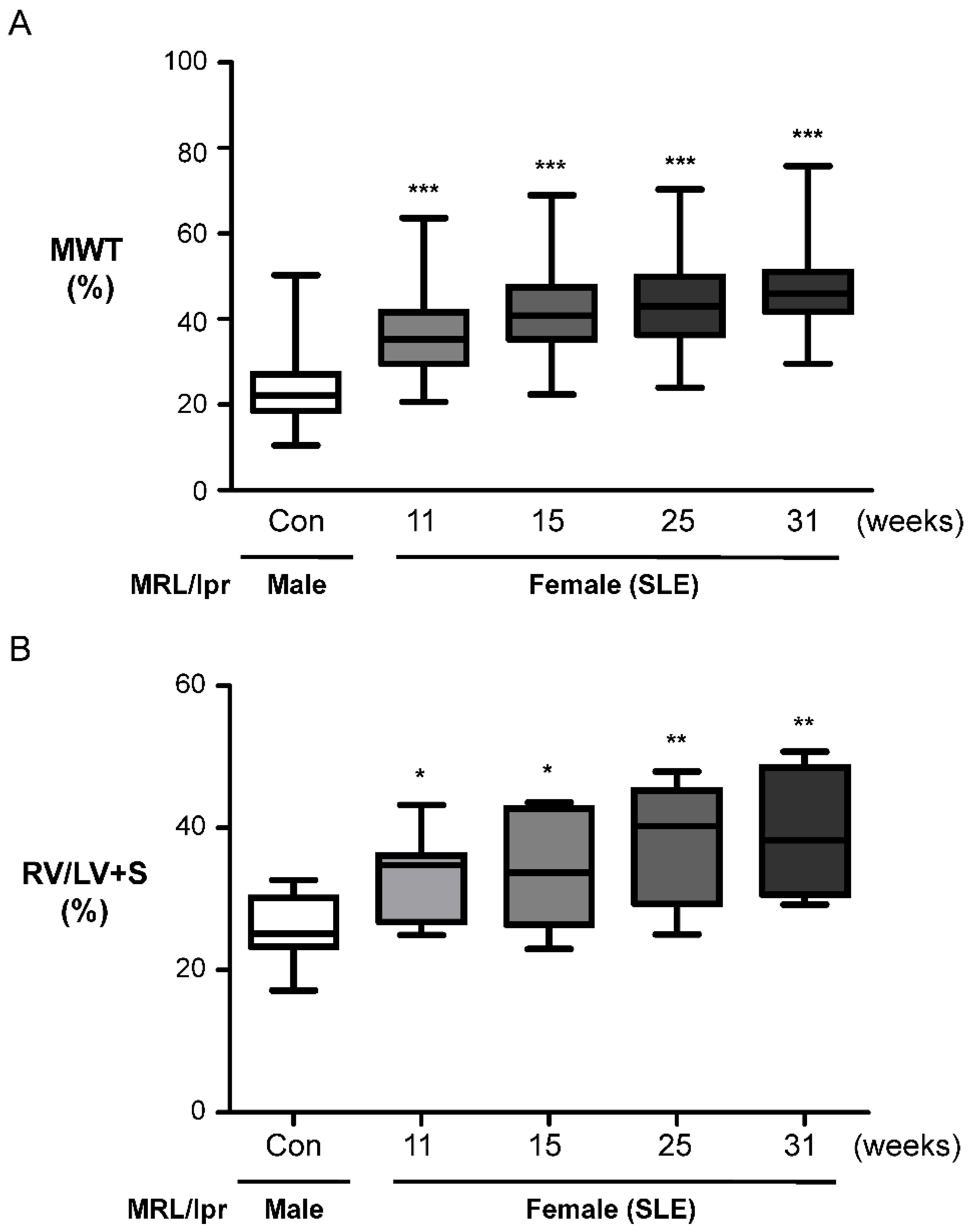
2.2. Female MRL/lpr (SLE) Mice Show Pulmonary Arterial Remodeling and Right Ventricular Hypertrophy
2.3. Female MRL/lpr Mice Show Partial Muscularization of the Pulmonary Arteries
2.4. Cat S Inhibitor Upregulates PPARγ and Suppresses Cat S Expression
2.5. Cat S Inhibitor Upregulates PPARγ and Suppresses Cat S Expression to Prevent the Pulmonary Arterial Remodeling and Right Ventricular Hypertrophy in Experimental SLE
3. Discussion
4. Materials and Methods
4.1. Mice and Experimental Protocol
4.2. Hemodynamic Measurements and Cardiovascular Evaluation
4.3. Immunohistochemical Analysis
4.4. Assessment of Media Wall Thickness (MWT)
4.5. Western Blot Analysis
4.6. Cathepsin S Activity Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2008, 118, 2372–2379. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Kovacs, G.; Hoeper, M.M.; Badagliacca, R.; Berger, R.M.F.; Brida, M.; Carlsen, J.; Coats, A.J.S.; Escribano-Subias, P.; Ferrari, P.; et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022, 43, 3618–3731. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Liu, J.; Parsons, L.; Hassoun, P.M.; McGoon, M.; Badesch, D.B.; Miller, D.P.; Nicolls, M.R.; Zamanian, R.T. Characterization of connective tissue disease-associated pulmonary arterial hypertension from REVEAL: Identifying systemic sclerosis as a unique phenotype. Chest 2010, 138, 1383–1394. [Google Scholar] [CrossRef]
- Haas, C. Pulmonary hypertension associated with systemic lupus erythematosus. Bull. Acad. Natl. Med. 2004, 188, 985–997; discussion 997. [Google Scholar] [PubMed]
- Oktay, A.; Oto, A. Pulmonary hypertension in systemic lupus erythematosus. Br. Med. J. 1983, 287, 1629. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qian, J.; Li, M.; Zhang, X.; Wang, Q.; Zhao, J.; Tian, Z.; Wei, W.; Zuo, X.; Zhang, M.; Zhu, P.; et al. Long-term prognosis of patients with systemic lupus erythematosus-associated pulmonary arterial hypertension: CSTAR-PAH cohort study. Eur. Respir. J. 2019, 53, 1800081. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.T.; Wang, P.; Guan, S.Y.; Li, H.M.; Li, X.M.; Wang, B.; Pan, H.F. Prevalence of pulmonary hypertension in systemic lupus erythematosus: A meta-analysis. Ir. J. Med. Sci. 2018, 187, 723–730. [Google Scholar] [CrossRef]
- Sasaki, N.; Kamataki, A.; Sawai, T. A histopathological study of pulmonary hypertension in connective tissue disease. Allergol. Int. 2011, 60, 411–417. [Google Scholar] [CrossRef]
- Amarnani, R.; Yeoh, S.A.; Denneny, E.K.; Wincup, C. Lupus and the Lungs: The Assessment and Management of Pulmonary Manifestations of Systemic Lupus Erythematosus. Front. Med. 2020, 7, 610257. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, R.; Kiely, D.G.; Peacock, A.J.; Corris, P.A.; Gibbs, J.S.; Vrapi, F.; Das, C.; Elliot, C.A.; Johnson, M.; DeSoyza, J.; et al. Connective tissue disease-associated pulmonary arterial hypertension in the modern treatment era. Am. J. Respir. Crit. Care Med. 2009, 179, 151–157. [Google Scholar] [CrossRef]
- Kommireddy, S.; Bhyravavajhala, S.; Kurimeti, K.; Chennareddy, S.; Kanchinadham, S.; Prasad, I.R.V.; Rajasekhar, L. Pulmonary arterial hypertension in systemic lupus erythematosus may benefit by addition of immunosuppression to vasodilator therapy: An observational study. Rheumatology 2015, 54, 1673–1679. [Google Scholar] [CrossRef]
- Sanges, S.; Savale, L.; Lamblin, N.; Remy-Jardin, M.; Humbert, M.; Sobanski, V. Efficacy of immunosuppressants with bridge vasodilator therapy in severe lupus erythematosus-associated pulmonary arterial hypertension. ESC Heart Fail. 2019, 6, 1322–1325. [Google Scholar] [CrossRef]
- Khanna, D.; Zhao, C.; Saggar, R.; Mathai, S.C.; Chung, L.; Coghlan, J.G.; Shah, M.; Hartney, J.; McLaughlin, V. Long-Term Outcomes in Patients With Connective Tissue Disease-Associated Pulmonary Arterial Hypertension in the Modern Treatment Era: Meta-Analyses of Randomized, Controlled Trials and Observational Registries. Arthritis Rheumatol. 2021, 73, 837–847. [Google Scholar] [CrossRef]
- Riese, R.J.; Mitchell, R.N.; Villadangos, J.A.; Shi, G.P.; Palmer, J.T.; Karp, E.R.; De Sanctis, G.T.; Ploegh, H.L.; Chapman, H.A. Cathepsin S activity regulates antigen presentation and immunity. J. Clin. Investig. 1998, 101, 2351–2363. [Google Scholar] [CrossRef]
- Rupanagudi, K.V.; Kulkarni, O.P.; Lichtnekert, J.; Darisipudi, M.N.; Mulay, S.R.; Schott, B.; Gruner, S.; Haap, W.; Hartmann, G.; Anders, H.J. Cathepsin S inhibition suppresses systemic lupus erythematosus and lupus nephritis because cathepsin S is essential for MHC class II-mediated CD4 T cell and B cell priming. Ann. Rheum. Dis. 2015, 74, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Guo, J.; Zhang, X.; Sukhova, G.K.; Libby, P.; Shi, G.P. Cysteine protease cathepsins in cardiovascular disease: From basic research to clinical trials. Nat. Rev. Cardiol. 2018, 15, 351–370. [Google Scholar] [CrossRef]
- Turk, V.; Turk, B.; Turk, D. Lysosomal cysteine proteases: Facts and opportunities. EMBO J. 2001, 20, 4629–4633. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Chen, C.C.; Hsu, L.A.; Chang, G.J.; Ko, Y.H.; Chen, C.F.; Chen, M.Y.; Yang, S.H.; Pang, J.H. Degradation of the internal elastic laminae in vein grafts of rats with aortocaval fistulae: Potential impact on graft vasculopathy. Am. J. Pathol. 2009, 174, 1837–1846. [Google Scholar] [CrossRef][Green Version]
- Chang, C.J.; Hsu, H.C.; Ho, W.J.; Chang, G.J.; Pang, J.S.; Chen, W.J.; Huang, C.C.; Lai, Y.J. Cathepsin S promotes the development of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L1–L13. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, Y.; He, L. Predictive factors for concomitant pulmonary arterial hypertension at diagnosis of systemic lupus erythematosus in a Chinese population. Int. J. Rheum. Dis. 2022, 25, 76–82. [Google Scholar] [CrossRef]
- Lee, J.; Jang, S.; Choi, M.; Kang, M.; Lim, S.G.; Kim, S.Y.; Jang, S.; Ko, J.; Kim, E.; Yi, J.; et al. Overexpression of cathepsin S exacerbates lupus pathogenesis through upregulation TLR7 and IFN-alpha in transgenic mice. Sci. Rep. 2021, 11, 16348. [Google Scholar] [CrossRef]
- Kawato, Y.; Fukahori, H.; Nakamura, K.; Kanno, A.; Kubo, K.; Hiramitsu, M.; Matsuda, T.; Hanada, Y.; Furukawa, T.; Nakajima, Y.; et al. Potential benefit of the cathepsin S inhibitor, ASP1617, as a treatment for systemic lupus erythematosus. Eur. J. Pharmacol. 2022, 919, 174826. [Google Scholar] [CrossRef]
- Chapman, H.A.; Riese, R.J.; Shi, G.P. Emerging roles for cysteine proteases in human biology. Annu. Rev. Physiol. 1997, 59, 63–88. [Google Scholar] [CrossRef]
- Yen, T.H.; Yang, H.Y.; Yeh, Y.H.; Chu, P.H.; Wen, C.J.; Fu, J.F.; Wang, I.K.; Liang, C.C.; Chang, C.T.; Chen, K.H.; et al. Aliskiren attenuates proteinuria in mice with lupus nephritis by a blood pressure-independent mechanism. Lupus 2013, 22, 180–189. [Google Scholar] [CrossRef]
- Yu, C.C.; Yang, C.W.; Wu, M.S.; Ko, Y.C.; Huang, C.T.; Hong, J.J.; Huang, C.C. Mycophenolate mofetil reduces renal cortical inducible nitric oxide synthase mRNA expression and diminishes glomerulosclerosis in MRL/lpr mice. J. Lab. Clin. Med. 2001, 138, 69–77. [Google Scholar] [CrossRef]
- Durcan, L.; O’Dwyer, T.; Petri, M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet 2019, 393, 2332–2343. [Google Scholar] [CrossRef]
- Rahman, A.; Isenberg, D.A. Systemic lupus erythematosus. N. Engl. J. Med. 2008, 358, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Koelink, P.J.; Bloemendaal, F.M.; Vos, A.C.; D’Haens, G.R.; van den Brink, G.R.; Wildenberg, M.E. Autophagy Contributes to the Induction of Anti-TNF Induced Macrophages. J. Crohns Colitis 2016, 10, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Cao, X.; Zhou, Y.; Nagpure, B.V.; Wu, Z.Y.; Hu, L.F.; Yang, Y.; Sethi, G.; Moore, P.K.; Bian, J.S. Hydrogen sulfide inhibits ATP-induced neuroinflammation and Abeta1-42 synthesis by suppressing the activation of STAT3 and cathepsin S. Brain Behav. Immun. 2018, 73, 603–614. [Google Scholar] [CrossRef]
- Stoeckle, C.; Quecke, P.; Ruckrich, T.; Burster, T.; Reich, M.; Weber, E.; Kalbacher, H.; Driessen, C.; Melms, A.; Tolosa, E. Cathepsin S dominates autoantigen processing in human thymic dendritic cells. J. Autoimmun. 2012, 38, 332–343. [Google Scholar] [CrossRef]
- Brown, R.; Nath, S.; Lora, A.; Samaha, G.; Elgamal, Z.; Kaiser, R.; Taggart, C.; Weldon, S.; Geraghty, P. Cathepsin S: Investigating an old player in lung disease pathogenesis, comorbidities, and potential therapeutics. Respir. Res. 2020, 21, 111. [Google Scholar] [CrossRef] [PubMed]
- Burns-Kurtis, C.L.; Olzinski, A.R.; Needle, S.; Fox, J.H.; Capper, E.A.; Kelly, F.M.; McQueney, M.S.; Romanic, A.M. Cathepsin S expression is up-regulated following balloon angioplasty in the hypercholesterolemic rabbit. Cardiovasc. Res. 2004, 62, 610–620. [Google Scholar] [CrossRef]
- Cheng, X.W.; Kuzuya, M.; Sasaki, T.; Arakawa, K.; Kanda, S.; Sumi, D.; Koike, T.; Maeda, K.; Tamaya-Mori, N.; Shi, G.P.; et al. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am. J. Pathol. 2004, 164, 243–251. [Google Scholar] [CrossRef][Green Version]
- Legchenko, E.; Chouvarine, P.; Borchert, P.; Fernandez-Gonzalez, A.; Snay, E.; Meier, M.; Maegel, L.; Mitsialis, S.A.; Rog-Zielinska, E.A.; Kourembanas, S.; et al. PPARgamma agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med. 2018, 10, eaao0303. [Google Scholar] [CrossRef]
- Ameshima, S.; Golpon, H.; Cool, C.D.; Chan, D.; Vandivier, R.W.; Gardai, S.J.; Wick, M.; Nemenoff, R.A.; Geraci, M.W.; Voelkel, N.F. Peroxisome proliferator-activated receptor gamma (PPARgamma) expression is decreased in pulmonary hypertension and affects endothelial cell growth. Circ. Res. 2003, 92, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, M. PPARgamma and the pathobiology of pulmonary arterial hypertension. Adv. Exp. Med. Biol. 2010, 661, 447–458. [Google Scholar]
- Gizard, F.; Bruemmer, D. Transcriptional Control of Vascular Smooth Muscle Cell Proliferation by Peroxisome Proliferator-Activated Receptor-gamma: Therapeutic Implications for Cardiovascular Diseases. PPAR Res. 2008, 2008, 429123. [Google Scholar] [CrossRef]
- Hsueh, W.A.; Jackson, S.; Law, R.E. Control of vascular cell proliferation and migration by PPAR-gamma: A new approach to the macrovascular complications of diabetes. Diabetes Care 2001, 24, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, T.R.; Bonegio, R.G.; Weitzner, Z.; Gharakhanian, R.; Rifkin, I.R. Peroxisome proliferator-activated receptor gamma agonists in the prevention and treatment of murine systemic lupus erythematosus. Immunology 2014, 142, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Luo, S.; Zhan, Y.; Lu, Q. The roles of PPARgamma and its agonists in autoimmune diseases: A comprehensive review. J. Autoimmun. 2020, 113, 102510. [Google Scholar] [CrossRef]
- Zhao, W.; Berthier, C.C.; Lewis, E.E.; McCune, W.J.; Kretzler, M.; Kaplan, M.J. The peroxisome-proliferator activated receptor-gamma agonist pioglitazone modulates aberrant T cell responses in systemic lupus erythematosus. Clin. Immunol. 2013, 149, 119–132. [Google Scholar] [CrossRef]
- Aprahamian, T.; Bonegio, R.G.; Richez, C.; Yasuda, K.; Chiang, L.K.; Sato, K.; Walsh, K.; Rifkin, I.R. The peroxisome proliferator-activated receptor gamma agonist rosiglitazone ameliorates murine lupus by induction of adiponectin. J. Immunol. 2009, 182, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Sonkar, S.K.; Singh, P.K.; Chandra, S.; Sonkar, G.K.; Bhosale, V.; Sharma, S. Cathepsin S as an early biomarker for cardiovascular disease in chronic kidney disease patients. J. Bras. Nefrol. 2022, 44, 329–335. [Google Scholar] [CrossRef]
- Steubl, D.; Kumar, S.V.; Tato, M.; Mulay, S.R.; Larsson, A.; Lind, L.; Riserus, U.; Renders, L.; Heemann, U.; Carlsson, A.C.; et al. Circulating cathepsin-S levels correlate with GFR decline and sTNFR1 and sTNFR2 levels in mice and humans. Sci. Rep. 2017, 7, 43538. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Huang, C.C.; Wang, I.K.; Chang, C.T.; Chen, K.H.; Weng, C.H.; Lin, J.L.; Hung, C.C.; Yang, C.W.; Yen, T.H. Impact of renal survival on the course and outcome of systemic lupus erythematosus patients treated with chronic peritoneal dialysis. Ther. Apher. Dial. 2010, 14, 35–42. [Google Scholar] [CrossRef]
- Jadhav, P.K.; Schiffler, M.A.; Gavardinas, K.; Kim, E.J.; Matthews, D.P.; Staszak, M.A.; Coffey, D.S.; Shaw, B.W.; Cassidy, K.C.; Brier, R.A.; et al. Discovery of Cathepsin S Inhibitor LY3000328 for the Treatment of Abdominal Aortic Aneurysm. ACS Med. Chem. Lett. 2014, 5, 1138–1142. [Google Scholar] [CrossRef]
- Lai, Y.J.; Kao, W.W.; Yeh, Y.H.; Chen, W.J.; Chu, P.H. Lumican deficiency promotes pulmonary arterial remodeling. Transl. Res. 2021, 237, 63–81. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, T.-H.; Ho, W.-J.; Yeh, Y.-H.; Lai, Y.-J. Cathepsin S Inhibition Suppresses Experimental Systemic Lupus Erythematosus-Associated Pulmonary Arterial Remodeling. Int. J. Mol. Sci. 2022, 23, 12316. https://doi.org/10.3390/ijms232012316
Yen T-H, Ho W-J, Yeh Y-H, Lai Y-J. Cathepsin S Inhibition Suppresses Experimental Systemic Lupus Erythematosus-Associated Pulmonary Arterial Remodeling. International Journal of Molecular Sciences. 2022; 23(20):12316. https://doi.org/10.3390/ijms232012316
Chicago/Turabian StyleYen, Tzung-Hai, Wan-Jing Ho, Yung-Hsin Yeh, and Ying-Ju Lai. 2022. "Cathepsin S Inhibition Suppresses Experimental Systemic Lupus Erythematosus-Associated Pulmonary Arterial Remodeling" International Journal of Molecular Sciences 23, no. 20: 12316. https://doi.org/10.3390/ijms232012316
APA StyleYen, T.-H., Ho, W.-J., Yeh, Y.-H., & Lai, Y.-J. (2022). Cathepsin S Inhibition Suppresses Experimental Systemic Lupus Erythematosus-Associated Pulmonary Arterial Remodeling. International Journal of Molecular Sciences, 23(20), 12316. https://doi.org/10.3390/ijms232012316





