Abstract
Intervertebral disc degeneration (IVDD), for which obesity and genetics are known risk factors, is a chronic process that alters the structure and function of the intervertebral discs (IVD). Circulating leptin is positively correlated with body weight and is often measured to elucidate the pathogenesis of IVD degeneration. In this study, we examined the associations of LEP single nucleotide polymorphisms (SNPs) genetic and environmental effects with IVDD. A total of 303 Taiwanese patients with IVDD (mean age, 58.6 ± 12.7 years) undergoing cervical discectomy for neck pain or lumbar discectomy for back pain were enrolled. Commercially available enzyme-linked immunosorbent assay (ELISA) kits measured the circulating plasma leptin levels. TaqMan SNP genotyping assays genotyped the LEP SNPs rs2167270 and rs7799039. Leptin levels were significantly increased in obese individuals (p < 0.001) and non-obese or obese women (p < 0.001). In the dominant model, recoded minor alleles of rs2167270 and rs7799039 were associated with higher leptin levels in all individuals (p = 0.011, p = 0.012). Further, the association between these LEP SNPs and leptin levels was significant only in obese women (p = 0.025 and p = 0.008, respectively). There was an interaction effect between sex and obesity, particularly among obese women (interaction p = 0.04 and 0.02, respectively). Our findings demonstrate that these SNPs have sex-specific associations with BMI in IVDD patients, and that obesity and sex, particularly among obese women, may modify the LEP transcription effect.
1. Introduction
Disc degeneration is a multifaceted chronic process that alters the structure and function of intervertebral discs (IVD) [1]. Degenerated discs occur in 40% of individuals under 30 years of age, and in more than 90% of those over 50 [2]. IVD degeneration (IVDD) may lead to disc herniation, radiculopathy, myelopathy, spinal stenosis, and/or degenerative spondylolisthesis, and can cause acute or chronic pain. IVDD manifests as different cellular and biochemical alterations, including degradation of the extracellular matrix (ECM), the buildup of cellular waste products, and an increase in the expression of pro-inflammatory cytokines [3]. Risk factors for IVDD include aging, genetics, nutrition, toxins, metabolic disorders, low-grade infections, neurogenic inflammation, autoimmune diseases, and mechanical factors [4]. Obesity is a prevalent condition in both middle- and high-income countries, and results from genetic and environmental factors. It is recognized as a systemic inflammatory state mediated by adipokines [5] and is also a known mechanical risk factor for IVDD. Furthermore, IVDD is an indicator of obesity that decreases life expectancy by ≥20% of the ideal value.
Leptin is a hormone that suppresses food intake and increases energy expenditure by binding to and activating its specific receptor in the hypothalamus [6,7]. The adipokine leptin (a 16-kDa peptide hormone) was first discovered in 1994 by Zhang et al. [8]. Leptin is released from white adipose tissue (WAT), and circulating leptin is therefore positively correlated with body fat and body mass [9,10], which increases with age and is higher in females than in males [11]. It is also commonly measured to elucidate the pathogenesis of IVDD [12,13,14,15].
Genetics is an important factor in determining the individual risk of developing disc degeneration. Traditionally, occupation, physical activity, mechanical injury, smoking, repetitive loads, gender, and vibration are dominant risk factors for accelerated degeneration [16,17,18,19,20,21]. While the influence of genetics is unclear [20], many twin studies have identified positive familial aggregation, suggesting a degree of genetic influence. In a study by Simmons et al. [22], the results showed that 44.6% of patients who underwent surgery for degenerated discs had a positive family history of degeneration, compared to 25.4% who did not. Sambrook et al. reported similar results in a study involving 172 monozygotic and 154 dizygotic twins [23]. This suggests that in addition to environmental risk factors, genetics is also an important factor in determining disc degeneration variation, which implies that disc degeneration development is possibly determined by a complex combination of factors, with gene–environment and gene–gene interactions that uniquely determine the degeneration progression in each individual [24]. In this study, we examined whether the genetic and environmental effects of LEP SNPs are associated with IVDD.
2. Results
2.1. Clinical and Biochemical Characteristics
Among the 303 IVDD patients recruited for analysis, the results of the association of age, BMI, smoking, and leptin levels demonstrated that lower smoking frequencies and higher leptin levels were observed in women (Table 1). Furthermore, the obesity statuses showed that leptin levels were significantly higher in obese individuals (19.91 ± 19.11) compared with non-obese individuals (p < 0.001); in non-obese women (10.19 ± 7.52) compared with non-obese men (p < 0.001); and in obese women (26.59 ± 21.54) compared with obese men (p < 0.001). In the baseline characteristics regarding sex and obesity of the study subjects, the frequency of smoking was significantly lower in non-obese women (Table 2).

Table 1.
Baseline characteristics of study subjects.

Table 2.
Baseline characteristics of study subjects in relation to sex and obesity status.
2.2. Associations of LEP Polymorphisms with Respective Circulation Levels in Different Sex and Obesity Statuses
The association analyses in the additive and dominant models were adjusted for age, sex, BMI, and smoking status according to the different group selections. In the dominant model, the recoded minor alleles of rs2167270 (GA + AA) and rs7799039 (AG + GG) were associated with higher leptin levels in all individuals (12.87 ± 17.25, p = 0.011 and 12.28 ± 16.42, p = 0.012). The levels among women were 19.93 ± 21.42, p = 0.049 and 19.05 ± 20.48, p = 0.02, respectively (Table 3). We further stratified the individuals into four groups to analyze the association between LEP polymorphisms and leptin levels with respect to sex and obesity status. A subgroup analysis of sex according to obesity status showed that in the dominant model, the associations between LEP polymorphisms and leptin levels were significant only among obese women (rs2167270, 33.25 ± 27.83, p = 0.025; rs7799039, 33.23 ± 26.85, p = 0.008; Table 4). When subgroup analysis was performed on obesity according to sex, the associations between LEP polymorphisms and leptin levels were also only significant among obese women in the dominant model (Supplemental Table S1).

Table 3.
Association of LEP SNPs with leptin levels in relation to sex and obesity status.

Table 4.
Association between LEP SNPs and leptin levels in sex according to obesity status.
2.3. Interaction Analysis
The results demonstrated an interaction effect between sex and obesity within the LEP polymorphism and leptin level association in the dominant model of rs2167270 and rs7799039, particularly among obese women (interaction p = 0.04 and 0.02, respectively; Figure 1a,b). However, there was no significant interaction effect in the group of obesity according to sex (interaction p = 0.46 and 0.28, respectively; Supplemental Figure S1a,b).
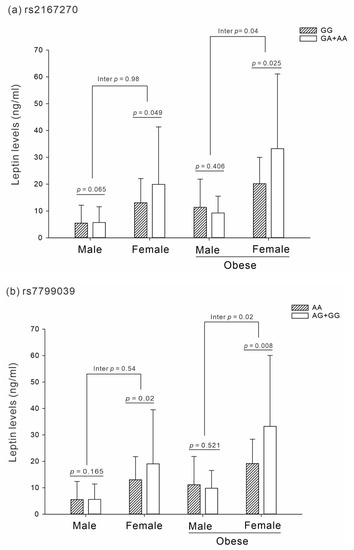
Figure 1.
Association and interaction analysis between LEP SNPs and leptin levels in sex according to obesity status (p adjusted for age and smoking status). (a) Interaction effect of rs2167270 (p = 0.04). (b) Interaction effect of rs7799039 (p = 0.02).
3. Discussion
Our study provides evidence that leptin levels are associated with LEP SNPs in patients with IVDD, particularly among obese women. Leptin is an adipokine found in adipose tissue and is mostly produced in white adipose tissue, although it is also produced elsewhere such as in IVD cells [14,25,26,27,28]. Leptin has pleiotropic functions, contributes to obesity-associated chronic low-grade inflammation, and plays an important role in IVDD pathophysiology [29].
Leptin plays an important role in innate immunity, and has been shown to have direct pro-inflammatory and catabolic effects on cartilage in experimental models. It also stimulates the production of pro-inflammatory cytokines, which mediate the signaling of, inter alia, macrophages, monocytes, and dendritic cells. Several studies have demonstrated that leptin and IVDD are influenced by both sex and obesity. Krishnamoorthy et al. used a dietary mouse model to test the hypothesis that chronic consumption of diets high in advanced glycation end-products (AGEs) results in sex-specific IVD structural disruption and functional changes. They found that a high-AGE diet resulted in AGE accumulation in IVDs, as well as increased IVD compressive stiffness, decreased torque range, and increased torque failure, particularly in females [30]. Natelson et al. used in vivo diabetic and dietary mouse models to investigate whether obesity and type 2 diabetes result in spinal pathology in a sex-specific manner. They found that obesity and diabetes due to impaired leptin signaling contribute to pathological changes in the vertebrae as well as an immature IVD phenotype, particularly in females, suggesting a sex-dependent role of leptin [31]. Our previous study on IVDs from 182 patients with IVDD (mean age, 57 years) demonstrated that BMI was positively correlated with the histologic degeneration score, the plasma leptin level, and the ratio of leptin and MMP-1 immunostaining grade [32].
The etiology of IVDD as a multifactorial disease includes genetic predisposition and exposure to environmental factors, of which genetic predispositions over the past decade have been shown to be more dominant. While certain genetic factors of IVDD have been identified, most of them are unknown [33,34,35,36], and thus, the genetic mechanisms underlying IVDD remain poorly understood. Genetic modulations associated with human disc degeneration or back pain are separated by gene function, such as structural enzymes that cleave extracellular-matrix molecules and inflammatory mediators [37]. In fact, many SNPs have been reported in several studies to be associated with IVDD, such as MMP1 and MMP3 in the degeneration of different matrix components [38]; COL1, 2, 9, and 11 in the degradation or loss of collagen [33,39,40,41,42]; VDR and CLIP in the degradation or loss of proteoglycan [43,44]; and IL-1, IL-6 and COX2 in the increase of inflammation [45,46].
Herein, we found that two LEP SNPs were associated with leptin levels in patients with IVDD, which had not been reported prior to our study. However, as described above, leptin is strongly associated with IVDD because of its role in pro-inflammation [30,31,32]. Moreover, the two SNPs identified in our study had previously been extensively reported to be strongly associated with diabetes mellitus (DM) and obesity [47,48,49,50,51]. LEP rs7799039 promoter polymorphism is close to the specificity protein-1 (SP-1) transcription factor binding site [52]. Hoffstedt et al. reported that nuclear extracts derived from human adiposities could bind a DNA fragment spanning the 2548G/A polymorphic site [53]. Thus, it is possible that leptin rs7799039 polymorphism could affect LEP gene transcription and expression, thereby affecting leptin synthesis and secretion from adipose tissue [53,54]. Aly et al. conducted a study to assess the potential role of leptin and its polymorphisms as predictive markers of diabetes associated with obesity. They concluded that increased leptin levels could predict insulin resistance in obese patients. Moreover, obese subjects with the mutant genotype LEP gene (rs2167270) G > A showed a significantly higher susceptibility rate for DM than those with the wild type [47]. Dasgupta et al. evaluated the association between obesity and leptin gene polymorphisms and levels in a South Indian population. They found that the LEP SNPs rs7799039 and rs2167270 were independently and significantly associated with the risk of obesity [51].
DM and obesity are common risk factors for IVDD. Our findings imply that LEP SNPs may affect IVDD due to the mechanical stress of obesity and leptin expression induced by inflammation in adipose tissue. Interestingly, our interaction analysis found that obesity status and sex had a significant interactive effect in LEP SNPs related to leptin levels in patients with IVDD. We previously reported that the LEP SNPs rs7799039 and rs2167270 were significantly associated with leptin levels in obese women. Further univariate analysis demonstrated that both LEP SNPs and inflammation markers, such as CRP and E-selectin, are independently associated with leptin levels [55]. Moreover, Bains et al. conducted a case–control study in an Indian population, and reported that rs7799039 significantly increased the risk of DM in females with a BMI ≥ 23 [48]. Pawlik et al. also examined the association between leptin gene polymorphisms and the development of gestational diabetes mellitus, and found that the LEP rs2167270 A allele was significantly associated with GDM in women [50].
In this study, we observed a relationship between leptin variants and IVDD risk among women, which raised new questions regarding the mechanisms by which leptin and leptin gene variants might affect IVDD pathways. The mechanisms underlying sex heterogeneity observed in the aforementioned studies remain unclear. However, there are two factors that provide possible explanations. Firstly, there are varying adipokine levels between sexes, where women have higher levels of adiponectin and leptin compared to men, which thereby likely contributed to the null finding among women [56]. Secondly, the body fat distribution variations between the sexes may affect leptin levels and their effects on IVDD risk. Moreover, previous studies have suggested that sex-specific leptin levels and IVDD risk associations may be involved in the functional cross-talk between leptin and estrogen systems [31].
Several studies have described the sex-specific distribution of adipose tissue. Females have more abundant subcutaneous white adipose tissue (sWAT), whereas males have more abdominal-visceral depots [57,58]. sWAT is mainly located in the gluteal and femoral regions, and is associated with optimal metabolic health. For instance, sWAT expansion in humans is linked with improved insulin sensitivity, diminished lipolysis rate, decreased circulation of cytokines, and augmented levels of adipokines [59]. Notably, the protective role of sWAT in females seems to be age-dependent, as postmenopausal women suffer from fat redistribution where the fat depots from subcutaneous regions are transferred to the visceral regions [60]. Thus, sex hormones may play a critical role in sex-specific fat distribution and overall metabolic health. Among the sex hormones, estrogen is particularly important, as it has been shown that its decreased circulation contributes to increased adiposity, insulin resistance, low metabolic rate, and adipose tissue inflammation [61,62,63]. Moreover, the decrease in estrogen levels that comes with menopause is associated with higher risk of metabolic complications and body weight gain. Such anti-obesity effects of estrogen and its role in energy homeostasis are well established. Although Hong et al. demonstrated that male mice, compared to female mice, were more susceptible to increased body fat [62], other studies have shown that women tend to experience weight gain after menopause [64]. Estrogen has also been reported to be a key determinant of serum leptin levels and central leptin sensitivity. In diabetic Akita female mice carrying the Ins2 mutation, ERa ablation was found to exacerbate hyperphagia by further decreasing central leptin signals and downregulating POMC gene expression [65]. OVX rats displayed dramatic increases in serum leptin levels, which were associated with significant changes in body weight gain. After E2 replacement, the serum leptin levels decreased [66,67]. Both ob/ob and db/db mice treated with E2 for four weeks showed body weight loss, diminished fat mass, hypophagia, and energy expenditure. This was accompanied by elevated hypothalamic pSTAT3 levels and increased POMC immune reactivity [68]. These obesity markers were also significantly correlated with serum leptin levels, which suggests that circulating leptin could mediate the regulatory effects of estrogen signaling on adipose tissue homeostasis [69]. Similarly, estrogen has been demonstrated to have an effect on BAT thermogenesis, thermoregulation, cold adaptation, and energy expenditure [63,70,71]. Therefore, we suggest that the association among obese women of the LEP SNPs rs2167270 and rs7799039 with higher leptin levels in IVDD risk may be due to the post-menopausal loss of estrogen exposure and the loss of estrogen’s protective function in the downregulation of leptin expression.
There were several limitations to our study. Firstly, there was a relatively low number of genotyped subjects, and thus, replication of our results in a second cohort would support the strength of the study. Furthermore, independent association studies with larger sample sizes would not only corroborate our results, but would also allow for more definitive conclusions to be drawn. Secondly, the study sample included only Taiwanese individuals, and thus, the results cannot be generalized to other ethnicities. Given the variability of LEP variants and leptin levels between ethnicities, further research on different populations is required.
4. Materials and Methods
4.1. Study Population
This prospective study was approved by the local research ethics committee (IRB No. 05-XD31-061). Informed consent was obtained from all patients. During the period from March 2017 to June 2018, this study enrolled a total of 303 cervical and lumbar patients (mean age, 58.6 ± 12.7 years) undergoing cervical discectomy for neck pain with pain radiating to the upper limbs, or treated with lumbar discectomy for back pain with radicular pain to the legs. The exclusion criteria were fracture of the spine, spinal stenosis, spondylolisthesis, malignancies involving the spine, and poliomyelitis. The criteria for IVD degeneration were: (1) neck or back pain requiring visits to a physician; (2) pain problems that hampered or prevented daily activities; and (3) multiple episodes of pain. BMI ≥ 25 kg/m2 was classified as obese.
4.2. Enzyme-Linked Immunosorbent Assay
Venous blood was collected in the morning after an overnight fast. Plasma samples were obtained via centrifugation at 3000× g for 15 min at 4 °C. Immediately after centrifugation, plasma samples were frozen and stored at −80 °C until the time of analysis. Circulating plasma levels of leptin were measured using commercially available ELISA kits (R&D, Minneapolis, MN, USA).
4.3. Genomic DNA Extraction and Genotyping
Genomic DNA was extracted as reported previously [72]. Two SNPs around LEPs, rs7799039 (HGVS nomenclature: NC_000007.13:g.127878783A > G) and rs2167270 (HGVS nomenclature: NM_000230.2:c.-39G > A), were selected. Genotyping was performed using TaqMan SNP with genotyping assays (Thermo Fisher SCIENTIFIC, Waltham, MA USA).
4.4. Statistical Analysis
An independent samples t-test was performed for continuous variables. A Chi-square test was used to analyze categorical variables. The continuous variables, expressed as mean ± standard deviation, were tested using one-way analysis of variance (ANOVA). Tests of normality were conducted for all quantitative traits. Moreover, the leptin levels were logarithmically transformed before statistical analysis to adhere to a normality assumption. A p of <0.05 according to a two-sided test was considered statistically significant. Linear regression coefficients with 95% confidence intervals were calculated for leptin levels and the predicted confounders. Allelic frequencies for each SNP were estimated through gene counting, and the polymorphism distribution was tested for Hardy–Weinberg equilibrium using the Chi-square test. The stratified association analysis according to sex and obesity was performed using one-way ANOVA in additive and dominant genetic models. The statistical analysis was performed using IBM SPSS Statistics (version 22; IBM) unless otherwise specified. Furthermore, we investigated the sex- and obesity-specific effects of leptin level variants. The resultant significant polymorphisms were included in the interaction analysis using a linear regression model of SVS Win32 (version 7.3.1; Golden Helix, Bozeman, MT, USA) to determine the impact of dependent variables.
5. Conclusions
To our knowledge, this is the first study on the possible association between LEP rs7799039 and rs2167270 polymorphisms within a Taiwanese population. This study found that the frequency of IVDD is expected to be higher in the A allele of rs2167270 and the G allele of rs7799039 carriers. Our previous study disclosed that BMI is significantly associated with the HDS (histological degeneration score) [32], indicating that obesity is associated with IVDD severity. In addition, serum leptin levels in patients with IVDD are significantly related to BMI. As the number of enrolled patients with IVDD increased in the present study, the association of serum leptin levels with BMI became more significant. Based on these findings, serum leptin levels may indirectly affect IVDD, suggesting a possible influence of the LEP variant on IVDD. It also indicated that being overweight may have a modifying effect on SNP associations, which leads to a loss of the protective effect attributed to the alleles on the studied genes. However, based on a recent review by Curic, we cannot conclude whether detrimental and beneficial effects of the LEP variant exist in IVDD [73]. We suggest that detailed functional studies should be performed to investigate and understand the role of these SNPs in patients with IVDD, as well as the SNPs’ association with serum leptin levels.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012275/s1.
Author Contributions
Conceptualization, M.-S.T. and H.-T.H.; methodology, H.-H.C. and M.-S.T.; software and validation, M.-S.T. and M.-H.L.; formal analysis, M.-S.T. and M.-H.L.; resources, H.-T.H. and H.-H.C.; data curation, M.-S.T. and H.-H.C.; writing—original draft preparation, H.-T.H., H.-H.C. and M.-S.T.; writing—review and editing, M.-S.T.; visualization, H.-T.H. and H.-H.C.; project administration, M.-S.T. and H.-T.H.; supervision, M.-S.T.; funding acquisition, M.-S.T. and H.-T.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD-TPE-111-12(1/2)); grants from the Ministry of Science and Technology (MOST 109-2314-B-303-021-MY2, MOST 111-2314-B-303-002) to M.-S.T; and grants from the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (TCRD-TPE-109-56(1/3),(2/3),(3/3)) to H.-T.H.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Research Ethics Committee of Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (approval number: 05-XD31-061).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We greatly appreciate the technical support from the Core Laboratory of the Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, and expert statistical analysis assistance from I-Shiang Tzeng and Tsung-Han Hsieh.
Conflicts of Interest
No potential conflict of interest relevant to this article were reported.
References
- Adams, M.A.; Roughley, P.J. What is intervertebral disc degeneration, and what causes it? Spine 2006, 31, 2151–2161. [Google Scholar] [CrossRef]
- Cheung, K.M.; Karppinen, J.; Chan, D.; Ho, D.W.; Song, Y.Q.; Sham, P.; Cheah, K.S.; Leong, J.C.; Luk, K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine 2009, 34, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Smith, S.; Fairbank, J.C. Nutrition of the intervertebral disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Hadjipavlou, A.G.; Tzermiadianos, M.N.; Bogduk, N.; Zindrick, M.R. The pathophysiology of disc degeneration: A critical review. J. Bone Jt. Surg. Br. 2008, 90, 1261–1270. [Google Scholar] [CrossRef]
- Sharma, A. The Role of Adipokines in Intervertebral Disc Degeneration. Med. Sci. 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Woods, A.J.; Stock, M.J. Leptin activation in hypothalamus. Nature 1996, 381, 745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Bribiescas, R.G.; Hickey, M.S. Population variation and differences in serum leptin independent of adiposity: A comparison of Ache Amerindian men of Paraguay and lean American male distance runners. Nutr. Metab. 2006, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Kelesidis, T.; Kelesidis, I.; Chou, S.; Mantzoros, C.S. Narrative review: The role of leptin in human physiology: Emerging clinical applications. Ann. Intern. Med. 2010, 152, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Linseisen, J.; Wolfram, G.; Himmerich, S.; Gedrich, K.; Pollmächer, T.; Himmerich, H. Leptin plasma levels in the general population: Influence of age, gender, body weight and medical history. Protein Pept. Lett. 2010, 17, 1436–1440. [Google Scholar] [CrossRef]
- Li, Z.; Liang, J.; Wu, W.K.; Yu, X.; Yu, J.; Weng, X.; Shen, J. Leptin activates RhoA/ROCK pathway to induce cytoskeleton remodeling in nucleus pulposus cells. Int. J. Mol. Sci. 2014, 15, 1176–1188. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Wu, W.K.; Yu, X.; Liang, J.; Qiu, G.; Liu, J. Leptin induces cyclin D1 expression and proliferation of human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK pathways. PLoS ONE 2012, 7, e53176. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Ingram, J.A.; Hoelscher, G.L.; Hanley, E.N., Jr. Leptin expression by annulus cells in the human intervertebral disc. Spine J. 2007, 7, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhao, C.; Cao, L.; Zhang, K.; Sun, W.; Xie, Y.; Li, H.; Zhao, J. Leptin induces terminal differentiation of rat annulus fibrosus cells via activation of MAPK signaling. Anat. Rec. 2013, 296, 1806–1812. [Google Scholar] [CrossRef] [PubMed]
- Luoma, K.; Riihimäki, H.; Raininko, R.; Luukkonen, R.; Lamminen, A.; Viikari-Juntura, E. Lumbar disc degeneration in relation to occupation. Scand. J. Work. Environ. Health 1998, 24, 358–366. [Google Scholar] [CrossRef]
- Videman, T.; Sarna, S.; Battié, M.C.; Koskinen, S.; Gill, K.; Paananen, H.; Gibbons, L. The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine 1995, 20, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Cinotti, G.; Della Rocca, C.; Romeo, S.; Vittur, F.; Toffanin, R.; Trasimeni, G. Degenerative changes of porcine intervertebral disc induced by vertebral endplate injuries. Spine 2005, 30, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Battié, M.C.; Videman, T.; Gill, K.; Moneta, G.B.; Nyman, R.; Kaprio, J.; Koskenvuo, M. 1991 Volvo Award in clinical sciences. Smoking and lumbar intervertebral disc degeneration: An MRI study of identical twins. Spine 1991, 16, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Battié, M.C.; Videman, T. Lumbar disc degeneration: Epidemiology and genetics. J. Bone Jt. Surg. Am. 2006, 88 (Suppl. S2), 3–9. [Google Scholar] [CrossRef]
- Yuan, H.Y.; Tang, Y.; Liang, Y.X.; Lei, L.; Xiao, G.B.; Wang, S.; Xia, Z.L. Matrix metalloproteinase-3 and vitamin d receptor genetic polymorphisms, and their interactions with occupational exposure in lumbar disc degeneration. J. Occup. Health 2010, 52, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Simmons, E.D., Jr.; Guntupalli, M.; Kowalski, J.M.; Braun, F.; Seidel, T. Familial predisposition for degenerative disc disease. A case-control study. Spine 1996, 21, 1527–1529. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, P.N.; MacGregor, A.J.; Spector, T.D. Genetic influences on cervical and lumbar disc degeneration: A magnetic resonance imaging study in twins. Arthritis Rheum. 1999, 42, 366–372. [Google Scholar] [CrossRef]
- Kalichman, L.; Hunter, D.J. The genetics of intervertebral disc degeneration. Associated genes. Jt. Bone Spine 2008, 75, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Segar, A.; Urban, J.; Fairbank, J.C. Adipokines and the Intervertebral Disc: A Biochemical Link Exists between Obesity, Intervertebral Disc Degeneration and Low Back Pain. Spine J. 2016, 16, S225. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Liu, D.; Li, H.; Jiang, L.S.; Dai, L.Y. Expression of leptin and its functional receptor on disc cells: Contribution to cell proliferation. Spine 2008, 33, E858–E864. [Google Scholar] [CrossRef]
- Segar, A.H.; Fairbank, J.C.T.; Urban, J. Leptin and the intervertebral disc: A biochemical link exists between obesity, intervertebral disc degeneration and low back pain-an in vitro study in a bovine model. Eur. Spine J. 2019, 28, 214–223. [Google Scholar] [CrossRef]
- Krishnamoorthy, D.; Hoy, R.C.; Natelson, D.M.; Torre, O.M.; Laudier, D.M.; Iatridis, J.C.; Illien-Jünger, S. Dietary advanced glycation end-product consumption leads to mechanical stiffening of murine intervertebral discs. Dis. Model. Mech. 2018, 11, dmm036012. [Google Scholar] [CrossRef]
- Natelson, D.M.; Lai, A.; Krishnamoorthy, D.; Hoy, R.C.; Iatridis, J.C.; Illien-Jünger, S. Leptin signaling and the intervertebral disc: Sex dependent effects of leptin receptor deficiency and Western diet on the spine in a type 2 diabetes mouse model. PLoS ONE 2020, 15, e0227527. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.T.; Yue, C.T.; Teng, M.S.; Tzeng, I.S.; Li, T.C.; Tai, P.A.; Huang, K.F.; Chen, C.Y.; Ko, Y.L. Immuohistochemical score of matrix metalloproteinase-1 may indicate the severity of symptomatic cervical and lumbar disc degeneration. Spine J. 2020, 20, 124–137. [Google Scholar] [CrossRef]
- Feng, Y.; Egan, B.; Wang, J. Genetic Factors in Intervertebral Disc Degeneration. Genes Dis. 2016, 3, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hanaei, S.; Abdollahzade, S.; Khoshnevisan, A.; Kepler, C.K.; Rezaei, N. Genetic aspects of intervertebral disc degeneration. Rev. Neurosci. 2015, 26, 581–606. [Google Scholar] [CrossRef] [PubMed]
- Kitis, S.; Coskun, Z.M.; Tasdemir, P.; Tuncez, E.; Zamani, A.G.; Acar, A. Analysis of genetic polymorphisms associated with intervertebral disc degeneration. Cell. Mol. Biol. 2018, 64, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.A.; Dos Santos, A.A.; Peluso, C.; Barbosa, C.P.; Bianco, B.; Rodrigues, L.M.R. Influence of lifestyle characteristics and VDR polymorphisms as risk factors for intervertebral disc degeneration: A case-control study. Eur. J. Med. Res. 2018, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, I.; Lötsch, J. Current evidence for a modulation of low back pain by human genetic variants. J. Cell. Mol. Med. 2009, 13, 1605–1619. [Google Scholar] [CrossRef]
- Goupille, P.; Jayson, M.I.; Valat, J.P.; Freemont, A.J. Matrix metalloproteinases: The clue to intervertebral disc degeneration? Spine 1998, 23, 1612–1626. [Google Scholar] [CrossRef]
- Jones, G.; White, C.; Sambrook, P.; Eisman, J. Allelic variation in the vitamin D receptor, lifestyle factors and lumbar spinal degenerative disease. Ann. Rheum. Dis. 1998, 57, 94–99. [Google Scholar] [CrossRef]
- Roughley, P.; Martens, D.; Rantakokko, J.; Alini, M.; Mwale, F.; Antoniou, J. The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur. Cell. Mater. 2006, 11, 1–7. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Osada, R.; Kanamori, M.; Ishihara, H.; Ohmori, K.; Matsui, H.; Kimura, T. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine 1999, 24, 2456–2460. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Nakata, K.; Tsumaki, N.; Miyamoto, S.; Matsui, Y.; Ebara, S.; Ochi, T. Progressive degeneration of articular cartilage and intervertebral discs. An experimental study in transgenic mice bearing a type IX collagen mutation. Int. Orthop. 1996, 20, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Pękala, P.A.; Jasińska, M.; Taterra, D.; Skoczen, K.M.; Jarosz, A.; Konopka, T.; Loukas, M.; Walocha, J.A.; Tomaszewski, K.A.; Lis, G. Vitamin D receptor gene polymorphism influence on lumbar intervertebral disc degeneration. Clin. Anat. 2022, 35, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Kawaguchi, Y.; Chiba, K.; Mikami, Y.; Kizawa, H.; Oya, T.; Mio, F.; Mori, M.; Miyamoto, Y.; Masuda, I.; et al. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat. Genet. 2005, 37, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Eskola, P.J.; Kjaer, P.; Daavittila, I.M.; Solovieva, S.; Okuloff, A.; Sorensen, J.S.; Wedderkopp, N.; Ala-Kokko, L.; Männikkö, M.; Karppinen, J.I. Genetic risk factors of disc degeneration among 12-14-year-old Danish children: A population study. Int. J. Mol. Epidemiol. Genet. 2010, 1, 158–165. [Google Scholar] [PubMed]
- Valdes, A.M.; Hassett, G.; Hart, D.J.; Spector, T.D. Radiographic progression of lumbar spine disc degeneration is influenced by variation at inflammatory genes: A candidate SNP association study in the Chingford cohort. Spine 2005, 30, 2445–2451. [Google Scholar] [CrossRef]
- Aly, O.; Zaki, H.H.; Herzalla, M.R.; Fathy, A.; Raafat, N.; Hafez, M.M. Gene polymorphisms of Patatin-like phospholipase domain containing 3 (PNPLA3), adiponectin, leptin in diabetic obese patients. PLoS ONE 2020, 15, e0234465. [Google Scholar] [CrossRef]
- Bains, V.; Kaur, H.; Badaruddoza, B. Association analysis of polymorphisms in LEP (rs7799039 and rs2167270) and LEPR (rs1137101) gene towards the development of type 2 diabetes in North Indian Punjabi population. Gene 2020, 754, 144846. [Google Scholar] [CrossRef]
- Daniels, T.E.; Sadovnikoff, A.I.; Ridout, K.K.; Lesseur, C.; Marsit, C.J.; Tyrka, A.R. Associations of maternal diet and placenta leptin methylation. Mol. Cell. Endocrinol. 2020, 505, 110739. [Google Scholar] [CrossRef]
- Pawlik, A.; Teler, J.; Maciejewska, A.; Sawczuk, M.; Safranow, K.; Dziedziejko, V. Adiponectin and leptin gene polymorphisms in women with gestational diabetes mellitus. J. Assist. Reprod. Genet. 2017, 34, 511–516. [Google Scholar] [CrossRef]
- Dasgupta, S.; Salman, M.; Siddalingaiah, L.B.; Lakshmi, G.L.; Xaviour, D.; Sreenath, J. Genetic variants in leptin: Determinants of obesity and leptin levels in South Indian population. Adipocyte 2015, 4, 135–140. [Google Scholar] [CrossRef]
- Gong, D.W.; Bi, S.; Pratley, R.E.; Weintraub, B.D. Genomic structure and promoter analysis of the human obese gene. J. Biol. Chem. 1996, 271, 3971–3974. [Google Scholar] [CrossRef] [PubMed]
- Hoffstedt, J.; Eriksson, P.; Mottagui-Tabar, S.; Arner, P. A polymorphism in the leptin promoter region (-2548 G/A) influences gene expression and adipose tissue secretion of leptin. Horm. Metab. Res. 2002, 34, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Schwartz, M.W. Genetics and pathophysiology of human obesity. Annu. Rev. Med. 2003, 54, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.M.; Jhang, J.Y.; Wu, S.; Teng, M.S.; Hsu, L.A.; Ko, Y.L. Modification effect of sex and obesity on the correlation of LEP polymorphisms with leptin levels in Taiwanese obese women. Mol. Genet. Genomic. Med. 2020, 8, e1113. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Wu, K.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Plasma adiponectin and soluble leptin receptor and risk of colorectal cancer: A prospective study. Cancer Prev. Res. 2013, 6, 875–885. [Google Scholar] [CrossRef]
- Lemieux, S.; Prud’homme, D.; Bouchard, C.; Tremblay, A.; Després, J.P. Sex differences in the relation of visceral adipose tissue accumulation to total body fatness. Am. J. Clin. Nutr. 1993, 58, 463–467. [Google Scholar] [CrossRef]
- Porter, J.W.; Barnas, J.L.; Welly, R.; Spencer, N.; Pitt, J.; Vieira-Potter, V.J.; Kanaley, J.A. Age, Sex, and Depot-Specific Differences in Adipose-Tissue Estrogen Receptors in Individuals with Obesity. Obesity 2020, 28, 1698–1707. [Google Scholar] [CrossRef]
- Stinkens, R.; Brouwers, B.; Jocken, J.W.; Blaak, E.E.; Teunissen-Beekman, K.F.; Hesselink, M.K.; van Baak, M.A.; Schrauwen, P.; Goossens, G.H. Exercise training-induced effects on the abdominal subcutaneous adipose tissue phenotype in humans with obesity. J. Appl. Physiol. 2018, 125, 1585–1593. [Google Scholar] [CrossRef]
- Bloor, I.D.; Symonds, M.E. Sexual dimorphism in white and brown adipose tissue with obesity and inflammation. Horm. Behav. 2014, 66, 95–103. [Google Scholar] [CrossRef]
- Martínez de Morentin, P.B.; González-García, I.; Martins, L.; Lage, R.; Fernández-Mallo, D.; Martínez-Sánchez, N.; Ruíz-Pino, F.; Liu, J.; Morgan, D.A.; Pinilla, L.; et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell. Metab. 2014, 20, 41–53. [Google Scholar] [CrossRef]
- Hong, J.; Stubbins, R.E.; Smith, R.R.; Harvey, A.E.; Núñez, N.P. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr. J. 2009, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.H.; Perfield, J.W., 2nd; Strissel, K.J.; Obin, M.S.; Greenberg, A.S. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology 2009, 150, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Stefanick, M.L.; Kritz-Silverstein, D.; Fineberg, S.E.; Waclawiw, M.A.; James, M.K.; Greendale, G.A. Effect of postmenopausal hormone therapy on body weight and waist and hip girths. Postmenopausal Estrogen-Progestin Interventions Study Investigators. J. Clin. Endocrinol. Metab. 1997, 82, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Hirosawa, M.; Minata, M.; Harada, K.H.; Hitomi, T.; Krust, A.; Koizumi, A. Ablation of estrogen receptor alpha (ERalpha) prevents upregulation of POMC by leptin and insulin. Biochem. Biophys. Res. Commun. 2008, 371, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Meli, R.; Pacilio, M.; Raso, G.M.; Esposito, E.; Coppola, A.; Nasti, A.; Di Carlo, C.; Nappi, C.; Di Carlo, R. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology 2004, 145, 3115–3121. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakaya, S.; Kumai, T.; Watanabe, M.; Tateishi, T.; Shimizu, H.; Kobayashi, S. Effects of estrogen on serum leptin levels and leptin mRNA expression in adipose tissue in rats. Horm. Res. 2001, 56, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Mezei, G.; Nie, Y.; Rao, Y.; Choi, C.S.; Bechmann, I.; Leranth, C.; Toran-Allerand, D.; Priest, C.A.; Roberts, J.L.; et al. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat. Med. 2007, 13, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Hur, J.Y.; Seo, H.S.; Jeong, Y.A.; Lee, J.K.; Oh, M.J.; Kim, T.; Saw, H.S.; Kim, S.H. The ratio of estrogen receptor alpha to estrogen receptor beta in adipose tissue is associated with leptin production and obesity. Steroids 2007, 72, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reis, F.; He, Y.; Park, J.W.; DiVittorio, J.R.; Sivakumar, N.; van Veen, J.E.; Maesta-Pereira, S.; Shum, M.; Nichols, I.; et al. Estrogen-sensitive medial preoptic area neurons coordinate torpor in mice. Nat. Commun. 2020, 11, 6378. [Google Scholar] [CrossRef]
- Yokota-Nakagi, N.; Takahashi, H.; Kawakami, M.; Takamata, A.; Uchida, Y.; Morimoto, K. Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats. Nutrients 2020, 12, 907. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.-S.; Hsu, L.-A.; Wu, S.; Chou, H.-H.; Chang, C.-J.; Sun, Y.-Z.; Juan, S.-H.; Ko, Y.-L. Mediation analysis reveals a sex-dependent association between ABO gene variants and TG/HDL-C ratio that is suppressed by sE-selectin level. Atherosclerosis 2013, 228, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Curic, G. Intervertebral Disc and Adipokine Leptin-Loves Me, Loves Me Not. Int. J. Mol. Sci. 2020, 22, 375. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).