Does Intrauterine Injection of hCG Improve IVF Outcome? A Systematic Review and a Meta-Analysis
Abstract
1. Introduction
1.1. Search Strategy
1.2. Study Selection
1.3. Study Outcomes
1.4. Data Extraction
1.5. Assessment of Both the Risk of Bias and Publication Bias
1.6. Statistical Analysis
1.7. Subgroup Analysis
2. Results and Discussion
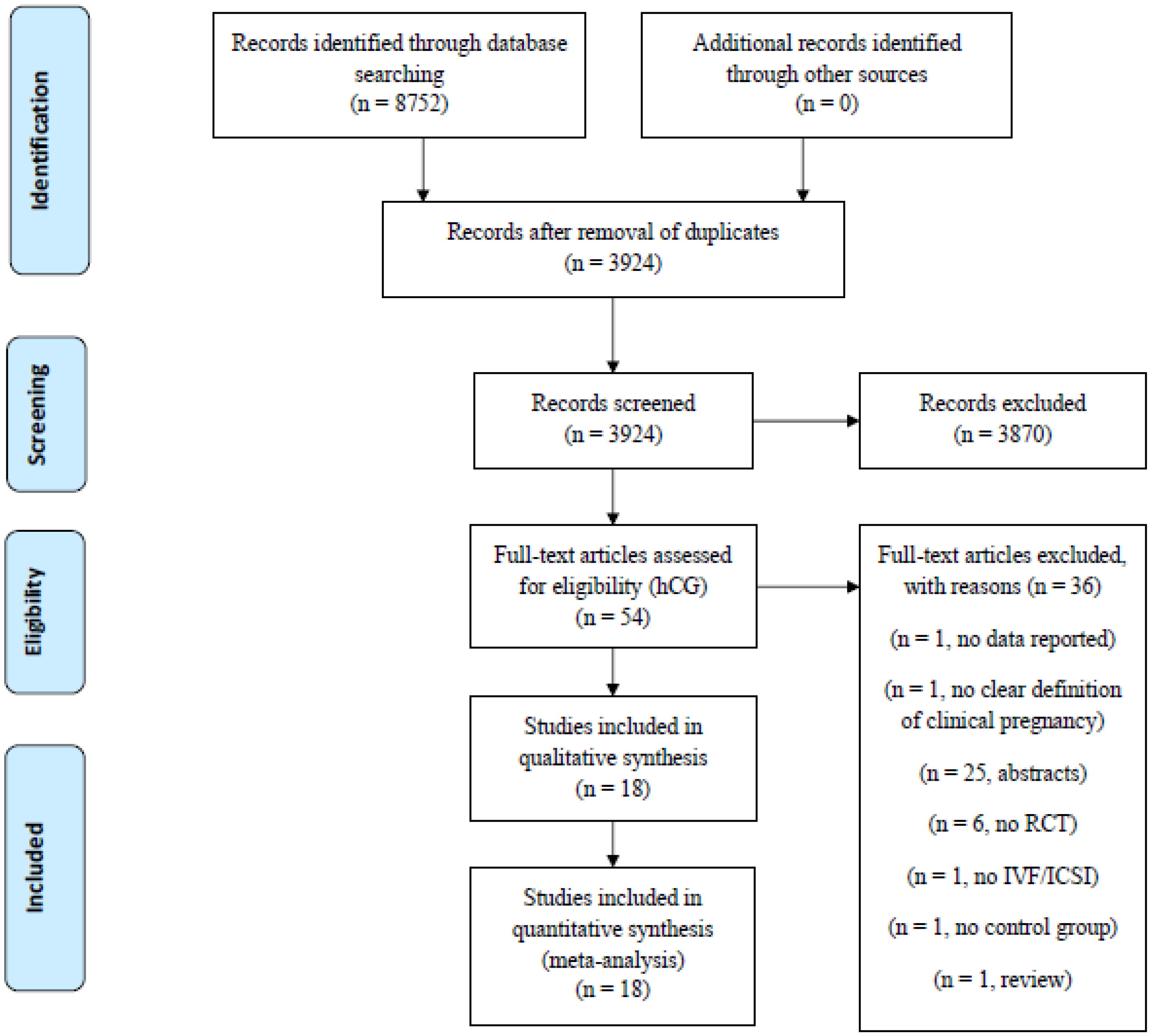
2.1. Study Selection and Characteristics
2.2. Risk of Bias within and across Study
2.3. Summary of Findings
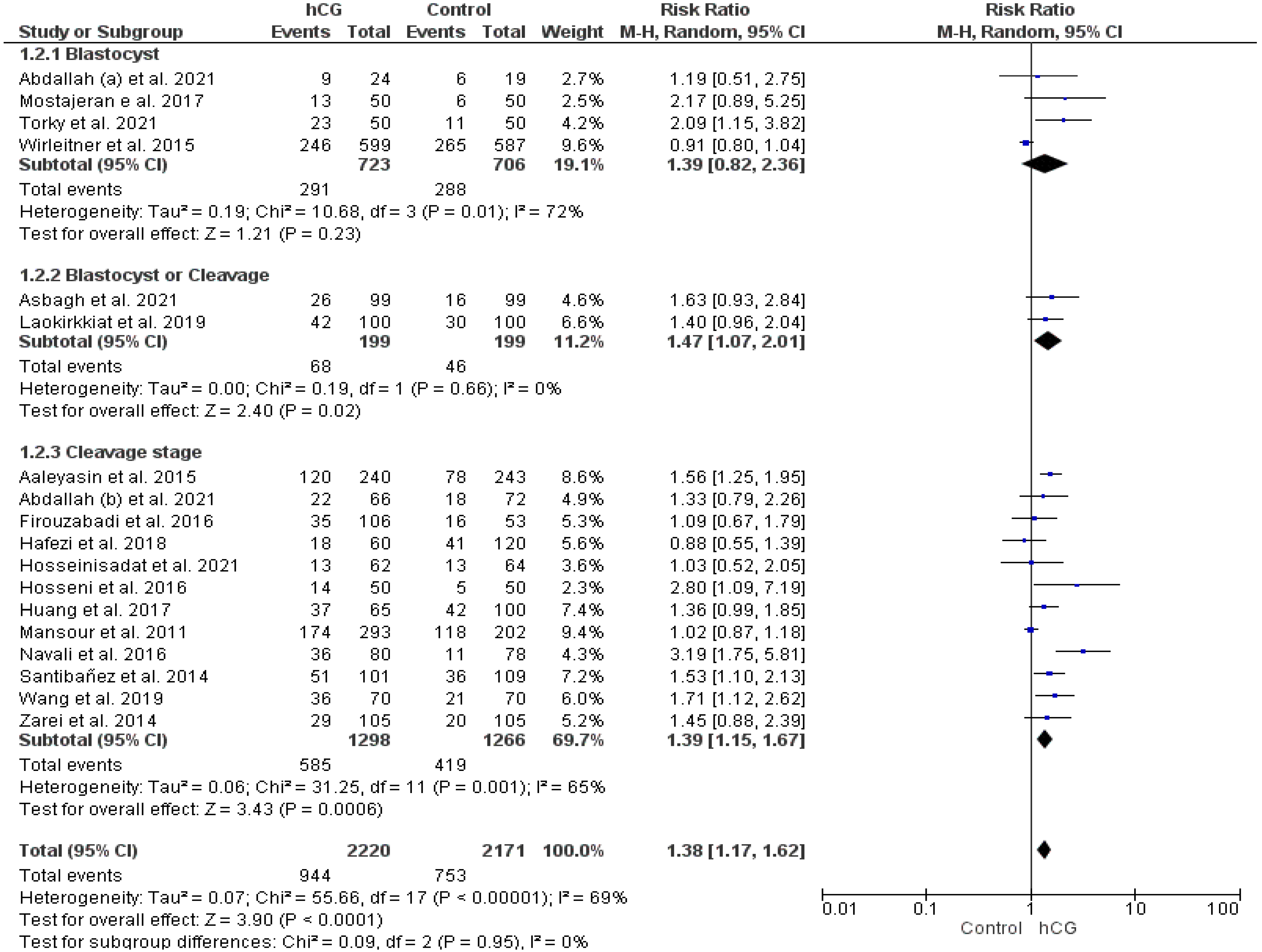
2.3.1. Clinical Pregnancy Rate
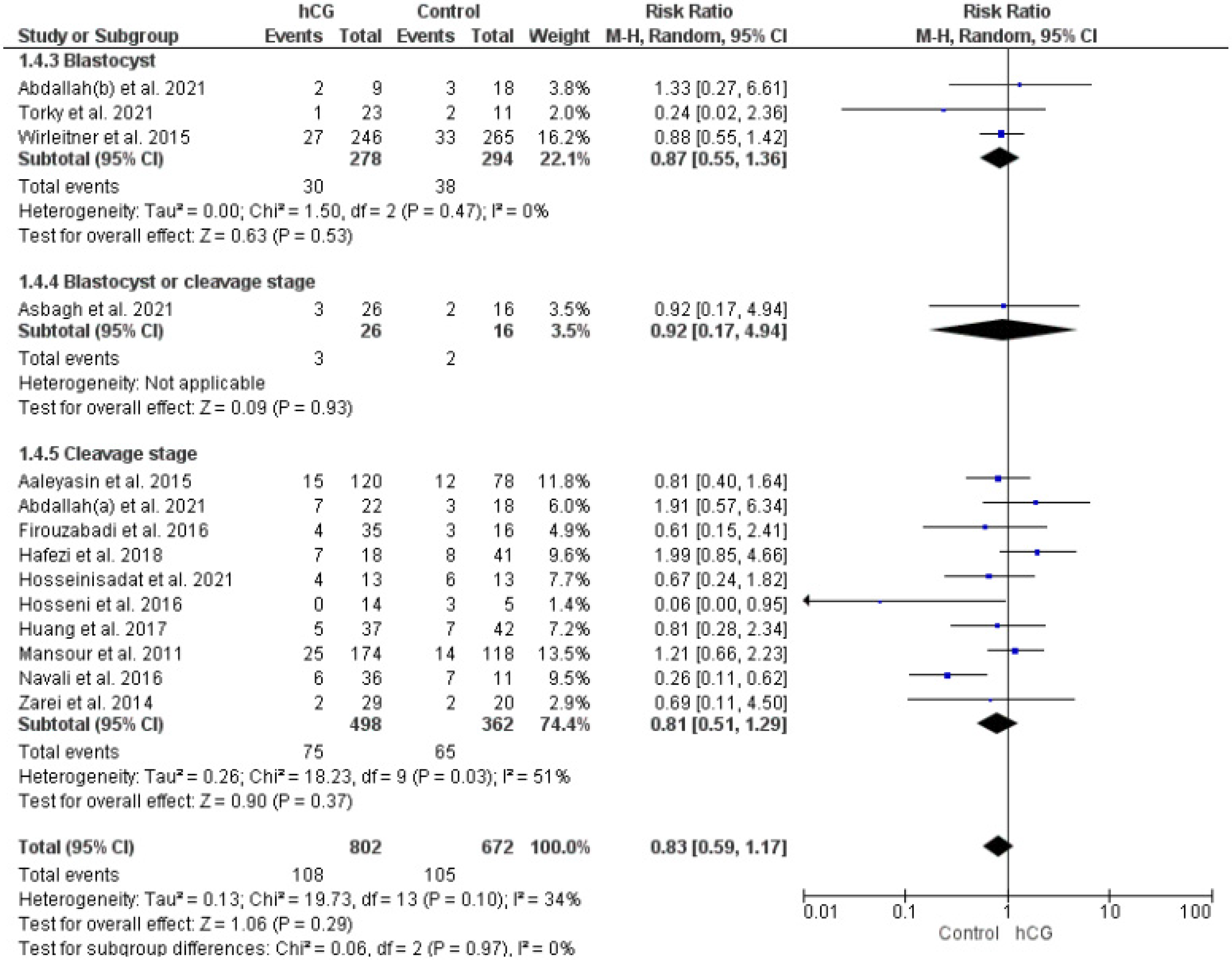
2.3.2. Miscarriage Rate
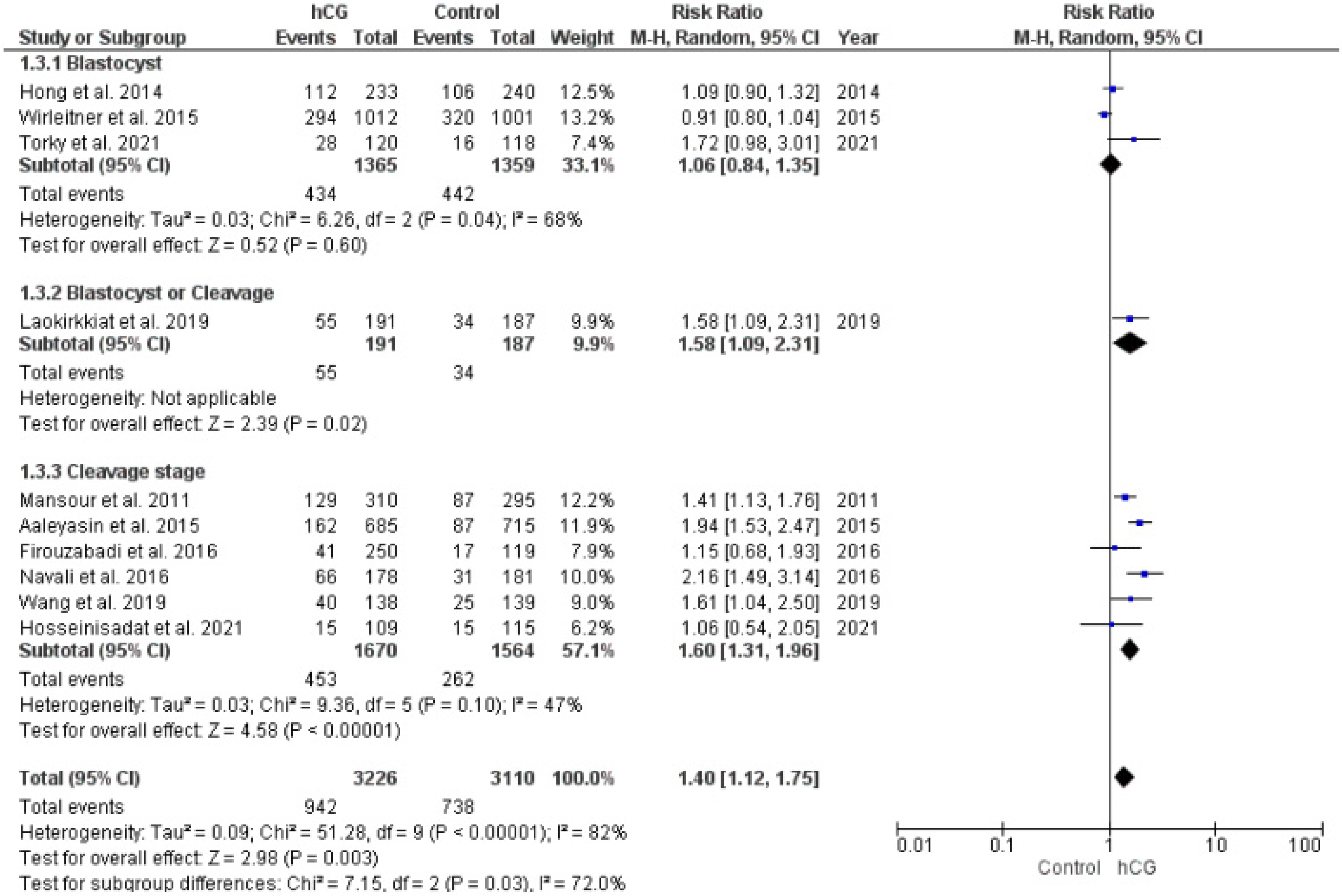
2.3.3. Implantation Rate
2.3.4. Live Birth Rate
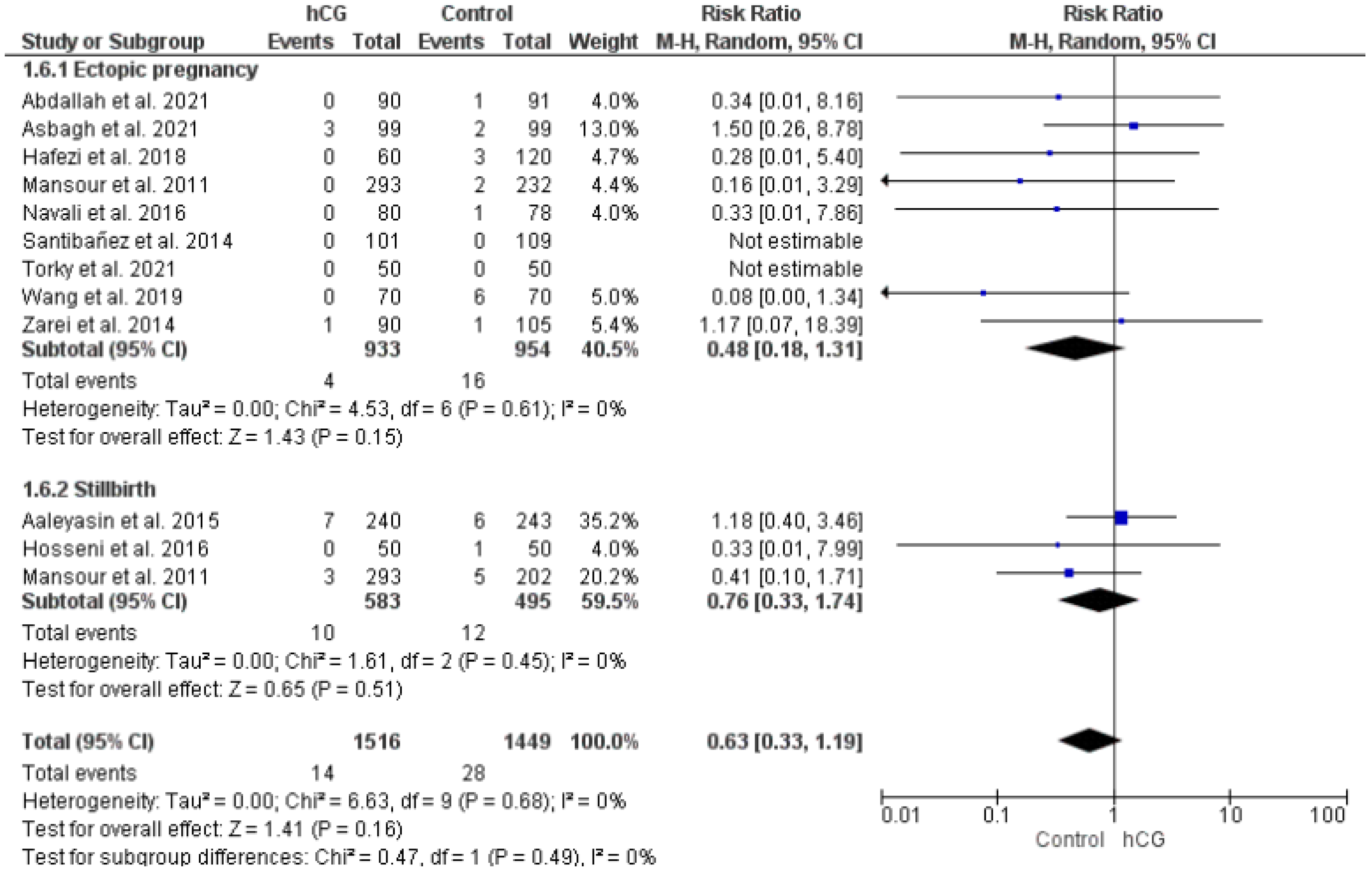
2.3.5. Ectopic Pregnancy and Stillbirth
2.4. Synthesis of Results
2.4.1. Summary of Evidence
2.4.2. Limitations
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achache, H.; Revel, A. Endometrial Receptivity Markers, the Journey to Successful Embryo Implantation. Hum. Reprod. Update 2006, 12, 731–746. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, E.R.; Schust, D.J.; Fisher, S.J. Implantation and the Survival of Early Pregnancy. N. Engl. J. Med. 2001, 345, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Schoolcraft, W.B.; Surrey, E.S.; Gardner, D.K. Embryo Transfer: Techniques and Variables Affecting Success. Fertil. Steril. 2001, 76, 863–870. [Google Scholar] [CrossRef]
- Laufer, N.; Simon, A. Recurrent Implantation Failure: Current Update and Clinical Approach to an Ongoing Challenge. Fertil. Steril. 2012, 97, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Busnelli, A.; Papaleo, E.; Del Prato, D.; La Vecchia, I.; Iachini, E.; Paffoni, A.; Candiani, M.; Somigliana, E. A Retrospective Evaluation of Prognosis and Cost-Effectiveness of IVF in Poor Responders According to the Bologna Criteria. Hum. Reprod. 2015, 30, 315–322. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nastri, C.O.; Lensen, S.F.; Gibreel, A.; Raine-Fenning, N.; Ferriani, R.A.; Bhattacharya, S.; Martins, W.P. Endometrial Injury in Women Undergoing Assisted Reproductive Techniques. Cochrane Database Syst. Rev. 2015, 22, CD009517. [Google Scholar] [CrossRef]
- Sfakianoudis, K.; Simopoulou, M.; Nitsos, N.; Lazaros, L.; Rapani, A.; Pantou, A.; Koutsilieris, M.; Nikas, Y.; Pantos, K. Successful Implantation and Live Birth Following Autologous Platelet-Rich Plasma Treatment for a Patient with Recurrent Implantation Failure and Chronic Endometritis. Vivo 2019, 33, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Andrisani, A.; Alviggi, C.; Vitale, S.G.; Valenti, G.; Sapia, F.; Favilli, A.; Martins, W.P.; Raine-Ferring, N.; Polanski, L.; et al. Endometrial Scratching for Infertile Women Undergoing a First Embryo Transfer: A Systematic Review and Meta-Analysis of Published and Unpublished Data from Randomized Controlled Trials. Fertil. Steril. 2019, 111, 734–746.e2. [Google Scholar] [CrossRef]
- Kamath, M.S.; Kirubakaran, R.; Sunkara, S.K. Granulocyte-Colony Stimulating Factor Administration for Subfertile Women Undergoing Assisted Reproduction. Cochrane Database Syst. Rev. 2020, 1, CD013226. [Google Scholar] [CrossRef]
- Hong, K.H.; Forman, E.J.; Werner, M.D.; Upham, K.M.; Gumeny, C.L.; Winslow, A.D.; Kim, T.J.; Scott, R.T. Endometrial Infusion of Human Chorionic Gonadotropin at the Time of Blastocyst Embryo Transfer Does Not Impact Clinical Outcomes: A Randomized, Double-Blind, Placebo-Controlled Trial. Fertil. Steril. 2014, 102, 1591–1595.e2. [Google Scholar] [CrossRef][Green Version]
- Aaleyasin, A.; Aghahosseini, M.; Rashidi, M.; Safdarian, L.; Sarvi, F.; Najmi, Z.; Mobasseri, A.; Amoozgar, B. In Vitro Fertilization Outcome Following Embryo Transfer with or without Preinstillation of Human Chorionic Gonadotropin into the Uterine Cavity: A Randomized Controlled Trial. Gynecol. Obs. Investig. 2015, 79, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Perrier d’Hauterive, S.; Berndt, S.; Tsampalas, M.; Charlet-Renard, C.; Dubois, M.; Bourgain, C.; Hazout, A.; Foidart, J.-M.; Geenen, V. Dialogue between Blastocyst HCG and Endometrial LH/HCG Receptor: Which Role in Implantation? Gynecol. Obs. Investig. 2007, 64, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Paiva, P.; Hannan, N.J.; Hincks, C.; Meehan, K.L.; Pruysers, E.; Dimitriadis, E.; Salamonsen, L.A. Human Chorionic Gonadotrophin Regulates FGF2 and Other Cytokines Produced by Human Endometrial Epithelial Cells, Providing a Mechanism for Enhancing Endometrial Receptivity. Hum. Reprod. 2011, 26, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Licht, P.; Russu, V.; Lehmeyer, S.; Wildt, L. Molecular Aspects of Direct LH/HCG Effects on Human Endometrium—Lessons from Intrauterine Microdialysis in the Human Female in Vivo. Reprod. Biol. 2001, 1, 10–19. [Google Scholar] [PubMed]
- Santibañez, A.; García, J.; Pashkova, O.; Colín, O.; Castellanos, G.; Sánchez, A.P.; De la Jara, J.F. Effect of Intrauterine Injection of Human Chorionic Gonadotropin before Embryo Transfer on Clinical Pregnancy Rates from in Vitro Fertilisation Cycles: A Prospective Study. Reprod. Biol. Endocrinol. 2014, 12, 9. [Google Scholar] [CrossRef]
- Zarei, A.; Parsanezhad, M.E.; Younesi, M.; Alborzi, S.; Zolghadri, J.; Samsami, A.; Amooee, S.; Aramesh, S. Intrauterine Administration of Recombinant Human Chorionic Gonadotropin before Embryo Transfer on Outcome of in Vitro Fertilization/Intracytoplasmic Sperm Injection: A Randomized Clinical Trial. Iran. J. Reprod. Med. 2014, 12, 1–6. [Google Scholar]
- Wirleitner, B.; Schuff, M.; Vanderzwalmen, P.; Stecher, A.; Okhowat, J.; Hradecký, L.; Kohoutek, T.; Králícková, M.; Spitzer, D.; Zech, N.H. Intrauterine Administration of Human Chorionic Gonadotropin Does Not Improve Pregnancy and Life Birth Rates Independently of Blastocyst Quality: A Randomised Prospective Study. Reprod. Biol. Endocrinol. 2015, 13, 70. [Google Scholar] [CrossRef]
- Dehghani Firouzabadi, R.; Janati, S.; Razi, M.H. The Effect of Intrauterine Human Chorionic Gonadotropin Injection before Embryo Transfer on the Implantation and Pregnancy Rate in Infertile Patients: A Randomized Clinical Trial. Int. J. Reprod. Biomed. 2016, 14, 657–664. [Google Scholar] [CrossRef]
- Osman, A.; Pundir, J.; Elsherbini, M.; Dave, S.; El-Toukhy, T.; Khalaf, Y. The Effect of Intrauterine HCG Injection on IVF Outcome: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2016, 33, 350–359. [Google Scholar] [CrossRef]
- Craciunas, L.; Tsampras, N.; Raine-Fenning, N.; Coomarasamy, A. Intrauterine Administration of Human Chorionic Gonadotropin (HCG) for Subfertile Women Undergoing Assisted Reproduction. Cochrane Database Syst. Rev. 2018, 10, CD011537. [Google Scholar] [CrossRef]
- Gao, M.; Jiang, X.; Li, B.; Li, L.; Duan, M.; Zhang, X.; Tian, J.; Qi, K. Intrauterine Injection of Human Chorionic Gonadotropin before Embryo Transfer Can Improve in Vitro Fertilization-Embryo Transfer Outcomes: A Meta-Analysis of Randomized Controlled Trials. Fertil. Steril. 2019, 112, 89–97.e1. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, M.; Madani, T.; Arabipoor, A.; Zolfaghari, Z.; Sadeghi, M.; Ramezanali, F. The Effect of Intrauterine Human Chorionic Gonadotropin Flushing on Live Birth Rate after Vitrified-Warmed Embryo Transfer in Programmed Cycles: A Randomized Clinical Trial. Arch. Gynecol. Obs. 2018, 297, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Laokirkkiat, P.; Thanaboonyawat, I.; Boonsuk, S.; Petyim, S.; Prechapanich, J.; Choavaratana, R. Increased Implantation Rate after Intrauterine Infusion of a Small Volume of Human Chorionic Gonadotropin at the Time of Embryo Transfer: A Randomized, Double-Blind Controlled Study. Arch. Gynecol. Obs. 2019, 299, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Deng, H.; Ye, H. Intrauterine injection of human chorionic gonadotropin improves pregnancy outcome in patients with repeated implantation failure in frozen-thawed embryo transfer. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, K.S.; Makhlouf, A.; Badran, E.; El-Nashar, I.M.; Al-Hussaini, T.K.; Farghaly, T.; Mohamed, H.S.; Mol, B.W.; Abdelmagied, A.M. Intrauterine Injection of HCG before Embryo Transfer: A Parallel, Double-Blind Randomized Trial. Reprod. Biomed. Online 2021, 43, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Asbagh, P.A.; Asbagh, F.A.; Karimi, M.; Quchani, S.H.; Hajialiakbar, V. The Effect of Intrauterine Infusion of Human Gonadotropin (HCG) on the Outcome of Embryo Transfer Cycles in Infertile Women: A Randomized Clinical Trial. Int. J. Pediatr.-Masshad 2021, 9, 13429–13436. [Google Scholar] [CrossRef]
- Hosseinisadat, R.; Saeed, L.; Ashourzadeh, S.; Heidari, S.S.; Habibzadeh, V. Effects of Human Chorionic Gonadotropin Intrauterine Injection on Oocyte Retrieval Day on Assisted Reproductive Techniques Outcomes: An RCT. Int. J. Reprod. Biomed. 2021, 19, 773–780. [Google Scholar] [CrossRef]
- Torky, H.; El-Desouky, E.-S.; El-Baz, A.; Aly, R.; El-Taher, O.; Shata, A.; Hussein, A.; Marie, H.; Deif, O.; Eldemery, A.; et al. Effect of Intra Uterine Granulocyte Colony Stimulating Factor vs. Human Chorionic Gonadotropin at Ovum Pick up Day on Pregnancy Rate in IVF/ICSI Cases With Recurrent Implantation Failure. JBRA Assist. Reprod. 2022, 26, 274–279. [Google Scholar] [CrossRef]
- Tyler, B.; Walford, H.; Tamblyn, J.; Keay, S.D.; Mavrelos, D.; Yasmin, E.; Al Wattar, B.H. Interventions to Optimize Embryo Transfer in Women Undergoing Assisted Conception: A Comprehensive Systematic Review and Meta-Analyses. Hum. Reprod. Update 2022, 28, 480–500. [Google Scholar] [CrossRef]
- Scherer, R.W.; Saldanha, I.J. How Should Systematic Reviewers Handle Conference Abstracts? A View from the Trenches. Syst. Rev. 2019, 8, 264. [Google Scholar] [CrossRef]
- ASRM. Blastocyst Culture and Transfer in Clinically Assisted Reproduction: A Committee Opinion. Fertil. Steril. 2018, 110, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomised Trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil. Steril. 2017, 108, 393–406. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019; ISBN 978-1-119-53661-1. [Google Scholar]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Hosseini, R.S.; Farzadi, L.; Abdollahi, S.; Nouri, M.; Ghasemzadeh, A.; Hamdi, K.; Soleimanpour, H. Effect of Intrauterine Injection of Human Chorionic Gonadotropin Before Frozen-Thawed Embryo Transfer on Implantation and Clinical Pregnancy Rate: A Randomized Controlled Trial. Int. J. Womens Health Reprod. Sci. 2016, 4, 189–193. [Google Scholar] [CrossRef]
- Huang, P.; Wei, L.; Li, X. A Study of Intrauterine Infusion of Human Chorionic Gonadotropin (HCG) before Frozen-Thawed Embryo Transfer after Two or More Implantation Failures. Gynecol. Endocrinol. 2017, 33, 67–69. [Google Scholar] [CrossRef]
- Mansour, R.; Tawab, N.; Kamal, O.; El-Faissal, Y.; Serour, A.; Aboulghar, M.; Serour, G. Intrauterine Injection of Human Chorionic Gonadotropin before Embryo Transfer Significantly Improves the Implantation and Pregnancy Rates in in Vitro Fertilization/Intracytoplasmic Sperm Injection: A Prospective Randomized Study. Fertil. Steril. 2011, 96, 1370–1374.e1. [Google Scholar] [CrossRef]
- Mostajeran, F.; Godazandeh, F.; Ahmadi, S.M.; Movahedi, M.; Jabalamelian, S.A. Effect of Intrauterine Injection of Human Chorionic Gonadotropin before Embryo Transfer on Pregnancy Rate: A Prospective Randomized Study. J. Res. Med. Sci. 2017, 22, 6. [Google Scholar] [CrossRef]
- Navali, N.; Gassemzadeh, A.; Farzadi, L.; Abdollahi, S.; Nouri, M.; Hamdi, K.; Mallah, F.; Jalilvand, F. Intrauterine Administration of HCG Immediately after Oocyte Retrieval and the Outcome of ICSI: A Randomized Controlled Trial. Hum. Reprod. 2016, 31, 2520–2526. [Google Scholar] [CrossRef]
- Clarke, J.F.; van Rumste, M.M.E.; Farquhar, C.M.; Johnson, N.P.; Mol, B.W.J.; Herbison, P. Measuring Outcomes in Fertility Trials: Can We Rely on Clinical Pregnancy Rates? Fertil. Steril. 2010, 94, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- d’Hauterive, S.P.; Close, R.; Gridelet, V.; Mawet, M.; Nisolle, M.; Geenen, V. Human Chorionic Gonadotropin and Early Embryogenesis: Review. Int. J. Mol. Sci. 2022, 23, 1380. [Google Scholar] [CrossRef]
- Tsampalas, M.; Gridelet, V.; Berndt, S.; Foidart, J.-M.; Geenen, V.; Perrier d’Hauterive, S. Human Chorionic Gonadotropin: A Hormone with Immunological and Angiogenic Properties. J. Reprod. Immunol. 2010, 85, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, A.; Brachwitz, N.; Sohr, S.; Engeland, K.; Langwisch, S.; Dolaptchieva, M.; Alexander, T.; Taran, A.; Malfertheiner, S.F.; Costa, S.-D.; et al. Human Chorionic Gonadotropin Attracts Regulatory T Cells into the Fetal-Maternal Interface during Early Human Pregnancy. J. Immunol. 2009, 182, 5488–5497. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Liu, F.; Zhai, J.; Liu, X.; Zhang, Q.; Zhang, B. Alteration of Th17 and Foxp3+ Regulatory T Cells in Patients with Unexplained Recurrent Spontaneous Abortion before and after the Therapy of HCG Combined with Immunoglobulin. Exp. Med. 2017, 14, 1114–1118. [Google Scholar] [CrossRef]
- Srivastava, A.; Sengupta, J.; Kriplani, A.; Roy, K.K.; Ghosh, D. Profiles of Cytokines Secreted by Isolated Human Endometrial Cells under the Influence of Chorionic Gonadotropin during the Window of Embryo Implantation. Reprod. Biol. Endocrinol. 2013, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Han, S.W.; Lei, Z.M.; Rao, C.V. Treatment of Human Endometrial Stromal Cells with Chorionic Gonadotropin Promotes Their Morphological and Functional Differentiation into Decidua. Mol. Cell. Endocrinol. 1999, 147, 7–16. [Google Scholar] [CrossRef]
- Sales, K.J.; Grant, V.; Catalano, R.D.; Jabbour, H.N. Chorionic Gonadotrophin Regulates CXCR4 Expression in Human Endometrium via E-Series Prostanoid Receptor 2 Signalling to PI3K-ERK1/2: Implications for Fetal-Maternal Crosstalk for Embryo Implantation. Mol. Hum. Reprod. 2011, 17, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Dokras, A.; Sargent, I.L.; Barlow, D.H. Human Blastocyst Grading: An Indicator of Developmental Potential? Hum. Reprod. 1993, 8, 2119–2127. [Google Scholar] [CrossRef]
- Riboldi, M.; Barros, B.; Piccolomini, M.; Alegretti, J.R.; Motta, E.L.A.; Serafini, P.C. Does the Intrauterine Administration of RhCG before Vitrified Blastocysts Transfer Improves the Potential of Pregnancies When Using Blastocysts of Inferior Morphological Grading? Fertil. Steril. 2013, 100, S289. [Google Scholar] [CrossRef]

| Reference | Country | Population | Intervention | Comparators | Embryo Stage |
|---|---|---|---|---|---|
| Aaleyasin et al., 2015 [11] | Iran | N = 483 <40 years old | n = 240 500 IU of hCG, 5–7 min before ET | n = 243 50 μL tissue culture media, 5–7 min prior to ET | Cleavage stage |
| Abdallah et al., 2022 [25] | Egypt | N = 181 18–43 years old, at least one good-quality embryo to transfer | n = 90 hCG (500 IU in 0.1 mL of tissue culture media) 4 min before ET | n = 91 Culture media (0.1 mL) | Clevage stage; Blastocyst stage |
| Asbagh et al., 2021 [26] | Iran | N = 198 <40 years old, ≥1 implantation failures | n = 99 500 IU of hCG, 15 min before ET | n = 99 No intervention | Clevage stage; Blastocyst stage |
| Dehghani Firouzabadi et al., 2016 [18] | Iran | N = 159 20–40 years old | n = 106 500 IU hCG, approx 7 min before ET 1000 IU hCG, approx 7 min before ET | n = 53 No intervention | Cleavage stage |
| Hafezi et al., 2018 [22] | Iran | N = 180 <40 years old, 1st FET and ≥1 implantation failures (fresh IVF/ICSI cycle) | n = 60 500 IU of hCG, 7–10 min before ET | n = 60 40 μL of culture medium, 7–10 min before ET n = 60 No intervention | Cleavage stage |
| Hong et al., 2014 [10] | USA | N = 300 <43 years old | n = 148 500 IU of hCG, less than 3 min before ET | n = 152 ET media, before ET | Blastocyst stage |
| Hosseinisadat et al., 2021 [27] | Iran | N = 126 <40 years old | n = 62 1000 IU of hCG | n = 64 No intervention | Cleavage stage |
| Hosseini et al., 2016 [37] | Iran | N = 100 <40 years old | n = 50 500 IU of hCG, 7 min before ET | n = 50 No intervention | Cleavage stage |
| Huang et al., 2017 [38] | China | N = 165 ≤38 years old, ≥2 implantation failures | n = 65 1000 IU of hCG, 3 days before ET | n = 50 Physiological saline before ET n = 50 No intervention | Cleavage stage |
| Laokirkkiat et al., 2017 [23] | Thailand | N = 200 18–43 years old | n = 100 500 IU of hCG, 4 min before ET | n = 100 10 μL of culture medium, 4 min before ET | Cleavage stage; Blastocyst |
| Mansour et al., 2011 [39] | Egypt | N = 445 <40 years old | n = 243 100 IU of hCG vs. 200 IU of hCG vs. 500 IU of hCG, 7 min before ET | n = 202 No intervention | Cleavage stage |
| Mostajeran et al., 2017 [40] | Iran | N = 100 20–40 years old | n = 50 700 IU of hCG, 5–10 min before ET | n = 50 No intervention | Blastocyst |
| Navali et al., 2016 [41] | Iran | N = 158 ≤41 years old | n = 80 500 IU hCG in up to 0.5 mL normal saline, immediately after oocyte retrieval | n = 78 0.5 mL normal saline, immediately after oocyte retrieval | Cleavage stage |
| Santibañez et al., 2014 [15] | Mexico | N = 210 <40 years old | n = 101 500 IU of hCG, before the ET | n = 109 Same culture media without hCG | Cleavage stage |
| Torky et al., 2021 [28] | Egypt | N = 100 20–39 years old, ≥3 implantation failures of good quality embryo | n = 50 5000UI c, at the time of ovum pick-up | n = 50 Saline solution (placebo), at the time of ovum pick-up | Blastocyst |
| Wang et al., 2019 [24] | China | N = 140 Implantation failure definition: (1) embryo transfer + frozen embryo transfer ≥3 transfer cycles; (2) cumulative number of transferred embryos ≥4; (3) each time at least 1 high-quality embryo was transferred | n = 70 500 UI hCG + G2 fluid, 3 min before ET | n = 70 G2 fluid | Cleavage stage |
| Wirleitner et al., 2015 [17] | Austria | N = 1186 ≤43 years old, ≤2 implantation failure | n = 89 500 IU hCG: 2 days before ET n = 510 500 IU hCG3 min before ET | n = 93 40 μL culture medium: 2 days before ET n = 494 40 μL culture medium 3 min before ET | Blastocyst |
| Zarei et al., 2014 [16] | Iran | N = 210 18–40 years old | n = 105 250 μg (equivalent to 6500 UI) of recombinant hCG, 12 min before ET | n = 105 Normal saline (0.5 mL), 12 min before ET | Cleavage stage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conforti, A.; Longobardi, S.; Carbone, L.; Iorio, G.G.; Cariati, F.; Campitiello, M.R.; Strina, I.; Palese, M.; D’Hooghe, T.; Alviggi, C. Does Intrauterine Injection of hCG Improve IVF Outcome? A Systematic Review and a Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 12193. https://doi.org/10.3390/ijms232012193
Conforti A, Longobardi S, Carbone L, Iorio GG, Cariati F, Campitiello MR, Strina I, Palese M, D’Hooghe T, Alviggi C. Does Intrauterine Injection of hCG Improve IVF Outcome? A Systematic Review and a Meta-Analysis. International Journal of Molecular Sciences. 2022; 23(20):12193. https://doi.org/10.3390/ijms232012193
Chicago/Turabian StyleConforti, Alessandro, Salvatore Longobardi, Luigi Carbone, Giuseppe Gabriele Iorio, Federica Cariati, Maria Rosaria Campitiello, Ida Strina, Michela Palese, Thomas D’Hooghe, and Carlo Alviggi. 2022. "Does Intrauterine Injection of hCG Improve IVF Outcome? A Systematic Review and a Meta-Analysis" International Journal of Molecular Sciences 23, no. 20: 12193. https://doi.org/10.3390/ijms232012193
APA StyleConforti, A., Longobardi, S., Carbone, L., Iorio, G. G., Cariati, F., Campitiello, M. R., Strina, I., Palese, M., D’Hooghe, T., & Alviggi, C. (2022). Does Intrauterine Injection of hCG Improve IVF Outcome? A Systematic Review and a Meta-Analysis. International Journal of Molecular Sciences, 23(20), 12193. https://doi.org/10.3390/ijms232012193







